Collagenase Production from Aspergillus serratalhadensis URM 7866 Using Industrial By-Products: Purification and Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism
2.2. Solid State Fermentation (SSF)
2.3. Determination of Collagenolytic Activity and Total Protein
2.4. Purification Steps of Collagenolytic Enzymes
2.4.1. Organic Solvent Precipitation
2.4.2. Chromatography
2.4.3. Electrophoresis
2.5. Effect of pH and Temperature on Enzyme Activity and Stability
2.6. Effect of Inhibitors on Activity
2.7. Effect of Metal Ions on Collagenolytic Activity
2.8. Effect of Surfactants on Collagenolytic Activity
2.9. Fourier Transform Infrared Spectroscopy (FTIR)
2.10. Data Analysis
3. Results and Discussion
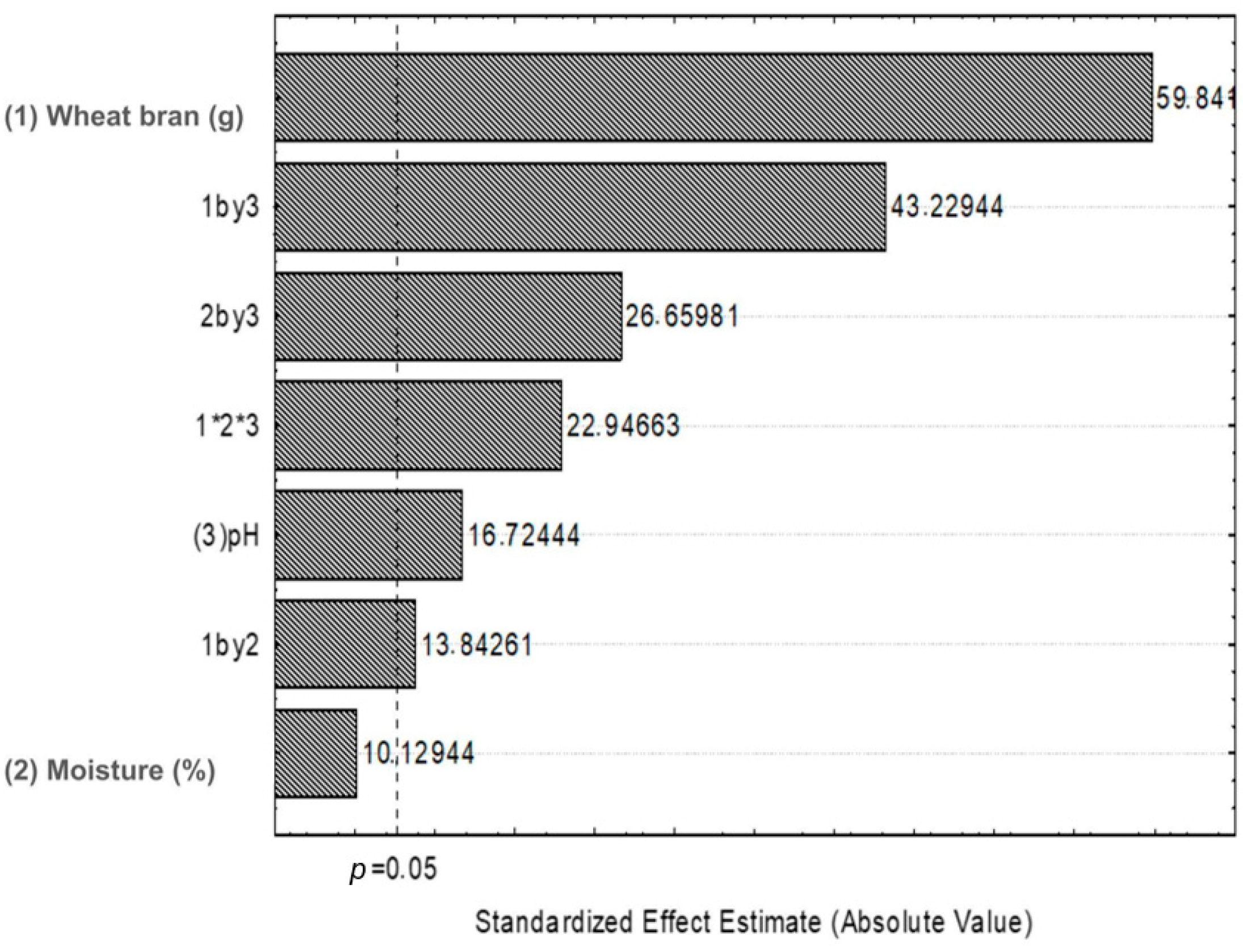
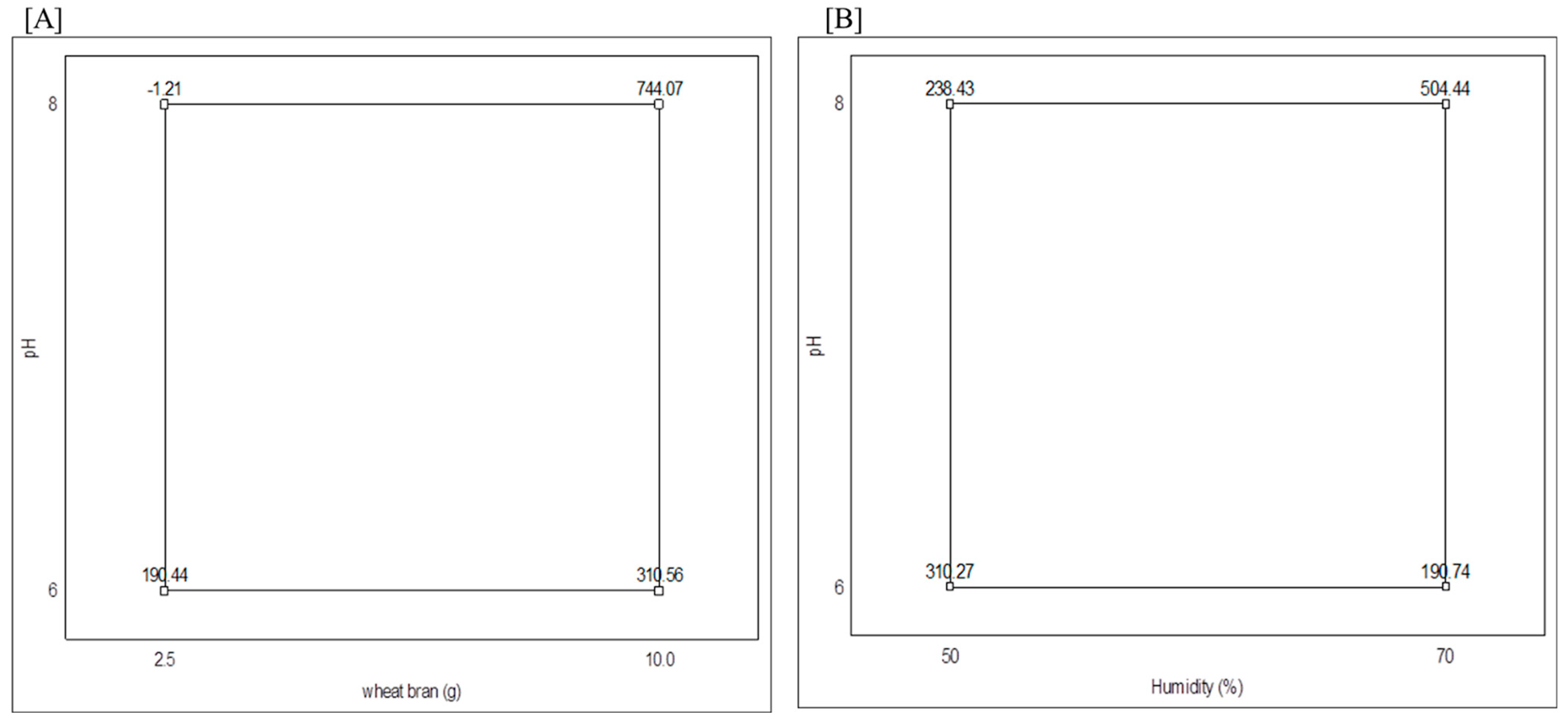
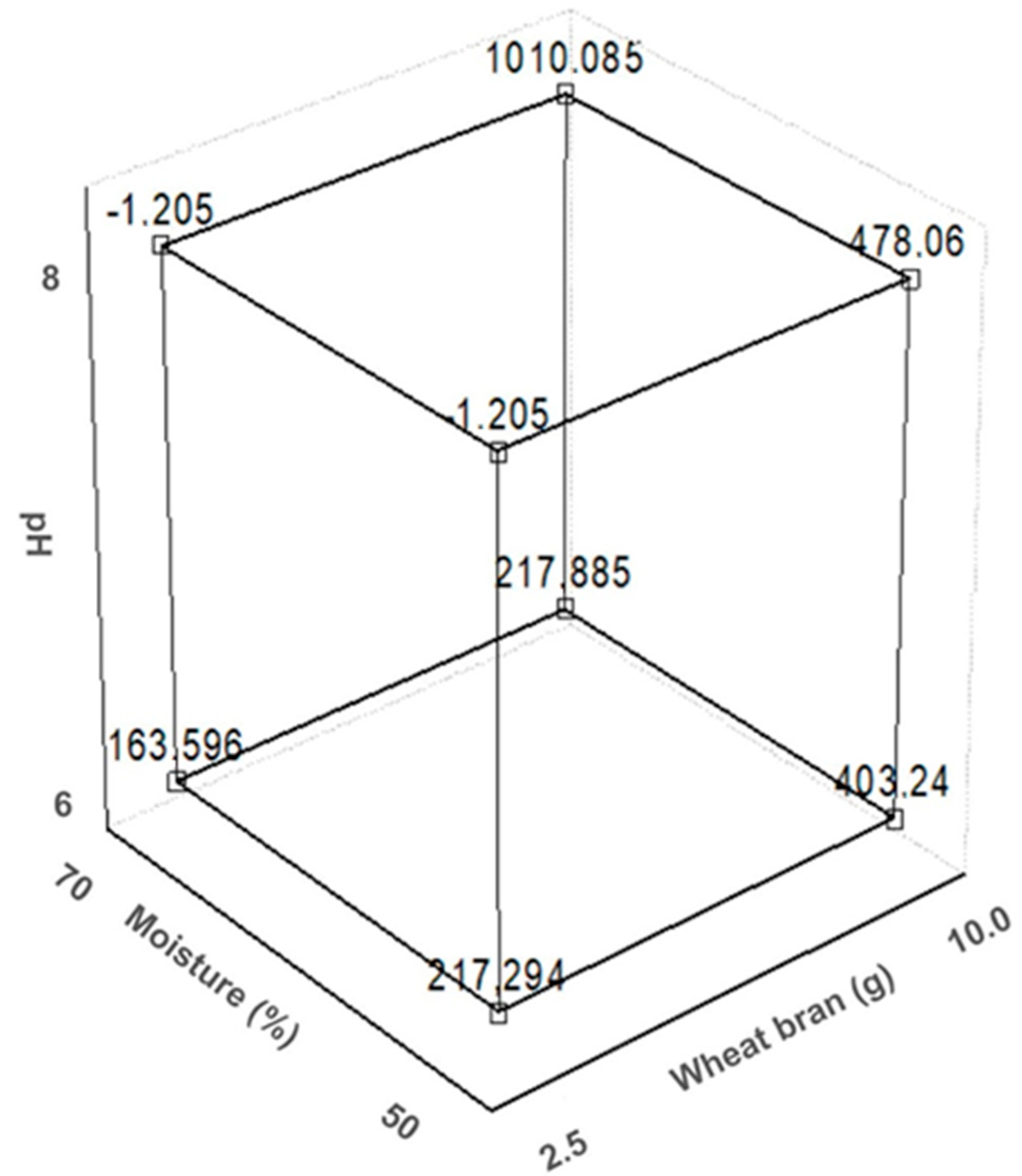
3.1. Collagenase Production
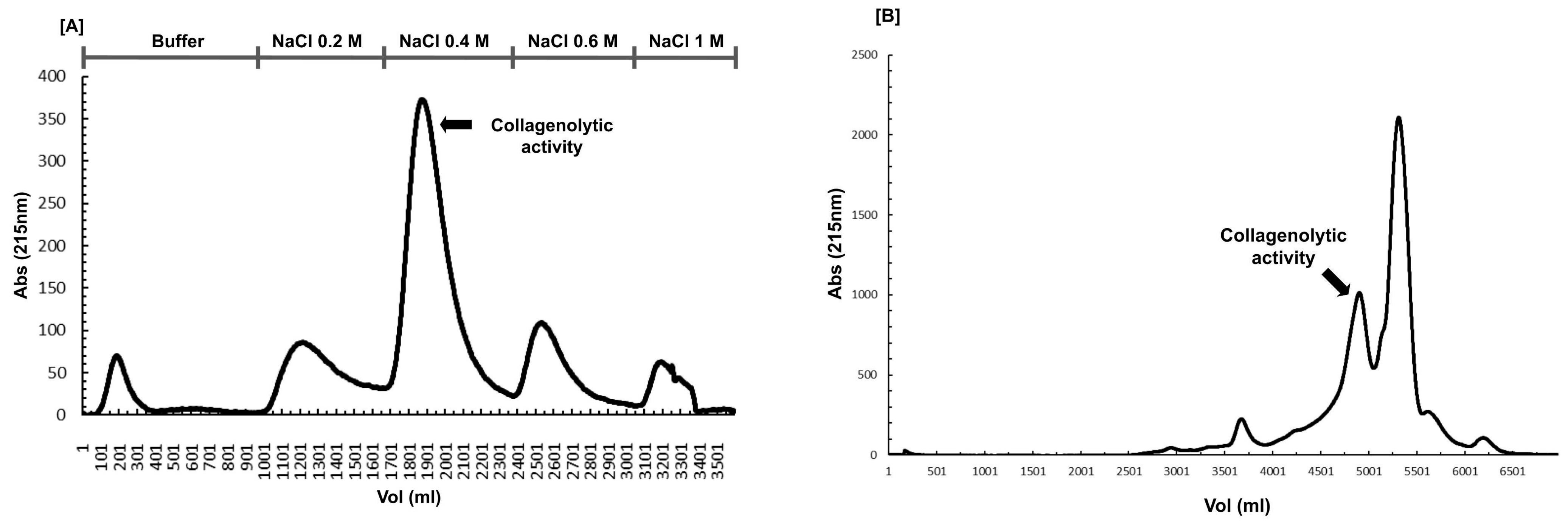
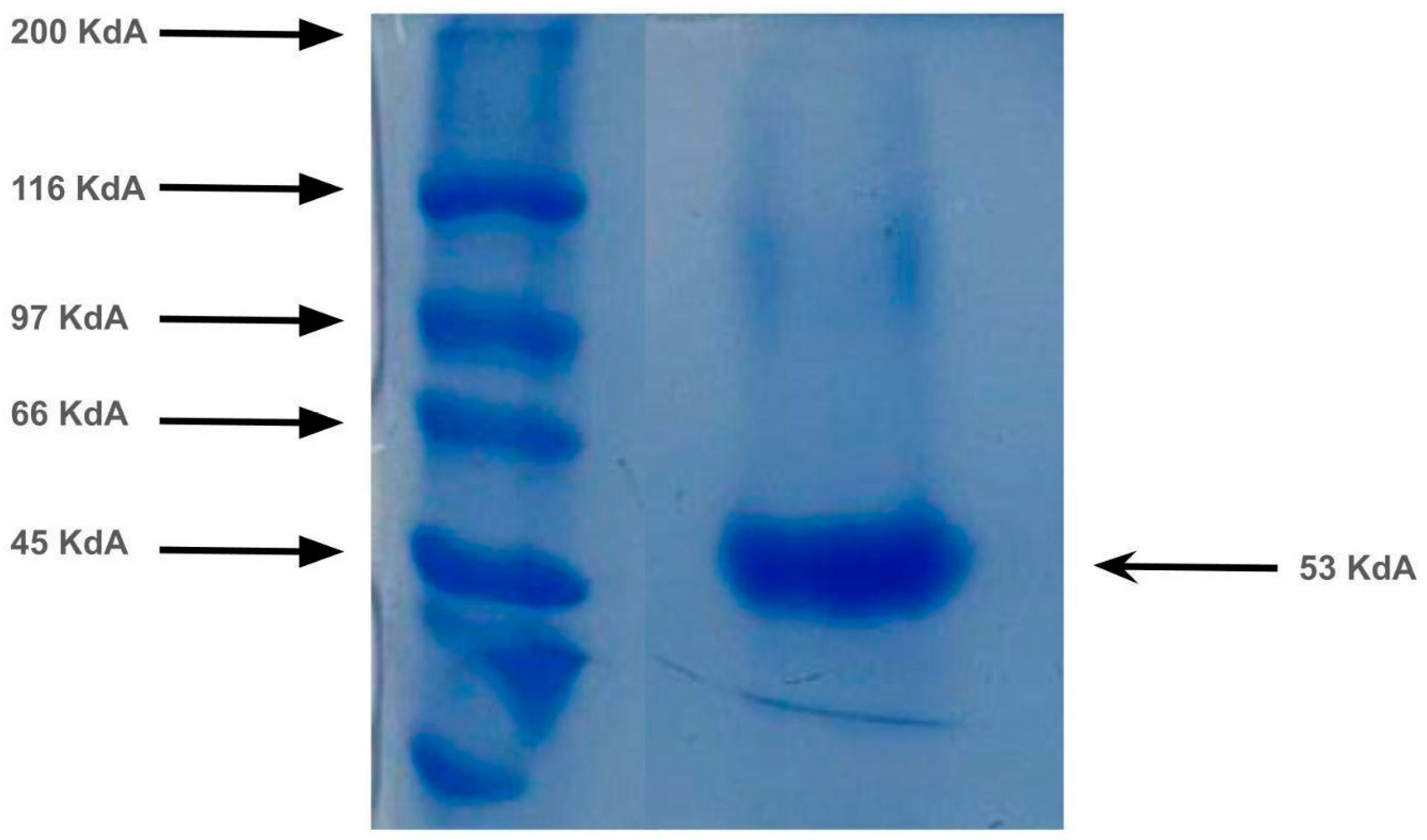
3.2. Collagenase Purification
3.3. Enzyme Characterization
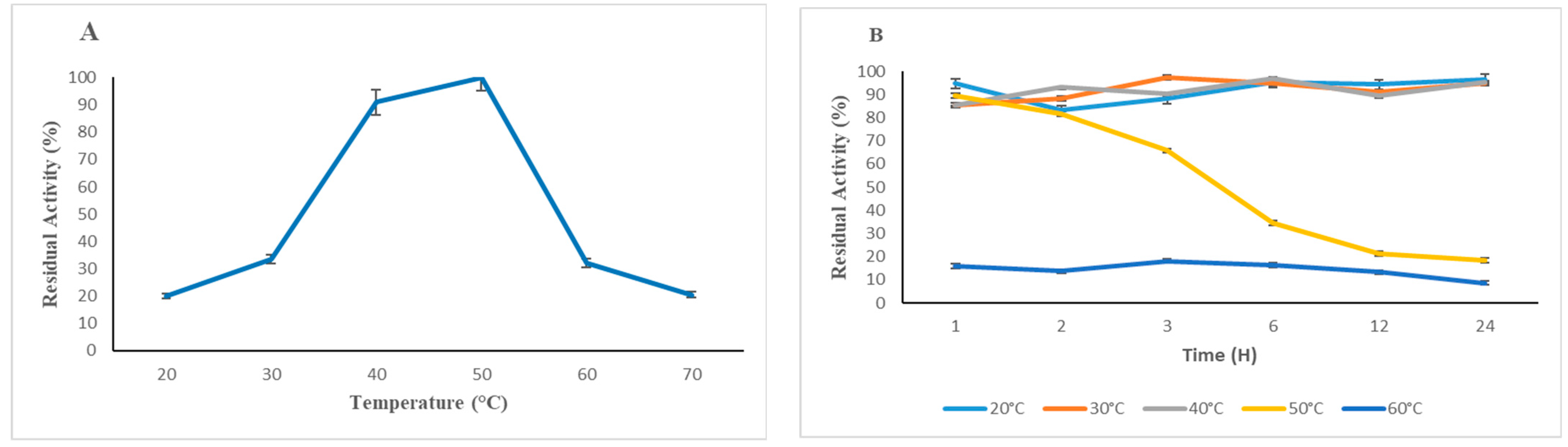
3.3.1. Effects of Temperature and pH on Enzymatic Activity and Stability
3.3.2. Effect of Inhibitors and Surfactants on Collagenolytic Activity
3.3.3. Effect of Metal Ions on Collagenolytic Activity
3.4. Fourier Transform Infrared Spectroscopy (FTIR) Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arasu, V.M.; Choi, K.C.; Sathya, I.; Soundharrajan, I.; Rejiniemon, T.S.; Ponnuswamy, V.R.; Balamuralikrishnan, A.; Pri-yanka, A. Perspectives of microbial enzyme biocatalyst in food industries. In Microbial Oxidative Enzymes: Biotechnological Applications; De Gruyter: Berlin, Germany, 2023; pp. 257–279. [Google Scholar] [CrossRef]
