Identification of Dominant Microbes and Their Successions During Solid-State Fermentation of Luzhou-Flavour Liquor Based on High-Throughput Sequencing Following Culture
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Luzhou-Flavour Liquor Manufacturing Process and Sample Collection
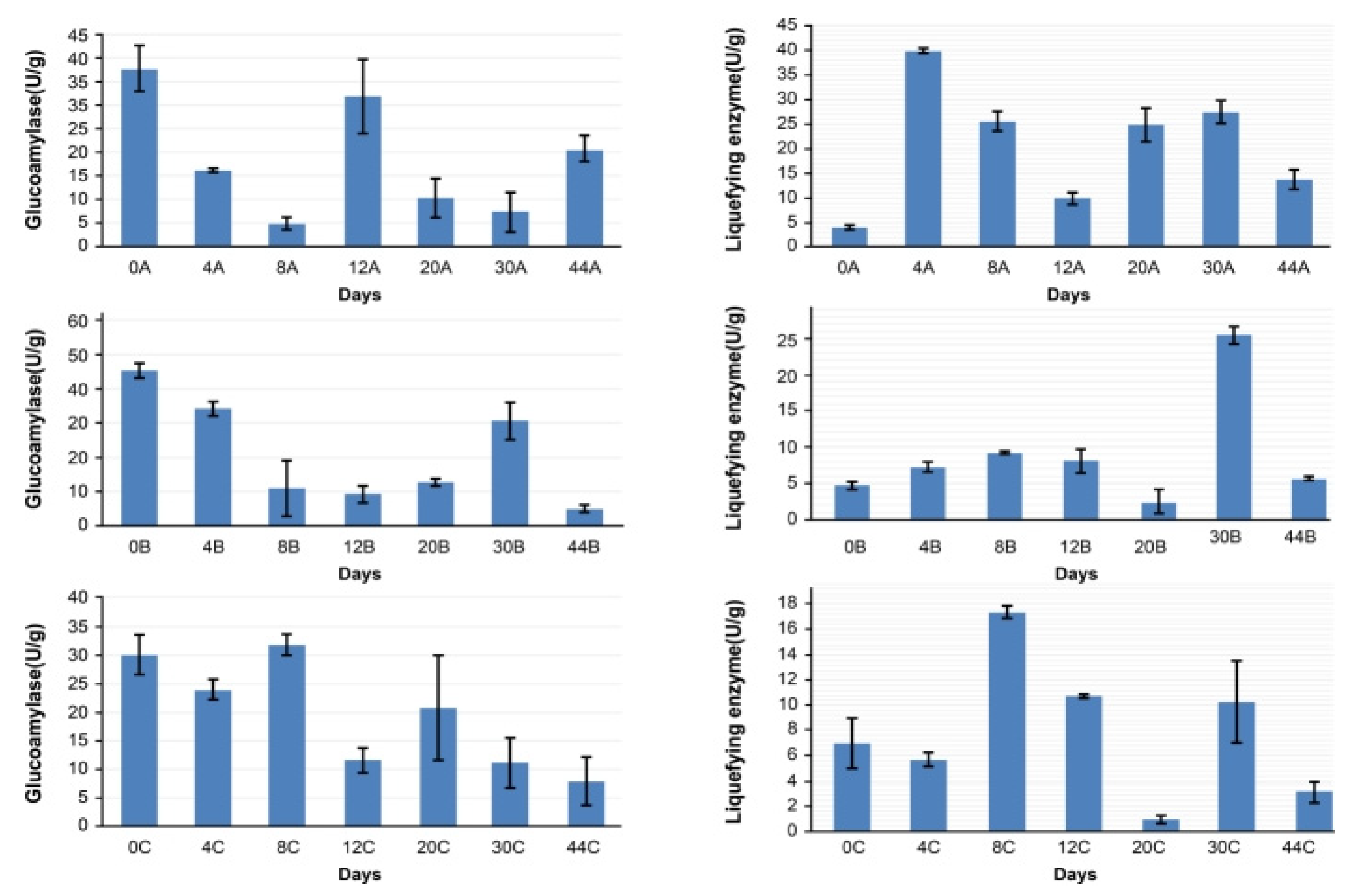
2.3. Analysis of Glucoamylase and Liquefying Enzyme Activity and Volatile Compound Content in Grains During Fermentation
2.4. High-Throughput Sequencing (HTS) Analysis
2.4.1. Extraction of Total DNA from Samples for HTS
2.4.2. Bioinformatics Analysis
Data Quality Control
Read Merging and Optimisation
3. Results
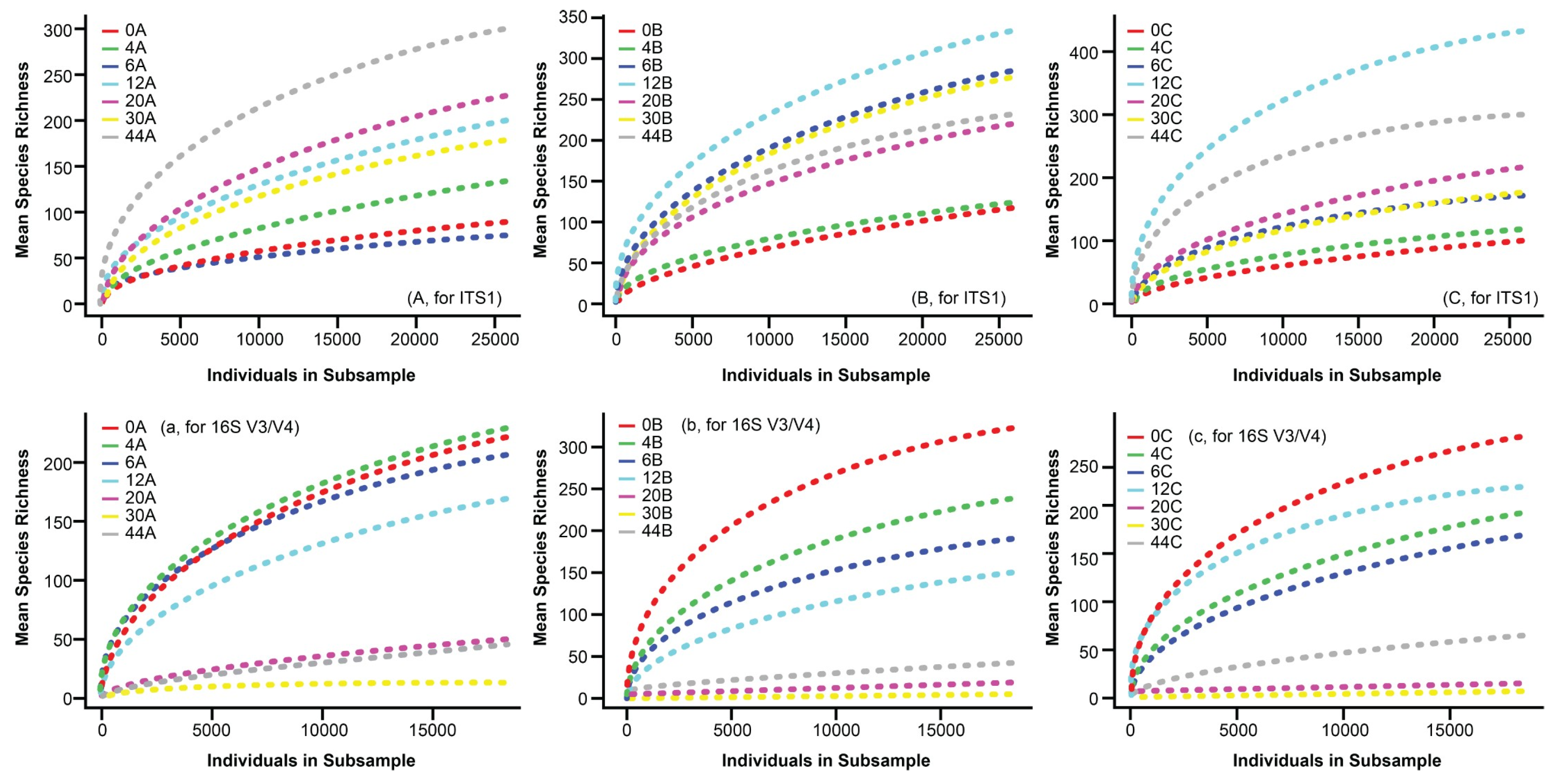
3.1. HTS Rarefaction Curves
3.2. HTS Shannon–Wiener Curve
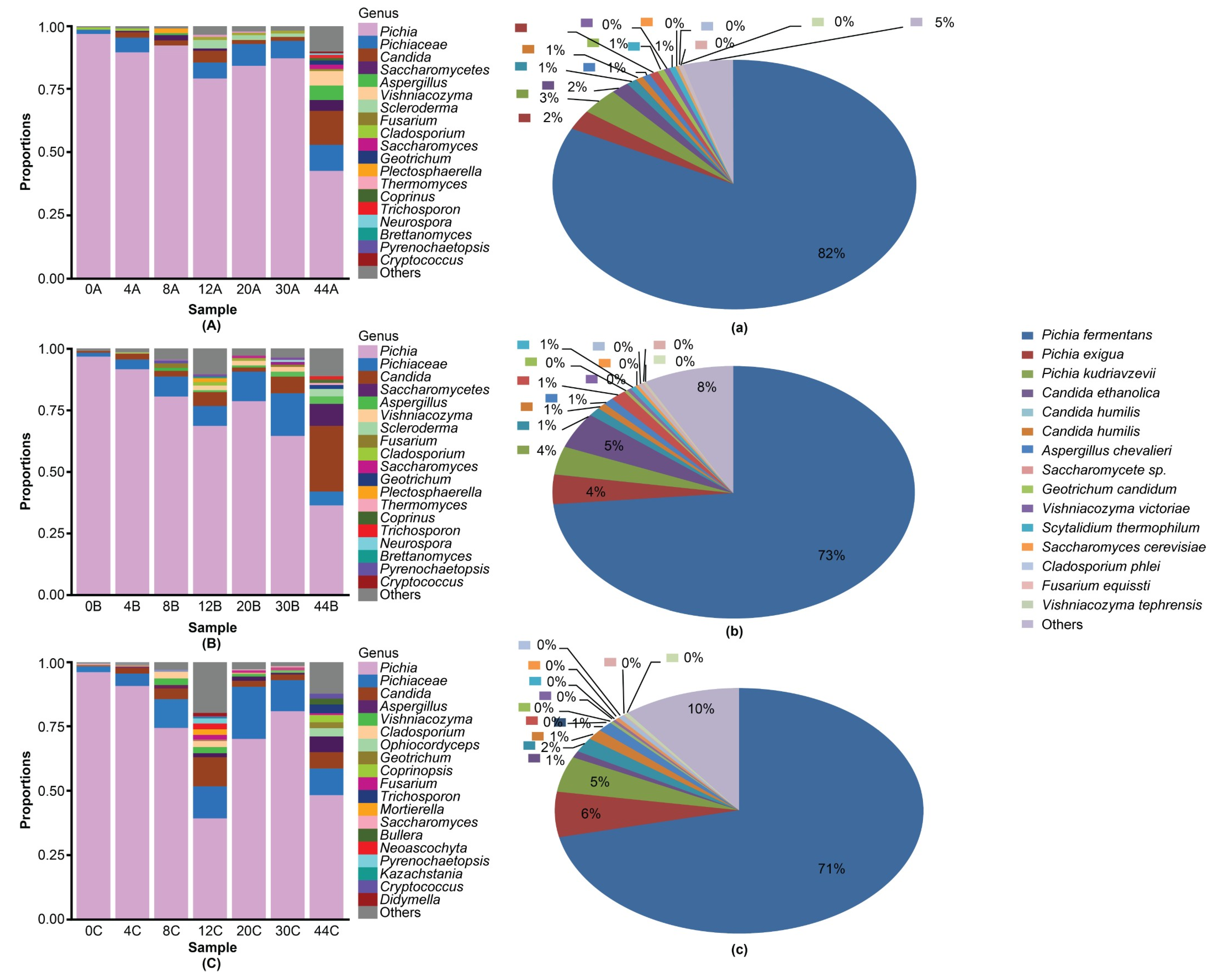
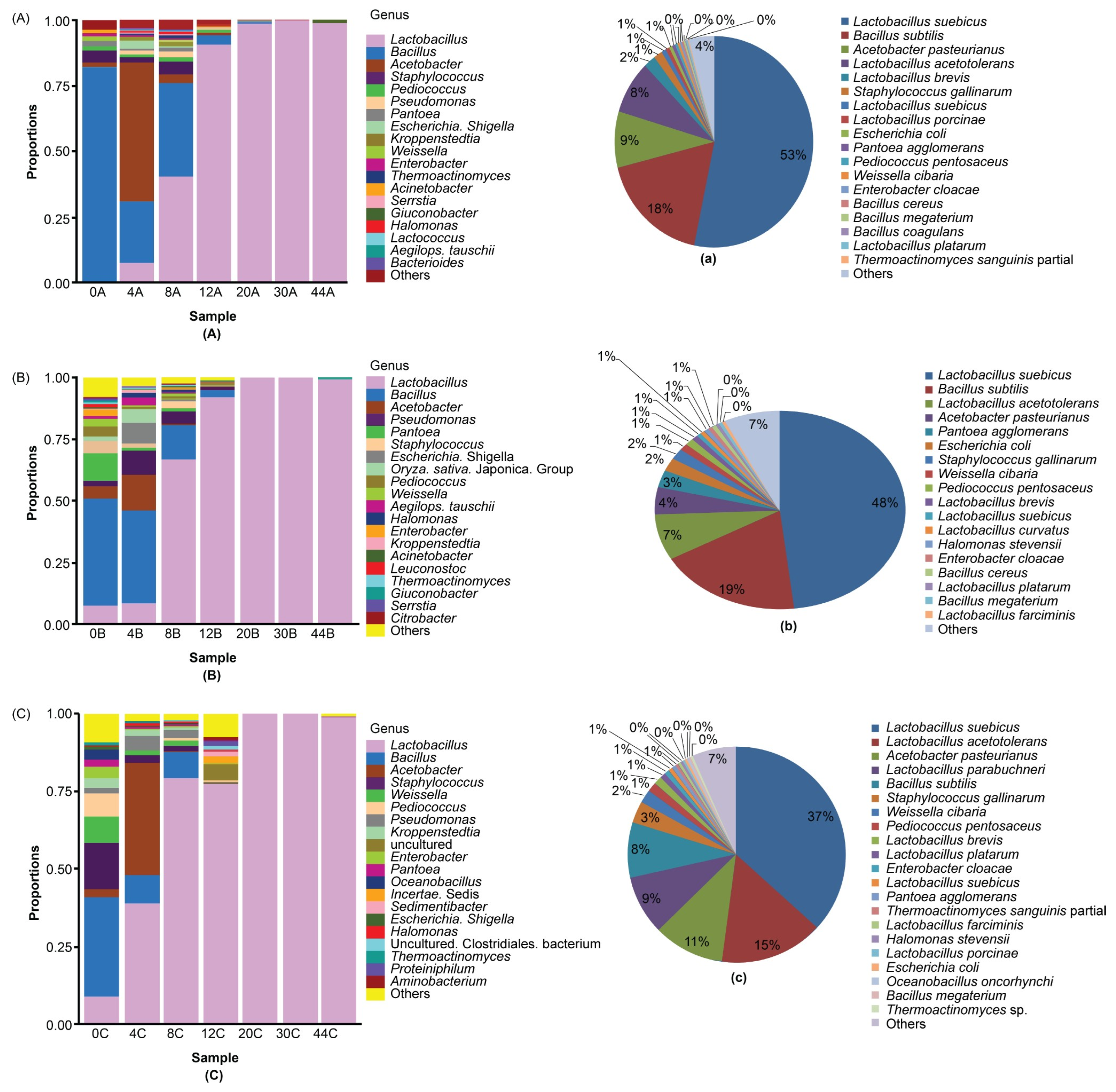
3.3. Dominant Microbes in the Fermented Grain Layers Based on HTS
3.4. Tree-Structure Analysis of Multiple Sample Similarity Based on the UniFrac of HTS
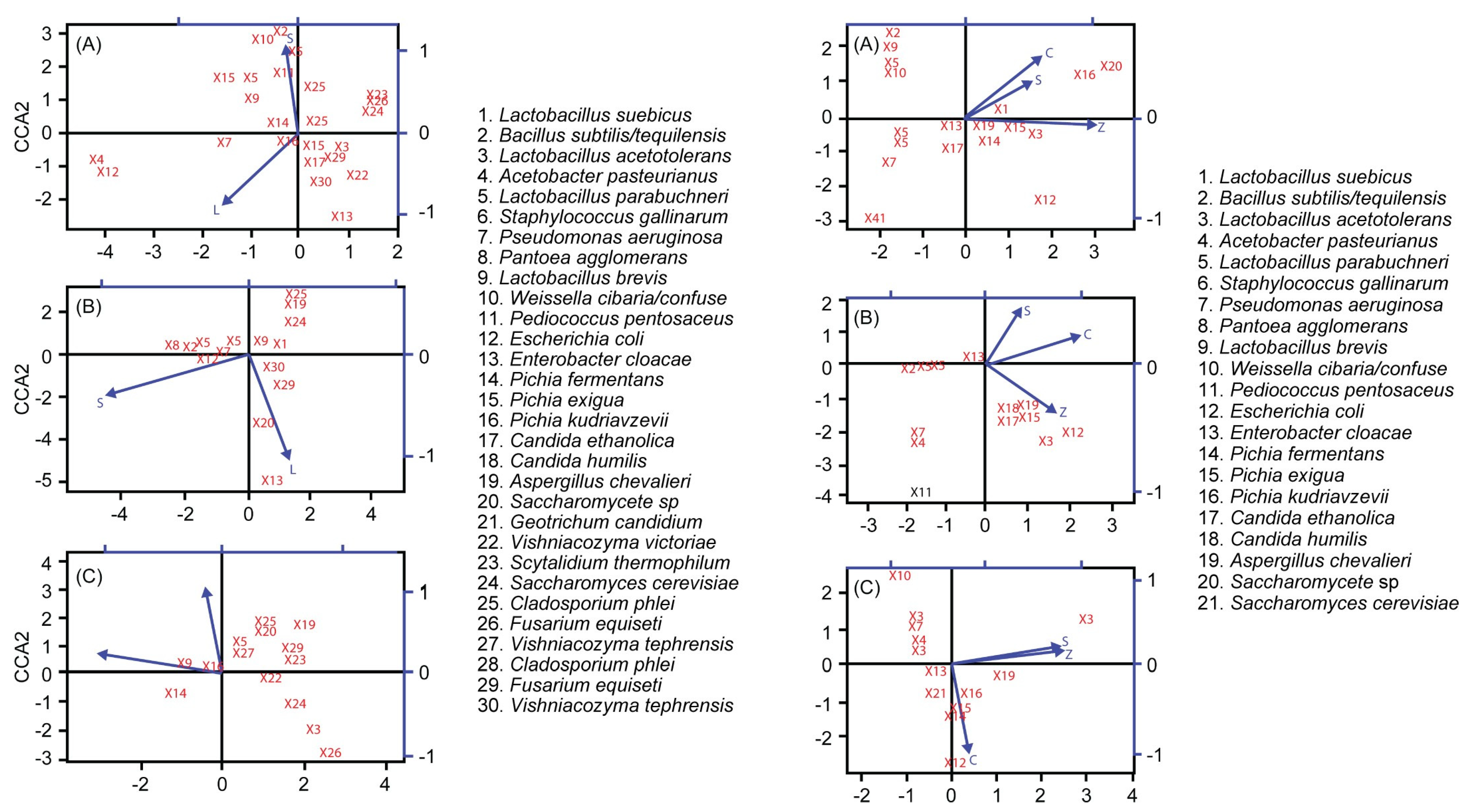
3.5. Principal Component Analysis (PCA)
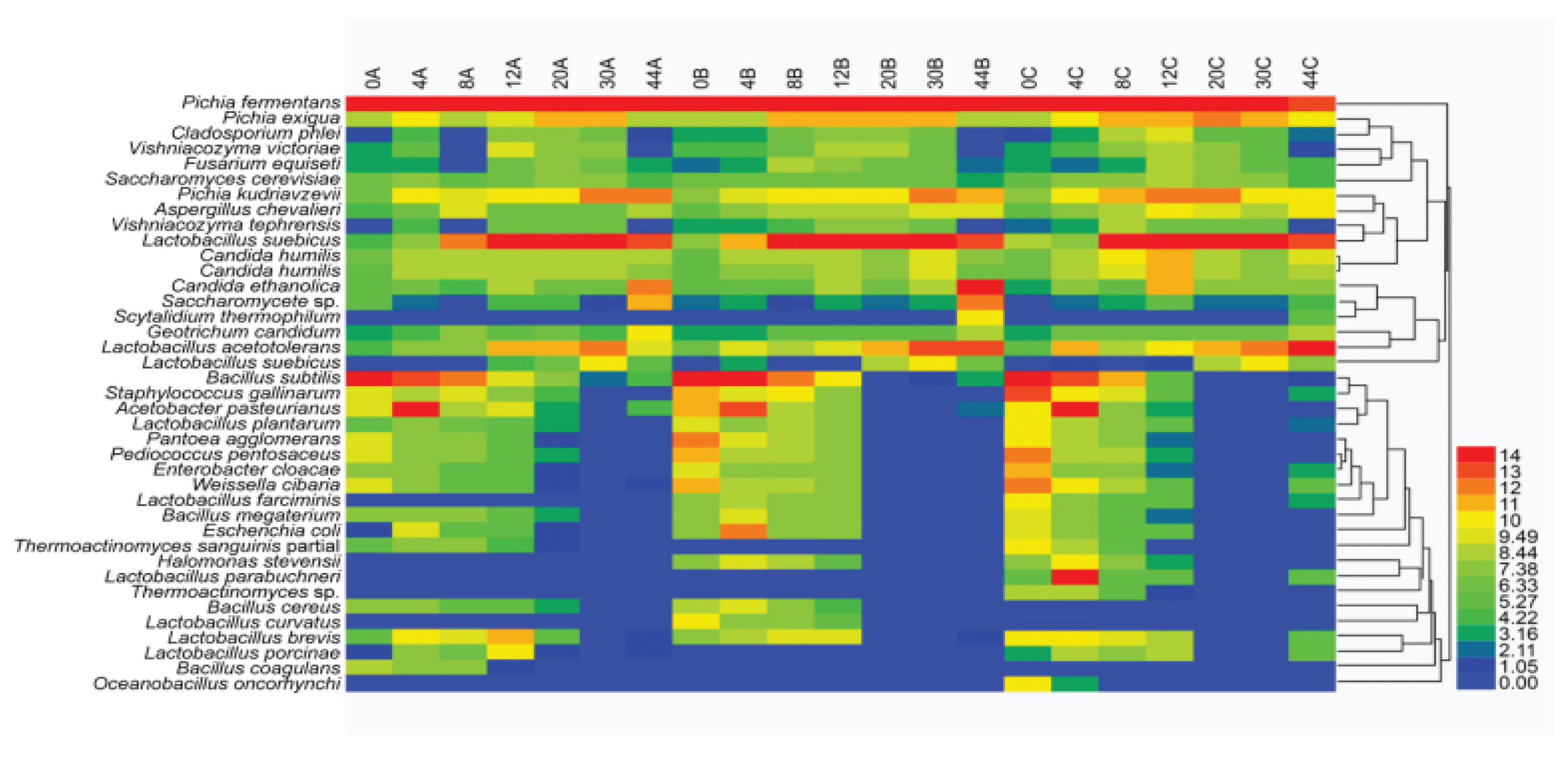
3.6. Population Succession of Dominant Microorganisms During Liquor Fermentation Based on HTS
3.7. Correlations Between Dominant Strains, Enzyme Activity, and Volatile Component Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Q.; Lin, B.; Tang, J.; An, L.; Jiang, W.; Li, R.; Zhang, G.; Yang, Q.; Yang, S.; Chen, S. Studying on Genetic Diversity and Metabolic Differences of Saccharomyces cerevisiae in Baijiu. Eur. Food Res. Technol. 2024, 250, 1619–1640. [Google Scholar] [CrossRef]
