Abstract
The dual environmental challenges of karst areas lie in organic solid waste’s (OSW) massive generation scale and diffuse dispersion, which accelerate bedrock exposure and soil contamination, while simultaneously representing an underutilized resource for soil amendments through optimized composting. Bio-enhanced composting of multi-source OSW yields compounds with dual redox/adsorption capabilities, effectively improving soil quality and restoring ecological balance. The recycling and circular utilization of OSW resources become particularly critical in karst regions with vulnerable soil ecosystems, where sustainable resource management is urgently needed to maintain ecological balance. This review elucidates the ecological impacts of multi-source OSW compost applications on soil environments in ecologically fragile karst regions, specifically elucidating the mechanisms of heavy metals (HMs) migration–transformation and organic contaminant degradation (with emphasis on emerging pollutants), and the functional role of microbial carbon pumps in these processes. Furthermore, establishing a sustainable “multi-source OSW−compost−organic matter (adsorption and redox sites)−microorganisms−pollution remediation” cycle creates a green, low-carbon microenvironment for long-term soil remediation. Finally, this study evaluates the application prospects of the refined composting technology utilizing multi-objective regulation for OSW resource recycling and utilization in karst areas. This review provides critical insights for optimizing soil remediation strategies in karst ecosystems through organic waste valorization.
1. Introduction
In China, approximately 20% of the land area is characterized by karst landscapes, predominantly concentrated in the southwestern regions of Guangxi, Guizhou, and Yunnan [1]. Karst areas have poor soil and fragile ecosystems due to geological features and rock weathering. With urbanization and population growth, urban and rural domestic waste and agricultural OSW are increasing [2]. Improper disposal of these organic solid waste can cause severe organic and heavy metal pollution to the soil. They can rapidly contaminate the karst groundwater system through leakage and runoff. There is a strong connectivity between surface water and groundwater [3,4,5], thereby posing a threat to human health [6]. Meanwhile, the accumulation and degradation of OSW may accelerate the process of rocky desertification, potentially disrupting the original carbon cycle balance [7].
There has been an accumulation of large quantities of heavy metals (Cr, Cu, Ca, Ni, Pb, Zn, and the quasi-heavy metal As, etc.) and emerging organic pollutants (including microplastics, persistent organic pollutants (POPs), etc.) in karst soils [8,9,10]. Enrichment and accumulation of heavy metals in the soil can enter the food chain through plant uptake or biological ingestion. Emerging contaminants can cause serious harm to human life through ingestion, respiration, etc. [1,11]. Therefore, soil enhancement and ecological restoration in karst areas are urgent challenges to be overcome in order to reduce the safety risks associated with these contaminations.
Compost, the process of using microorganisms to decompose multi-source organic waste and convert them into stable, humus-rich organic fertilizers. It is one of the most effective methods of environmentally sound and resourceful treatment of municipal waste, agricultural and forestry waste, and livestock manure. Compost is highly efficient, stable and sustainable in OSW use [12,13,14]. Organic matter (OM) components can modify heavy metals (HMs) fate and behavior in soil through multiple mechanisms, including the following: co-precipitation, complexation–adsorption, (de)methylation, rhizosphere modification, and redox reactions [15,16,17,18]. The dominant flora in composting such as Bacillus, Pseudomonas, and Streptomyces can secrete degrading enzymes (e.g., esterases, hydrolases, etc.) to degrade different organic pollutants in OSW and soils [19,20]. Thermophilic composting cleaves C-C bonds and generates oxygen-containing functional groups (e.g., carbonyl, carboxyl, hydroxyl), enhancing biodegradability of organic pollutants under elevated temperature and oxygen conditions [21]. Compost enhances soil organic carbon (SOC) by stimulating microbial activity, restructuring microbial communities, and modifying carbon chemical stability. This facilitates short-term carbon release and long-term sequestration [22,23], influencing soil carbon cycling while contributing to climate change mitigation [24,25].
Based on the multiple disposal methods of OSW, compost is the best complement to karst soil: it synergistically solves the two major problems of soil pollution and ecological remediation, so that the OSW compost can directly reduce its accumulation pressure in karst depressions, fissures, and other sensitive landscapes, while efficiently and orderly meeting the soil needs and returning to the soil (Figure 1). Therefore, to advance comprehension and application of compost in karst soil remediation, this paper executes the following: (1) summarizes karst soil pollution background (heavy metals/organic pollutants); (2) elucidates in situ mechanisms of compost-mediated HM immobilization and organic pollutant degradation; (3) proposes the multi-source OSW–Compost–OM/Microorganisms–Pollution Remediation. This framework supports socioeconomic green transition and low-carbon sustainability through pollution reduction. (Figure 2).
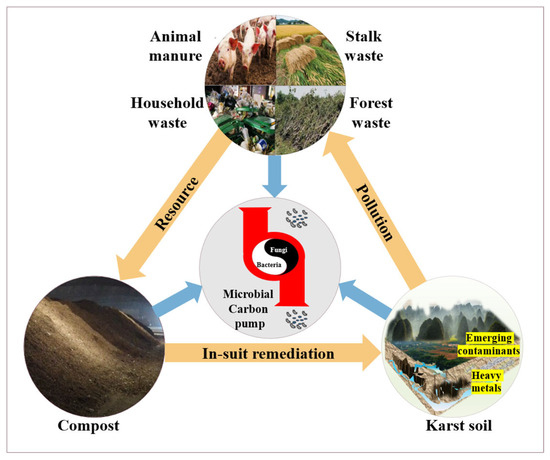
Figure 1.
Circular utilization of multi-source OSW in karst area—the “resource-pollution” duality of OSW. By resourcefully utilizing it to produce compost and applying it to the in situ soil remediation in karst areas, this technology is of great significance for achieving the coordinated goals of pollution control, increased production, and carbon sequestration.
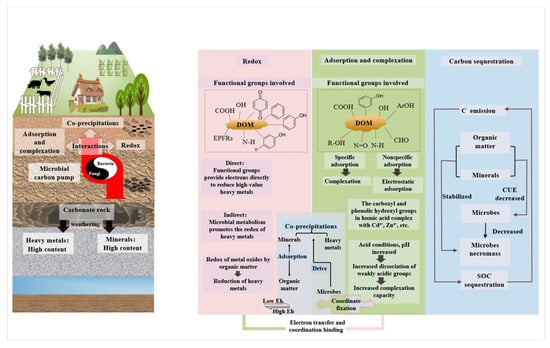
Figure 2.
Reaction mechanisms of compost with HMs in soils and carbon sequestration in karst regions.
2. Heavy Metals in Karst Soils
HMs are typically defined as metals with an atomic mass greater than 20 and a density exceeding 4.5 g/cm3. In the context of soil pollution, HMs commonly include Cr, Cu, Cd, Ni, Pb, Zn, and Hg. From a biological perspective, heavy metals refer to certain metallic elements that may pose a threat to animals and plants even at low concentrations [26]. Unlike aqueous pollutants, HMs in soil resist leaching and microbial decomposition, resulting in persistent contamination that is more environmentally detrimental and challenging to remediate [27,28].
2.1. Background of Heavy Metal Pollution in Karst Areas
Karst areas typically feature clay enriched terra rossa (red soils), whose variable thicknesses contribute to elevated HM concentrations in these soils [29,30]. Several hypotheses have been proposed to explain the formation of this “terra rossa” layer: (1) pedogenic accumulation of insoluble relic phases—predominantly aluminum iron oxides and silica—derived from in situ chemical weathering of underlying carbonate bedrock; (2) karst-specific differential dissolution of carbonate matrices triggers concomitant enrichment of allochthonous siliceous/clayey particulates via groundwater mediated transport; (3) subsequent weathering of allochthonous detrital inputs—particularly silt clay fraction aeolian dust and fluvial overbank deposits—mediates secondary pedogenic processes [31,32]. A consensus exists among researchers that heavy metal (HM) accumulation in karst soils originates primarily from lithogenic inheritance, with particularly elevated concentrations observed in lateritic terra rossa formations. Thus, the weathering and soil-forming processes of the parent rocks are crucial factors contributing to the high background values of HMs in karst soils [31,33,34,35]. Yang et al. demonstrated that while the heavy metal content of carbonate rocks is generally lower than that of clastic rocks, except for Cd, the heavy metal content of carbonate soils is higher due to secondary enrichment [32]. In addition to the secondary enrichment of parent materials and natural soil formation processes, anthropogenic activities—including industrial and mining operations, urbanization, and agricultural practices—have significantly intensified HM contamination in karst regions.
2.2. Sources of Heavy Metal Pollution
The ecological environment of karst regions is particularly fragile and highly susceptible to human disturbances. In addition to natural sources such as enrichment from parent materials, anthropogenic activities—fueled by economic development and improved living standards—have substantially increased heavy metal emissions into the environment. As previously discussed, HMs in karst soils exhibit elevated background concentrations due to secondary enrichment during the weathering and pedogenesis of carbonate and limestone parent rocks. Consequently, the soil’s capacity to tolerate additional heavy metal inputs is limited [31,36,37]. Exogenous HMs are heavily deposited in soils, often exceeding thresholds that threaten plant and animal health [38]. For example, the karst regions of southwestern China are rich in mineral resources. However, extensive mining and smelting activities have resulted in the release of heavy metal-laden pollutants into the environment, thereby exacerbating soil contamination by adding to the naturally elevated background levels of HMs [32]. Furthermore, as human lifestyles improve, domestic waste production has surged. Current waste disposal methods, such as landfills and incineration, release HM contaminants into the environment through wind, surface runoff, and groundwater infiltration [39]. Additionally, leaded gasoline, brake linings (Cu source), and tires (Zn source) constitute major anthropogenic sources of heavy metal pollution [40]. In agriculture, sewage irrigation is common in water-scarce developing countries, introducing HMs into farmland soils [41]. Sewage water, while providing irrigation and nutrients, also delivers significant amounts of HMs [42]. The use of pesticides and phosphate fertilizers further exacerbates the issue, as these are significant sources of Cd, Pb, Cu, Hg, and As [43,44].
