Chromium-Driven Changes in Heavy Metal Resistance Genes During Pig Manure Composting
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Experimental Setup and Implementation
2.3. Determination of Physicochemical Properties and Cr
2.4. DNA Extraction and High-Throughput qPCR
2.5. Bioinformatics Analysis and Data Processing
3. Results and Discussion
3.1. Changes in Physicochemical Properties During Composting
3.1.1. Temperature
3.1.2. pH and EC
3.1.3. Organic Matter and Total Nitrogen
3.1.4. Dissolved Organic Carbon
3.1.5. NH4+-N and NO3−-N
3.2. Germination Index
3.3. Changes in Cr During Composting
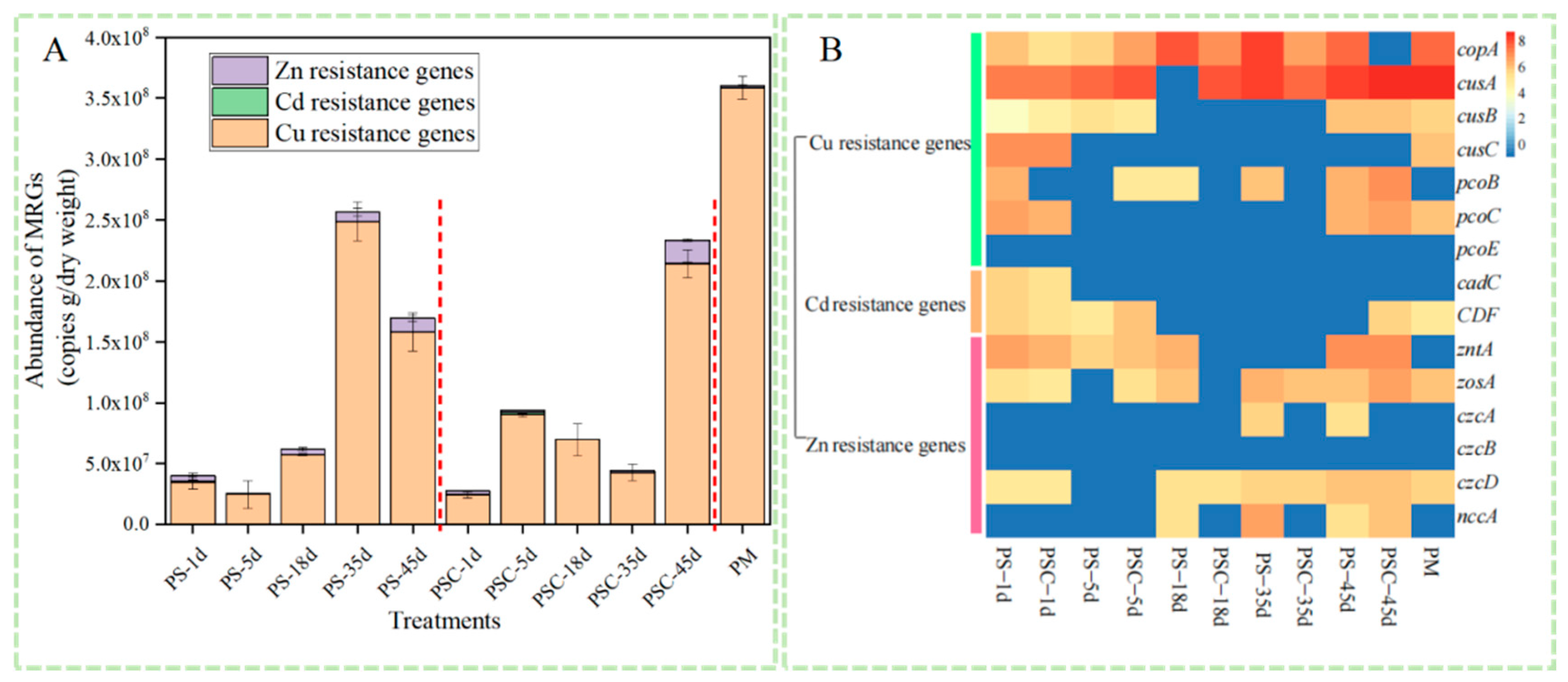
3.4. Changes in Heavy Metal Resistance Genes During Composting
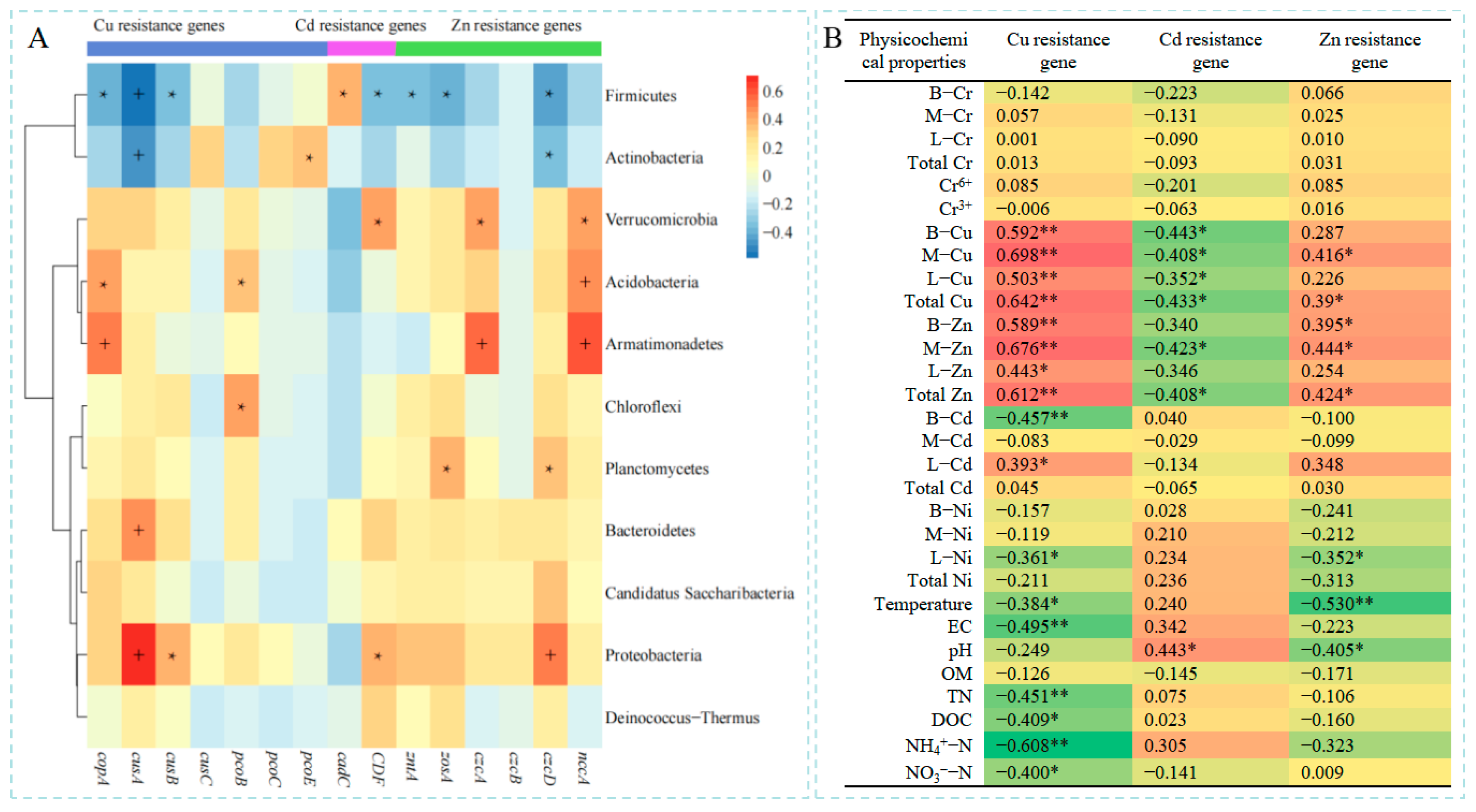
3.5. Influencing Factors of the Dynamics of Heavy Metal Resistance Genes During Composting
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Liu, Y.W.; Cheng, D.M.; Xue, J.M.; Weaver, L.; Wakelin, S.A.; Feng, Y.; Li, Z.J. Changes in microbial community structure during pig manure composting and its relationship to the fate of antibiotics and antibiotic resistance genes. J. Hazard. Mater. 2020, 389, 122082. [Google Scholar] [CrossRef]
- Zhao, J.; He, J.J.; Han, Y.R.; Liang, J.W.; Chen, Y.C.; Zhang, Y.M.; Liu, J.Z.; Huang, Q.Z.; Xu, W.H. Manure application increases soil doxycycline and zinc levels and resistance gene abundance. Ecotoxicol. Environ. Saf. 2025, 290, 117806. [Google Scholar] [CrossRef]
- Chen, X.M.; Du, Z.; Song, X.Y.; Wang, L.Q.; Wei, Z.M.; Jia, L.M.; Zhao, R. Evaluating the occurrence frequency of horizontal gene transfer induced by different degrees of heavy metal stress. J. Clean. Prod. 2023, 382, 135371. [Google Scholar] [CrossRef]
- Tian, J.Y.; Du, Y.B.; Yu, C.H.; Liu, W.Q.; Zou, R.H.; Zhao, Y.F.; Zhang, T.; Jiang, Y.C.; Tian, Z.J. The influences of heavy metals on soil microbial C, N, P cycling and heavy metal resistance under different fertilization regimes. Environ. Pollut. 2025, 370, 125915. [Google Scholar] [CrossRef]
