Banana Pseudostem By-Product: A Sustainable Source of Prebiotics and Protection for Probiotic Lactic Acid Bacteria Under Gastrointestinal Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Obtaining the BPS By-Product
2.2. Preparation of BPS Flour
2.3. Characterization of the BPS Flour
2.3.1. Determination of Total Starch and Resistant Starch in the BPS Flour
2.3.2. Analyses of Lignin and Carbohydrates in the BPS Flour
2.3.3. Characterization of Bioactive Compounds and Antioxidant Activity of BPS Flour
2.4. Starch Extraction from BPS By-Product
2.5. Microbiological Analysis
2.5.1. Strains
2.5.2. Pre-Inoculum Preparation
2.5.3. Fermentation with Different Carbon Sources
2.5.4. Growth of Probiotic Microorganisms in CFCM
2.6. LAB Carbohydrate Fermentation and Enzyme Activities Profiles
2.7. INFOGEST Static Model for Simulation of Gastrointestinal Digestion
2.8. Statistical Analysis
3. Results and Discussion
3.1. Characterization of BPS By-Products
3.2. Effects of Prebiotics on LAB Count
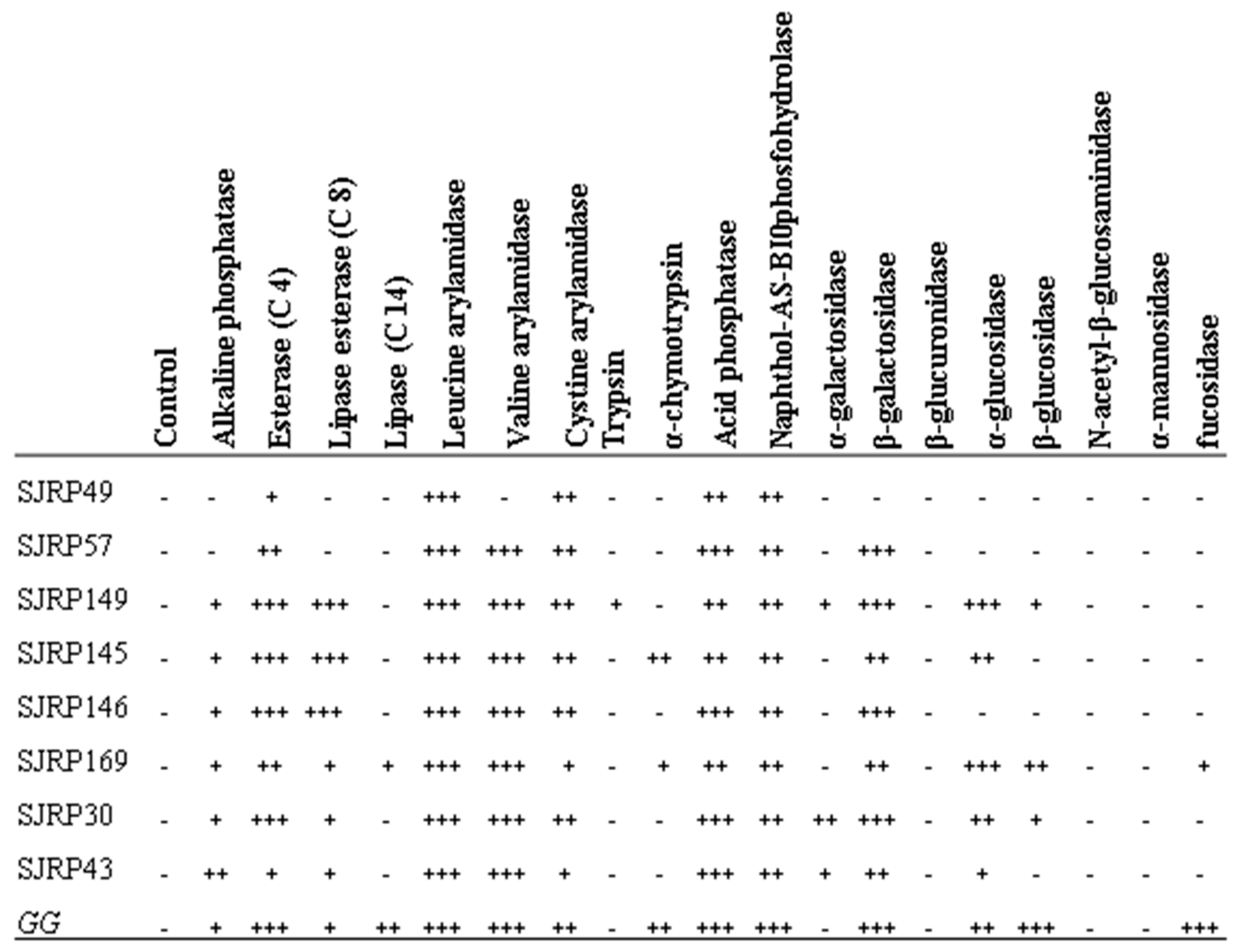
3.3. Enzyme Production Assay Using API ZYM Kit
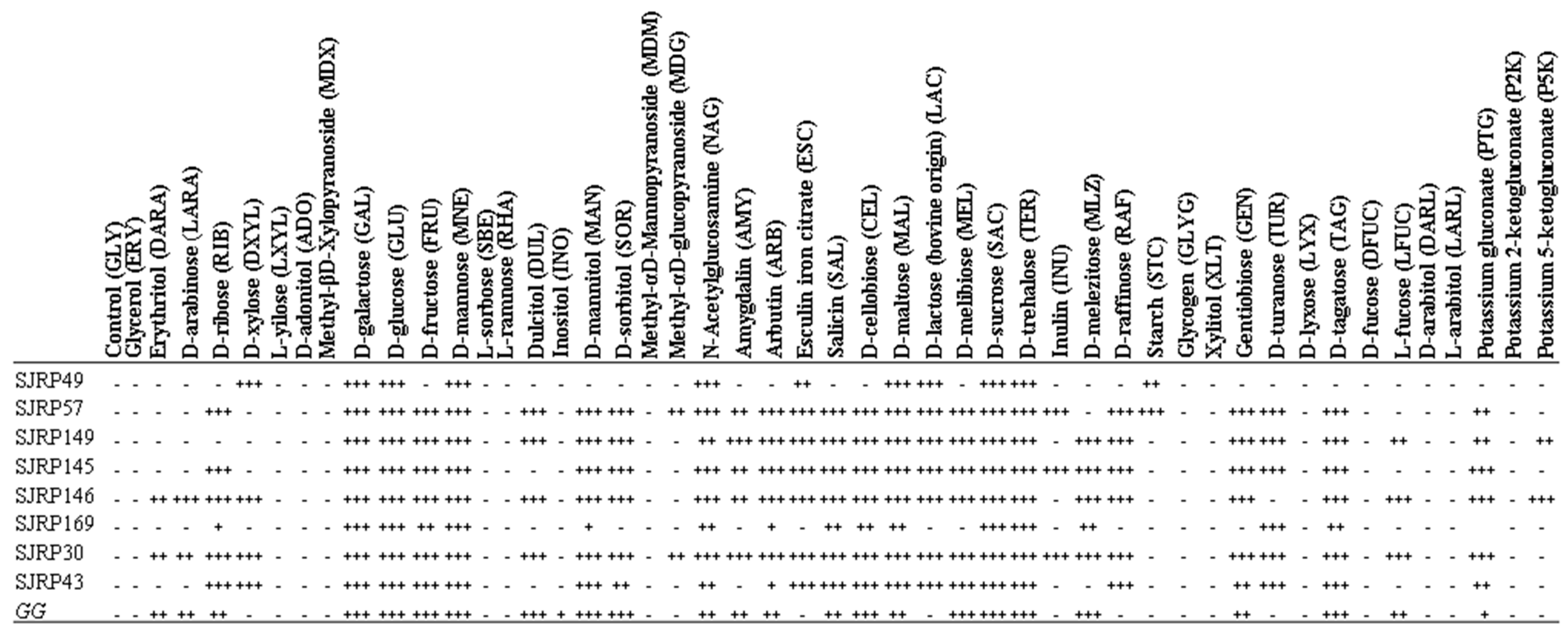
3.4. Carbohydrate Utilization Assay Using API 50 CHL Kit
3.5. In Vitro Simulation of Gastrointestinal Digestion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Freitas, C. Production of Xylo-Oligosaccharides by Different Treatments of Banana Pseudostem and Their Prebiotic Evaluation via Probiotic Bacteria Growth and Their Metabolites. Ph.D. Thesis, Universidade Estadual Paulista (UNESP), Rio Claro, Brazil, 2024. [Google Scholar]
- Huang, J.; Wang, J.; Liu, S. Advanced fermentation techniques for lactic acid production from agricultural waste. Fermentation 2023, 9, 765. [Google Scholar] [CrossRef]
- Xavier, J.R.; Nallamuthu, I.; Murugan, M.P.; Chauhan, O.P. Optimisation of lactic acid production using cost effective agro residue for food applications. Sustain. Food Technol. 2024, 2, 741–749. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. Medium-Term Outlook: Prospects for Global Production and Trade in Bananas and Tropical Fruits 2019 to 2028; FAO: Rome, Italy, 2020. [Google Scholar]
- Yasin, M.; Gangan, S.; Panchal, S.K. Banana Peels: A Genuine Waste or a Wonderful Opportunity? Appl. Sci. 2025, 15, 3195. [Google Scholar] [CrossRef]