2.3. Importance of Remediation of Heavy Metal Pollution
Remediating HMs contamination in karst soils significantly reduces bioavailable Cr and Pb concentrations, mitigating their health threats via food chain bioaccumulation and hepatorenal toxicity. By lowering the levels of these contaminants, remediation reduces the risk of heavy metal intake and enhances food safety [45,46,47]. While soil remediation is not directly linked to global warming, the process and resulting soil quality improvements can indirectly mitigate climate change. As one of Earth’s largest carbon pools, soil boosts CO2 sequestration potential through structural and organic amendments that enhance carbon storage capacity [48]. Although this carbon sequestration effect is modest, it constitutes a meaningful contribution to climate change mitigation. Beyond climate benefits, remediating heavy metal pollution in karst soils delivers significant ecological gains; it creates healthier habitats for plants, animals, and microorganisms, which promotes biodiversity recovery and enhances ecosystem stability. Ultimately, these improvements elevate overall soil quality [49,50].
3. Compost-Mediated Heavy Metal Environmental Behavior in Karst Soil Systems
The retention of HMs in soil is governed by a variety of reaction mechanisms, including adsorption, precipitation, complexation, (de)methylation, oxidation, and reduction [51]. Remediation of HM pollution can be achieved by employing different techniques based on these mechanisms. Generally, remediation strategies can be categorized into “ex situ” techniques (such as extraction), and “in situ” techniques (such as fixation) [52,53]. As an in situ fixation technique, compost enhances HMs remediation in contaminated soils via OM-mediated adsorption, complexation, redox reactions, and co-precipitation (Figure 2) [54].
3.1. Adsorption and Complexation
OM in compost can adsorb HMs or complex with HMs. The immobilization of heavy metal ions by organic matter can be classified into specific and non-specific retention mechanisms. Specific adsorption (complexation) involves the formation of chemical or coordination bonds between organic matter and heavy metal ions, thereby generating stable complexes. In contrast, non-specific adsorption (electrostatic adsorption) refers to the electrostatic interaction between metal ions and organic matter, which helps to achieve charge balance but does not form chemical bonds [55].
3.1.1. Electrostatic Adsorption
OM in compost fixes heavy metals through electrostatic adsorption, a process mainly dependent on highly electronegative functional groups such as carboxyl, hydroxyl, and phenolic hydroxyl groups (Table 1). During the composting process, these functional groups undergo dissociation due to pH changes, causing the organic matter surface to carry a negative charge. This promotes the adsorption of heavy metal ions through electrostatic attraction [56]. In composts with added hydrochar, the −COO− groups formed by the dissociation of carboxylic acids can directly interact with Zn2+, thereby reducing the bioavailable form of zinc by 19% [57]. The charge characteristics of organic matter in compost are influenced by environmental pH. Under low pH (<5.5), protonation by H+ increases the surface’s positive charge, which hinders the adsorption of heavy metal ions. In contrast, alkaline environments promote the dissociation of functional groups, thereby increasing surface negative charge density and enhancing electrostatic attraction for metal ions. For example, under alkaline conditions, the adsorption capacity of corn stalk-based manure for Cd2+ can reach up to 227.27 mg/g (Table 2). This is attributed to the ionization of abundant C=O and H−O bonds present in the organic matter under such conditions [58]. Biochar possesses a permanent negative surface charge and can function as an “electron bridge,” thereby enhancing the electrostatic interactions between organic matter and heavy metals during the composting process. The application of straw-derived biochar at a rate of 8%, in conjunction with vermicomposting, has been shown to reduce the bioavailability of Cd in sewage sludge by 40.99%, primarily through the elevation of system pH and cation exchange capacity (CEC) [59].
The humification of organic matter in compost directly affects electrostatic adsorption efficiency through the evolution of its components and structure. Large molecules such as lignocellulose degrade into small organic acids and further condense into humic acid (HA). Due to its large molecular weight and high density of functional groups, humic acid has a significantly superior electrostatic adsorption capacity compared to uncomposted OM. Experiments on electric-field-assisted composting have shown that the electric field accelerates the proliferation of actinomycetes, promoting humification and increasing the content of humic acid by 69% (Table 2). At the same time, the contents of Cu, Zn, As, and Cd in the humic acid bound state increased by 34%, 41%, 29%, and 135.1%, respectively [60].

Table 1.
Main functional groups of OM in compost and their electrostatic adsorption characteristics.
Table 1.
Main functional groups of OM in compost and their electrostatic adsorption characteristics.
| Functional Group Type | Dissociation Constant (pKa) | Charged State (pH > 7) | Typical Combination of Heavy Metals | Reference |
|---|---|---|---|---|
| Carboxyl (−COOH) | 3.0–4.5 | −COO− (negative electricity) | Cu2+, Zn2+ | [57] |
| Phenolic hydroxyl group (−OH) | 9.5–10.5 | −O− (negative electricity) | Pb2+, Cd2+ | [61] |
| Amidogen (−NH2) | 8.0–10.0 | −NH3+ (positive electricity) | Cr6+, As5+ | [62] |

Table 2.
Comparison of the promoting effects of different exogenous enhancement techniques on the electrostatic adsorption of heavy metals by OM in compost.
Table 2.
Comparison of the promoting effects of different exogenous enhancement techniques on the electrostatic adsorption of heavy metals by OM in compost.
| Type of Technology | Mechanism of Action | Typical Increase in Heavy Metal Adsorption Capacity | Reference |
|---|---|---|---|
| Electric-field-assisted | Electrostatic-potential-driven ion migration and microbial regulation. | Cd: 135.1% | [60] |
| Hydrochar adding | Porous adsorption and functional group complexation. | Cu: 36.3% | [57] |
| Straw biochar (8%) | Increase pH and CEC. | Cd: 40.99% | [59] |
| Insect manure additive | High density oxygen containing functional groups | Cd: 227.27 mg/g | [58] |
3.1.2. The Complexation Mechanism of OM-HMs
Guo et al. [63] investigated the complexation of OM with metal ions during the composting process. The results demonstrated that the binding mechanisms between dissolved organic matter (DOM) and Cu(II) were comparable to those observed for Pb(II). During composting, the primary binding sites for DOM interacting with both Cu(II) and Pb(II) were identified in the spectral regions associated with C−O stretching vibrations in alcohols, ethers, and esters (1150–1000 cm−1). However, compost-derived DOM exhibited a higher affinity for Cu(II) compared to Pb(II). The binding interactions between DOM and HMs exhibit variability across different compost types. Cui et al. [64] identified four distinct fluorescent DOM components in sewage sludge compost: a polyphenolic component; two humic-like components with varying degrees of humification; a highly humified humic-like component. Stern–Volmer modeling demonstrated that all components exhibited complexation capacities with Zn, Cu, and Cr, but not with Pb. This finding contrasts with the results reported in Guo’s study [63]. This may be due to the fact that the source of compost materials, the degree of humification, and the methods for separation/identification of DOM components have a significant impact on the results. There is an urgent need to carry out standardized comparative studies or establish more universal comprehensive models. During the complexation process of Cr(III) in DOM and landfill leachate, N=O, C=O, −COOH, C−N, Ar−OH, and C−O in DOM act as the binding sites for heavy metal complexation. These groups are generally regarded as the main binding sites in DOM. Moreover, the complexation affinity of N=O and C−N towards Cr(III) is stronger than that of −COOH, Ar−OH, C=O, and C−O of polysaccharides [65].
In addition to the composting process and the type of compost, the incorporation of chemical agents into the compost matrix has been shown to enhance the complexation efficiency of HMs. Calcium peroxide (CP), a stable solid peroxide compound, is commonly employed in organic waste treatment systems to generate hydroxyl radicals (OH) and create alkaline conditions during anaerobic fermentation. This dual action effectively decomposes recalcitrant organic compounds, including lignocellulosic biomass, thereby enhancing humification processes through the accelerated formation of humic substances [66]. The addition of calcium peroxide to sludge composting promotes the formation of humic acid and shortens the composting cycle, increasing the complexation stability constant of humus with copper (from log KM = 4.05–4.45 to log KM = 4.22–5.13). The complexation of copper ions with humic acid only involves the C=O in amide (I) [67]. Combined with the research results of Huang and Tang et al. [68,69], this also indicates that some non-fluorescent substances are also involved in the complexation of Cu(II), but the specific components of these substances still need to be confirmed.