- Li, W.; Han, M.; Zhang, H.; Zhang, Q.; Lang, Y.; Hu, S.; Li, X.; Sun, B. Exploring the Differences in Sauce-Flavour Daqu from Different Regions and Its Contribution to Baijiu Brewing Based on Microbial Communities and Flavours. Int. J. Food Sci. Technol. 2024, 59, 7357–7371. [Google Scholar] [CrossRef]
- Xia, Y.; Luo, H.; Wu, Z.; Zhang, W. Microbial Diversity in Jiuqu and Its Fermentation Features: Saccharification, Alcohol Fermentation and Flavors Generation. Appl. Microbiol. Biotechnol. 2023, 107, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Zou, W.; Shen, C.H.; Yang, J.G. Basic Flavor Types and Component Characteristics of Chinese Traditional Liquors: A Review. J. Food Sci. 2020, 85, 4096–4107. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sun, B.; Fan, G.; Teng, C.; Xiong, K.; Zhu, Y.; Li, J.; Li, X. The Brewing Process and Microbial Diversity of Strong Flavour Chinese Spirits: A Review. J. Inst. Brew. 2017, 123, 5–12. [Google Scholar] [CrossRef]
- Gao, J.; Liu, G.; Li, A.; Liang, C.; Ren, C.; Xu, Y. Domination of Pit Mud Microbes in the Formation of Diverse Flavour Compounds During Chinese Strong Aroma-Type Baijiu Fermentation. LWT 2021, 137, 110442. [Google Scholar] [CrossRef]
- Xiang, W.; Li, K.; Liu, S.; Xing, Y.; Li, M.; Che, Z. Microbial Succession in the Traditional Chinese Luzhou-Flavor Liquor Fermentation Process as Evaluated by SSU rRNA Profiles. World J. Microbiol. Biotechnol. 2013, 29, 559–567. [Google Scholar] [CrossRef]
