Enhancing the Nutritional Value of Foods Through Probiotics and Dietary Fiber from Fruit and Berry Pomace
Abstract
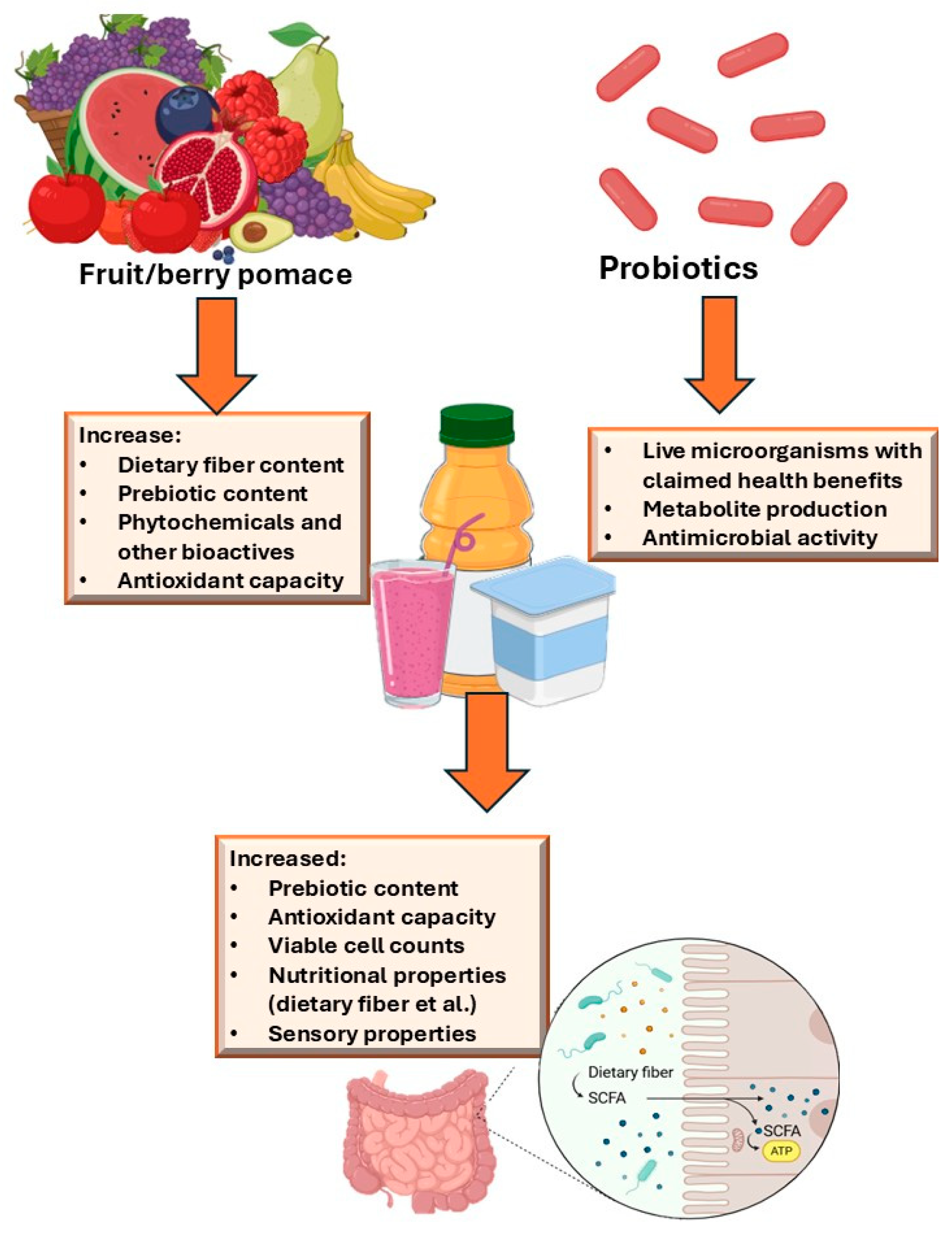
1. Introduction
2. Methodology
3. Probiotics and Their Benefits
4. Criteria for Probiotic Selection
5. Physiological and Technological Properties of Dietary Fiber from Fruit/Berry Processing By-Products
5.1. Physiological Effects of Dietary Fiber from Fruit and Berry Processing By-Products
5.2. Technological Properties of Dietary Fiber from Fruit/Berry Processing By-Products
5.3. Impact of Modification on the Prebiotic and Technological Properties of By-Products
6. Berry and Fruit By-Products for Application with Probiotics in Functional Foods
| Food Product | By-Products | Microorganisms | Outcomes | Reference |
|---|---|---|---|---|
| Fermented goat milk | Grape pomace extract | L. acidophilus LA-5, L. rhamnosus HN001 | ↑ Total phenolic compound content; ↑ viability of L. acidophilus; ↑ sensory scores of flavor, color, and overall acceptability | [121] |
| Fermented skim milk | Str. thermophilus TA040, L. acidophilus LAC4 | ↑ Total phenolic compound content; ↑ viability of Str. thermophilus and L. acidophilus | [120] | |
| Yogurt | Grape pomace | L. acidophilus, B. bifidum | ↑ Total phenolic compound content; ↑ antioxidant activity; ↓ syneresis; ↑ viable cell counts | [72] |
| Fermented grape juice | L. casei subsp. rhamnosus ATCC 7469 | ↑ Growth of LAB population | [122] | |
| Low-fat symbiotic yogurt gel | L. acidophilus LA-5, B. bifidum BB-12 | ↑ Viable cell counts; ↓ syneresis; ↑ steady and dynamic rheological properties; ↑ firmness; ↑ overall acceptability | [71] | |
| Yogurt | Apple pomace | L. acidophilus, Str. thermophilus, B. bifidum | ↑ Total phenolic compound content; ↑ antioxidant activity; ↓ syneresis of enriched yogurts; ↓ colon cancer cells’ viability | [123] |
| Cheese | Lac. lactis LL16 | ↑ Viability of Lac. lactis LL16; ↑ overall sensory acceptance | [124] | |
| Fermented soy drink | Loigalactobacillus bifermentans MIUG BL-16 | ↑ Total phenolic compound content; ↑ antioxidant activity; ↑ viable cell counts | [74] | |
| Gummy supplements | L. plantarum LUHS135, L. paracasei LUHS244 | ↑ Total phenolic compound content; ↑ antioxidant activity | [125] | |
| Yogurt | Orange pomace | Str. thermophilus, L. acidophilus, L. bulgaricus | ↓ Syneresis; ↓ firmness; ↑ consistency index | [126] |
| Ice cream | Fruit (grape, apricot, and apple) and grain (rice, corn, sunflower, and barley)-based by-products | L. acidophilus ATCC 4357D-5, B. animalis subsp. lactis ATCC 27536 | ↑ Survival of the probiotic strains | [127] |
| Milk | Peach pomace fiber | L. acidophilus, B. animalis subsp. lactis | ↑ Total phenolic compound content; ↑ antioxidant activity; ↑ titratable acidity; ↓ pH | [128] |
| Fermented soymilk | Passion fruit by-products | Str. thermophilus TH-4, L. acidophilus LA-5, L. rhamnosus LGG, L. fermentum PCC, and L. reuteri RC-14, Str. thermophilus ST-M6 and TA-40 | ↑ Folate content; ↑ growth of probiotics | [114] |
| Millet probiotic beverage | Pineapple pomace | L. rhamnosus LGG | ↑ Total phenolic compound content; ↑ antioxidant activity; ↑ sensory score | [129] |
| Petit suisse cheese | Blueberry pomace | Lac. lactis subsp. cremoris, Lac. lactis subsp. lactis, Lac. lactis subsp. lactis biovar. diacetylactis, Leuc. mesenteroides subsp. cremoris, Leuc. pseudomesenteroides, L. acidophilus LA5, B. animalis subsp. lactis BB12 | ↑ Fiber content; ↑ total phenolic compound content; ↑ antioxidant activity; ↑ sensory score | [130] |
| Ice cream | Passion fruit peel | L. acidophilus TISTR 2365 | ↑ Growth of probiotics; ↑ overrun value; ↑ sensory score; ↓ melting rate; ↓ pH; ↓ color intensity | [131] |
| Butter spread product | Soluble DF extracted from cranberry and sea buckthorn berry pomace | L. reuteri 182, L. paracasei subsp. paracasei ATCC® BAA-52, L. plantarum F1 | ↑ Viscosity; ↑ probiotic viability | [132] |
| Low-fat yogurt | Sour cherry pomace pectin-derived oligosaccharides | L. acidophilus DSM 20079 | ↑ Acidity; ↑ viscosity; ↑ probiotic viability; ↓ pH; ↓ syneresis; ↑ sensory properties; ↑ antioxidant activity | [22] |
| Yogurt | Carrot waste extract | L. plantarum | ↑ Total phenolic compound content; ↑ antioxidant activity; ↑ survival of the probiotic strains | [133] |