3.2. Redox
Soil OM is rich in redox groups, such as quinone, phenolic hydroxyl, and amino. During Cr(VI)-DOM synergistic reduction, phenolic hydroxyl and amino groups in DOM oxidize to aldehydes/carboxyl groups while reducing Cr(VI) to Cr(III). This dual transformation effectively immobilizes chromium and degrades copresent organic toxins [70]. Compost-derived humic substances exhibit complex structures and abundant reactive moieties—including hydroxyl, phenolic, carboxyl groups, environmentally persistent free radicals (EPFRs), quinones, and semiquinones. These components collectively participate in redox reactions with sensitive elements (e.g., Ag, As, Cr, Hg), attenuating their soil biotoxicity [71,72]. OM as an electron shuttle, has different influences on the redox of heavy metals [73]. Gu et al. [74] found that the concentration of organic matter (the molar ratio of DOC to Hg(II)) affects the electron transfer efficiency during the redox reaction of Hg. When the concentration of organic matter is low (HA concentration = 0.2 mg/L), the reduction reaction is dominant. Conversely, when the concentration of organic matter is high (HA concentration >5 mg/L), the oxidation reaction is dominant. However, the mechanism of the reversal effect of the redox direction is not yet fully understood. Another researcher found that the accumulation capacity and biological toxicity of heavy metals in soil not only depend on the concentration of OM, but also their different forms and valence states affect their accumulation degree. The environmental impact and bioavailability of heavy metals vary with different valence states [75,76]. OM mediates electron transfer and facilitates the conversion of high valence metal ions into less toxic and stable forms. Dissolved humic substances act as electron shuttles that significantly accelerate extracellular electron transfer. This facilitates Fe(III)/Mn(IV) oxide reduction at rates approximately sevenfold greater than microbially driven reduction alone [77]. Beyond OM concentration, heavy metal speciation/valence and OM structural characteristics critically govern its redox capacity for heavy metals. Specifically, OM’s electron transfer capacity is determined by chemical properties including aromaticity and substituent types [78,79]. The electron acceptor ability (EAC) and electron donating ability (EDC) of the OM are positively correlated with the content of quinone and phenol moieties in the OM [80].
Humic substances act as effective redox agents for metal ion conversion. Their redox activity interfaces with microorganisms: iron-reducing, fermentative, and sulfate reducing bacteria utilize humus as a terminal electron acceptor, generating reduced humus. This reduced form is reoxidized by oxygen and subsequently regenerated through microbial reduction, establishing a continuous electron cycling loop [78,81]. Dong et al. [82] demonstrated that humic substances reduce Ag+ to silver nanoparticles (AgNPs) in natural environments, with AgNPs production rates exhibiting pH-dependent linear kinetics. Carboxyl groups were identified as the key functional moieties mediating this reduction process. Through comparison of different humic substance classes, Nie et al. [83] demonstrated that reducing functional groups (e.g., free radicals, phenolic hydroxyls, and alcoholic hydroxyls) facilitate AgNPs formation. In contrast, quinone/semiquinone moieties were identified as the dominant active groups mediating Hg(II). Wang et al. [84] verified that humus showed the highest reduction of Hg(II) (29 ± 3%) when the Hg/HS ratio was 1134 ng/mg. As a ubiquitous, redox-active component of soil organic matter (SOM), humic substances (HS) can remediate arsenic-contaminated soil by oxidizing As(III) to As(V). Experimental evidence identifies the semiquinone radical moieties within HS as an effective functional group responsible for this oxidation [85].
3.3. Co-Precipitation of OM and Minerals: Key to HMs Immobilization in Soil
Co-precipitation between compost-derived OM and soil minerals significantly controls heavy metal (HM) activity in soil. Under identical conditions, this process immobilizes more HM ions than surface complexation [17]. During compost-based remediation of metal-contaminated soils, dissolved organic carbon (DOC) co-precipitates with dissolved mineral ions derived from mineral surfaces, as demonstrated by research [86]. X-ray and Fe-specific spectra at 4.2 K and room temperature confirm DOM adsorption by ferrihydrite. Subsequent co-precipitation generates secondary minerals with distinct crystallinity and magnetic properties relative to pristine ferrihydrite. Carbon NMR analysis further reveals preferential interaction between iron minerals and O-alkyl C moieties in DOM. Critically, organic mineral co-precipitates represent the most stable state for HM stabilization. These co-precipitates exhibit reduced specific surface area (SSA), altered crystallographic facets, and contracted lattice spacing compared to pure minerals [17]. Within organo-ferrihydrite co-precipitates (OFCs), coexisting ferric hydroxide and organic fractions provide multifunctional HM binding sites (e.g., Fe−OH, R−OH, −COOH), constituting a key mechanism for compost-enhanced in situ HM immobilization [17,54]. In this study on the fixation of trivalent chromium by dissolved organic carbon (DOC) in composts of rice straw and rape straw, the fixation rate of trivalent chromium by OFCs exceeded 88% within the range of iron–carbon molar ratio (0.3–10) [17]. X-ray spectroscopy and chemical extraction identified adsorption and co-precipitation as concurrent molecular-scale mechanisms. As a key control parameter for HM sequestration, the initial mineral to organic ratio modulates adsorption capacity in organic mineral complexes. The co-precipitation complex, within the range of iron-to-carbon ratio from 0.1 to 10, shows an increasing immobilization effect on heavy metals as the iron-to-carbon ratio decreases. However, at the critical threshold of an iron-to-carbon ratio of 0.1, due to the organic saturation effect, its efficiency drops significantly. Mak and Lo et al. [87] suggested that it may be due to the fact that the DOC increase results in the formation of more metal–organic complexes, leading to the clogging of metal binding sites on the mineral surface. In the co-precipitation complex, enhanced organic interactions of iron or aluminum, inhibited nucleation and crystallization of iron/aluminum (hydro)oxides, and isomorphic substitution of coexisting metal cations jointly regulate the adsorption behavior of heavy metals [88,89]. Du et al. [90] compared the adsorption performance of Pb and Cd formed under both adsorption and co-precipitation in Fe/Al(hydro)oxide-HA. It was also found that the co-precipitated hydrotalcite-HA composites have higher carbon content, smaller specific surface area and faster Pb adsorption rate.
3.4. Metal Isotopes as “Monitors” of Heavy Metals
As a powerful geochemical tool, metal isotopes trace HM sources and quantify their environmental behavior (fate, transport, transformation), simultaneously delivering mechanistic constraints for process-based modeling [91,92,93]. Advanced analytical techniques—particularly atomic spectroscopy and multi-collector ICP-MS (MC-ICP-MS)—enable direct analysis of metal isotope signatures, providing mechanistic insights into HM cycling processes such as redox transformations [94,95], which can better reflect the migratory behaviors and contributions of HMs from different sources.
In their study of Fe cycling dynamics and associated HM accumulation in karst soils, Qi et al. employed δ56 Fe compositions of carbonate-derived Fe to analyze key drivers of Fe transport [96]. This approach elucidated linkages between the formation and transformation of Fe (hydr)oxides and HM sequestration in these soils. For heavy metal-contaminated farmland areas in karst regions, Kong et al. [97] conducted experimental analyses of surface soils and soil profiles to assess the spatial distribution (both laterally and vertically) of heavy metal contamination (Pb, Zn, Cu, Cd, As), revealing critical patterns of contamination. Zhang et al. [98] analyzed the soil Fe content and Fe isotope rows in native vegetation, abandoned farmland, and cropland, and found that the Fe oxidation–precipitation process was the main factor leading to higher δ56 Fe content in deeper soils than in upper soils. Apparently, metal isotopes have been well applied in the study of heavy metal morphology and fate in karst areas. There are still some drawbacks: (1) The application of metal isotopes for source assignment is prone to misinterpretation. Consequently, stable isotopes are typically applicable only for identifying and quantifying pollution sources in scenarios involving three or more sources, making it difficult to account for pollution originating from a single source; (2) When HMs undergo complexation and adsorption, redox, microbial metabolism, and other biotic and abiotic reactions, the mechanisms of fractionation at the molecular scale are not clear; (3)The pretreatment protocols for different environmental samples (soil, atmosphere, water, solid waste sediment, etc.) do not exactly match the existing ones, which can lead to errors in the experimental process [99,100,101,102].
4. Composts Renew the Soil Microbial Carbon Pump
As the largest carbon reservoir in terrestrial ecosystems, soil organic carbon (SOC) dynamics are subject to dual regulation by soil microorganisms [103]. Microorganisms serve as primary agents in the terrestrial carbon cycle via in vitro modification and in vivo turnover metabolic pathways (Figure 2). In combination with the stabilization mechanism of microbial residues by renewal of burial [104], microorganisms act as both drivers and participants in the terrestrial carbon cycle. As drivers, they decompose aboveground biomass, transferring carbon belowground, and regulate soil organic carbon decomposition and cycling. As participants, their life cycle processes release assimilated carbon into the soil pool, ultimately storing it as long-term microbial-derived organic carbon [105,106]. Microbial carbon use efficiency (CUE)—defined as the proportion of assimilated carbon allocated to biomass synthesis—is a key regulator of soil organic carbon (SOC) transformation [105]. A high CUE signifies that more carbon flows into microbial biomass formation and subsequent residue production, which promotes the long-term sequestration of SOC through the stabilization of microbial residues [107,108].
Notably, the mineral-rich soil environments in karst regions, formed by weathering of parent rocks, provide unique conditions for microbial residue stabilization. Studies have shown that minerals can achieve physicochemical protection of microbial residues by forming organic–mineral bonds with OM [109,110]. Although soil aggregates and mineral content were negatively correlated with microbial CUE (potentially increasing metabolic carbon loss), the formation of organic–mineral bonds not only compensated for such loss but also significantly enhanced the soil carbon sink capacity [111]. However, the impacts of compost, as an exogenous OM input, on the formation of karst soil aggregates, mineral association mechanisms, and the coupling between microbial CUE and residue stabilization remain poorly understood. Urgent in-depth investigations are required to elucidate its potential for regulating carbon sink dynamics in karst ecosystems [109].
Microorganisms, as the core drivers of the composting process, play a key role in organic waste conversion and soil carbon sequestration. Organic waste from various sources sustain rich microbial communities throughout composting, with population densities reaching up to 109 CFU/g in the mesophilic phase and ranging from 106 to 109 CFU/g in the thermophilic phase [112]. This microbial activity has a significant impact on soil ecosystems. Tian et al. [113] showed that moderate application of livestock manure compost in paddy fields can significantly enhance soil microbial activity and promote the proliferation of functional microbial communities such as bacteria, archaea, and ammonia-oxidizing bacteria (AOB).