- Li, K.; Zhang, Q.; Zhong, X.T.; Jia, B.H.; Yuan, C.H.; Liu, S.; Che, Z.M.; Xiang, W.L. Microbial Diversity and Succession in the Chinese Luzhou-Flavor Liquor Fermenting Cover Lees as Evaluated by SSU rRNA Profiles. Indian J. Microbiol. 2013, 53, 425–431. [Google Scholar] [CrossRef]
- Liang, H.; Luo, Q.; Zhang, A.; Wu, Z.; Zhang, W. Comparison of Bacterial Community in Matured and Degenerated Pit Mud from Chinese Luzhou-Flavour Liquor Distillery in Different Regions. J. Inst. Brew. 2016, 122, 48–54. [Google Scholar] [CrossRef]
- Fu, J.; Chen, L.; Yang, S.; Li, Y.; Jin, L.; He, X.; He, L.; Ao, X.; Liu, S.; Liu, A.; et al. Metagenome and Analysis of Metabolic Potential of the Microbial Community in Pit Mud Used for Chinese Strong-Flavor Liquor Production. Food Res. Int. 2021, 143, 110294. [Google Scholar] [CrossRef]
- Liu, K.Y.; Wang, Q.; Chen, Z.; Mao, D.M.; Liang, Z.W. Correlation Analysis Between Amino Acids and Bacterial Communities of Wuliangye-Flavour Liquor Fermentation in Aged Fermentation Pit. Int. Food Res. J. 2022, 29, 892–899. [Google Scholar] [CrossRef]
- Wu, X.; Pan, D.; Xia, Q.; Sun, Y.; Geng, F.; Cao, J.; Zhou, C. The Combination of High-Throughput Sequencing and LC-MS/MS Reveals the Mechanism of Staphylococcus Inoculation on Bacterial Community Succession and Taste Development During the Processing of Dry-Cured Bacon. J. Sci. Food Agric. 2023, 103, 7187–7198. [Google Scholar] [CrossRef] [PubMed]