| Yogurt | Wolfberry DF | L. casei CGMCC1.5956, L. plantarum subsp. plantarum CGMCC 1.5953 | ↓ Syneresis; ↑ acidity; ↑ sensory properties; ↑ viscosity | [21] |
| Yogurt | Orange, mandarin, and lemon pomaces | L. acidophilus LA-5, B. animalis subsp. lactis BB12, Str. thermophilus | ↑ Acidity; ↑ growth of probiotics; ↑ stability and enhanced texture; ↑ sensorial quality; ↑ antioxidant activity | [134] |
| Yogurt | Mango peel powder and banana peel powder | L. casei 431®, L. rhamnosus LGG®, B. subsp. lactis Bb-12® | ↑ Viable cell counts; ↑ fat, ash, and protein contents; ↑ total phenolic contents; ↑ antioxidant activity; ↑ sugar contents; ↑ titratable acidity; ↓ pH | [20] |
7. Regulatory Overview of Pomace and Probiotics in Food Products
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| B. | Bifidobacterium |
| DF | Dietary fiber |
| EFSA | The European Food Safety Authority |
| EPS | Exopolysaccharide |
| EU | European Union |
| FAO | Food and Agriculture Organization |
| IDF | Insoluble dietary fiber |
| K. | Kluyveromyces |
| L. | Lactobacillus, Lacticaseibacillus, Lactiplantibacillus, Limosilactobacillus, and Levilactobacillus |
| LAB | Lactic acid bacteria |
| Lac. | Lactococcus |
| Leuc. | Leuconostoc |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MW | Molecular weight |
| QPS | Qualified Presumption of Safety |
| Quorum-quenching | |
| QS | Quorum sensing |
| QSI | Quorum sensing inhibition |
| SCFA | Short chain fatty acids |
| SDF | Soluble dietary fiber |
| Str. | Streptococcus |
| WHO | World Health Organization |
References
- Ali, T.; Ali, J. Factors Affecting the Consumers’ Willingness to Pay for Health and Wellness Food Products. J. Agric. Food Res. 2020, 2, 100076. [Google Scholar] [CrossRef]
- Probiotics Market Size, Share & Growth, Report 2031. Available online: https://www.transparencymarketresearch.com/probiotics-market.html (accessed on 19 March 2025).
- Cerk, K.; Aguilera-Gómez, M. Microbiota Analysis for Risk Assessment: Evaluation of Hazardous Dietary Substances and Its Potential Role on the Gut Microbiome Variability and Dysbiosis. EFSA J. 2022, 20, e200404. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO/WHO Expert Consultation on Evaluation of Health and Nutritional Properties of Probiotics in Food Including Powder Milk with Live Lactic acid bacteria 2002. Available online: https://openknowledge.fao.org/items/db384295-64d9-47e2-b65b-3c918efc5140 (accessed on 22 February 2025).
- Probiotics-International Scientific Association for Probiotics and Prebiotics (ISAPP). Available online: https://isappscience.org/for-scientists/resources/probiotics/ (accessed on 22 February 2025).
- Ranjha, M.M.A.N.; Shafique, B.; Batool, M.; Kowalczewski, P.Ł.; Shehzad, Q.; Usman, M.; Manzoor, M.F.; Zahra, S.M.; Yaqub, S.; Aadil, R.M. Nutritional and Health Potential of Probiotics: A Review. Appl. Sci. 2021, 11, 11204. [Google Scholar] [CrossRef]
- Mizock, B.A. Probiotics. Dis.-A-Mon. 2015, 61, 259–290. [Google Scholar] [CrossRef]
- Palanivelu, J.; Thanigaivel, S.; Vickram, S.; Dey, N.; Mihaylova, D.; Desseva, I. Probiotics in Functional Foods: Survival Assessment and Approaches for Improved Viability. Appl. Sci. 2022, 12, 455. [Google Scholar] [CrossRef]
- Latif, A.; Shehzad, A.; Niazi, S.; Zahid, A.; Ashraf, W.; Iqbal, M.W.; Rehman, A.; Riaz, T.; Aadil, R.M.; Khan, I.M.; et al. Probiotics: Mechanism of Action, Health Benefits and Their Application in Food Industries. Front. Microbiol. 2023, 14, 1216674. [Google Scholar] [CrossRef]
- Anderson, J.W.; Baird, P.; Davis, R.H., Jr.; Ferreri, S.; Knudtson, M.; Koraym, A.; Waters, V.; Williams, C.L. Health Benefits of Dietary Fiber. Nutr. Rev. 2009, 67, 188–205. [Google Scholar] [CrossRef]
- Agostoni, C.; Bresson, J.-L.; Fairweather-Tait, S.; Flynn, A.; Golly, I.; Korhonen, H.; Lagiou, P.; Løvik, M.; Marchelli, R.; Martin, A.; et al. Scientific Opinion on Dietary Reference Values for Carbohydrates and Dietary Fibre. EFSA J. 2010, 8, 1462. [Google Scholar] [CrossRef]
- Health Claims: Fruits, Vegetables, and Grain Products That Contain Fiber, Particularly Soluble Fiber, and Risk of Coronary Heart Disease. Available online: https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-101/subpart-E/section-101.77 (accessed on 22 February 2025).