From the perspective of carbon cycles, the transformation of OM in compost mainly undergoes two key processes: degradation and humification. In recent years, the humification mechanism has attracted much attention due to its prominent role in carbon sequestration [114]. Several studies have confirmed the positive effect of compost on soil organic carbon (SOC) accumulation. Michaud et al. reported that municipal OSW compost can enhance soil SOC content by 32–69% [115]. Malone et al. [116], further quantified this effect through a meta-analysis of 38 studies and found that compost application can increase urban soil SOC by 3.1–6.5%. These studies fully demonstrate that the contribution of multi-source organic solid waste to soil carbon sinks through humification pathways cannot be ignored. Meanwhile, the influence of different raw material compositions and composting processes on microbial community structure and carbon sequestration efficiency requires further systematic research, which is crucial for optimizing composting technology to maximize its environmental benefits.
5. Composts as “Targeted Therapy” for Emerging Contaminants in Karst Areas
Emerging contaminants are “chemical substances that have no regulation, are suspected to affect the environment or whose effects are unknown”. The escalating volume of household waste, coupled with the widespread application of pesticides in agriculture, often leads to improper disposal practices. This, in turn, introduces a significant burden of emerging contaminants into the soil, including microplastics (MPs) [117], polycyclic aromatic hydrocarbons (PAHs) [118], polychlorinated biphenyls (PCBs) [119], as well as caffeine and phthalate esters (PAEs) [120], among others. Research has shown that long-term accumulation of these organic pollutants in the body can impair organ function and may lead to skeletal, cardiovascular, and cerebrovascular diseases [121]. Therefore, in addition to reducing emerging contaminants in OSW during composting, the organic matter and microorganisms in compost are particularly important as they can be used to degrade emerging pollutants in the soil.
In the physical degradation mechanism of composting, mechanical stirring and regular turning of organic solid waste (OSW) can promote the homogenization of materials. At the same time, these operations break down large contaminants (such as microplastics) into smaller particles through continuous shear force and friction [122,123]. Critically, this particle size reduction exponentially increases specific surface area, thereby amplifying interfacial exposure to biotic/abiotic degradation agents. Consequently, the accelerated biodegradation manifests through intensified microbial colonization, enzymatic oxidation, and chemisorption by activated functional groups during subsequent composting stages [124]. Within the chemical degradation regime of composting, recalcitrant contaminants in organic solid waste (OSW)—including microplastics, per- and polyfluoroalkyl substances (PFAS), PAHs, PAEs—undergo thermally activated oxidative transformation. The combined effects of elevated temperatures (>50 °C) and sustained humidity create a reactive microenvironment that facilitates homolytic cleavage of covalent bonds (e.g., C−C, C−F, C−O) [125]. This primary degradation step yields bioavailable low-molecular-weight intermediates, which subsequently enter metabolic mineralization pathways [126]. For instance, PFAS compounds experience stepwise defluorination via oxidative chain-shortening mechanisms, ultimately generating short-chain perfluoroalkyl acids. These intermediates are then mineralized to terminal products (CO2, H2O, F−) through microbially mediated β-oxidation and defluorinase activity [20,127]. Biodegradation is the main mechanism for the degradation of emerging contaminants in the process and involves the metabolic activities of microorganisms such as bacteria and fungi. In the composting environment, abundant microbial communities directly act on pollutant molecules by secreting extracellular enzymes (e.g., esterases, oxidases, etc.) to decompose them into small-molecule organic or inorganic substances [19,128]. For instance, during PAEs degradation, specialized microbial strains (e.g., Pseudarthrobacter defluvii E5) secrete esterases that catalyze the hydrolysis of ester bonds within PAE molecules. This enzymatic cleavage yields primary metabolites—monoalkyl phthalate esters and corresponding alcohols—which are subsequently mineralized by microbial consortia [20,129]. In addition, the synergistic effect between microorganisms also promoted the degradation of pollutants. Throughout the composting process, multiple microbial communities form a complex ecological network through cross-feeding of metabolites and exchange of signaling molecules. This interconnected structure enhances the functional resilience of the community and the diversity of collective catabolism, thereby improving the ability to degrade pollutants [130]. During PAEs biodegradation, metabolically specialized populations engage in sequential metabolic collaboration: primary hydrolyzers (e.g., esterase-producing bacteria) initiate ester bond cleavage, while secondary mineralizers subsequently metabolize the resulting monoester and alcohol intermediates to achieve complete pollutant mineralization [131].
The composting system operates through a synergistic triad of interconnected degradation mechanisms wherein physical fragmentation, chemical transformation, and biological processing exhibit dynamic interdependencies. Physical comminution (e.g., shear-induced particle size reduction) enhances contaminant bioavailability by increasing specific surface area and mass transfer efficiency, thereby expanding reactive interfaces for subsequent abiotic and biotic attack. Concurrently, chemical transformations activated by thermophilic conditions (>55 °C) cleave critical molecular bonds (C−C, C−O, C−F) via thermal hydrolysis/oxidation, generating polar intermediates with heightened microbial accessibility that serve as preferential substrates for metabolic processing. Biological mediation then achieves terminal mineralization through enzymatic cascades (e.g., oxygenase-mediated PAHs ring opening, defluorinase-driven PFAS degradation), converting pollutants into inorganic constituents (CO2, H2O, minerals) [132]. This mechanistic hierarchy is further reinforced by biological feedback loops, where microbial exudates accelerate physicochemical weathering through biosurfactant production, establishing a self-amplifying degradation architecture that maximizes contaminant destruction efficiency.
In summary, the degradation of emerging contaminants during composting is a multi-mechanism synergistic process. By optimizing the composting conditions (e.g., temperature, humidity, pH, etc.), the degradation efficiency can be further improved and the effective removal of emerging contaminants can be achieved [133]. Based on the characteristics of karst regions, future research directions include the following: (1) screening for efficient degrading bacteria that are resistant to mineral stress, constructing synthetic microbial communities, and enhancing the simultaneous degradation capacity of multiple pollutants [134,135]; (2) utilizing the calcium-rich feature of karst areas to design mineral–microbe coupled carriers, thereby improving in situ immobilized degradation efficiency [136]; (3) developing in situ tracing techniques (such as isotope labeling) to distinguish the biodegradation pathways of new pollutants [137]. This will provide a precise and regionally adapted development path for composting technology to target the management of emerging pollutants.
6. Conclusions
Compost represents a critical approach for the efficient resource utilization of organic solid waste, facilitating the conversion of diverse organic sources into soil conditioners enriched with functional OM. This OM exhibits important environmental functions, such as redox, adsorption, complexation, and chelation, enabling the effective transformation or stabilization of heavy metals and organic contaminants. In calcareous karst ecosystems, the humification of microbial residues, along with their interaction with minerals, can substantially enhance soil organic carbon levels. Consequently, compost demonstrates significant applicability and advantages in karst regions. Building on compost’s “waste-to-resource” potential for soil microecological improvement, this study proposes a technical pathway: “multi-source organic waste, resource-oriented composts, functional organic matter/microorganisms, soil remediation”. This technological model enables the sustainable recycling of organic waste in karst areas and simultaneously facilitates pollution remediation.
Looking to the future, to further enhance the application efficiency of composting technology in environmental remediation, research should focus on the following directions. First, establish a standard model for the complexation of DOM with heavy metals to solve the inconsistent conclusions in current research. Second, deeply reveal the regulatory mechanism of OM concentration thresholds on the direction of redox reactions and clarify their applicable scope under different environmental conditions. Third, develop a mineral–microbe coupling carrier system suitable for karst areas to enhance its remediation efficiency in special geological backgrounds. Fourth, build an intelligent composting system to co-treat heavy metals, greenhouse gases, and emerging pollutants, and enhance overall environmental benefits. Fifth, develop dual-function fertilizers combining passivation and carbon sequestration, and assess their potential in carbon trading to transform composting from a pollution control tool into a key approach for soil remediation and carbon sink creation.
When incorporating compost remediation into the comprehensive management system of rocky desertification, the core value lies in integrating “waste treatment-pollution remediation-soil fertility improvement” into a single closed loop. Ultimately, it establishes a green remediation model for karst areas, characterized by the principles of “treating pollution with waste and enhancing soil fertility through waste utilization.”