- Metin, B.; Pehlivanoğlu, H.; Yildirim Servi, E.Y.; Arıcı, M. Bacterial Dynamics of Hardaliye, a Fermented Grape Beverage, Determined by High-Throughput Sequencing. J. Agric. Sci.-Tarim Bilim. Derg. 2023, 29, 756–764. [Google Scholar] [CrossRef]
- Dou, X.; Han, P.J.; Liu, L.; Zhang, Y.H.; He, J.M.; Zhou, X.X.; Wu, Y.Y.; Bai, F.Y.; Yang, J.G. Study on Isolation and Identification and Population Succession Law of Bacterial in Fermented Grains During the Brewing of Luzhou-Flavour Liquor. Sci. Technol. Food Ind. 2017, 30, 169–174. [Google Scholar] [CrossRef]
- Yang, J.G.; Su, C.; Dou, X.; Guo, J.; Zhang, Q.; Zhang, S.; Ao, Z.; Shen, C. Analysis of Yeast Succession During the Fermentation of Luzhou-flavor Liquor and Its Effect on the Formation of Selected Flavor Components. Food Sci. 2018, 39, 166–172, (In Chinese with English Abstract). [Google Scholar] [CrossRef]
- Yang, J.G.; Dou, X.; Ma, Y.Y. Diversity and Dynamic Succession of Microorganisms During Daqu Preparation for Luzhou-Flavour Liquor Using Second-Generation Sequencing Technology. J. Inst. Brew. 2018, 124, 498–507. [Google Scholar] [CrossRef]
- Yang, J.G.; Dou, X.; Han, P.J.; Bai, F.Y.; Zhou, J.; Zhang, S.Y.; Qin, H.; Ma, Y.Y. Microbial Diversity in Daqu During Production of Luzhou Flavored Liquor. J. Am. Soc. Brew. Chem. 2017, 75, 136–144. [Google Scholar] [CrossRef]
- Sun, W.; Xiao, H.; Peng, Q.; Zhang, Q.; Li, X.; Han, Y. Analysis of Bacterial Diversity of Chinese Luzhou-Flavor Liquor Brewed in Different Seasons by Illumina MiSeq Sequencing. Ann. Microbiol. 2016, 66, 1293–1301. [Google Scholar] [CrossRef]