- Ho, L.H.; Tan, T.C.; Abdul, A.N.A.; Bhat, R. In vitro starch digestibility of bread with banana (Musa acuminata X balbisiana ABB cv. Awak) Pseudo-Stem Flour hydrocolloids. Food Biosci. 2015, 12, 10–17. [Google Scholar]
- Ghutke, T.D.; Bhalerao, V.P.; Mendhe, A.R.; Kadam, R.G. Recycling of banana pseudostem waste, it’s effective method for decomposition and nutrient analysis. Int. J. Adv. Biochem. Res. 2024, 8, 286–292. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Salminen, S.J.; Scott, K.; Stanton, C.; Cani, P.D.; Verbeke, K.; Reid, G. The International Scientific Association and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; Jung, S.C.; Kwak, K.; Kim, J.S. The Role of Prebiotics in Modulating Gut Microbiota: Implications for Human Health. Int. J. Mol. Sci. 2024, 25, 4834. [Google Scholar] [CrossRef]
- Silva, A.S.; Casarotti, S.N.; Penna, A.L.B. Trends and challenges for the application of probiotic lactic acid bacteria in functional foods. Ciênc. Rural. 2024, 54, e20230014. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Handbook of Prebiotics, 1st ed.; CRC Press: Boca Raton, FL, USA, 2008; 504p. [Google Scholar]
- Govaert, M.; Rotsaert, C.; Vannieuwenhuyse, C.; Duysburgh, C.; Medlin, S.; Marzorati, M.; Jarrett, H. Survival of probiotic bacterial cells in the upper gastrointestinal tract and the effect of the surviving population on the colonic microbial community activity and composition. Nutrients 2024, 16, 2791. [Google Scholar] [CrossRef]
- Ross, F.C.; Patangia, D.; Grimaud, G.; Lavelle, A.; Dempsey, E.M.; Ross, R.P.; Stanton, C. The interplay between diet and the gut microbiome: Implications for health and disease. Nat. Rev. Microbiol. 2024, 22, 671–686. [Google Scholar] [CrossRef]
- Abdeen, S.K.; Mastandrea, I.; Stinchcombe, N.; Puschhof, J.; Elinav, E. Diet-microbiome interactions in cancer. Cancer Cell 2025, 43, 680–707. [Google Scholar] [CrossRef]
- Chandrasekaran, P.; Weiskirchen, S.; Weiskirchen, R. Effects of probiotics on gut microbiota: An overview. Int. J. Mol. Sci. 2024, 25, 6022. [Google Scholar] [CrossRef]
- Bistas, K.G.; Tabet, J. The benefits of prebiotics and probiotics on mental health. Cureus 2023, 15, e43217. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Rejón, Ó.A.; Gonzalez-Figueredo, C.; Quintero-Covarrubias, A.R.; Saldaña-Jáuregui, A. Growth of Lactiplantibacillus plantarum BG112 in batch and continuous culture with Camellia sinensis as prebiotic. Fermentation 2024, 10, 487. [Google Scholar] [CrossRef]