Author Contributions
Writing—original draft, C.H.; supervision, H.Z. and B.X.; writing—review and editing, X.Z.; investigation, Z.W.; funding acquisition, X.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work is financially supported by the National Natural Science Foundation of China (No. 52270142).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Chen, H.; Teng, Y.; Lu, S.; Wang, Y.; Wang, J. Contamination features and health risk of soil heavy metals in China. Sci. Total Environ. 2015, 512–513, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.; Pandey, A.; Zhang, Z.; Awasthi, M.K.; Bhatia, S.K.; Taherzadeh, M.J. Organic solid waste biorefinery: Sustainable strategy for emerging circular bioeconomy in China. Ind. Crops Prod. 2020, 153, 112568. [Google Scholar] [CrossRef]
- Li, Q.; Hervé, J.M.; Ye, W. Introduction. In Geometric Method for Type Synthesis of Parallel Manipulators; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–21. [Google Scholar][Green Version]
- Xiao, B.; Bai, X.; Zhao, C.; Tan, Q.; Li, Y.; Luo, G.; Wu, L.; Chen, F.; Li, C.; Ran, C.; et al. Responses of carbon and water use efficiencies to climate and land use changes in China’s karst areas. J. Hydrol. 2023, 617, 128968. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, H.; Fu, Z.; Wang, F.; Wang, K. Towards hydrological connectivity in the karst hillslope critical zone: Insight from using water isotope signals. J. Hydrol. 2023, 617, 128926. [Google Scholar] [CrossRef]
- Velázquez, J.A.; García-Meneses, P.M.; Esse, C.; Saavedra, P.; Trosino, R.M.; Alfonzo, R.B.; Mazari-Hiriart, M. Spatially explicit vulnerability analysis of contaminant sources in a karstic watershed in southeastern Mexico. Appl. Geogr. 2022, 138, 102606. [Google Scholar] [CrossRef]
- Li, B.; Li, T.; Wu, P.; Yang, L.; Long, J.; Liu, P.; Li, T. Transport of pollutants in groundwater of domestic waste landfills in karst regions and its engineering control technologies. J. Environ. Manag. 2023, 347, 119245. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wu, X.; Zhang, J.; Huang, X. Distribution and migration characteristics of microplastics in farmland soils, surface water and sediments in Caohai Lake, southwestern plateau of China. J. Clean. Prod. 2022, 366, 132912. [Google Scholar] [CrossRef]
- Bolan, N.; Sarkar, B.; Vithanage, M.; Singh, G.; Tsang, D.C.W.; Mukhopadhyay, R.; Ramadass, K.; Vinu, A.; Sun, Y.; Ramanayaka, S.; et al. Distribution, behaviour, bioavailability and remediation of poly- and per-fluoroalkyl substances (PFAS) in solid biowastes and biowaste-treated soil. Environ. Int. 2021, 155, 106600. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Cheng, H. Application of Stochastic Models in Identification and Apportionment of Heavy Metal Pollution Sources in the Surface Soils of a Large-Scale Region. Environ. Sci. Technol. 2013, 47, 3752–3760. [Google Scholar] [CrossRef] [PubMed]
- Rivas, F.J. Polycyclic aromatic hydrocarbons sorbed on soils: A short review of chemical oxidation based treatments. J. Hazard. Mater. 2006, 138, 234–251. [Google Scholar] [CrossRef] [PubMed]
- Fernández, J.M.; Plaza, C.; Hernández, D.; Polo, A. Carbon mineralization in an arid soil amended with thermally-dried and composted sewage sludges. Geoderma 2007, 137, 497–503. [Google Scholar] [CrossRef]
- Huang, D.-L.; Zeng, G.-M.; Feng, C.-L.; Hu, S.; Jiang, X.-Y.; Tang, L.; Su, F.-F.; Zhang, Y.; Zeng, W.; Liu, H.-L. Degradation of Lead-Contaminated Lignocellulosic Waste by Phanerochaete chrysosporium and the Reduction of Lead Toxicity. Environ. Sci. Technol. 2008, 42, 4946–4951. [Google Scholar] [CrossRef] [PubMed]
- Semple, K.T.; Reid, B.J.; Fermor, T.R. Impact of composting strategies on the treatment of soils contaminated with organic pollutants. Environ. Pollut. 2001, 112, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Nagpal, A.K. Soil amendments: A tool to reduce heavy metal uptake in crops for production of safe food. Rev. Environ. Sci. Bio/Technol. 2018, 17, 187–203. [Google Scholar] [CrossRef]
- He, L.; Zhong, H.; Liu, G.; Dai, Z.; Brookes, P.C.; Xu, J. Remediation of heavy metal contaminated soils by biochar: Mechanisms, potential risks and applications in China. Environ. Pollut. 2019, 252, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Xia, X.; Liu, J.; Wang, J.; Hu, Y. Molecular Mechanisms of Chromium(III) Immobilization by Organo–Ferrihydrite Co-precipitates: The Significant Roles of Ferrihydrite and Carboxyl. Environ. Sci. Technol. 2020, 54, 4820–4828. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Tan, C.; He, Y.; Chen, Y.; Wan, Z.; Fu, T.; Wu, Y. Rhizosphere activity induced mobilization of heavy metals immobilized by combined amendments in a typical lead/zinc smelter-contaminated soil. Chemosphere 2023, 313, 137556. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhu, J.; Sun, M.; Gu, M.; Xie, X.; Ying, T.; Zhang, Z.; Zhong, W. Biodegradation of combined pollutants of polyethylene terephthalate and phthalate esters by esterase-integrated Pseudomonas sp. JY-Q with surface-co-displayed PETase and MHETase. Synth. Syst. Biotechnol. 2025, 10, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Feng, N.-X.; Li, D.-W.; Zhang, F.; Bin, H.; Huang, Y.-T.; Xiang, L.; Liu, B.-L.; Cai, Q.-Y.; Li, Y.-W.; Xu, D.-L.; et al. Biodegradation of phthalate acid esters and whole-genome analysis of a novel Streptomyces sp. FZ201 isolated from natural habitats. J. Hazard. Mater. 2024, 469, 133972. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, T.G.; Bester, K. Organic Micropollutant Degradation in Sewage Sludge during Composting under Thermophilic Conditions. Environ. Sci. Technol. 2010, 44, 5086–5091. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Jia, Z.; Jiao, X.; Wang, J.; Huang, X. Long-term manure applications to increase carbon sequestration and macroaggregate-stabilized carbon. Soil Biol. Biochem. 2022, 174, 108827. [Google Scholar] [CrossRef]
- Payen, F.T.; Sykes, A.; Aitkenhead, M.; Alexander, P.; Moran, D.; MacLeod, M. Soil organic carbon sequestration rates in vineyard agroecosystems under different soil management practices: A meta-analysis. J. Clean. Prod. 2021, 290, 125736. [Google Scholar] [CrossRef]
- Swati, A.; Hait, S. Greenhouse Gas Emission During Composting and Vermicomposting of Organic Wastes—A Review. CLEAN—Soil Air Water 2018, 46, 1700042. [Google Scholar] [CrossRef]
- Cui, P.; Chen, Z.; Zhao, Q.; Yu, Z.; Yi, Z.; Liao, H.; Zhou, S. Hyperthermophilic composting significantly decreases N2O emissions by regulating N2O-related functional genes. Bioresour. Technol. 2019, 272, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, X.; Yu, L.; Wang, T.; Wang, J.; Liu, T. Review of soil heavy metal pollution in China: Spatial distribution, primary sources, and remediation alternatives. Resour. Conserv. Recycl. 2022, 181, 106261. [Google Scholar] [CrossRef]
- Zhang, H.; Yuan, X.; Xiong, T.; Wang, H.; Jiang, L. Bioremediation of co-contaminated soil with heavy metals and pesticides: Influence factors, mechanisms and evaluation methods. Chem. Eng. J. 2020, 398, 125657. [Google Scholar] [CrossRef]
- Yang, S.; Zhao, J.; Chang, S.X.; Collins, C.; Xu, J.; Liu, X. Status assessment and probabilistic health risk modeling of metals accumulation in agriculture soils across China: A synthesis. Environ. Int. 2019, 128, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.J.; Ji, H.B.; Ouyang, Z.; Zhou, D.Q.; Zhen, L.P.; Li, T.Y. Preliminary study on weathering and pedogenesis of carbonate rock. Sci. China Ser. D-Earth Sci. 1999, 42, 572–581. [Google Scholar] [CrossRef]
- Ji, H.; Wang, S.; Ouyang, Z.; Zhang, S.; Sun, C.; Liu, X.; Zhou, D. Geochemistry of red residua underlying dolomites in karst terrains of Yunnan-Guizhou Plateau: I. The formation of the Pingba profile. Chem. Geol. 2004, 203, 1–27. [Google Scholar] [CrossRef]
- Wen, Y.; Li, W.; Yang, Z.; Zhang, Q.; Ji, J. Enrichment and source identification of Cd and other heavy metals in soils with high geochemical background in the karst region, Southwestern China. Chemosphere 2020, 245, 125620. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Yang, Z.; Filippelli, G.M.; Ji, J.; Ji, W.; Liu, X.; Wang, L.; Yu, T.; Wu, T.; Zhuo, X.; et al. Distribution and secondary enrichment of heavy metal elements in karstic soils with high geochemical background in Guangxi, China. Chem. Geol. 2021, 567, 120081. [Google Scholar] [CrossRef]
- Ahmadi, M.; Akhbarizadeh, R.; Haghighifard, N.J.; Barzegar, G.; Jorfi, S. Geochemical determination and pollution assessment of heavy metals in agricultural soils of south western of Iran. J. Environ. Health Sci. Eng. 2019, 17, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Ji, H.; Li, D.; Zhang, F.; Wang, S. Material source analysis and element geochemical research about two types of representative bauxite deposits and terra rossa in western Guangxi, southern China. J. Geochem. Explor. 2013, 133, 68–87. [Google Scholar] [CrossRef]