- Yan, S.B.; Wang, S.C.; Wei, G.G.; Zhang, K.G. Investigation of the Main Parameters During the Fermentation of Chinese Luzhou-Flavour Liquor. J. Inst. Brew. 2015, 121, 145–154. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R. Rpackage, Version 1.17-3. Vegan: Community Ecology Package; R Foundation: Vienna, Austria, 2010.
- Narayan, A.; Jain, K.; Shah, A.R.; Madamwar, D. An Efficient and Cost-Effective Method for DNA Extraction from Athalassohaline Soil Using a Newly Formulated Cell Extraction Buffer. 3 Biotech 2016, 6, 62. [Google Scholar] [CrossRef]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J.; et al. Introducing Mothur: Open-Source, Platform-Independent, Community-Supported Software for Describing and Comparing Microbial Communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C.; Haas, B.J.; Clemente, J.C.; Quince, C.; Knight, R. Uchime Improves Sensitivity and Speed of Chimera Detection. Bioinformatics 2011, 27, 2194–2200. [Google Scholar] [CrossRef] [PubMed]
- Amato, K.R.; Yeoman, C.J.; Kent, A.; Righini, N.; Carbonero, F.; Estrada, A.; Gaskins, H.R.; Stumpf, R.M.; Yildirim, S.; Torralba, M.; et al. Habitat Degradation Impacts Black Howler Monkey (Alouatta pigra) Gastrointestinal Microbiomes. ISME J. 2013, 7, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sheng, H.F.; He, Y.; Wu, J.Y.; Jiang, Y.X.; Tam, N.F.Y.; Zhou, H.W. Comparison of the Levels of Bacterial Diversity in Freshwater, Intertidal Wetland, and Marine Sediments by Using Millions of Illumina Tags. Appl. Environ. Microbiol. 2012, 78, 8264–8271. [Google Scholar] [CrossRef]