- Hussain, S.; Jõudu, I.; Bhat, R. Dietary Fiber from Underutilized Plant Resources—A Positive Approach for Valorization of Fruit and Vegetable Wastes. Sustainability 2020, 12, 5401. [Google Scholar] [CrossRef]
- Wadhwa, M.; Bakshi, S.P.M. Utilization of Fruit and Vegetable Wastes as Livestock Feed and as Substrates for Generation of Other Value-Added Products. RAP Publ. 2013, 4, 67. [Google Scholar]
- Banerjee, J.; Singh, R.; Vijayaraghavan, R.; MacFarlane, D.; Patti, A.F.; Arora, A. Bioactives from Fruit Processing Wastes: Green Approaches to Valuable Chemicals. Food Chem. 2017, 225, 10–22. [Google Scholar] [CrossRef]
- Coleman, C.M.; Ferreira, D. Oligosaccharides and Complex Carbohydrates: A New Paradigm for Cranberry Bioactivity. Molecules 2020, 25, 881. [Google Scholar] [CrossRef]
- EUR-Lex-52015DC0614-EN-EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52015DC0614 (accessed on 22 February 2025).
- Creating a Sustainable Food System: The EU’s Strategy|Topics|European Parliament. Available online: https://www.europarl.europa.eu/topics/en/article/20200519STO79425/creating-a-sustainable-food-system-the-eu-s-strategy (accessed on 22 February 2025).
- Balogun, O.; Kang, H.W. Bioactivities and Applications of Fruit Byproducts and Their Phytochemicals: A Mini Review. Food Rev. Int. 2024, 40, 3964–4004. [Google Scholar] [CrossRef]
- Zahid, H.F.; Ranadheera, C.S.; Fang, Z.; Ajlouni, S. Functional and Healthy Yogurts Fortified with Probiotics and Fruit Peel Powders. Fermentation 2022, 8, 469. [Google Scholar] [CrossRef]
- Fan, X.; Shi, Z.; Xu, J.; Li, C.; Li, X.; Jiang, X.; Du, L.; Tu, M.; Zeng, X.; Wu, Z.; et al. Characterization of the Effects of Binary Probiotics and Wolfberry Dietary Fiber on the Quality of Yogurt. Food Chem. 2023, 406, 135020. [Google Scholar] [CrossRef] [PubMed]
- Parastouei, K.; Khodaiyan, F.; Hosseini, S.S. Developing Novel Synbiotic Low-Fat Yogurt Using Sour Cherry Pomace Pectin-Derived Oligosaccharides: Lactobacillus acidophilus Survival, Quality and Antioxidant Properties. J. Food Sci. Technol. 2024, 62, 1–10. [Google Scholar] [CrossRef]
- Soccol, C.R.; Vandenberghe, L.D.S.; Spier, M.R.; Medeiros, A.B.P.; Yamaguishi, C.T.; Lindner, J.D.D.; Pandey, A.; Thomaz-Soccol, V. The Potential of Probiotics: A Review. Food Technol. Biotechnol. 2010, 48, 413–435. [Google Scholar]
- Guo, Q.; Kang, J.; Bai, Y.; Xu, F. Dietary Fiber: Chemistry, Structure, and Properties. J. Chem. 2018, 2018, 1328797. [Google Scholar] [CrossRef]
- He, C.; Sampers, I.; Raes, K. Dietary Fiber Concentrates Recovered from Agro-Industrial by-Products: Functional Properties and Application as Physical Carriers for Probiotics. Food Hydrocoll. 2021, 111, 106175. [Google Scholar] [CrossRef]
- How, Y.H.; Nyam, K.L. Reutilization of Fruit Waste as Potential Prebiotic for Probiotic or Food-Grade Microorganisms in Food Applications: A Review. Probiotics Antimicrob. Proteins 2024, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Nickerson, M.T.; Korber, D.R. Valorization of Berry Pomace for Extraction of Polyphenol Compounds and Its Co-Encapsulation with Probiotic Bacteria. Food Biosci. 2024, 62, 105124. [Google Scholar] [CrossRef]
- Iqbal, A.; Schulz, P.; Rizvi, S.S.H. Valorization of Bioactive Compounds in Fruit Pomace from Agro-Fruit Industries: Present Insights and Future Challenges. Food Biosci. 2021, 44, 101384. [Google Scholar] [CrossRef]
- Diez-Sánchez, E.; Quiles, A.; Hernando, I. Use of Berry Pomace to Design Functional Foods. Food Rev. Int. 2023, 39, 3204–3224. [Google Scholar] [CrossRef]
- Elleuch, M.; Bedigian, D.; Roiseux, O.; Besbes, S.; Blecker, C.; Attia, H. Dietary Fibre and Fibre-Rich by-Products of Food Processing: Characterisation, Technological Functionality and Commercial Applications: A Review. Food Chem. 2011, 124, 411–421. [Google Scholar] [CrossRef]
- Tejada-Ortigoza, V.; Garcia-Amezquita, L.E.; Serna-Saldívar, S.O.; Welti-Chanes, J. Advances in the Functional Characterization and Extraction Processes of Dietary Fiber. Food Eng. Rev. 2016, 8, 251–271. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S. Classification, Technological Properties, and Sustainable Sources. In Dietary Fiber: Properties, Recovery, and Applications; Academic Press: London, UK, 2019; pp. 27–58. [Google Scholar] [CrossRef]
- Comunian, T.A.; Silva, M.P.; Souza, C.J.F. The Use of Food By-Products as a Novel for Functional Foods: Their Use as Ingredients and for the Encapsulation Process. Trends Food Sci. Technol. 2021, 108, 269–280. [Google Scholar] [CrossRef]
- Ahmad, T.; Esposito, F.; Cirillo, T. Valorization of Agro-Food by-Products: Advancing Sustainability and Sustainable Development Goals 2030 through Functional Compounds Recovery. Food Biosci. 2024, 62, 105194. [Google Scholar] [CrossRef]
- Shokryazdan, P.; Faseleh Jahromi, M.; Liang, J.B.; Ho, Y.W. Probiotics: From Isolation to Application. J. Am. Coll. Nutr. 2017, 36, 666–676. [Google Scholar] [CrossRef]
- Srinivash, M.; Krishnamoorthi, R.; Mahalingam, P.U.; Malaikozhundan, B.; Keerthivasan, M. Probiotic Potential of Exopolysaccharide Producing Lactic Acid Bacteria Isolated from Homemade Fermented Food Products. J. Agric. Food Res. 2023, 11, 100517. [Google Scholar] [CrossRef]
- Bagci, U.; Ozmen Togay, S.; Temiz, A.; Ay, M. Probiotic Characteristics of Bacteriocin-Producing Enterococcus Faecium Strains Isolated from Human Milk and Colostrum. Folia Microbiol. 2019, 64, 735–750. [Google Scholar] [CrossRef]