- Guo, H.H.; Xue, S.H.; Nasir, M.; Gu, J.; Lv, J.L. Impacts of cadmium addition on the alteration of microbial community and transport of antibiotic resistance genes in oxytetracycline contaminated soil. J. Environ. Sci. 2021, 99, 51–58. [Google Scholar] [CrossRef]
- Shehata, E.; Liu, Y.; Feng, Y.; Cheng, D.; Li, Z. Changes in arsenic and copper bioavailability and oxytetracycline degradation during the composting process. Molecules 2019, 24, 4240. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Z.; Wen, Q.; Ji, Y. Variation of heavy metal speciation, antibiotic degradation, and potential horizontal gene transfer during pig manure composting under different chlortetracycline concentration. Environ. Sci. Pollut. Res. 2021, 28, 1224–1234. [Google Scholar] [CrossRef]
- Yen, C.C.; Chen, K.Y.; Ahmed, M.M.M.; Syu, C.H.; Liu, Y.T.; Hsieh, Y.C.; Jien, S.H.; Tzou, Y.M. Photochemical oxidation of Cr(III) to Cr(VI) in the presence of Fe(III): Influence of Fe(III) concentration and UV wavelength. J. Hazard. Mater. 2025, 485, 136852. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.N.; Gu, J.; Wang, X.J.; Song, W.; Zhang, K.Y.; Zhang, X.; Lu, C.Y.; Liu, J.Y. Effects of chromium(III) on enzyme activities and bacterial communities during swine manure composting. Bioresour. Technol. 2017, 243, 693–699. [Google Scholar] [CrossRef]
- Cheng, D.M.; Xiong, J.S.; Chen, J.Y.; Chang, H.Q.; Wong, J.W.C. Effect of biochar addition on antibiotic and heavy metal resistance genes during sewage sludge composting. J. Environ. Chem. Eng. 2025, 13, 115732. [Google Scholar] [CrossRef]
- Liu, Y.; Feng, Y.; Cheng, D.; Xue, J.; Wakelin, S.; Li, Z. Dynamics of bacterial composition and the fate of antibiotic resistance genes and mobile genetic elements during the co-composting with gentamicin fermentation residue and lovastatin fermentation residue. Bioresour. Technol. 2018, 261, 249–256. [Google Scholar] [CrossRef]