- Lugo-Zarate, L.; Jiménez, O.A.S.; González-Olivares, L.G.; Pérez-Escalante, E.; Castañeda, O.A.; Pedraza-Chaverri, J.; Medina-Campos, O.N.; Herrera, H.M.G.; Delgado-Olivares, L. Potential of whey protein-fortified blackberry juice in transporting and protecting lactic acid bacteria: A proteolytic profile analysis and antioxidant activity. Fermentation 2025, 11, 252. [Google Scholar] [CrossRef]
- Wanyo, P.; Chamsai, T.; Toontom, N.; Nghiep, L.K.; Tudpor, K. Evaluation of in vitro digested mulberry leaf tea kombucha: A functional fermented beverage with antioxidant, anti-inflammatory, antihyperglycemic, and antihypertensive potentials. Fermentation 2025, 11, 258. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. NREL/TP-510-42618 Analytical Procedure—Determination of Structural Carbohydrates and Lignin in Biomass; Laboratory Analytical Procedure (LAP); NREL: Golden, CO, USA, 2012; 17p. [Google Scholar]
- Moretti, M.M.S.; Bocchini-Martins, D.A.; Nunes, C.C.C.; Villena, M.A.; Perrone, O.M.; Silva, R.; Boscolo, M.; Gomes, E. Pretreatment of sugarcane bagasse with microwaves irradiation and its effects on the structure and on enzymatic hydrolysis. Appl. Energy 2014, 122, 189–195. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar]
- Rufino, M.S.M.; Alves, R.E.; Brito, E.S.; Morais, S.M.; Sampaio, C.G.; Pérez-Jiménez, J.; Saura-Calixto, F.D. Metodologia Científica: Determinação da Atividade Antioxidante Total em Frutas pela Captura do Radical Livre DPPH; Embrapa—Cot_127; Embrapa: Fortaleza, Brazil, 2007; pp. 1–4. [Google Scholar]
- Pulido, R.; Bravo, L.; Saura-Calixto, F. Antioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assay. J. Agric. Food Chem. 2000, 48, 3396–3402. [Google Scholar] [CrossRef]
- Utrilla-Coello, R.G.; Rodríguez-Huezo, M.E.; Carrillo-Navas, H.; Hernández-Jaimes, C.; Vernon-Carter, E.J.; Alvarez-Ramirez, J. In vitro digestibility, physicochemical, thermal and rheological properties of banana starches. Carbohydr. Polym. 2014, 101, 154–162. [Google Scholar] [CrossRef]
- Jeronymo-Ceneviva, A.B.; Paula, A.T.; Silva, L.F.; Todorov, S.D.; Franco, B.D.G.M.; Penna, A.L.B. Probiotic Properties of Lactic Acid Bacteria Isolated from Water-Buffalo Mozzarella Cheese. Probiotics Antimicrob. Proteins 2014, 6, 141–156. [Google Scholar] [CrossRef]