- Peng, S.; Xue, Z.; Wang, S.; Xu, W.; Wang, F.; Fu, R.; Wei, F. Microbial proteases and their applications. Front. Microbiol. 2023, 14, 1236368–1236382. [Google Scholar] [CrossRef] [PubMed]
- Arya, P.; Jani, S.A.; Rajput, K.N.; Raval, V.H. Thermostable alkaline proteases from bacteria: A review. SSRN Electron. J. 2020. [Google Scholar] [CrossRef]
- Mokrani, S.; Nabti, E.H. Recent status in production, biotechnological applications, commercial aspects, and future prospects of microbial enzymes: A comprehensive review. Int. J. Agric. Sci. Food Technol. 2024, 10, 6–20. [Google Scholar] [CrossRef]
- Blieva, R.K.; Kalieva, A.K.; Suleimenova, Z.B.; Zhakipbekova, A.S.; Tapenbayeva, I.E. Screening of Aspergillus fungi for extracellular protease and collagenase production. Rep. Natl. Acad. Sci. Kazakhst. 2020, 2, 36–40. [Google Scholar] [CrossRef]
- Barbosa, R.N.; Bezerra, J.D.P.; Santos, A.C.S.; Melo, R.F.R.; Houbraken, J.; Oliveira, N.T.; Souza-Motta, C.M. Brazilian tropical dry forest (Caatinga) in the spotlight: An overview of species of Aspergillus, Penicillium and Talaromyces (Eurotiales) and the description of P. vascosobrinhous sp. nov. Acta Bot. Bras. 2020, 34, 409–429. [Google Scholar] [CrossRef]
- Bernal, S.P.F.; Gritti, M.A.; Santos, V.P.D.; Ottoni, J.R.; de Oliveira, V.M.; Peichoto, M.E.; Passarini, M.R.Z. Pharmaceutical biotechnological potential of filamentous fungi isolated from textile industry. Arch. Microbiol. 2021, 203, 3933–3944. [Google Scholar] [CrossRef]