- Henseler, J.; Sarstedt, M. Goodness-of-fit indices for partial least squares path modeling. Comput. Stattistics 2013, 28, 565–580. [Google Scholar] [CrossRef]
- Lalremruati, M.; Devi, A.S. Duration of composting and changes in temperature, pH and C/N ratio during composting: A review. Agric. Rev. 2023, 44, 350–356. [Google Scholar] [CrossRef]
- Li, T.; Yan, Z.X.; Sun, Y.; Hu, X.; Peng, C.L.; Zhao, S.J.; Xu, D.B.; Liu, D.Y.; Shen, Q.R. Inorganic and organic additives differently regulate compost microbiomes in response to heavy metals immobilization. Chem. Eng. J. 2024, 502, 158087. [Google Scholar] [CrossRef]
- Jain, M.S.; Daga, M.; Kalamdhad, A.S. Variation in the key indicators during composting of municipal solid organic wastes. Sustain. Environ. Res. 2019, 29, 9. [Google Scholar] [CrossRef]
- Yang, Y.M.; Zhang, X.F.; Yang, Z.F.; Xi, B.D.; Liu, H.L. Turnover and loss of nitrogenous compounds during composting of food wastes. Front. Environ. Sci. Eng. China 2008, 2, 251–256. [Google Scholar] [CrossRef]
- Abdellah, Y.A.Y.; Shi, Z.J.; Luo, Y.S.; Hou, W.T.; Yang, X.; Wang, R.L. Effects of different additives and aerobic composting factors on heavy metal bioavailability reduction and compost parameters: A meta-analysis. Environ. Pollut. 2022, 307, 119549. [Google Scholar] [CrossRef]
- Zhu, L.; Huang, C.H.; Li, L.P.; Wang, S.M.; Wu, X.X.; Shan, G.C.; Tian, Y. Innovative insights into organic nitrogen degradation through protein family domains analysis in chicken and pig manure composting using metagenomic sequencing. Bioresour. Technol. 2024, 406, 131048. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Zheng, L.L.; Zheng, X.Y.; Yang, Y.; Xiao, D.; Wang, Y.Q.; Sheng, Z.W.; Ai, B.L. Insights from meta-analysis on carbon to nitrogen ratios in aerobic composting of agricultural residues. Bioresour. Technol. 2024, 413, 131416. [Google Scholar] [CrossRef]
- Chen, H.X.; Dou, J.F.; Xu, H.B. Remediation of Cr(VI)-contaminated soil with co-composting of three different biomass solid wastes. J. Soils Sediments 2017, 18, 897–905. [Google Scholar] [CrossRef]