- Casarotti, S.N.; Carneiro, B.M.; Todorov, S.D.; Nero, L.A.; Rahal, P.; Penna, A.L.B. In vitro assessment of safety and probiotic potential characteristics of Lactobacillus strains isolated from water buffalo mozzarella cheese. Ann. Microbiol. 2017, 67, 289–301. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food-an international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Bornscheuer, U.T. Microbial carboxyl esterases: Classification, properties and application in biocatatysis. FEMS Microbiol. Rev. 2002, 26, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Rosicka-Kaczmarek, J.; Komisarczyk, A.; Nebesny, E.; Makowski, B. The influence of arabinoxylans on the quality of grain industry products. Eur. Food Res. Technol. 2016, 242, 295–303. [Google Scholar] [CrossRef]
- Chen, Y.; Teng, W.; Wang, J.; Wang, Y.; Zhang, Y.; Cao, J. The intestinal delivery systems of ferulic acid: Absorption, metabolism, influencing factors, and potential applications. Food Front. 2024, 5, 1126–1144. [Google Scholar] [CrossRef]
- Johnson, E.M.; Jayabalan, L.R.; Patra, S.K.; Suh, J.-W. Self-assembled fructo-oligosaccharide conjugated ferulic acid microparticle: Anticancer, anti-inflammatory and immunomodulatory effects. J. Drug Deliv. Sci. Tech. 2024, 92, 105339. [Google Scholar] [CrossRef]
- Martiniakova, M.; Sarocka, A.; Penzes, N.; Biro, R.; Kovacova, V.; Mondockova, V.; Sevcikova, A.; Ciernikova, S.; Omelka, R. Protective role of dietary polyphenols in the management and treatment of type 2 diabetes mellitus. Nutrients 2025, 17, 275. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, T.; Robert-Baudouy, J. Bacterial aminopeptidases: Properties and functions. FEMS Microbiol. Rev. 1996, 18, 319–344. [Google Scholar] [CrossRef]
- Liu, Y.; Nawazish, H.; Farid, M.S.; Abdul, Q.K.; Habiba, U.E.; Muzamil, M.; Tanveer, M.; Sienkiewicz, M.; Lichota, A.; Łopusiewicz, Ł. Health-promoting effects of Lactobacillus acidophilus and its technological applications in fermented food products and beverages. Fermentation 2024, 10, 380. [Google Scholar] [CrossRef]
- Ruiz-Ramírez, Y.; Valadez-Blanco, R.; Calderón-García, C.; Chikindas, M.L.; Ponce-Alquicira, E. Probiotic and functional potential of lactic acid bacteria isolated from pulque and evaluation of their safety for food applications. Front. Microbiol. 2023, 14, 1241581. [Google Scholar] [CrossRef]