- Unban, K.; Kodchasee, P.; Shetty, K.; Khanongnuch, C. Tannin-Tolerant and Extracellular Tannase Producing Bacillus Isolated from Traditional Fermented Tea Leaves and Their Probiotic Functional Properties. Foods 2020, 9, 490. [Google Scholar] [CrossRef]
- Rodrigues, N.P.A.; Garcia, E.F.; de Souza, E.L. Selection of Lactic aczid bacteria with Promising Probiotic Aptitudes from Fruit and Ability to Survive in Different Food Matrices. Braz. J. Microbiol. 2021, 52, 2257–2269. [Google Scholar] [CrossRef]
- Alameri, F.; Tarique, M.; Osaili, T.; Obaid, R.; Abdalla, A.; Masad, R.; Al-Sbiei, A.; Fernandez-Cabezudo, M.; Liu, S.Q.; Al-Ramadi, B.; et al. Lactic acid bacteria Isolated from Fresh Vegetable Products: Potential Probiotic and Postbiotic Characteristics Including Immunomodulatory Effects. Microorganisms 2022, 10, 389. [Google Scholar] [CrossRef] [PubMed]
- Abdalla, A.K.; Ayyash, M.M.; Olaimat, A.N.; Osaili, T.M.; Al-Nabulsi, A.A.; Shah, N.P.; Holley, R. Exopolysaccharides as Antimicrobial Agents: Mechanism and Spectrum of Activity. Front. Microbiol. 2021, 12, 664395. [Google Scholar] [CrossRef]
- Ayyash, M.M.; Abdalla, A.K.; AlKalbani, N.S.; Baig, M.A.; Turner, M.S.; Liu, S.Q.; Shah, N.P. Invited Review: Characterization of New Probiotics from Dairy and Nondairy Products-Insights into Acid Tolerance, Bile Metabolism and Tolerance, and Adhesion Capability. J. Dairy Sci. 2021, 104, 8363–8379. [Google Scholar] [CrossRef]
- Oelschlaeger, T.A. Mechanisms of Probiotic Actions—A Review. Int. J. Med. Microbiol. 2010, 300, 57–62. [Google Scholar] [CrossRef]
- Thananimit, S.; Pahumunto, N.; Teanpaisan, R. Characterization of Short Chain Fatty Acids Produced by Selected Potential Probiotic Lactobacillus Strains. Biomolecules 2022, 12, 1829. [Google Scholar] [CrossRef]
- Cella, M.A.; Coulson, T.; MacEachern, S.; Badr, S.; Ahmadi, A.; Tabatabaei, M.S.; Labbe, A.; Griffiths, M.W. Probiotic Disruption of Quorum Sensing Reduces Virulence and Increases Cefoxitin Sensitivity in Methicillin-Resistant Staphylococcus aureus. Sci. Rep. 2023, 13, 4373. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Peng, Q.; Zhang, Y.; Tian, D.; Zhang, P.; Huang, Y.; Ma, L.; Qiao, Y.; Shi, B. A Novel Exopolysaccharide Produced by Lactobacillus coryniformis NA-3 Exhibits Antioxidant and Biofilm-Inhibiting Properties in Vitro. Food Nutr. Res. 2020, 64, 3744. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.K.; Abuqwider, J.; Mauriello, G. Anti-Quorum Sensing Activity of Probiotics: The Mechanism and Role in Food and Gut Health. Microorganisms 2023, 11, 793. [Google Scholar] [CrossRef]
- Prazdnova, E.V.; Gorovtsov, A.V.; Vasilchenko, N.G.; Kulikov, M.P.; Statsenko, V.N.; Bogdanova, A.A.; Refeld, A.G.; Brislavskiy, Y.A.; Chistyakov, V.A.; Chikindas, M.L. Quorum-Sensing Inhibition by Gram-Positive Bacteria. Microorganisms 2022, 10, 350. [Google Scholar] [CrossRef]
- Azami, S.; Arefian, E.; Kashef, N. Postbiotics of Lactobacillus casei Target Virulence and Biofilm Formation of Pseudomonas aeruginosa by Modulating Quorum Sensing. Arch. Microbiol. 2022, 204, 157. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Giri, S.K. Probiotic Functional Foods: Survival of Probiotics during Processing and Storage. J. Funct. Foods 2014, 9, 225–241. [Google Scholar] [CrossRef]
- Shen, F.; Zhuang, J.; Wang, Q.; Zhang, J.; Liu, T.; Ruan, S.; Du, J.; Zhong, H.; Zhao, M.; Feng, F. Screening of Novel Probiotics with Intestinal Peristalsis-Promoting Potential Based on in Vitro and in Vivo Investigations. Food Biosci. 2023, 53, 102681. [Google Scholar] [CrossRef]
- Sreepathi, N.; Kumari, V.B.C.; Huligere, S.S.; Al-Odayni, A.-B.; Lasehinde, V.; Jayanthi, M.K.; Ramu, R. Screening for Potential Novel Probiotic Levilactobacillus brevis RAMULAB52 with Antihyperglycemic Property from Fermented Carica papaya L. Front. Microbiol. 2023, 14, 1168102. [Google Scholar] [CrossRef] [PubMed]
- Alard, J.; Cudennec, B.; Boutillier, D.; Peucelle, V.; Descat, A.; Decoin, R.; Kuylle, S.; Jablaoui, A.; Rhimi, M.; Wolowczuk, I.; et al. Multiple Selection Criteria for Probiotic Strains with High Potential for Obesity Management. Nutrients 2021, 13, 713. [Google Scholar] [CrossRef]
- Cuffia, F.; George, G.; Godoy, L.; Vinderola, G.; Reinheimer, J.; Burns, P. In Vivo Study of the Immunomodulatory Capacity and the Impact of Probiotic Strains on Physicochemical and Sensory Characteristics: Case of Pasta Filata Soft Cheeses. Food Res. Int. 2019, 125, 108606. [Google Scholar] [CrossRef] [PubMed]
- Tarique, M.; Abdalla, A.; Masad, R.; Al-Sbiei, A.; Kizhakkayil, J.; Osaili, T.; Olaimat, A.; Liu, S.Q.; Fernandez-Cabezudo, M.; al-Ramadi, B.; et al. Potential Probiotics and Postbiotic Characteristics Including Immunomodulatory Effects of Lactic acid bacteria Isolated from Traditional Yogurt-like Products. LWT 2022, 159, 113207. [Google Scholar] [CrossRef]
- Terpou, A.; Papadaki, A.; Lappa, I.K.; Kachrimanidou, V.; Bosnea, L.A.; Kopsahelis, N. Probiotics in Food Systems: Significance and Emerging Strategies Towards Improved Viability and Delivery of Enhanced Beneficial Value. Nutrients 2019, 11, 1591. [Google Scholar] [CrossRef]
- Division, N. Probiotics in Food. 2006. Available online: https://openknowledge.fao.org/server/api/core/bitstreams/382476b3-4d54-4175-803f-2f26f3526256/content (accessed on 22 February 2025).