- Shahid, M.; Shamshad, S.; Rafiq, M.; Khalid, S.; Bibi, I.; Niazi, N.K.; Dumat, C.; Rahid, M.I. Chromium speciation, bioavailability, uptake, toxicity and detoxification in soil-plant system: A review. Chemosphere 2017, 178, 513–533. [Google Scholar] [CrossRef]
- Chen, L.; Yi, Z.G.; Chen, Y.N.; Li, Y.P.; Jiang, H.J.; Wang, J.; Chen, Y.R.; Nie, Y.Q.; Luo, M.W.; Wang, Q.R.Y.; et al. Improved humification and Cr(VI) immobilization by CaO2 and Fe3O4 during composting. Bioresour. Technol. 2024, 413, 131479. [Google Scholar] [CrossRef]
- Wu, J.N.; Lv, Z.W.; Zheng, Z.Q.; Fu, Y.P.; Li, J. A transformation and auxiliary extraction of Cr during electrokinetic removal of Cr-contaminated multilayer composite soil chamber. Environ. Geochem. Health 2024, 46, 450. [Google Scholar] [CrossRef]
- Wang, C.; Jia, Y.X.; Li, J.P.; Li, P.; Wang, Y.; Yan, F.F.; Wu, M.H.; Fang, W.Z.; Xu, F.; Qiu, Z.P. Influence of microbial augmentation on contaminated manure composting: Metal immobilization, matter transformation, and bacterial response. J. Hazard. Mater. 2023, 441, 129762. [Google Scholar] [CrossRef]
- Yin, Y.N.; Gu, J.; Wang, X.J.; Song, W.; Zhang, K.Y.; Sun, W.; Zhang, X.; Zhang, Y.J.; Li, H.C. Effects of copper addition on copper resistance, antibiotic resistance genes, and intl1 during swine manure composting. Front. Microbil. 2017, 8, 344. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.Y.; Zhang, L.H.; Zhang, J.C.; Ren, L.H.; Zhou, Y.Y.; Zheng, Y.Y.; Luo, L.; Yang, Y.; Huang, H.L.; Chen, A.W. Physicochemical features, metal availability and enzyme activity in heavy metal-polluted soil remediated by biochar and compost. Sci. Total Environ. 2020, 701, 134751. [Google Scholar] [CrossRef] [PubMed]
- Argudín, M.A.; Hoefer, A.; Butaye, P. Heavy metal resistance in bacteria from animals. Res. Vet. Sci. 2019, 122, 132–147. [Google Scholar] [CrossRef]