- Souza, B.M.S.; Borgonovi, T.F.; Casarotti, S.N.; Todorov, S.D.; Penna, A.L.B. Lactobacillus casei and Lactobacillus fermentum strains isolated from mozzarella cheese: Probiotic potential, safety, acidifying kinetic parameters and viability under gastrointestinal tract conditions. Probiotics Antimicrob. Proteins 2019, 11, 382–396. [Google Scholar] [CrossRef] [PubMed]
- Cebrián, R.; Baños, A.; Valdivia, E.; Pérez-Pulido, R.; Martínez-Bueno, M.; Maqueda, M. Characterization of functional, safety, and probiotic properties of Enterococcus faecalis UGRA10, a new AS-48-producer strain. Food Microbiol. 2012, 30, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Pillai, G.S.; Morya, S.; Khalid, W.; Khalid, M.Z.; Almalki, R.S.; Siddeeg, A. Banana pseudostem: An undiscovered fiber enriched sustainable functional food. J. Nat. Fibers 2024, 21, 2304004. [Google Scholar] [CrossRef]
- Kumari, P.; Gaur, S.S.; Tiwari, R.K. Banana and its by-products: A comprehensive review on its nutritional composition and pharmacological benefits. eFood 2023, 4, e110. [Google Scholar] [CrossRef]
- Capra, M.L.; Tibaldo, M.M.; Vinderola, G.; Reinheimer, J.A.; Quiberoni, A. Technological and probiotic characterisation of Lactobacillus casei/paracasei strains and their phage-resistant mutants. Int. Dairy J. 2014, 37, 39–47. [Google Scholar] [CrossRef]
- Michalak, M.; Gustaw, K.; Waśko, A.; Polak-Berecka, M. Composition of lactic acid bacteria during spontaneous curly kale (Brassica oleracea var. sabellica) fermentation. Microbiol. Res. 2018, 206, 121–130. [Google Scholar] [CrossRef]
- Gao, S.; Sun, R.; Singh, R.; Yu So, S.; Chan, C.T.Y.; Savidge, T.; Hu, M. The role of gut microbial β-glucuronidase in drug disposition and development. Drug Discov. Today 2022, 27, 103316. [Google Scholar] [CrossRef]
- Ahmad, N.; Zakaria, M.R. Oligosaccharide from hemicellulose. In Lignocellulose for Future Bioeconomy, 1st ed.; Ariffin, H., Sapuan, S.M., Hassan, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 135–152. [Google Scholar]
- Baptista, D.P.; Salgaço, M.K.; Sivieri, K.; Gigante, M.L. Use of static and dynamic in vitro models to simulate Prato cheese gastrointestinal digestion: Effect of Lactobacillus helveticus LH-B02 addition on peptides bioaccessibility. LWT Food Sci. Tech. 2020, 134, 110229. [Google Scholar] [CrossRef]