- Cizeikiene, D.; Jagelaviciute, J. Investigation of Antibacterial Activity and Probiotic Properties of Strains Belonging to Lactobacillus and Bifidobacterium Genera for Their Potential Application in Functional Food and Feed Products. Probiotics Antimicrob. Proteins 2021, 13, 1387–1403. [Google Scholar] [CrossRef]
- Alizadeh Behbahani, B.; Jooyandeh, H.; Taki, M.; Falah, F. Evaluation of the Probiotic, Anti-Bacterial, Anti-Biofilm, and Safety Properties of Lacticaseibacillus paracasei B31-2. LWT 2024, 207, 116676. [Google Scholar] [CrossRef]
- Hoffmann, A.; Kleniewska, P.; Pawliczak, R. Antioxidative Activity of Probiotics. Arch. Med. Sci. 2019, 17, 792–804. [Google Scholar] [CrossRef]
- Li, S.; Zhao, Y.; Zhang, L.; Zhang, X.; Huang, L.; Li, D.; Niu, C.; Yang, Z.; Wang, Q. Antioxidant Activity of Lactobacillus plantarum Strains Isolated from Traditional Chinese Fermented Foods. Food Chem. 2012, 135, 1914–1919. [Google Scholar] [CrossRef]
- Chuah, L.-O.; Foo, H.L.; Loh, T.C.; Mohammed Alitheen, N.B.; Yeap, S.K.; Abdul Mutalib, N.E.; Abdul Rahim, R.; Yusoff, K. Postbiotic Metabolites Produced by Lactobacillus plantarum Strains Exert Selective Cytotoxicity Effects on Cancer Cells. BMC Complement. Altern. Med. 2019, 19, 114. [Google Scholar] [CrossRef] [PubMed]
- Elham, N.; Naheed, M.; Elahe, M.; Hossein, M.M.; Majid, T. Selective Cytotoxic Effect of Probiotic, Paraprobiotic and Postbiotics of L. casei Strains against Colorectal Cancer Cells: Invitro Studies. Braz. J. Pharm. Sci. 2022, 58, e19400. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, S.; Fernández-Pacheco, P.; Seseña, S.; Pintado, C.; Palop, M.L. Selection of Probiotic Lactobacillus Strains with Antimicrobial Activity to Be Used as Biocontrol Agents in Food Industry. LWT 2021, 143, 111142. [Google Scholar] [CrossRef]
- Rastogi, S.; Mittal, V.; Singh, A. Selection of Potential Probiotic Bacteria from Exclusively Breastfed Infant Faeces with Antagonistic Activity Against Multidrug-Resistant ESKAPE Pathogens. Probiotics Antimicrob. Proteins 2021, 13, 739–750. [Google Scholar] [CrossRef]
- Murugu, J.; Narayanan, R. Production, Purification, and Characterization of a Novel Exopolysaccharide from Probiotic Lactobacillus amylovorus: MTCC 8129. Indian J. Microbiol. 2024, 64, 1355–1365. [Google Scholar] [CrossRef]
- Zahid, H.F.; Ranadheera, C.S.; Fang, Z.; Ajlouni, S. Utilization of Mango, Apple and Banana Fruit Peels as Prebiotics and Functional Ingredients. Agriculture 2021, 11, 584. [Google Scholar] [CrossRef]
- Spizzirri, U.G.; Carullo, G.; Aiello, F.; Paolino, D.; Restuccia, D. Valorisation of Olive Oil Pomace Extracts for a Functional Pear Beverage Formulation. Int. J. Food Sci. Technol. 2021, 56, 5497–5505. [Google Scholar] [CrossRef]
- Gerardi, C.; D’amico, L.; Migoni, D.; Santino, A.; Salomone, A.; Carluccio, M.A.; Giovinazzo, G. Strategies for Reuse of Skins Separated from Grape Pomace as Ingredient of Functional Beverages. Front. Bioeng. Biotechnol. 2020, 8, 535807. [Google Scholar] [CrossRef]
- Mall, U.P.; Patel, V.H. Carrot Pomace Powder: A Promising Source of Polyphenols and Prebiotics for Improving Gut Health. Nutrire 2024, 49, 9. [Google Scholar] [CrossRef]
- Mobasserfar, R.; Shiri, A.; Mofid, V.; Shahidi Noghabi, M.; Gharibzahedi, S.M.T. Grape Pomace High-Methoxyl Pectin: A New Prebiotic Stabilizer for Low-Fat Synbiotic Yogurt Gels—Optimization and Characterization. Int. J. Biol. Macromol. 2024, 282, 137139. [Google Scholar] [CrossRef]
- Navaz, S.H.; Yam, B.A.Z. Influence of Red Grape Pomace Powder on Physiochemical, Antioxidant, Nutritional, Microbial, and Sensory Properties of Probiotic Yoghurt. Indian. J. Dairy Sci. 2021, 74, 224–231. [Google Scholar] [CrossRef]
- Raikos, V.; Ni, H.; Hayes, H.; Ranawana, V. Antioxidant Properties of a Yogurt Beverage Enriched with Salal (Gaultheria shallon) Berries and Blackcurrant (Ribes nigrum) Pomace during Cold Storage. Beverages 2018, 5, 2. [Google Scholar] [CrossRef]
- Vlad, C.C.; Păcularu-Burada, B.; Vasile, A.M.; Milea, Ș.A.; Bahrim, G.-E.; Râpeanu, G.; Stănciuc, N. Upgrading the Functional Potential of Apple Pomace in Value-Added Ingredients with Probiotics. Antioxidants 2022, 11, 2028. [Google Scholar] [CrossRef] [PubMed]
- Opperman, C.; Majzoobi, M.; Farahnaky, A.; Shah, R.; Van, T.T.H.; Ratanpaul, V.; Blanch, E.W.; Brennan, C.; Eri, R. Beyond Soluble and Insoluble: A Comprehensive Framework for Classifying Dietary Fibre’s Health Effects. Food Res. Int. 2025, 206, 115843. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, H.; Pajari, A.M. Dietary Fiber—A Scoping Review for Nordic Nutrition Recommendations 2023. Food Nutr. Res. 2023, 67, 9979. [Google Scholar] [CrossRef]
- Ghorbani, Z.; Noormohammadi, M.; Kazemi, A.; Poustchi, H.; Pourshams, A.; Martami, F.; Hashemian, M.; Malekzadeh, R.; Hekmatdoost, A. Higher Intakes of Fiber, Total Vegetables, and Fruits May Attenuate the Risk of All-Cause and Cause-Specific Mortality: Findings from a Large Prospective Cohort Study. Nutr. J. 2023, 22, 60. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, J.; Zhang, Y.; Qi, H.; Wang, P. Associations between Dietary Fiber Intake and Mortality from All Causes, Cardiovascular Disease and Cancer: A Prospective Study. J. Transl. Med. 2022, 20, 344. [Google Scholar] [CrossRef]
- Cruz-Requena, M.; Escobedo-García, S.; Salas-Tovar, J.A.; Mora-Cura, Y.; Chávez-González, M.L.; Castillo-Reyes, F.; Flores-Gallegos, A.C.; Rodríguez-Herrera, R. Definitions and Regulatory Perspectives of Dietary Fibers. In Dietary Fiber: Properties, Recovery, and Applications; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–25. [Google Scholar]
- Soliman, G.A. Dietary Fiber, Atherosclerosis, and Cardiovascular Disease. Nutrients 2019, 11, 1155. [Google Scholar] [CrossRef]
- Dhingra, D.; Michael, M.; Rajput, H.; Patil, R.T. Dietary Fibre in Foods: A Review. J. Food Sci. Technol. 2011, 49, 255. [Google Scholar] [CrossRef]