- Halamic, J.; Peh, Z.; Miko, S.; Galovic, L.; Sorsa, A. Geochemical Atlas of Croatia: Environmental implications and geodynamical thread. J. Geochem. Explor. 2012, 115, 36–46. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, W.; Wang, L.; Zhang, X.; Wen, Y.; Bostick, B.C.; Wen, Y.; He, X.; Zhang, L.; Zhuo, X.; et al. New strategy for exploring the accumulation of heavy metals in soils derived from different parent materials in the karst region of southwestern China. Geoderma 2022, 417, 115806. [Google Scholar] [CrossRef]
- Liu, P.F.; Wu, Z.Q.; Luo, X.R.; Wen, M.L.; Huang, L.L.; Chen, B.; Zheng, C.J.; Zhu, C.; Liang, R. Pollution assessment and source analysis of heavy metals in acidic farmland of the karst region in southern China—A case study of Quanzhou County. Appl. Geochem. 2020, 123, 104764. [Google Scholar] [CrossRef]
- Gao, Y.J.; Jia, J.L.; Xi, B.D.; Cui, D.Y.; Tan, W.B. Divergent response of heavy metal bioavailability in soil rhizosphere to agricultural land use change from paddy fields to various drylands. Environ. Sci. Process. Impacts 2021, 23, 417–428. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Cui, J.; Jiang, G.; Chen, X.; Wang, L.; Fang, C. Soil Heavy Metal Pollution Assessment Near the Largest Landfill of China. Soil Sediment Contam. Int. J. 2013, 22, 390–403. [Google Scholar] [CrossRef]
- Zwolak, A.; Sarzyńska, M.; Szpyrka, E.; Stawarczyk, K. Sources of Soil Pollution by Heavy Metals and Their Accumulation in Vegetables: A Review. Water Air Soil Pollut. 2019, 230, 164. [Google Scholar] [CrossRef]
- Mahanta, M.J.; Bhattacharyya, K.G. Total concentrations, fractionation and mobility of heavy metals in soils of urban area of Guwahati, India. Environ. Monit. Assess. 2011, 173, 221–240. [Google Scholar] [CrossRef] [PubMed]
- Chaganti, V.N.; Ganjegunte, G.; Niu, G.; Ulery, A.; Flynn, R.; Enciso, J.M.; Meki, M.N.; Kiniry, J.R. Effects of treated urban wastewater irrigation on bioenergy sorghum and soil quality. Agric. Water Manag. 2020, 228, 105894. [Google Scholar] [CrossRef]
- Luo, W.; Lu, Y.; Giesy, J.P.; Wang, T.; Shi, Y.; Wang, G.; Xing, Y. Effects of land use on concentrations of metals in surface soils and ecological risk around Guanting Reservoir, China. Environ. Geochem. Health 2007, 29, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, C. Natural and Human Factors Affect the Distribution of Soil Heavy Metal Pollution: A Review. Water Air Soil Pollut. 2020, 231, 350. [Google Scholar] [CrossRef]
- Li, X.; Liu, H.; Meng, W.; Liu, N.; Wu, P. Accumulation and source apportionment of heavy metal(loid)s in agricultural soils based on GIS, SOM and PMF: A case study in superposition areas of geochemical anomalies and zinc smelting, Southwest China. Process Saf. Environ. Prot. 2022, 159, 964–977. [Google Scholar] [CrossRef]
- Luo, G.; Han, Z.; Xiong, J.; He, Y.; Liao, J.; Wu, P. Heavy metal pollution and ecological risk assessment of tailings in the Qinglong Dachang antimony mine, China. Environ. Sci. Pollut. Res. 2021, 28, 33491–33504. [Google Scholar] [CrossRef] [PubMed]
- Hemati, S.; Heidari, M.; Momenbeik, F.; Khodabakhshi, A.; Fadaei, A.; Farhadkhani, M.; Mohammadi-Moghadam, F. Co-occurrence of polycyclic aromatic hydrocarbons and heavy metals in various environmental matrices of a chronic petroleum polluted region in Iran; Pollution characterization, and assessment of ecological and human health risks. J. Hazard. Mater. 2024, 478, 135504. [Google Scholar] [CrossRef] [PubMed]
- Weyhenmeyer, G.A. Toward a fundamental understanding of ecosystem metabolism responses to global warming. One Earth 2024, 7, 1886–1898. [Google Scholar] [CrossRef]
- Zuo, Y.; Li, Y.; Chen, H.; Ran, G.; Liu, X. Effects of multi-heavy metal composite pollution on microorganisms around a lead-zinc mine in typical karst areas, southwest China. Ecotoxicol. Environ. Saf. 2023, 262, 115190. [Google Scholar] [CrossRef] [PubMed]
- Che, S.; Wang, J.; Zhou, Y.; Yue, C.; Zhou, X.; Xu, Y.; Tian, S.; Cao, Z.; Wei, X.; Li, S.; et al. The adsorption and fixation of Cd and Pb by the microbial consortium weakened the toxic effect of heavy metal-contaminated soil on rice. Chem. Eng. J. 2024, 497, 154684. [Google Scholar] [CrossRef]
- Bolan, N.; Kunhikrishnan, A.; Thangarajan, R.; Kumpiene, J.; Park, J.; Makino, T.; Kirkham, M.B.; Scheckel, K. Remediation of heavy metal(loid)s contaminated soils—To mobilize or to immobilize? J. Hazard. Mater. 2014, 266, 141–166. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.-M.; Fu, R.-B.; Wang, J.-X.; Shi, Y.-X.; Guo, X.-P. Chemical stabilization remediation for heavy metals in contaminated soils on the latest decade: Available stabilizing materials and associated evaluation methods—A critical review. J. Clean. Prod. 2021, 321, 128730. [Google Scholar] [CrossRef]
- Yang, X.; Liu, L.; Tan, W.; Liu, C.; Dang, Z.; Qiu, G. Remediation of heavy metal contaminated soils by organic acid extraction and electrochemical adsorption. Environ. Pollut. 2020, 264, 114745. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Han, H.; Gao, C.; Wang, Y.; Dong, B.; Xu, Z. Organic amendments for in situ immobilization of heavy metals in soil: A review. Chemosphere 2023, 335, 139088. [Google Scholar] [CrossRef] [PubMed]
- Sposito, G. The Surface Chemistry of Soils; Oxford University Press: Oxford, UK, 1984. [Google Scholar]
- Ahmad, Z.; Gao, B.; Mosa, A.; Yu, H.; Yin, X.; Bashir, A.; Ghoveisi, H.; Wang, S. Removal of Cu(II), Cd(II) and Pb(II) ions from aqueous solutions by biochars derived from potassium-rich biomass. J. Clean. Prod. 2018, 180, 437–449. [Google Scholar] [CrossRef]
- Long, Y.; Zhu, N.; Zhu, Y.; Shan, C.; Jin, H.; Cao, Y. Hydrochar drives reduction in bioavailability of heavy metals during composting via promoting humification and microbial community evolution. Bioresour. Technol. 2024, 395, 130335. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Darma, A.I.; Yang, J.; Wang, X.; Shakouri, M. Protaetia brevitarsis larvae produce frass that can be used as an additive to immobilize Cd and improve fertility in alkaline soils. J. Hazard. Mater. 2024, 472, 134379. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.M.; Zhang, Z.; Chu, Z.X.; Dong, P.; Liang, S.; Deng, R. Temporal changes of heavy metals in sludge under the condition of straw charcoal assisted earthworm composting. J. Environ. Eng. Technol. 2024, 14, 528–537. [Google Scholar]
- Cao, Y.; Wang, X.; Zhang, X.; Misselbrook, T.; Bai, Z.; Ma, L. An electric field immobilizes heavy metals through promoting combination with humic substances during composting. Bioresour. Technol. 2021, 330, 124996. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, Y.; Wang, S.; Long, S.; Wu, Y.; Chen, Z. Sources, transfers and the fate of heavy metals in soil-wheat systems: The case of lead (Pb)/zinc (Zn) smelting region. J. Hazard. Mater. 2023, 441, 129863. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sun, M.; Zhang, L.; Finlay, R.D.; Liu, R.; Lian, B. Widespread bacterial responses and their mechanism of bacterial metallogenic detoxification under high concentrations of heavy metals. Ecotoxicol. Environ. Saf. 2022, 246, 114193. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.-J.; He, X.-S.; Li, C.-W.; Li, N.-X. The binding properties of copper and lead onto compost-derived DOM using Fourier-transform infrared, UV–vis and fluorescence spectra combined with two-dimensional correlation analysis. J. Hazard. Mater. 2019, 365, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Zhang, X.; Wang, Y.; Zhang, X.; Lv, J. Binding characteristics of heavy metal contaminations and sewage sludge DOM products: Determination based on complexing-variance partitioning analysis. Chem. Eng. J. 2024, 489, 151387. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, Y.; Tan, W.; Zhang, J.; Gong, X.; Li, Y.; Hui, K.; Chen, H.; Xi, B. New insight into the functional group mechanism and structure-activity relationship of the complexation between DOM and Cr(III) in landfill leachate. J. Hazard. Mater. 2024, 466, 133210. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Huang, Q.-S.; Wei, W.; Sun, J.; Dai, X.; Ni, B.-J. Improving the treatment of waste activated sludge using calcium peroxide. Water Res. 2020, 187, 116440. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.Y.; Chen, Z.Q.; Wen, Q.X.; Wu, Y.Q.; Fu, Q.Q. Effect of calcium peroxide dosage on organic matter degradation, humification during sewage sludge composting and application as amendment for Cu (II)-polluted soils. J. Hazard. Mater. 2022, 439, 129592. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Li, D.; Shah, G.M.; Chen, W.; Wang, W.; Xu, Y.; Huang, H. Hyperthermophilic pretreatment composting significantly accelerates humic substances formation by regulating precursors production and microbial communities. Waste Manag. 