- Da Rocha, W.R.V.; Agamez-Montalvo, G.S.; Feitosa, V.A.; Machado, S.E.F.; De Souza Lima, G.M.; Pessoa, A., Jr.; Alves, H.S. Screening and optimizing fermentation production of L-asparaginase by Aspergillus terreus strain S-18 isolated from the Brazilian Caatinga Biome. J. Appl. Microbiol. 2019, 126, 1426–1437. [Google Scholar] [CrossRef]
- Barzkar, N.; Babich, O.; Sukhikh, S.; Venmathi Maran, B.A.; Tamadoni Jahromi, S.; Luwor, R.B.; Sorsa, T.; Das, R. Exploring the sources and potential applications of marine collagenases. Biocatal. Agric. Biotechnol. 2024, 58, 103150. [Google Scholar] [CrossRef]
- Wang, Z.-Z.; Wang, K.; Xu, L.-F.; Su, C.; Gong, J.-S.; Shi, J.-S.; Ma, X.-D.; Xie, N.; Qian, J.-Y. Unlocking the potential of collagenases: Structures, functions, and emerging therapeutic horizons. BioDesign Res. 2024, 6, 0050. [Google Scholar] [CrossRef]
- Gao, B.; Tan, C.; Roshani, D.; Yang, R.; Lv, Z.; Li, P.; Shang, N. Microbial collagenases: An updated review on their characterization, degradation mechanisms, and current applications. Crit. Rev. Food Sci. Nutr. 2024, 1–25. [Google Scholar] [CrossRef]
- Luo, J.; Zhang, Z.; Zeng, Y.; Dong, Y.; Ma, L. Co-encapsulation of collagenase type I and silibinin in chondroitin sulfate coated multilayered nanoparticles for targeted treatment of liver fibrosis. Carbohydr Polym. 2021, 263, 117964. [Google Scholar] [CrossRef]
- Silva, R.L.; de Oliveira, C.M.; Souza-Motta, A.L.F.; Porto, T.S. Novel prote-ase from Aspergillus tamarii URM4634: Produção e caracterização utilizando substratos agroindustriais baratos por fermentação em estado sólido. Adv. Enzima Res. 2016, 4, 125–143. [Google Scholar] [CrossRef]