- Calatayud, M.; Van den Abbeele, P.; Ghyselinck, J.; Marzorati, M.; Rohs, E.; Birkett, A. Comparative Effect of 22 Dietary Sources of Fiber on Gut Microbiota of Healthy Humans in Vitro. Front. Nutr. 2021, 8, 700571. [Google Scholar] [CrossRef]
- Nevara, G.A.; Muhammad, S.K.S.; Zawawi, N.; Mustapha, N.A.; Karim, R. Dietary Fiber: Fractionation, Characterization and Potential Sources from Defatted Oilseeds. Foods 2021, 10, 754. [Google Scholar] [CrossRef]
- Popoola-Akinola, O.O.; Raji, T.J.; Olawoye, B. Lignocellulose, Dietary Fibre, Inulin and Their Potential Application in Food. Heliyon 2022, 8, e10459. [Google Scholar] [CrossRef]
- Elshahed, M.S.; Miron, A.; Aprotosoaie, A.C.; Farag, M.A. Pectin in Diet: Interactions with the Human Microbiome, Role in Gut Homeostasis, and Nutrient-Drug Interactions. Carbohydr. Polym. 2021, 255, 117388. [Google Scholar] [CrossRef] [PubMed]
- Mishra, B.P.; Mishra, J.; Paital, B.; Rath, P.K.; Jena, M.K.; Reddy, B.V.V.; Pati, P.K.; Panda, S.K.; Sahoo, D.K. Properties and Physiological Effects of Dietary Fiber-Enriched Meat Products: A Review. Front. Nutr. 2023, 10, 1275341. [Google Scholar] [CrossRef] [PubMed]
- Raninen, K.; Lappi, J.; Mykkänen, H.; Poutanen, K. Dietary Fiber Type Reflects Physiological Functionality: Comparison of Grain Fiber, Inulin, and Polydextrose. Nutr. Rev. 2011, 69, 9–21. [Google Scholar] [CrossRef]
- Ghinea, C.; Leahu, A. Valorisation of Apple (Malus domestica) Wastes. In Mediterranean Fruits Bio-Wastes; Springer International Publishing: Berlin/Heidelberg, Germany, 2022; pp. 325–348. [Google Scholar]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Manning, T.S.; Gibson, G.R. Prebiotics. Best. Pract. Res. Clin. Gastroenterol. 2004, 18, 287–298. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, T.; Wang, X.; Lü, X. Apple Pomace as a Potential Valuable Resource for Full-Components Utilization: A Review. J. Clean. Prod. 2021, 329, 129676. [Google Scholar] [CrossRef]
- Vásquez, P.; Stucken, K.; Garcia-Martin, A.; Ladero, M.; Bolivar, J.M.; Bernal, C. Enzymatic Production, Physicochemical Characterization, and Prebiotic Potential of Pectin Oligosaccharides from Pisco Grape Pomace. Int. J. Biol. Macromol. 2024, 281, 136302. [Google Scholar] [CrossRef]
- Spadoni Andreani, E.; Karboune, S. Comparison of Enzymatic and Microwave-Assisted Alkaline Extraction Approaches for the Generation of Oligosaccharides from American Cranberry (Vaccinium macrocarpon) Pomace. J. Food Sci. 2020, 85, 2443–2451. [Google Scholar] [CrossRef]
- Tejada-Ortigoza, V.; Garcia-Amezquita, L.E.; Campanella, O.H.; Hamaker, B.R.; Welti-Chanes, J. Extrusion Effect on in Vitro Fecal Fermentation of Fruit Peels Used as Dietary Fiber Sources. LWT 2022, 153, 112569. [Google Scholar] [CrossRef]
- Huang, Y.L.; Ma, Y.S.; Tsai, Y.H.; Chang, S.K.C. In Vitro Hypoglycemic, Cholesterol-Lowering and Fermentation Capacities of Fiber-Rich Orange Pomace as Affected by Extrusion. Int. J. Biol. Macromol. 2019, 124, 796–801. [Google Scholar] [CrossRef]
- Chen, Z.H.; Yuan, X.H.; Tu, T.T.; Wang, L.; Mao, Y.H.; Luo, Y.; Qiu, S.Y.; Song, A.X. Characterization and Prebiotic Potential of Polysaccharides from Rosa roxburghii Tratt Pomace by Ultrasound-Assisted Extraction. Int. J. Biol. Macromol. 2024, 268, 131910. [Google Scholar] [CrossRef]
- Bamigbade, G.B.; Subhash, A.J.; Tarique, M.; al-Ramadi, B.; Abu-Jdayil, B.; Kamal-Eldin, A.; Nyström, L.; Ayyash, M. Date Pomace Polysaccharide: Ultrasonic-Assisted Deep Eutectic Solvent Extraction, Physicochemical Properties, Biological Activities, Gut Microbiota Modulation, and Rheological Properties. Chem. Biol. Technol. Agric. 2024, 11, 79. [Google Scholar] [CrossRef]
- Bachari, S.; Ghaderi-Ghahfarokhi, M.; Gavlighi, H.A.; Zarei, M. Ultrasonic Depolymerization of Pomegranate Peel Pectin: Effect of Sonication Time on Antioxidant, α-Amylase Inhibitory, and Prebiotic Properties. Food Chem. X 2024, 24, 101901. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Hou, Y.; Xie, L.; Zhang, H.; Liu, C.; Chen, T. Effects of Microwave on the Potential Microbiota Modulating Effects of Agro-Industrial by-Product Fibers among Different Individuals. LWT 2023, 178, 114621. [Google Scholar] [CrossRef]
- Qin, X.; Dong, X.; Tang, J.; Chen, Y.; Xie, J.; Cheng, Y.; Zheng, B.; Hu, X.; Yu, Q. Comparative Analysis of Dietary Fibers from Grapefruit Peel Prepared by Ultrafine Grinding: Structural Properties, Adsorption Capacities, in Vitro Prebiotic Activities. Food Biosci. 2023, 56, 103163. [Google Scholar] [CrossRef]
- Wang, S.; Xia, J.; De Paepe, K.; Zhang, B.; Fu, X.; Huang, Q.; Van de Wiele, T. Ultra-High Pressure Treatment Controls In Vitro Fecal Fermentation Rate of Insoluble Dietary Fiber from Rosa roxburghii Tratt Pomace and Induces Butyrogenic Shifts in Microbiota Composition. J. Agric. Food Chem. 2021, 69, 10638–10647. [Google Scholar] [CrossRef]
- Bamigbade, G.B.; Subhash, A.J.; Al-Ramadi, B.; Kamal-Eldin, A.; Gan, R.Y.; Liu, S.Q.; Ayyash, M. Gut Microbiota Modulation, Prebiotic and Bioactive Characteristics of Date Pomace Polysaccharides Extracted by Microwave-Assisted Deep Eutectic Solvent. Int. J. Biol. Macromol. 2024, 262, 130167. [Google Scholar] [CrossRef]
- Jiang, G.; Wu, Z.; Ramachandra, K.; Zhao, C.; Ameer, K. Changes in Structural and Chemical Composition of Insoluble Dietary Fibers Bound Phenolic Complexes from Grape Pomace by Alkaline Hydrolysis Treatment. Food Sci. Technol. 2021, 42, e50921. [Google Scholar] [CrossRef]
- Kang, Y.R.; Chang, Y.H. Structural Characterization and Prebiotic Activity of Rhamnogalacturonan-I Rich Pumpkin Pectic Polysaccharide Extracted by Alkaline Solution. Int. J. Biol. Macromol. 2024, 270, 132311. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, Y. Physicochemical and Functional Properties of Coconut (Cocos nucifera L) Cake Dietary Fibres: Effects of Cellulase Hydrolysis, Acid Treatment and Particle Size Distribution. Food Chem. 2018, 257, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.P.; Wee, W.Y.; Wan, K.W.; Loh, K.M.; Lee, K.C. Improvement of Prebiotic Activity of Guava Purée By-Products through Cellulase Treatment. Food Biotechnol. 2022, 36, 38–57. [Google Scholar] [CrossRef]