2019, 92, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Zhuang, L.; Yu, Z.; Liu, X.; Wang, Y.; Wen, P.; Zhou, S. Insight into complexation of Cu(II) to hyperthermophilic compost-derived humic acids by EEM-PARAFAC combined with heterospectral two dimensional correlation analyses. Sci. Total Environ. 2019, 656, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Tang, Y.; Sun, J.; Dong, B.; Zuxin, X. Tracking of the conversion and transformation pathways of dissolved organic matter in sludge hydrothermal liquids during Cr(VI) reduction using FT-ICR MS. J. Hazard. Mater. 2024, 466, 133566. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Tan, W.; Peng, J.; Dang, Q.; Zhang, H.; Xi, B. Biowaste-source-dependent synthetic pathways of redox functional groups within humic acids favoring pentachlorophenol dechlorination in composting process. Environ. Int. 2020, 135, 105380. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.-X.; Gai, S.; Cheng, K.; Yang, F. Roles of humic substances redox activity on environmental remediation. J. Hazard. Mater. 2022, 435, 129070. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, T.; Gao, C.; Xie, Y.; Zhang, A. Use of extracellular polymeric substances as natural redox mediators to enhance denitrification performance by accelerating electron transfer and carbon source metabolism. Bioresour. Technol. 2022, 345, 126522. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Bian, Y.; Miller, C.L.; Dong, W.; Jiang, X.; Liang, L. Mercury reduction and complexation by natural organic matter in anoxic environments. Proc. Natl. Acad. Sci. USA 2011, 108, 1479–1483. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Zheng, W.; Luo, M.; Kuang, C.; Tang, X. Characterization of copper (II) chemical forms and heavy metal distribution in chemical looping gasification of municipal solid waste. J. Energy Inst. 2021, 96, 140–147. [Google Scholar] [CrossRef]
- Li, Y.; Gong, X.; Xiong, J.; Sun, Y.; Shu, Y.; Niu, D.; Lin, Y.; Wu, L.; Zhang, R. Different dissolved organic matters regulate the bioavailability of heavy metals and rhizosphere microbial activity in a plant-wetland soil system. J. Environ. Chem. Eng. 2021, 9, 106823. [Google Scholar] [CrossRef]
- Jiang, J.; Kappler, A. Kinetics of Microbial and Chemical Reduction of Humic Substances: Implications for Electron Shuttling. Environ. Sci. Technol. 2008, 42, 3563–3569. [Google Scholar] [CrossRef] [PubMed]
- Klüpfel, L.; Piepenbrock, A.; Kappler, A.; Sander, M. Humic substances as fully regenerable electron acceptors in recurrently anoxic environments. Nat. Geosci. 2014, 7, 195–200. [Google Scholar] [CrossRef]
- Ratasuk, N.; Nanny, M.A. Characterization and Quantification of Reversible Redox Sites in Humic Substances. Environ. Sci. Technol. 2007, 41, 7844–7850. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Xi, B.; Wang, G.; Jiang, J.; He, X.; Mao, X.; Gao, R.; Huang, C.; Zhang, H.; Li, D.; et al. Increased Electron-Accepting and Decreased Electron-Donating Capacities of Soil Humic Substances in Response to Increasing Temperature. Environ. Sci. Technol. 2017, 51, 3176–3186. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.N.; Wang, X.N.; Wu, J.; Lu, Y.Z.; Fu, L.; Zhang, F.; Lau, T.C.; Zeng, R.J. Humic substances as electron acceptors for anaerobic oxidation of methane driven by ANME-2d. Water Res. 2019, 164, 114935. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Liu, G.; Zhou, J.; Wang, J.; Jin, R. Transformation of silver ions to silver nanoparticles mediated by humic acid under dark conditions at ambient temperature. J. Hazard. Mater. 2020, 383, 121190. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Zhu, K.; Zhao, S.; Dai, Y.; Tian, H.; Sharma, V.K.; Jia, H. Interaction of Ag+ with soil organic matter: Elucidating the formation of silver nanoparticles. Chemosphere 2020, 243, 125413. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Fei, Z.; Wu, Q.; Yin, R. Evaluation of the effects of Hg/DOC ratios on the reduction of Hg(II) in lake water. Chemosphere 2020, 253, 126634. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Bauer, I.; Paul, A.; Kappler, A. Arsenic Redox Changes by Microbially and Chemically Formed Semiquinone Radicals and Hydroquinones in a Humic Substance Model Quinone. Environ. Sci. Technol. 2009, 43, 3639–3645. [Google Scholar] [CrossRef] [PubMed]
- Schwertmann, U.; Wagner, F.; Knicker, H. Ferrihydrite–Humic Associations. Soil Sci. Soc. Am. J. 2005, 69, 1009–1015. [Google Scholar] [CrossRef]
- Mak, M.S.H.; Lo, I.M.C. Influences of redox transformation, metal complexation and aggregation of fulvic acid and humic acid on Cr(VI) and As(V) removal by zero-valent iron. Chemosphere 2011, 84, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Fritzsche, A.; Schröder, C.; Wieczorek, A.K.; Händel, M.; Ritschel, T.; Totsche, K.U. Structure and composition of Fe–OM co-precipitates that form in soil-derived solutions. Geochim. Cosmochim. Acta 2015, 169, 167–183. [Google Scholar] [CrossRef]
- Kleber, M.; Eusterhues, K.; Keiluweit, M.; Mikutta, C.; Mikutta, R.; Nico, P.S. Chapter One—Mineral–Organic Associations: Formation, Properties, and Relevance in Soil Environments. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 130, pp. 1–140. [Google Scholar]
- Du, H.; Huang, Q.; Lei, M.; Tie, B. Sorption of Pb(II) by Nanosized Ferrihydrite Organo-Mineral Composites Formed by Adsorption versus Coprecipitation. ACS Earth Space Chem. 2018, 2, 556–564. [Google Scholar] [CrossRef]
- Li, W.; Gou, W.; Li, W.; Zhang, T.; Yu, B.; Liu, Q.; Shi, J. Environmental applications of metal stable isotopes: Silver, mercury and zinc. Environ. Pollut. 2019, 252, 1344–1356. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, S.; Chen, Y.; Wang, L.; Long, Z.; Hughes, S.S.; Ni, S.; Cheng, X.; Wang, J.; Li, T.; et al. Tracing Pb and Possible Correlated Cd Contamination in Soils by Using Lead Isotopic Compositions. J. Hazard. Mater. 2020, 385, 121528. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Pan, X.D.; Peng, C.; Chen, J.Y.; Li, J.; Zeng, J. Tracking contaminants in groundwater flowing across a river bottom within a complex karst system: Clues from hydrochemistry, stable isotopes, and tracer tests. J. Environ. Manag. 2023, 342, 118099. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Song, H.; Shang, J.; Liu, X.; Qi, S.; Li, H. Spectroscopic methods for isotope analysis of heavy metal atoms: A review. Spectrochim. Acta Part B At. Spectrosc. 2023, 207, 106740. [Google Scholar] [CrossRef]
- Greber, N.D.; van Zuilen, K. Multi-collector Inductively Coupled Plasma Mass Spectrometry: New Developments and Basic Concepts for High-precision Measurements of Mass-dependent Isotope Signatures. Chimia 2022, 76, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Qi, M.; Liu, C.; Gao, T.; Wang, Z.; Song, K.; Liu, Y.; Xia, Y. Coupled iron and heavy metal accumulation in karst soils in Southwestern China: Iron isotope perspective. J. Hazard. Mater. 2024, 480, 136105. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Guo, Q.; Wei, R.; Strauss, H.; Zhu, G.; Li, S.; Song, Z.; Chen, T.; Song, B.; Zhou, T.; et al. Contamination of heavy metals and isotopic tracing of Pb in surface and profile soils in a polluted farmland from a typical karst area in southern China. Sci. Total Environ. 2018, 637–638, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Guilin, H.; Liu, M.; Zhang, S.; Wang, L.; Zhu, G. Environmental implications of agricultural abandonment on Fe cycling: Insight from iron forms and stable isotope composition in karst soil, southwest China. Environ. Res. 2022, 215, 114377. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Han, G.; Zeng, J.; Liang, B.; Zhu, G.; Zhao, Y. Development of copper contamination trajectory on the soil systems: A review on the application for stable copper isotopes. Environ. Technol. Innov. 2024, 36, 103795. [Google Scholar] [CrossRef]
- Han, R.; Liu, W.; Xu, Z. The constraint of soil Zn isotope compositions by diverse land utilizations: Evidence from geochemical fingerprint in a typical karst area. Catena 2024, 240, 108005. [Google Scholar] [CrossRef]
- Wang, P.; Hu, J.; Liu, T.; Liu, J.; Ma, S.; Ma, W.; Li, J.; Zheng, H.; Lu, R. Advances in the application of metallic isotopes to the identification of contaminant sources in environmental geochemistry. J. Hazard. Mater. 2023, 458, 131913. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.J.; Han, D.M.; Song, X.F.; Liu, S.H. Sources and migration of heavy metals in a karst water system under the threats of an abandoned Pb-Zn mine, Southwest China. Environ. Pollut. 2021, 277, 116774. [Google Scholar] [CrossRef] [PubMed]
- Bossio, D.A.; Cook-Patton, S.C.; Ellis, P.W.; Fargione, J.; Sanderman, J.; Smith, P.; Wood, S.; Zomer, R.J.; von Unger, M.; Emmer, I.M.; et al. The role of soil carbon in natural climate solutions. Nat. Sustain. 2020, 3, 391–398. [Google Scholar] [CrossRef]
- Liang, C.; Amelung, W.; Lehmann, J.; Kästner, M. Quantitative assessment of microbial necromass contribution to soil organic matter. Glob. Change Biol. 2019, 25, 3578–3590. [Google Scholar] [CrossRef] [PubMed]