- Chavira, R.; Burnett, T.J.; Hageman, J.H. Ensaio de proteinases com Azocoll. Anal. Bioquímica. 1984, 136, 446–450. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Medição de proteína usando ácido bicinconínico. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Nickerson, J.L.; Baghalabadi, V.; Dang, Z.; Miller, V.A.; Little, S.L.; Doucette, A.A. Organic solvent-based protein precipitation for robust proteome purification ahead of mass spectrometry. J. Vis. Exp. 2022, 180, 63503. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Kango, N.; Jana, U.K.; Choukade, R. Fungal enzymes: Sources and biotechnological applications. In Fungal Enzymes: Sources and Biotechnological Applications; Springer: Singapore, 2019; pp. 515–538. [Google Scholar] [CrossRef]
- Jin, G.; Boeschoten, S.; Hageman, J.; Zhu, Y.; Wijffels, R.; Rinzema, A.; Xu, Y. Identifying variables influencing traditional food solid-state fermentation by statistical modeling. Foods 2024, 13, 1317. [Google Scholar] [CrossRef]
- de Souza, E.P.; da Silva Araújo, T.; Ramos, D.G.; da Silva Santos, H.L.; de Correia, A.C.C.; Bezerra, R.P.; Porto, A.L.F.; Converti, A.; de Araújo Viana Marques, D.; de Albuquerque, P.B.S.; et al. Production and partial characterization of collagenase from Rhizopus microsporus UCP 1296: Cytotoxic potential and controlled release strategy. J. Polym. Environ. 2025, 1–15. [Google Scholar] [CrossRef]
- Silva, O.S.; de Almeida, E.M.; de Melo, A.H.F.; Porto, T.S. Purification and characterization of a novel extracellular serine-protease with collagenolytic activity from Aspergillus tamarii URM4634. Int. J. Biol. Macromol. 2018, 117, 1081–1088. [Google Scholar] [CrossRef]