- Rodrigues, V.C.D.C.; Duque, A.L.R.F.; Fino, L.C.; Simabuco, F.M.; Sartoratto, A.; Cabral, L.; Noronha, M.F.; Sivieri, K.; Antunes, A.E.C. Modulation of the intestinal microbiota and the metabolites produced by the administration of ice cream and a dietary supplement containing the same probiotics. Br. J. Nutr. 2020, 124, 57–68. [Google Scholar] [CrossRef]
- Andrade, R.M.S.; Silva, S.; Costa, C.M.S.F.; Veiga, M.; Costa, E.; Ferreira, M.S.L.; Gonçalves, E.C.B.A.; Pintado, M.E. Potential prebiotic effect of fruit and vegetable byproducts flour using in vitro gastrointestinal digestion. Food Res. Int. 2020, 137, 109354. [Google Scholar] [CrossRef]
- Martino, M.E.; Bayjanov, J.R.; Caffrey, B.E.; Wels, M.; Joncour, P.; Hughes, S.; Gillet, B.; Kleerebezem, M.; van Hijum, S.A.F.T.; Leulier, F. Nomadic lifestyle of Lactobacillus plantarum revealed by comparative genomics of 54 strains isolated from different habitats. Environ. Microbiol. 2016, 18, 4974–4989. [Google Scholar] [CrossRef]
- Watson, D.; O’Connell Motherway, M.; Schoterman, M.H.C.; van Neerven, R.J.J.; Nauta, A.; Van Sinderen, D. Selective carbohydrate utilization by lactobacilli and bifidobacteria. J. Appl. Microbiol. 2013, 114, 1132–1146. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Xiong, T.; Peng, Z.; Xiao, Y.S.; Liu, Z.G.; Hu, M.; Xie, M.Y. Genomic analysis revealed adaptive mechanism to plant-related fermentation of Lactobacillus plantarum NCU116 and Lactobacillus spp. Genomics 2020, 112, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Panwar, D.; Kapoor, M. Transcriptional analysis of galactomannooligosaccharides utilization by Lactobacillus plantarum WCFS1. Food Microbiol. 2020, 86, 103336. [Google Scholar] [CrossRef]
- Macagnan, F.T.; Santos, L.R.; Roberto, B.S.; Moura, F.A.; Bizzani, M.; Silva, L.P. Biological properties of apple pomace, orange bagasse and passion fruit peel as alternative sources of dietary fibre. Bioact. Carbohydr. Diet. Fibre 2015, 6, 1–6. [Google Scholar] [CrossRef]
 = GG;
= GG;  = SJRP49;
= SJRP49;  = SJRP57;
= SJRP57;  = SJRP149;
= SJRP149;  = SJRP145; =
= SJRP145; =  SJRP146;
SJRP146;  = SJRP169;
= SJRP169;  = SJRP30;
= SJRP30;  = SJPR43.
= SJPR43.
 = GG;
= GG;  = SJRP49;
= SJRP49;  = SJRP57;
= SJRP57;  = SJRP149;
= SJRP149;  = SJRP145; =
= SJRP145; =  SJRP146;
SJRP146;  = SJRP169;
= SJRP169;  = SJRP30;
= SJRP30;  = SJPR43.
= SJPR43.
