1. Introduction
Stinging nettle (
Urtica dioica) has been utilized as a wild herb for thousands of years.
Stinging nettle (Sn) is a perennial herbaceous plant belonging to the
Urticaceae family that has prickly leaves [
1]. The potential for Sn medicinal use has been recognized after the fundamental determination of its chemical structure and pharmacological features [
2]. Additionally, Sn has been recognized as a food or part of a food that has therapeutic properties and can prevent and treat some diseases [
3]. For example, Sn contains biochemically active substances such as phenols, flavonoids, tannins (high-molecular-weight polyphenols), volatile compounds, fatty acids, polysaccharides, isolectins, sterols, terpenes, proteins, vitamins, and minerals, which can help in reducing the free radicals and improving human health [
4]. Many studies have attempted to enrich various foods with Sn leaves or powder as a functional ingredient to enhance the nutritional value of foods, such as minerals, dietary fiber, vitamins, and other bioactive compounds [
5]. The health benefits of
Stinging nettle were mentioned by Kanani [
6], who indicated that nettle protected the liver from hepatotoxicity by lowering lipid peroxidation and increasing the antioxidant defense system activity in rats given carbon tetrachloride (CCl
4). The phenolic compounds contained in
Stinging nettle are the main reason for this antioxidant property.
Kefir is made by fermenting milk using a culture containing a mixture of “the kefir grains”. This mixed culture contains lactic acid bacteria (lactobacilli, lactococci, leuconostoc), acetic acid bacteria, and many yeast genera that stick to a polysaccharide matrix [
7]. The high nutritional value of kefir comes from its rich chemical composition, including more easily digestible proteins, prebiotic oligosaccharides, minerals, vitamins, and other biologically active metabolites such as organic acids and bacteriocins that can produce an effective antimicrobial function to benefit human health [
8,
9]. Kefir is rich in vitamins B and C, as well as vitamins A and K and carotenoids [
10], with thiamine, pyridoxine, and folic acid being particularly abundant [
11]. Kefir contains partially digestible proteins that help the body’s digestion and metabolism [
12] and high levels of threonine, serine, alanine, lysine, and ammonia compared to milk, as well as other essential amino acids [
10]. As for minerals, kefir is a good source of calcium and magnesium, and it is rich in phosphorus, which helps the body utilize carbohydrates, fats, and proteins [
13]. Trace elements such as copper, zinc, and iron are also found in kefir [
14].
There have been many studies on combining kefir with plant-based ingredients. For example, mint was added to ice cream fermented with kefir culture to investigate its effect on the properties of the ice cream [
15]. Another study by Montanuci et al. [
16] showed that adding inulin to kefir prepared using fermented skimmed milk increased the pH and syneresis and decreased its titratable acidity during storage. Those studies suggested that the addition of herbal ingredients has the possibility of altering the physicochemical properties, microbial activity, and antioxidant activity of kefir, with the potential to enhance its health benefits. Consequently, this study examined the effects of
Stinging nettle powder as a functional ingredient on the physicochemical, microbial, and nutritional value of kefir. This study provided the scientific answer to the question: does enrichment of kefir with Sn powder improve kefir’s nutritional value?