- Mbira, C. Influence of substrate concentration on enzymatic activity in biocatalysis. J. Chem. 2024, 3, 48–58. [Google Scholar] [CrossRef]
- Colla, L.M.; Reinehr, C.O.; Manfredini, P.G.; Cavanhi, V.A.F.; Costa, J.A.V. Simultaneous production of proteases and antioxidant biopeptides by solid-state fermentation. Sustain. Food Technol. 2023, 1, 874–885. [Google Scholar] [CrossRef]
- Al Shimmery, M.A.A.; Authman, S.H.; Abo Ksou, M.F. Production, purification and determination of molecular weight of coagulase enzyme from Staphylococcus aureus isolated from clinical samples. J. Med. Genet. Clin. Biol. 2024, 1, 359–370. [Google Scholar] [CrossRef]
- Sinitsyna, O.A.; Volkov, P.V.; Zorov, I.N.; Rozhkova, A.M.; Emshanov, O.V.; Romanova, Y.M.; Komarova, B.S.; Novikova, N.S.; Nifantiev, N.E.; Sinitsyn, A.P. Physico-chemical properties and substrate specificity of α-(1→3)-d-glucan degrading recombinant mutanase from Trichoderma harzianum expressed in Penicillium verruculosum. Appl. Environ. Microbiol. 2025, 91, e00226-24. [Google Scholar] [CrossRef]
- Novelli, P.K.; Barros, M.M.; Fleuri, L.F. Novel inexpensive fungi proteases: Production by solid state fermentation and characterization. Food Chem. 2016, 198, 119–124. [Google Scholar] [CrossRef]
- Costa-Junior, R.B.; Brandão-Costa, R.M.P.; Albuquerque, W.W.C.; Batista, J.M.S.; Pedrosa, R.B.; Porto, A.L.F. Collagenase produced from Aspergillus sp. (UCP 1276) using chicken feather industrial residue. Biomed. Chromatogr. 2016, 31, e3863. [Google Scholar] [CrossRef]