- Pyeon, S.; Park, J.; Bharti, D.; Lee, C.S.; Jun, W.; Yang, K.Y.; Nam, S.H. Hemicellulose Enriched Dietary Fiber from Asian Pear Pomace: Enzymatic Approach for Assessing Potential Prebiotic and Immunomodulatory Properties. Process Biochem. 2024, 147, 465–475. [Google Scholar] [CrossRef]
- Kang, Y.R.; Chang, Y.H. Preparation, Characterization, and Prebiotic Potential of Pectic Oligosaccharide Enzymatically Derived from Citric Acid-Extracted Pumpkin Pectic Polysaccharide. Food Hydrocoll. 2024, 155, 110238. [Google Scholar] [CrossRef]
- Li, Y.; Yu, Y.; Wu, J.; Xu, Y.; Xiao, G.; Li, L.; Liu, H. Comparison the Structural, Physicochemical, and Prebiotic Properties of Litchi Pomace Dietary Fibers before and after Modification. Foods 2022, 11, 248. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, Y.; Huang, X.; Cui, C.; Wang, W. Enhancing Functionality of Citrus Fibers from Peel and Pulp Pomace via Combined Alkaline Hydrogen Peroxide and Xylanase Modification. Food Hydrocoll. 2025, 168, 111526. [Google Scholar] [CrossRef]
- Markowiak-Kopeć, P.; Śliżewska, K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef]
- Rivas, M.Á.; Benito, M.J.; Ruíz-Moyano, S.; Martín, A.; Córdoba, M. de G.; Merchán, A.V.; Casquete, R. Improving the Viability and Metabolism of Intestinal Probiotic Bacteria Using Fibre Obtained from Vegetable By-Products. Foods 2021, 10, 2113. [Google Scholar] [CrossRef]
- Massa, N.M.L.; Menezes, F.N.D.D.; de Albuquerque, T.M.R.; de Oliveira, S.P.A.; dos Santos Lima, M.; Magnani, M.; de Souza, E.L. Effects of Digested Jabuticaba (Myrciaria jaboticaba (Vell.) Berg) by-Product on Growth and Metabolism of Lactobacillus and Bifidobacterium Indicate Prebiotic Properties. LWT 2020, 131, 109766. [Google Scholar] [CrossRef]
- Albuquerque, M.A.C.; Bedani, R.; LeBlanc, J.G.; Saad, S.M.I. Passion Fruit By-Product and Fructooligosaccharides Stimulate the Growth and Folate Production by Starter and Probiotic Cultures in Fermented Soymilk. Int. J. Food Microbiol. 2017, 261, 35–41. [Google Scholar] [CrossRef]
- Hotchkiss, A.T.; Renye, J.A.; White, A.K.; Nunez, A.; Guron, G.K.P.; Chau, H.; Simon, S.; Poveda, C.; Walton, G.; Rastall, R.; et al. Cranberry Arabino-Xyloglucan and Pectic Oligosaccharides Induce Lactobacillus Growth and Short-Chain Fatty Acid Production. Microorganisms 2022, 10, 1346. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, F.; Chai, Z.; Liu, M.; Battino, M.; Meng, X. Mixed Fermentation of Blueberry Pomace with L. rhamnosus GG and L. plantarum-1: Enhance the Active Ingredient, Antioxidant Activity and Health-Promoting Benefits. Food Chem. Toxicol. 2019, 131, 110541. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, C.; Zhang, H.; Qu, G.; Li, C.; Liu, L. Biotransformation of Polyphenols in Apple Pomace Fermented by β-Glucosidase-Producing Lactobacillus rhamnosus L08. Foods 2021, 10, 1343. [Google Scholar] [CrossRef]
- Lee, S.-B.; Cosmas, B.; Park, H.-D. The Antimutagenic and Antioxidant Activity of Fermented Milk Supplemented with Cudrania tricuspidata Powder. Foods 2020, 9, 1762. [Google Scholar] [CrossRef] [PubMed]
- Aditya, A.; Alvarado-Martinez, Z.; Nagarajan, V.; Peng, M.; Biswas, D. Antagonistic Effects of Phenolic Extracts of Chokeberry Pomace on E. coli O157: H7 but Not on Probiotic and Normal Bacterial Flora. J. Berry Res. 2019, 9, 459–472. [Google Scholar] [CrossRef]
- de Souza de Azevedo, P.O.; Aliakbarian, B.; Casazza, A.A.; LeBlanc, J.G.; Perego, P.; de Souza Oliveira, R.P. Production of Fermented Skim Milk Supplemented with Different Grape Pomace Extracts: Effect on Viability and Acidification Performance of Probiotic Cultures. PharmaNutrition 2018, 6, 64–68. [Google Scholar] [CrossRef]
- dos Santos, K.M.; de Oliveira, I.C.; Lopes, M.A.; Cruz, A.P.G.; Buriti, F.C.; Cabral, L.M. Addition of Grape Pomace Extract to Probiotic Fermented Goat Milk: The Effect on Phenolic Content, Probiotic Viability and Sensory Acceptability. J. Sci. Food Agric. 2017, 97, 1108–1115. [Google Scholar] [CrossRef]
- Dias, J.F.; Simbras, B.D.; Beres, C.; dos Santos, K.O.; Cabral, L.M.C.; Miguel, M.A.L. Acid Lactic Bacteria as a Bio-Preservant for Grape Pomace Beverage. Front. Sustain. Food Syst. 2018, 2, 392006. [Google Scholar] [CrossRef]
- Jovanović, M.; Petrović, M.; Miočinović, J.; Zlatanović, S.; Petronijević, J.L.; Mitić-Ćulafić, D.; Gorjanović, S. Bioactivity and Sensory Properties of Probiotic Yogurt Fortified with Apple Pomace Flour. Foods 2020, 9, 763. [Google Scholar] [CrossRef] [PubMed]
- Mileriene, J.; Serniene, L.; Kasparaviciene, B.; Lauciene, L.; Kasetiene, N.; Zakariene, G.; Kersiene, M.; Leskauskaite, D.; Viskelis, J.; Kourkoutas, Y.; et al. Exploring the Potential of Sustainable Acid Whey Cheese Supplemented with Apple Pomace and GABA-Producing Indigenous Lactococcus lactis Strain. Microorganisms 2023, 11, 436. [Google Scholar] [CrossRef]
- Lele, V.; Ruzauskas, M.; Zavistanaviciute, P.; Laurusiene, R.; Rimene, G.; Kiudulaite, D.; Tomkeviciute, J.; Nemeikstyte, J.; Stankevicius, R.; Bartkiene, E. Development and Characterization of the Gummy–Supplements, Enriched with Probiotics and Prebiotics. CyTA-J. Food 2018, 16, 580–587. [Google Scholar] [CrossRef]
- Acharjee, A.; Afrin, S.M.; Sit, N. Physicochemical, Textural, and Rheological Properties of Yoghurt Enriched with Orange Pomace Powder. J. Food Process Preserv. 2021, 45, e15193. [Google Scholar] [CrossRef]
- Ayar, A.; Siçramaz, H.; Öztürk, S.; Öztürk Yilmaz, S. Probiotic Properties of Ice Creams Produced with Dietary Fibres from By-Products of the Food Industry. Int. J. Dairy. Technol. 2018, 71, 174–182. [Google Scholar] [CrossRef]
- Meral, H.; Savaş, İ.; Çiçek, Ş.K.; Demirdöven, A. Peach Pomace: A Potential Probiotic Carrier for Fiber Enrichment in Milk. J. Food Meas. Charact. 2024, 18, 1933–1946. [Google Scholar] [CrossRef]
- Buvaneswaran, M.; Sunil, C.K.; Rawson, A.; Vidyalakshmi, R.; Venkatachalapathy, N. Physicochemical, Sugar Profile, Probiotic Viability, and E-Nose Aroma Profile of Probiotic Beverage Enriched with Pineapple Pomace. Food Humanit. 2023, 1, 1404–1412. [Google Scholar] [CrossRef]