2. Materials and Methods
2.1. Materials
The skimmed milk was purchased from the local supermarket in Melbourne, Australia (ALDI Stores, Melbourne, VIC, Australia). The stinging powder was purchased from Austral Herbs (Kentucky, NSW, Australia). The KEFIR 12 starter culture (eXact®, a mixed culture of bacteria and yeasts) was provided by Chr. Hansen (Bayswater, VIC, Australia). The media trypticase soy agar (TSA), de Man Rogosa Sharpe (MRS) agar and M17 agar, and bacteriological peptone were purchased from Thermo Fisher Scientific (Thermo Fisher Scientific, 168 Third Avenue, Waltham, MA, 02451, United States. Lines 130–131). Sodium hydroxide (NaOH), Folin–Ciocalteu phenol reagent (FCR), DPPH (2,2-diphenyl-1-picrylhydrazyl), gallic acid, Trolox standard, L-Cysteine HCl, porcine pepsin, pancreatin, mucin, pectin, and bile salts were purchased from Sigma-Aldrich (Castle Hill, NSW, Australia). Dicalcium phosphate (KCl), potassium dihydrogen phosphate (KH2PO4), sodium bicarbonate (NaHCO3), sodium chloride (NaCl), magnesium chloride hexahydrate (MgCl2(H2O)6), and ammonium carbonate ((NH4)2CO3) were purchased from Chem-Supply Pty Ltd. (Huntingdale, VIC, Australia). Hydrochloric acid (HCl), ethanol, methanol, tryptone, soluble starch, yeast extract, pectin, casein, magnesium sulfate heptahydrate (MgSO4(H2O)7), guar gum, calcium chloride (CaCl2), Tween 80, and all disposable materials were purchased through the University of Melbourne specialist store (Bio21 Institute, Parkville, VIC, Australia). The standard mixtures of SCFAs were purchased from Cayman chemical (Ann Arbor, MI, USA).
2.2. Methods
2.2.1. Preparing Kefir Samples
Kefir samples fortified with
Stinging nettle powder were prepared using skimmed milk pasteurized in a hot water bath at 80–85 °C for 30 min [
17]. The pasteurized milk was cooled to 42 °C and inoculated with freeze-dried KEFIR 12 probiotic culture (eXact
®) at a concentration of 0.2 g/L and shaken well, then left for 10 min to dissolve completely. Since UV light at 280 nm or less is considered germicidal to most types of microorganisms,
Stinging nettle powder was exposed to UV light for about 20 min [
18] before it was added at 0%, 0.25%, and 0.5%
w/
w to the inoculated milk samples and mixed well using the vortex machine (Ultra Turax T25 D S5, IKA, Königswinter, Germany). The applied concentrations of Sn (0.25% and 0.5%) were selected based on some preliminary trails which showed that using > 0.5% affected the kefir color very significantly, and it became very dark green. The inoculated milk was incubated in a thermostatic incubator at 30 °C ± 1 under aerobic conditions for 16 h until the pH reached 4.4–4.6 and then stored at 4 °C for 1, 14, and 21 days [
19]. Kefir samples were prepared in two trials using 2 L of milk in each trial, and all measurements were conducted in triplicate.
2.2.2. Preparation of the Agar Medium
The MRS agar was prepared following the supplier’s instructions. A specific weight of MRS agar was added to Milli-Q water (500 mL) and boiled on a hot plate with continuous stirring using a magnetic bar [
20]. The boiled MRS agar was autoclaved at 121 °C for 15 min (HANHSIN VD-3041 autoclave, Tullamarine, VIC, Australia) and cooled to 48 °C in a water bath before pouring into Petri dishes. The trypticase soy agar (TSA) and M17 agar were prepared following the same procedures.
2.2.3. Determination of Microbial Survival During Storage Time
The kefir samples were serially diluted (10
−1–10
−7) in sterile peptone water, and 0.1 mL of the appropriate dilutions was then spread plated on MRS, M17, and TSA agar. All plates were inoculated in duplicates, and the final counts were reported as a log CFU/g sample. The inoculated plates were incubated at 37 °C for 48 h under anaerobic conditions, using a BB 150 CO
2 incubator (Thermo Fisher Scientific, 168 Third Avenue, Waltham, MA, USA, 02451). The MRS, M17, and TSA media were designated to isolate and enumerate the
Lactobacillus sp.,
Lactococcus sp., and total plate count, respectively [
15].
2.2.4. Analysis of Physicochemical Properties
Color and pH Testing
The color of the kefir samples was measured using a portable colorimeter CR-400 (Minolta chromameter CR-400, Osaka, Japan), and the spectral color parameters L* (lightness), a* (green to red), and b* (blue to yellow) of CIELAB color were reported.
The pH determination was performed using a digital pH meter (HI5222, Hanna Instruments Pty Ltd., Melbourne, Australia) following the method of Kaur Sidhu et al. [
19].