 = time zero (0 h),
= time zero (0 h),  = after exposure to simulated oral (2 min, pH 7.0),
= after exposure to simulated oral (2 min, pH 7.0),  = gastric (2 h, pH 3.0), and =
= gastric (2 h, pH 3.0), and =  intestinal conditions (2 h, pH 7.0), SD are indicated. Different capital letters denote differences (p < 0.05) of the same phase for different strains. Different lowercase letters denote differences (p < 0.05) among phases for the same strain.
intestinal conditions (2 h, pH 7.0), SD are indicated. Different capital letters denote differences (p < 0.05) of the same phase for different strains. Different lowercase letters denote differences (p < 0.05) among phases for the same strain.
 = time zero (0 h),
= time zero (0 h),  = after exposure to simulated oral (2 min, pH 7.0),
= after exposure to simulated oral (2 min, pH 7.0),  = gastric (2 h, pH 3.0), and =
= gastric (2 h, pH 3.0), and =  intestinal conditions (2 h, pH 7.0), SD are indicated. Different capital letters denote differences (p < 0.05) of the same phase for different strains. Different lowercase letters denote differences (p < 0.05) among phases for the same strain.
intestinal conditions (2 h, pH 7.0), SD are indicated. Different capital letters denote differences (p < 0.05) of the same phase for different strains. Different lowercase letters denote differences (p < 0.05) among phases for the same strain.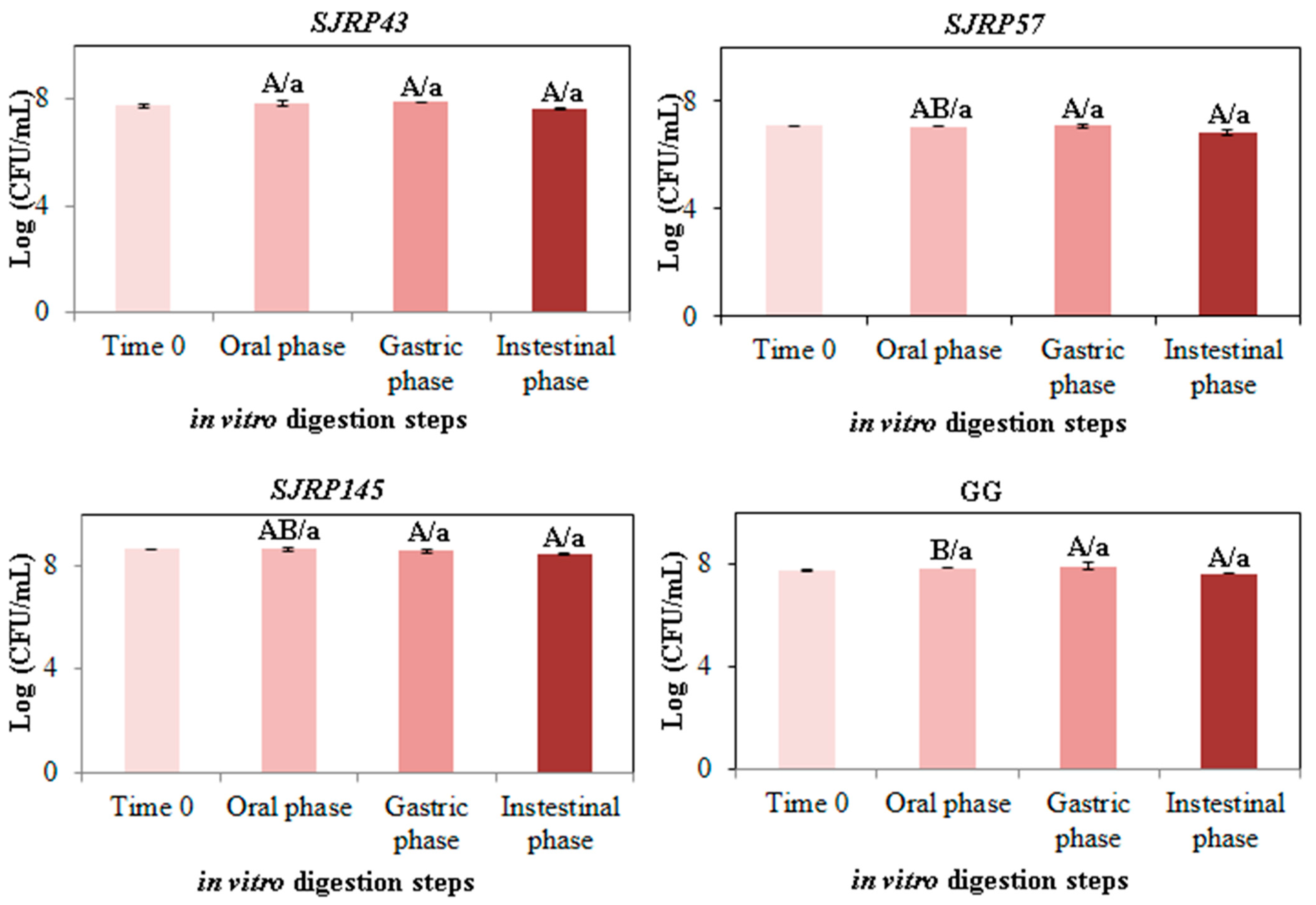
| Analysis | Flour Samples | Starch Samples | ||
|---|---|---|---|---|
| Carbohydrate composition | MBPS | NBPS | MBPS | NBPS |
| Starch (db-%) | 39.7 ± 1.1 a | 8.1 ± 1.4 b | 98.0 ± 0.2 a | 92.1 ± 0.6 b |
| Resistant starch (db-%) | 16.7 ± 0.1 a | 2.7 ± 0.0 b | 49.3 ± 0.7 a | 40.6 ± 3.0 b |
| Cellulose (db-%) | 27.0 ± 1.2 b | 52.4 ± 0.2 a | nd | nd |