- Noval Rivas, M.; Burton, O.T.; Wise, P.; Zhang, Y.Q.; Hobson, S.A.; Garcia Lloret, M.; Chehoud, C.; Kuczynski, J.; DeSantis, T.; Warrington, J.; et al. A Microbiota Signature Associated with Experimental Food Allergy Promotes Allergic Sensitization and Anaphylaxis. J. Allergy Clin. Immunol. 2013, 131, 201–212. [Google Scholar] [CrossRef]
- Wang, H.Y.; Zhang, X.J.; Zhao, L.P.; Xu, Y. Analysis and Comparison of the Bacterial Community in Fermented Grains During the Fermentation for Two Different Styles of Chinese Liquor. J. Ind. Microbiol. Biotechnol. 2008, 35, 603–609. [Google Scholar] [CrossRef]
- Zhang, W.; Qiao, Z.; Shigematsu, T.; Tang, Y.; Hu, C.; Morimura, S.; Kida, K. Analysis of the Bacterial Community in Zaopei During Production of Chinese Luzhou-Flavor Liquor. J. Inst. Brew. 2005, 111, 215–222. [Google Scholar] [CrossRef]
- Chen, S.; Huang, J.; Qin, H.; He, G.; Zhou, R.; Yang, Y.; Qiu, C.; Zhang, S. Evolving the Core Microbial Community in Pit Mud Based on Bioturbation of Fortified Daqu. Can. J. Microbiol. 2021, 67, 396–405. [Google Scholar] [CrossRef]
- Hong, M.; Diao, Q.Y.; Jiang, C.G.; Yan, G.L.; Tu, Y.; Zhang, N.F. Review for Effect of Lactobacillus buchneri on the Silage in the Journal Listing of OriProbe. Acta Pratacult. Sin. 2011, 5, 266–271. [Google Scholar]
- Ding, X.F.; Wu, C.D.; Zhang, L.Q.; Zheng, J.; Zhou, R.Q. Characterization of Eubacterial and Archaeal Community Diversity in the Pit Mud of Chinese Luzhou-Flavor Liquor by Nested PCR–DGGE. World J. Microbiol. Biotechnol. 