Titratable Acidity
The titratable acidity of kefir samples was analyzed following the method of Zahid et al. [
17]. A mixture of 10 g of yoghurt sample and 90 mL of Mili-Q water was titrated with 0.1 mol/L NaOH solution, and 200 μL of phenolphthalein (1%,
w/
v) was used as an indicator. The % lactic acid content was calculated using Equation (1):
F = Correction factor (=9 for lactic acid);
V1 = Volume of NaOH;
M = Molarity of NaOH solution;
V2 = Volume of sample used in the trituration.
Viscosity and Syneresis Determination
Viscosity was measured using the DV1 Digital Viscometer (Brookfield, John Morris Scientific, Deepdene, VIC, Australia) with spindle number 6 at 50 rpm [
21]. The spindle was rotated in 40 mL of kefir in a falcon tube, and the values were recorded in centipoise (cP) during the first 5 revolutions of the rotation from triplicate samples. The mean value was determined as the apparent viscosity of the kefir sample.
Determination of whey separation (syneresis) was based on the method of Ranadheera et al. [
22] where 20 ± 0.1 g of the kefir sample was carefully transferred into a funnel lined with Whatman Filter Paper No. 4 and left for 2 h. The result was calculated according to Equation (2) and expressed as % syneresis (
w/
w).
where W = Total weight of the falcon tube containing the sample,
Wt = Weight of the falcon tube after pouring out the sample,
Wr = Weight of residue on filter paper.
Determination of Antioxidant Capacity
The kefir sample (40 µL) was mixed with 1960 µL of 70% ethanol, vortexed, and left at room temperature for 15 min for extraction, followed by centrifugation for 10 min (1252.2×
g, 4 °C) (Fixed angle rotor FX6100, Allegra X-12R Centrifuge, Beckman Coulter, Inc., Brea, CA, USA). The supernatant was collected for further analysis. Based on the methodology of Zahid et al. [
17], the antioxidant activities in all ethanolic extracts were measured using the DPPH. The sample extract (40 µL) was mixed with 260 µL of 0.1 mM DPPH solution in ethanol. The mixture was incubated at room temperature for 30 min before measuring the absorbance at 517 nm using a Multiskan Go 96-well microplate reader (Thermo Fisher Scientific, Waltham, MA, USA). The standard curve (R
2 = 0.9915) was plotted using Trolox (0 µg/mL–100 µg/mL), and the final results were reported as mg Trolox equivalent (TE)/g sample.
Determination of Total Polyphenol Content
The sample extract prepared for antioxidant analysis was used for the total polyphenol assay. The total polyphenol contents (TPC) in the sample extracts and gallic acid standard solutions were determined by adding 25 μL of each into a 96-well microplate, mixing with 25 μL Folin–Ciocalteu phenol reagent already diluted in water at a 1:3 ratio (
v/v), and incubating for 15 min at room temperature [
17]. These incubated mixtures were mixed with 200 μL Milli-Q water and 25 μL of 10% (
w/
v) Na
2CO
3 and incubated for 60 min in the dark to allow color development. The absorbance was measured at 765 nm using a Multiskan Go microplate reader (Thermo Fisher Scientific, Waltham, MA, USA). The total phenolic content was calculated and expressed as mg gallic acid equivalents per gram sample (mg GAE/g) using the gallic acid standard curve.
2.2.5. In Vitro Digestion and Colonic Fermentation
In Vitro Digestion
Digestion in this experiment consisted of a gastric and small intestinal digestion phases, following the method of Hossain et al. [
25] and Minekus et al. [
23]. During the gastric digestion phase, 10 mL of the kefir sample was mixed with 7.5 mL of SGF stock solution, 1.6 mL of porcine pepsin (3200–4500 U/mg), and 5 µL of 0.3 M CaCl
2. The pH was adjusted to 3.0 using 1 M HCL and leveled to 20 mL with Milli-Q water, then incubated for 2 h at 37 °C in a shaking incubator (~120 rpm) (ZWYR-240, Labwit, Ashwood, Australia). The gastric digested samples were then mixed with 11 mL of simulated intestinal fluid, 5 mL of porcine pancreatin (800 U/mL), 2.5 mL fresh bile (160 mM), and 40 µL of 0.3 M CaCl
2. The pH was adjusted to 7.0 using NaOH (1 M) and leveled to 40 mL with Milli-Q water. The samples were flushed with N
2 for 30 s before being digested in a shaking incubator (~120 rpm) at 37 °C for 2 h. The digested samples were centrifuged at 6000 rpm, at 4 °C, for 10 min, and the residues were collected to continue the colonic fermentation.