| Hemicellulose * (db-%) | 25.4 ± 0.4 b | 33.8 ± 0.5 a | nd | nd |
| Insoluble lignin (%) | nd | nd | nd | nd |
| Soluble lignin (%) | nd | nd | nd | nd |
| Phenolic content (GAE/100 g) | 153.5 ± 0.1 b | 193.6 ± 0.1 a | nd | nd |
| Antioxidant activity | ||||
| ABTS (μmol Trolox/g) | 145.0 ± 0.2 a | 50.7 ± 0.1 b | nd | nd |
| DPPH (μmol Trolox/g) | 72.5 ± 0.1 b | 102.8 ± 0.4 a | nd | nd |
| FRAP (μmol sulfato ferroso/g) | 14.9 ± 0.0 a | 12.1 ± 0.1 b | nd | nd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moretti, M.M.d.S.; Borgonovi, T.F.; Todorov, S.D.; Penna, A.L.B. Banana Pseudostem By-Product: A Sustainable Source of Prebiotics and Protection for Probiotic Lactic Acid Bacteria Under Gastrointestinal Conditions. Fermentation 2025, 11, 476. https://doi.org/10.3390/fermentation11080476
Moretti MMdS, Borgonovi TF, Todorov SD, Penna ALB. Banana Pseudostem By-Product: A Sustainable Source of Prebiotics and Protection for Probiotic Lactic Acid Bacteria Under Gastrointestinal Conditions. Fermentation. 2025; 11(8):476. https://doi.org/10.3390/fermentation11080476
Chicago/Turabian StyleMoretti, Márcia Maria de Souza, Tais Fernanda Borgonovi, Svetoslav Dimitrov Todorov, and Ana Lúcia Barretto Penna. 2025. "Banana Pseudostem By-Product: A Sustainable Source of Prebiotics and Protection for Probiotic Lactic Acid Bacteria Under Gastrointestinal Conditions" Fermentation 11, no. 8: 476. https://doi.org/10.3390/fermentation11080476
APA StyleMoretti, M. M. d. S., Borgonovi, T. F., Todorov, S. D., & Penna, A. L. B. (2025). Banana Pseudostem By-Product: A Sustainable Source of Prebiotics and Protection for Probiotic Lactic Acid Bacteria Under Gastrointestinal Conditions. Fermentation, 11(8), 476. https://doi.org/10.3390/fermentation11080476