2014, 30, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhao, D.; Tian, S.; You, L.; Wang, S.; Feng, R.; Feng, X.; Zhang, Y.; Cui, X. Phylogenetic Diversity of Cultivable Bacteria During the Brewing Process of the Luzhou-Flavor Liquor in Yibin, Sichuan Province, China. Acta Microbiol. Sin. 2011, 51, 1351–1357. [Google Scholar] [CrossRef]
- Yumoto, I.; Hirota, K.; Nodasaka, Y.; Nakajima, K. Oceanobacillus oncorhynchi sp. nov., a Halotolerant Obligate Alkaliphile Isolated from the Skin of a Rainbow Trout (Oncorhynchus mykiss), and Emended Description of the Genus Oceanobacillus. Int. J. Syst. Evol. Microbiol. 2005, 55, 1521–1524. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Huang, J.; Zhou, R.; Wu, C.; Jin, Y. Effect of Fortified Daqu on the Microbial Community and Flavor in Chinese Strong-Flavor Liquor Brewing Process. Front. Microbiol. 2019, 10, 56. [Google Scholar] [CrossRef]
- Xiao, C.; Lu, Z.; Zhang, X.; Wang, S.; Li, D.; Shen, C.; Shi, J.; Xu, Z. Bacterial Community Succession in Fermented Grains of Luzhou-Flavor Baijiu. Acta Microbiol. Sin. 2019, 59, 195–204. [Google Scholar] [CrossRef]
- Li, P.; Lin, W.; Liu, X.; Wang, X.; Gan, X.; Luo, L.; Lin, W.T. Effect of Bioaugmented Inoculation on Microbiota Dynamics During Solid-State Fermentation of Daqu Starter Using Autochthonous of Bacillus, Pediococcus, Wickerhamomyces and Saccharomycopsis. Food Microbiol. 2017, 61, 83–92. [Google Scholar] [CrossRef]
- Liu, H.; Sun, B. Effect of Fermentation Processing on the Flavor of Baijiu. J. Agric. Food Chem. 2018, 66, 5425–5432. [Google Scholar] [CrossRef]