- Tao, F.; Huang, Y.Y.; Hungate, B.A.; Manzoni, S.; Frey, S.D.; Schmidt, M.W.I.; Reichstein, M.; Carvalhais, N.; Ciais, P.; Jiang, L.F.; et al. Microbial carbon use efficiency promotes global soil carbon storage. Nature 2023, 618, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Wieder, W.R.; Bonan, G.B.; Allison, S.D. Global soil carbon projections are improved by modelling microbial processes. Nat. Clim. Change 2013, 3, 909–912. [Google Scholar] [CrossRef]
- Hemingway, J.D.; Rothman, D.H.; Grant, K.E.; Rosengard, S.Z.; Eglinton, T.I.; Derry, L.A.; Galy, V.V. Mineral protection regulates long-term global preservation of natural organic carbon. Nature 2019, 570, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Qu, L.R.; Yang, L.M.; Liu, D.W.; Morrissey, E.; Miao, R.H.; Liu, Z.P.; Wang, Q.K.; Fang, Y.T.; Bai, E. Large-scale importance of microbial carbon use efficiency and necromass to soil organic carbon. Glob. Change Biol. 2021, 27, 2039–2048. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.L.; Zhang, W.; Nottingham, A.T.; Xiao, D.; Kuzyakov, Y.; Xu, L.; Chen, H.S.; Xiao, J.; Duan, P.P.; Tang, T.G.; et al. Lithological Controls on Soil Aggregates and Minerals Regulate Microbial Carbon Use Efficiency and Necromass Stability. Environ. Sci. Technol. 2024, 58, 21186–21199. [Google Scholar] [CrossRef] [PubMed]
- Xiao, K.-Q.; Zhao, Y.; Liang, C.; Zhao, M.; Moore, O.W.; Otero-Farina, A.; Zhu, Y.-G.; Johnson, K.; Peacock, C.L. Introducing the soil mineral carbon pump. Nat. Rev. Earth Environ. 2023, 4, 135–136. [Google Scholar] [CrossRef]
- Feng, X.; Qin, S.; Zhang, D.; Chen, P.; Hu, J.; Wang, G.; Liu, Y.; Wei, B.; Li, Q.; Yang, Y.; et al. Nitrogen input enhances microbial carbon use efficiency by altering plant-microbe-mineral interactions. Glob. Change Biol. 2022, 28, 4845–4860. [Google Scholar] [CrossRef] [PubMed]
- Jurado, M.M.; Suárez-Estrella, F.; López, M.J.; Vargas-García, M.C.; López-González, J.A.; Moreno, J. Enhanced turnover of organic matter fractions by microbial stimulation during lignocellulosic waste composting. Bioresour. Technol. 2015, 186, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Wang, L.; Li, Y.; Zhuang, K.; Li, G.; Zhang, J.; Xiao, X.; Xi, Y. Responses of microbial activity, abundance, and community in wheat soil after three years of heavy fertilization with manure-based compost and inorganic nitrogen. Agric. Ecosyst. Environ. 2015, 213, 219–227. [Google Scholar] [CrossRef]
- Bui, V.K.H.; Truong, H.B.; Hong, S.; Li, X.; Hur, J. Biotic and abiotic catalysts for enhanced humification in composting: A comprehensive review. J. Clean. Prod. 2023, 402, 136832. [Google Scholar] [CrossRef]
- Michaud, A.M.; Cambier, P.; Sappin-Didier, V.; Deltreil, V.; Mercier, V.; Rampon, J.-N.; Houot, S. Mass balance and long-term soil accumulation of trace elements in arable crop systems amended with urban composts or cattle manure during 17 years. Environ. Sci. Pollut. Res. 2020, 27, 5367–5386. [Google Scholar] [CrossRef] [PubMed]
- Malone, Z.; Berhe, A.A.; Ryals, R. Impacts of organic matter amendments on urban soil carbon and soil quality: A meta-analysis. J. Clean. Prod. 2023, 419, 138148. [Google Scholar] [CrossRef]
- Tang, X.; Yuan, X.; Lu, W.; Wen, X.; Wu, Y.; Chen, T. Microplastics in livestock manure and compost: Environmental distribution, degradation behavior, and their impact on antibiotic resistance gene dissemination. Chem. Eng. J. 2025, 513, 162881. [Google Scholar] [CrossRef]
- Yakovleva, E.; Gabov, D.; Shamrikova, E.; Korolev, M.; Panukov, A.; Zhangurov, E. Patterns of PAH distribution in karst sinkhole soils (Polar Urals). Environ. Res. 2025, 277, 121555. [Google Scholar] [CrossRef] [PubMed]
- Yan, M.; Peng, T.; Zhao, L.; Li, Q.; Wu, R.; Wang, Y.; Wu, Y.; Teng, Y.; Xiang, X.; Zeng, J.; et al. The roles of organic amendments and plant treatments in soil polychlorinated biphenyl dissipation under oxic and sequential anoxic–oxic conditions. Environ. Res. 2024, 262, 119943. [Google Scholar] [CrossRef] [PubMed]
- Timshina, A.S.; Robey, N.M.; Oldnettle, A.; Barron, S.; Mehdi, Q.; Cerlanek, A.; Townsend, T.G.; Bowden, J.A. Investigating the sources and fate of per- and polyfluoroalkyl substances (PFAS) in food waste compost. Waste Manag. 2024, 180, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Sheng, D.; Meng, X.; Wen, X.; Wu, J.; Yu, H.; Wu, M. Contamination characteristics, source identification, and source-specific health risks of heavy metal(loid)s in groundwater of an arid oasis region in Northwest China. Sci. Total Environ. 2022, 841, 156733. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Ren, X.N.; Rene, E.R.; Wang, Z.; Zhou, L.N.; Zhang, Z.Q.; Wang, Q. The degradation performance of different microplastics and their effect on microbial community during composting process. Bioresour. Technol. 2021, 332, 125133. [Google Scholar] [CrossRef] [PubMed]
- Gui, J.; Sun, Y.; Wang, J.; Chen, X.; Zhang, S.; Wu, D. Microplastics in composting of rural domestic waste: Abundance, characteristics, and release from the surface of macroplastics. Environ. Pollut. 2021, 274, 116553. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yang, J.; Wu, W.-M.; Zhao, J.; Song, Y.; Gao, L.; Yang, R.; Jiang, L. Biodegradation and Mineralization of Polystyrene by Plastic-Eating Mealworms: Part 2. Role of Gut Microorganisms. Environ. Sci. Technol. 2015, 49, 12087–12093. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Yang, C.; Cheng, Z.; Wang, H.; Mojiri, A.; Zhu, N.; Qian, X.; Shen, Y.; Wu, S.; Lou, Z. Exploring into a light-avoided environment: Mechanical-thermal coupled conditions responsible for the aging behavior of plastic waste in landfills. Water Res. 2023, 242, 120162. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.-W.; Zhang, T.; Fang, H.H.P.; He, J. Phthalates biodegradation in the environment. Appl. Microbiol. Biotechnol. 2008, 80, 183–198. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhang, Z.; Dai, Y.; Cun, D.; Cui, B.; Wang, Y.; Fan, Y.; Tang, H.; Qiu, L.; Wang, F.; et al. Biodegradation of phthalate acid esters by a versatile PAE-degrading strain Rhodococcus sp. LW-XY12 and associated genomic analysis. Int. Biodeterior. Biodegrad. 2022, 170, 105399. [Google Scholar] [CrossRef]
- Wu, F.; Guo, Z.; Cui, K.; Dong, D.; Yang, X.; Li, J.; Wu, Z.; Li, L.; Dai, Y.; Pan, T. Insights into characteristics of white rot fungus during environmental plastics adhesion and degradation mechanism of plastics. J. Hazard. Mater. 2023, 448, 130878. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Chen, Y.; Chen, C.; Feng, L.; Dong, Y.; Chen, J.; Lan, J.; Hou, H. High-efficiency degradation of phthalic acid esters (PAEs) by Pseudarthrobacter defluvii E5: Performance, degradative pathway, and key genes. Sci. Total Environ. 2021, 794, 148719. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wen, X.; Zhang, B.; Yang, Y. Diversity and assembly patterns of activated sludge microbial communities: A review. Biotechnol. Adv. 2018, 36, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, H.; Dai, C.; Wang, X.; Wang, L.; Xu, J.; Lu, Z. Microbial interactions enhanced environmental fitness and expanded ecological niches under dibutyl phthalate and cadmium co-contamination. Environ. Pollut. 2022, 306, 119362. [Google Scholar] [CrossRef] [PubMed]
- Xing, R.; Zhai, K.; Du, X.; Chen, X.; Chen, Z.; Zhou, S. Hybrid mechanism of microplastics degradation via biological and chemical process during composting. Bioresour. Technol. 2024, 408, 131167. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Du, H.; Zhou, M.-S.; Ji, Y.; Xie, Y.-Q.; Huang, H.-B.; Zhang, S.-H.; Li, F.; Xiang, L.; Cai, Q.-Y.; et al. Substrate-enzyme interactions and catalytic mechanism in a novel family VI esterase with dibutyl phthalate-hydrolyzing activity. Environ. Int. 2023, 178, 108054. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Teng, Y.; Wang, X.; Xu, Y.; Li, R.; Sun, Y.; Dai, S.; Hu, W.; Wang, H.; Li, Y.; et al. Nitrogen transfer and cross-feeding between Azotobacter chroococcum and Paracoccus aminovorans promotes pyrene degradation. ISME J. 2023, 17, 2169–2181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gilbert, J.A.; Liu, X.; Nie, L.; Xu, X.; Gao, G.; Lyu, L.; Ma, Y.; Fan, K.; Yang, T.; et al. SynCom-mediated herbicide degradation activates microbial carbon metabolism in soils. iMeta 2025, e70058. [Google Scholar] [CrossRef]
- Feng, Z.; Li, X. Microbially induced calcite precipitation and synergistic mineralization cementation mechanism of Pisha sandstone components. Sci. Total Environ. 2023, 866, 161348. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, D.; Lin, J.; Kumar, A.; Jia, K.; Tian, X.; Yu, Z.; Zhu, B. Priming effects induced by degradable microplastics in agricultural soils. Soil Biol. Biochem. 2023, 180, 109006. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).