- Hurtado-Romero, A.; Zepeda-Hernández, A.; Uribe-Velázquez, T.; Rosales-De la Cruz, M.F.; Raygoza-Murguía, L.V.; Garcia-Amezquita, L.E.; García-Cayuela, T. Utilization of Blueberry-Based Ingredients for Formulating a Synbiotic Petit Suisse Cheese: Physicochemical, Microbiological, Sensory, and Functional Characterization during Cold Storage. LWT 2023, 183, 114955. [Google Scholar] [CrossRef]
- Pimisa, R.; Bankeeree, W.; Maneerat, S.; Prasongsuk, S. Extraction and Characterization of Pectin from Passion Fruit Peel, and Its Application in Synbiotic Ice Cream: A Study from Phetchabun, Thailand. Future Foods 2024, 10, 100510. [Google Scholar] [CrossRef]
- Tamašauskaitė, L.; Minelgaitė, V.; Šipailienė, A.; Vinauskienė, R.; Eisinaitė, V.; Leskauskaitė, D. Bigel Matrix Loaded with Probiotic Bacteria and Prebiotic Dietary Fibers from Berry Pomace Suitable for the Development of Probiotic Butter Spread Product. Gels 2024, 10, 349. [Google Scholar] [CrossRef]
- Sharifi, Z.; Jebelli Javan, A.; Hesarinejad, M.A.; Parsaeimehr, M. Application of Carrot Waste Extract and Lactobacillus plantarum in Alyssum homalocarpum Seed Gum-Alginate Beads to Create a Functional Synbiotic Yogurt. Chem. Biol. Technol. Agric. 2023, 10, 3. [Google Scholar] [CrossRef]
- Alamoudi, S.A.; Saad, A.M.; Alsubhi, N.H.; Alrefaei, G.I.; Al-Quwaie, D.A.; Binothman, N.; Aljadani, M.; Alharbi, M.; Alanazi, H.; Babalghith, A.O.; et al. Upgrading the Physiochemical and Sensory Quality of Yogurt by Incorporating Polyphenol-Enriched Citrus Pomaces with Antioxidant, Antimicrobial, and Antitumor Activities. Front. Nutr. 2022, 9, 999581. [Google Scholar] [CrossRef]
- Domínguez Díaz, L.; Fernández-Ruiz, V.; Cámara, M. An International Regulatory Review of Food Health-Related Claims in Functional Food Products Labeling. J. Funct. Foods 2020, 68, 103896. [Google Scholar] [CrossRef]
- Precup, G.; Marini, E.; Zakidou, P.; Beneventi, E.; Consuelo, C.; Fernández-Fraguas, C.; Garcia Ruiz, E.; Laganaro, M.; Magani, M.; Mech, A.; et al. Novel Foods, Food Enzymes, and Food Additives Derived from Food by-Products of Plant or Animal Origin: Principles and Overview of the EFSA Safety Assessment. Front. Nutr. 2024, 11, 1390734. [Google Scholar] [CrossRef]
- Rao, M.; Bast, A.; de Boer, A. Valorized Food Processing By-Products in the EU: Finding the Balance between Safety, Nutrition, and Sustainability. Sustainability 2021, 13, 4428. [Google Scholar] [CrossRef]
- Garg, V.; Velumani, D.; Lin, Y.-C.; Haye, A. A Comprehensive Review of Probiotic Claims Regulations: Updates from Asia-Pacific, United States, and Europe. PharmaNutrition 2024, 30, 100423. [Google Scholar] [CrossRef]
- Qualified Presumption of Safety (QPS) | EFSA. Available online: https://www.efsa.europa.eu/en/topics/topic/qualified-presumption-safety-qps (accessed on 19 May 2025).
- Hoffmann, D.E. Health Claim Regulation of Probiotics in the USA and the EU: Is There a Middle Way? Benef. Microbes 2013, 4, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Schmelz, R.M. Regulation of Probiotic and Other Live Biologic Products. In Skin Microbiome Handbook: From Basic Research to Product Development; Scrivener Publishing LLC: Beverly, MA, USA, 2020; pp. 343–375. [Google Scholar] [CrossRef]
| DF Type | Physiological Properties | Technological Properties | Impact on Probiotics | References |
|---|---|---|---|---|
| Pectin | Lowers cholesterol, delays gastric emptying, improves glucose metabolism, stimulates bile acid excretion | High water-holding capacity, forms thermoreversible gels | Highly fermentable by gut microbes; enhances SCFA production and growth of Bifidobacterium and Lactobacillus in vitro | [32,75,76,81,82,83,84,85] |
| Inulin | Promotes mineral absorption, improves lipid metabolism, relieves constipation | Low viscosity, fat replacement, freeze–thaw stable | Highly fermentable; strong bifidogenic effect with increased SCFA production | [75,81,82,83,84,86,87] |
| Cellulose | Increases fecal bulk, reduces transit time, acts as a laxative | Insoluble, low water-holding capacity, texture modifier | Poorly fermentable; passes to colon largely intact, supporting microbial diversity indirectly | [81,82,84,87] |
| Hemicellulose | Supports bowel regularity, partial fermentation yields SCFAs | Moderate water-holding, viscosity development, could be used as a viscosity-increasing and stabilizing agent | Supports diverse microbiota via SCFA production | [81,82,84,88] |
| Lignin | Provides fecal bulking, reduces transit time, antioxidant activity | High oil-holding, textural integrity | Non-fermentable; negligible impact on probiotic bacteria | [76,81,84] |
| Oligosaccharides | Enhanced mineral absorption, reduced caloric density, improves gut barrier function | High solubility, low viscosity, sweetness enhancement | Strong prebiotic effect; selectively stimulates bifidobacteria | [75,76,81,82,83,84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jagelavičiūtė, J.; Bašinskienė, L.; Čižeikienė, D. Enhancing the Nutritional Value of Foods Through Probiotics and Dietary Fiber from Fruit and Berry Pomace. Fermentation 2025, 11, 481. https://doi.org/10.3390/fermentation11080481
Jagelavičiūtė J, Bašinskienė L, Čižeikienė D. Enhancing the Nutritional Value of Foods Through Probiotics and Dietary Fiber from Fruit and Berry Pomace. Fermentation. 2025; 11(8):481. https://doi.org/10.3390/fermentation11080481
Chicago/Turabian StyleJagelavičiūtė, Jolita, Loreta Bašinskienė, and Dalia Čižeikienė. 2025. "Enhancing the Nutritional Value of Foods Through Probiotics and Dietary Fiber from Fruit and Berry Pomace" Fermentation 11, no. 8: 481. https://doi.org/10.3390/fermentation11080481
APA StyleJagelavičiūtė, J., Bašinskienė, L., & Čižeikienė, D. (2025). Enhancing the Nutritional Value of Foods Through Probiotics and Dietary Fiber from Fruit and Berry Pomace. Fermentation, 11(8), 481. https://doi.org/10.3390/fermentation11080481