Fecal Slurry Preparation
Ethical approval (ID: 2024-29822-54940-5) was obtained from the Human Ethics Advisory Group at the University of Melbourne before commencing the colonic fermentation. The preparation of human fecal slurry was conducted based on the method of Tzounis et al. [
26]. The fresh fecal sample was collected from a 29-year-old healthy adult male donor without any history of antibiotics and probiotics intake in the past three months. About 10 g of that human feces was mixed with 90 g of sterilized pre-N
2 flushed phosphate buffer (pH = 7) in a stomacher bag and homogenized using a stomacher mixer (400 Circulator, Seward, AK, USA) for 2 min. Fecal slurry (10%
w/
w) was obtained by aseptically filtering the stomached liquid into a pre-N
2 flushed tube and refrigerated until used.
Colonic Fermentation
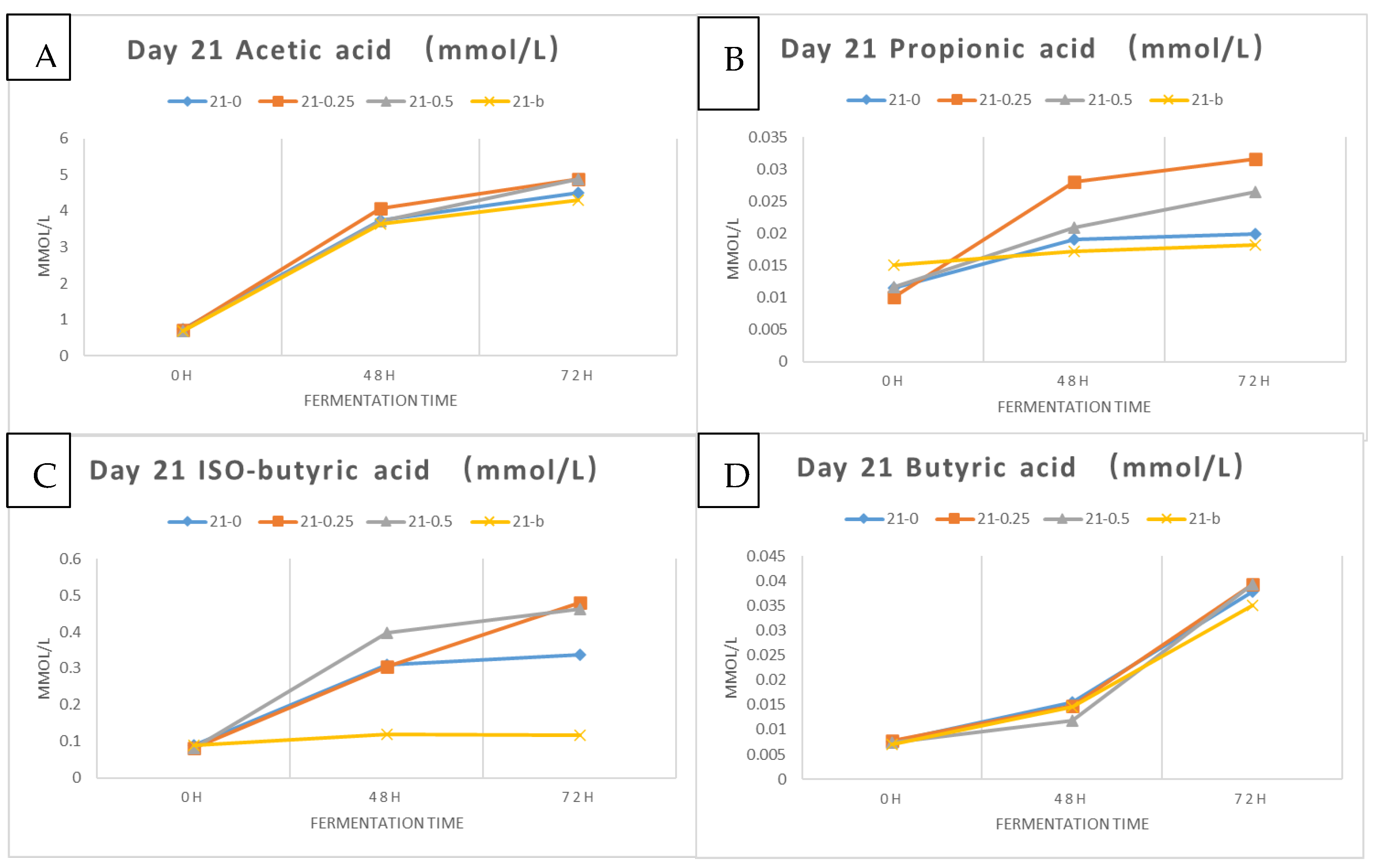
During the colonic fermentation phase, 5 mL of basal medium and 5 mL of fecal slurry were added to the residue (~5 g) obtained initially from the already performed intestinal digestion. The mixture was thoroughly mixed and then subjected to 1 min nitrogen flush to create anaerobic conditions. The samples were then placed in a shaking incubator at 37 °C, 120 rpm, and triplicate samples were collected at 0, 48, and 72 h for microbial activity testing and short-chain fatty acid (SCFA) analysis. The same procedures were repeated on days 1 and 21 of storage, and the blank sample was prepared using 5 mL fecal slurry and 5 mL basal medium only [