- Yu, X.; Ding, Z.; Ji, Y.; Zhao, J.; Liu, X.; Tian, J.; Wu, N.; Fan, Y. An operon consisting of a p-type atpase gene and a transcriptional regulator gene responsible for cadmium resistances in bacillus vietamensis 151-6 and bacillus marisflavi 151-25. BMC Microbiol. 2020, 20, 18. [Google Scholar] [CrossRef]
- Parsons, C.; Lee, S.; Jayeola, V.; Kathariou, S. Novel cadmium resistance determinant in Listeria monocytogenes. Appl. Environ. Microbiol. 2017, 83, e02580-16. [Google Scholar] [CrossRef]
- Song, W.; Qi, R.; Zhao, L.; Xue, N.; Wang, L.; Yang, Y. Bacterial community rather than metals shaping metal resistance genes in water, sediment and biofilm in lakes from arid northwestern China. Environ. Pollut. 2019, 254, 113041. [Google Scholar] [CrossRef]



| Property | Pig Manure | Sawdust |
|---|---|---|
| Moisture Content (%) | 43.04 ± 0.63 | - |
| pH | 8.51 ± 0.01 | - |
| EC (ms/cm) | 0.62 ± 0.02 | - |
| OM (%) | 70.21 ± 1.47 | 83.35 ± 0.98 |
| TN (%) | 1.76 ± 0.10 | 0.11 ± 0.01 |
| DOC (%) | 1.65 ± 0.17 | - |
| NH4+-N (g/kg) | 2.72 ± 0.01 | - |
| NO3−-N (mg/kg) | 70.43 ± 0.15 | - |
| GI (%) | 98.58 ± 7.80 | - |
| Gentamicin Content (mg/kg) | 556.35 ± 2.61 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, G.; Li, P.; Feng, Y.; Liu, Y. Chromium-Driven Changes in Heavy Metal Resistance Genes During Pig Manure Composting. Fermentation 2025, 11, 472. https://doi.org/10.3390/fermentation11080472
Zhao G, Li P, Feng Y, Liu Y. Chromium-Driven Changes in Heavy Metal Resistance Genes During Pig Manure Composting. Fermentation. 2025; 11(8):472. https://doi.org/10.3390/fermentation11080472
Chicago/Turabian StyleZhao, Guoqiang, Peng Li, Yao Feng, and Yuanwang Liu. 2025. "Chromium-Driven Changes in Heavy Metal Resistance Genes During Pig Manure Composting" Fermentation 11, no. 8: 472. https://doi.org/10.3390/fermentation11080472
APA StyleZhao, G., Li, P., Feng, Y., & Liu, Y. (2025). Chromium-Driven Changes in Heavy Metal Resistance Genes During Pig Manure Composting. Fermentation, 11(8), 472. https://doi.org/10.3390/fermentation11080472