- Costa, E.P.; Brandão-Costa, R.M.P.; Albuquerque, W.W.C.; Nascimento, T.P.; Conniff, A.E.S.; Cardoso, K.B.B.; Neves, A.G.D.; Batista, J.M.S.; Porto, A.L.F. Extracellular collagenase isolated from Streptomyces antibioticus UFPEDA 3421: Purification and biochemical characterization. Prep. Biochem. Biotechnol. 2023, 54, 260–271. [Google Scholar] [CrossRef]
- Li, Y.; Luo, F.; Peng, B.; Xu, B. Impact of typical surfactants on the collagenolytic and elastinolytic activities of proteases. J. Am. Leather Chem. Assoc. 2015, 110, 227–236. [Google Scholar]
- Sattar, H.; Aman, A.; Qader, S.A.U. Effect of metal ions, solvents and surfactants on the activity of protease from Aspergillus niger KIBGE-IB36. J. Basic Appl. Sci. 2017, 13, 491–495. [Google Scholar] [CrossRef]
- Ye, K.; Xie, T.; Wei, M.; Fan, Y.; Yu, J. Effects of Disulfide Bond and Ca2+ binding Residue on Recombinant Alkaline Protease from Aspergillus Sojae. J. Fujian Agric. For. Univ. 2019, 48, 813–818. [Google Scholar] [CrossRef]
- Jamal, H.S.; Raja, R.; Ahmed, S.; Yesiloz, G.; Ali, S.A. Immobilization of collagenase in inorganic hybrid nanoflowers with enhanced stability, proteolytic activity, and their anti- amyloid potential. Int. J. Biol. Macromol. 2024, 274, 133114. [Google Scholar] [CrossRef]
- Oh, K.-I.; Baiz, C.R. Empirical S=O stretch vibrational frequency map. J. Chem. Phys. 2019, 151, 234107. [Google Scholar] [CrossRef]
- Vijay, L.A.; Kannan, K. Analytical approaches in unveiling the compatibility of collagenase with additives in view of its colloidal delivery. J. Chil. Chem. Soc. 2019, 64, 4558–4564. [Google Scholar] [CrossRef]