25,
27]. Microbial viable counts were assessed following the same method mentioned in
Section 2.2.3 using MRS, M17, and TSA media.
2.2.6. Analysis of Short-Chain Fatty Acids
Sample Preparation
Kefir samples subjected to in vitro digestion and colonic fermentation were prepared for short-chain fatty acid analysis following the methods of Gu et al. [
28] and Loo et al. [
29]. Fermented samples (1.5 mL) were centrifuged at 5000 rpm for 15 min at 4°, and 1.0 mL of the supernatant was transferred into a new 10 mL plastic tube and mixed well with 3.5 mL of diluted acid (1% formic acid and 1% orthophosphoric acid), and 4-methylpentanoic acid (internal standard) at a final concentration of 1.59 mmol/L. A small volume (1.5 mL) of that final mixture was then transferred into a GC vial for GC-FID analysis.
Analyses of SCFAs Using GC-FID
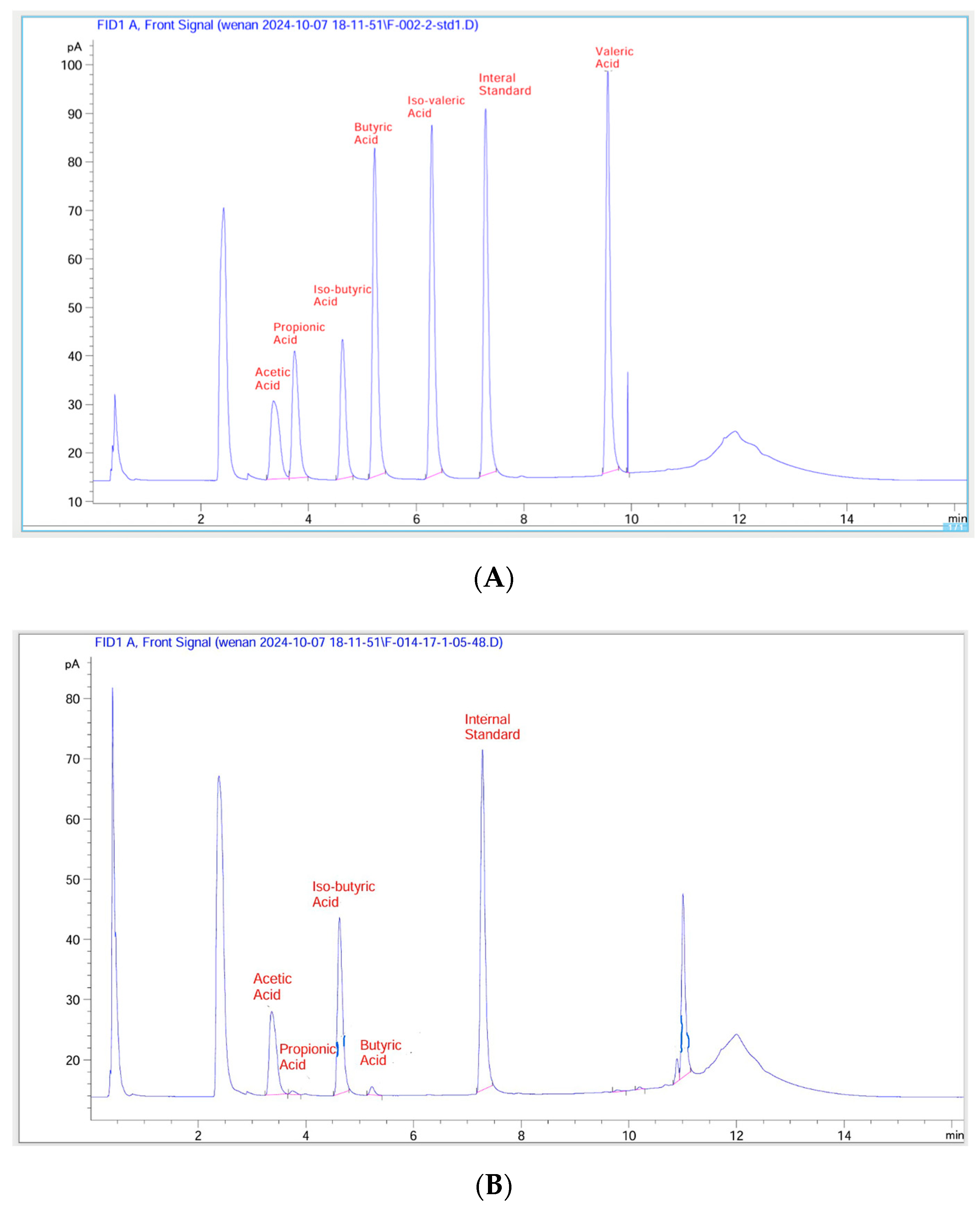
The analysis of short-chain fatty acids was conducted using GC-FID, according to the analytical methods of Gu et al. [