- Xiao, C.; Lu, Z.; Zhang, X.; Wang, S.; Ao, L.; Sen, C.; Shi, J.; Xu, Z. Succession of the Fungal Community on Fermented Grains of Luzhou-Flavor Baijiu Through Fermentation. Chin. J. Appl. Environ. Biol. 2018, 24, 1081–1086. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Xie, Z.; Dou, X.; Zhang, Y.; Zhou, X.; Pu, S. Identification of Dominant Microbes and Their Successions During Solid-State Fermentation of Luzhou-Flavour Liquor Based on High-Throughput Sequencing Following Culture. Fermentation 2025, 11, 501. https://doi.org/10.3390/fermentation11090501
Yang J, Xie Z, Dou X, Zhang Y, Zhou X, Pu S. Identification of Dominant Microbes and Their Successions During Solid-State Fermentation of Luzhou-Flavour Liquor Based on High-Throughput Sequencing Following Culture. Fermentation. 2025; 11(9):501. https://doi.org/10.3390/fermentation11090501
Chicago/Turabian StyleYang, Jiangang, Zaibin Xie, Xiao Dou, Yu Zhang, Xiaohui Zhou, and Shunchang Pu. 2025. "Identification of Dominant Microbes and Their Successions During Solid-State Fermentation of Luzhou-Flavour Liquor Based on High-Throughput Sequencing Following Culture" Fermentation 11, no. 9: 501. https://doi.org/10.3390/fermentation11090501
APA StyleYang, J., Xie, Z., Dou, X., Zhang, Y., Zhou, X., & Pu, S. (2025). Identification of Dominant Microbes and Their Successions During Solid-State Fermentation of Luzhou-Flavour Liquor Based on High-Throughput Sequencing Following Culture. Fermentation, 11(9), 501. https://doi.org/10.3390/fermentation11090501