| Factors | Coded Levels | ||
|---|---|---|---|
| −1 | 0 | +1 | |
| (1) Substrate concentration (%) | 2.5 | 5.0 | 10.0 |
| (2) Moisture (%) | 50.0 | 60.0 | 70.0 |
| (3) pH | 6.0 | 7.0 | 8.0 |
| Step | Protein Content (mg/mL) | Collagenolytic Activity (U/mL) | Specific Collagenolytic Activity (U/mg) | Yield (%) | Purification Factor |
|---|---|---|---|---|---|
| Crude extract | 3.52 ± 0.01 | 177.96 ± 0.09 | 50.55 | 100.00 | 1.00 |
| Acetone 70% precipitation | 0.13 ± 0.03 | 118.88 ± 0.07 | 914.52 | 3.69 | 18.09 |
| Purification in AKTA Pure | 0.11 ± 0.01 | 112.40 ± 0.05 | 1021.86 | 3.12 | 20.21 |
| Inhibitor (10 mM) | Relative Activity (%) | |
|---|---|---|
| Control | 100.00 ± 0.05 | |
| EDTA | 65.9 ± 0.01 | |
| PMSF | 18.1 ± 0.01 | |
| Pepstatin A | 99.9 ± 0.02 | |
| Iodoacetic Acid | 99.1 ± 0.02 | |
| Surfactants | (1%) | (10%) |
| SDS | 100.00 ± 0.09 | 100.00 ± 0.08 |
| Tween 80 | 90.25 ± 0.09 | 85.21 ± 0.07 |
| Tween 20 | 100.00 ± 0.1 | 91.71 ± 0.09 |
| Triton X-100 | 95.79 ± 0.06 | 89.79 ± 0.10 |
| Relative Activity (%) | ||
|---|---|---|
| Ion 5 mM | Ion 10 mM | |
| Control | 100 ± 0.05 | 100 ± 0.005 |
| K | 103 ± 0.30 | 98 ± 0.05 |
| NaCl | 134 ± 0.06 | 139 ± 0.02 |
| Mg | 100 ± 0.09 | 100 ± 0.10 |
| Cu | 62 ± 0.07 | 49 ± 0.06 |
| Ca | 118 ± 0.02 | 125± 0.08 |
| Fe | 60 ± 0.10 | 94 ± 0.09 |
| Zn | 52 ± 0.06 | 37 ± 0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lino, L.H.S.; Cardoso, K.B.B.; da Silva, P.G.O.C.; Silva, R.L.A.; de Miranda, M.E.L.C.; Macêdo, D.C.d.S.; Porto, A.L.F.; de Souza Motta, C.M.; da Cunha, M.N.C.; Nascimento, T.P.; et al. Collagenase Production from Aspergillus serratalhadensis URM 7866 Using Industrial By-Products: Purification and Characterization. Fermentation 2025, 11, 478. https://doi.org/10.3390/fermentation11080478