28]. The gas chromatography (GC) machine (7890B Agilent, Santa Clara, CA, USA) was coupled with a flame ionization detector (FID), an autosampler (7693 Agilent, CA, USA), and an autoinjector (G4513A Agilent, CA, USA). A SGE BP21 capillary column (12 × 0.53 mm internal diameter (ID) with 0.5 µm film thickness, SGE International, Ringwood, VIC, Australia, P/N 054473) and a retention gap kit (including a 2 × 0.53 mm ID guard column, P/N SGE RGK2) were attached. The carrier gas was helium with a flow rate of 14.4 mL/min. The detailed conditions used for GC-FID were as follows: 1 µL sample was injected; the oven temperature was set at 100 °C for 30 s, then increased to 180 °C at 6 °C/min for 1 min, and then increased to 200 °C at 20 °C/min for 10 min; the FID temperature was set at 240 °C; the inlet temperature was set at 200 °C; and the supplemental gases were nitrogen, hydrogen, and air at flow rates of 20, 30, and 300 mL/min, respectively. Short-chain fatty acids were calculated by substituting the sample peak area directly into the following standardized equation [
17]:
2.2.7. Statistical Analysis
The generated data were analyzed for significant differences between the treatments and sample mean ratings using a one-way analysis of variance (ANOVA) and Fisher’s least significant difference (LSD) (α = 0.05). All statistical analyses were conducted using the XLSTAT® (Addinsoft, New York, NY, USA) analysis add-in installed on Excel (Microsoft Corporation, Inc., Redmond, WA, USA).