Lino LHS, Cardoso KBB, da Silva PGOC, Silva RLA, de Miranda MELC, Macêdo DCdS, Porto ALF, de Souza Motta CM, da Cunha MNC, Nascimento TP, et al. Collagenase Production from Aspergillus serratalhadensis URM 7866 Using Industrial By-Products: Purification and Characterization. Fermentation. 2025; 11(8):478. https://doi.org/10.3390/fermentation11080478
Chicago/Turabian StyleLino, Luiz Henrique Svintiskas, Kethylen Barbara Barbosa Cardoso, Pietra Gícia Oliveira Cosmo da Silva, Raphael Luiz Andrade Silva, Maria Eduarda Luiz Coelho de Miranda, Daniel Charles dos Santos Macêdo, Ana Lúcia Figueiredo Porto, Cristina Maria de Souza Motta, Marcia Nieves Carneiro da Cunha, Thiago Pajéu Nascimento, and et al. 2025. "Collagenase Production from Aspergillus serratalhadensis URM 7866 Using Industrial By-Products: Purification and Characterization" Fermentation 11, no. 8: 478. https://doi.org/10.3390/fermentation11080478
APA StyleLino, L. H. S., Cardoso, K. B. B., da Silva, P. G. O. C., Silva, R. L. A., de Miranda, M. E. L. C., Macêdo, D. C. d. S., Porto, A. L. F., de Souza Motta, C. M., da Cunha, M. N. C., Nascimento, T. P., de Albuquerque Lima Duarte, C., Costa, R. M. P. B., & Viana Marques, D. d. A. (2025). Collagenase Production from Aspergillus serratalhadensis URM 7866 Using Industrial By-Products: Purification and Characterization. Fermentation, 11(8), 478. https://doi.org/10.3390/fermentation11080478








