Abstract
Two products fermented with lactic acid bacteria (LAB) were obtained using malted rice (FR) and mashed malted rice (FMR). Peptide, phenolic acids, and γ-aminobutyric acid (GABA) contents and neuroprotective activities were evaluated before and after simulated gastrointestinal digestion. GABA contents of fermented products were 45 and 51 mg 100 g−1, with a bioaccessibility of 51 and 45% for FR and FMR, respectively. Both fermented malted rice products exhibited inhibitory effects against tyrosinase, acetylcholinesterase, and prolyl oligopeptidase, with FR demonstrating the highest inhibitory activity. The neuroprotective properties were increased after digestion and could potentially be attributed to bioactive peptides generated by germination, fermentation, and digestion, as well as free phenolic acids. These findings suggest that fermented malted rice possesses promising biofunctional properties and may serve as suitable dietary options for individuals with gluten and lactose intolerance, offering additional neuroprotective benefits.
1. Introduction
Lactic acid bacteria (LAB) are microorganisms recognized for their positive effects on human health. In this regard, LAB fermentation produces substantial amounts of bioactive compounds such as peptides, exopolysaccharides (EPSs), etc. [1]. In addition, these bacteria release phenolic compounds from the food matrix due to the disruption of protein/fiber–phenolic compound interactions [2]. Several studies have indicated a potential link between fermented-food consumption and a reduced risk of developing dementia and Alzheimer’s disease. [3]. Moreover, Chen et al. (2024) [4] reported that fermented plant-based foods may have a positive impact on cognitive function by minimizing beta-amyloid-mediated neurodegeneration in humans. Acetylcholinesterase (AChE), tyrosinase (TYR), and prolyl oligopeptidase (POP) are key enzymes involved in the progression of neurodegenerative disorders [5]. AChE is responsible for hydrolyzing the neurotransmitter acetylcholine. Therefore, elevated AChE activity in the brain can impair cholinergic neurotransmission [6], contributing to the onset and progression of Alzheimer’s disease [7]. Conversely, elevated tyrosinase (TYR) activity can lead to an abnormal accumulation of neuromelanin within the brain [8], which has been associated with neurodegenerative processes [9]. Furthermore, the POP enzyme is involved in some neurodegenerative disorders, including Alzheimer’s and Parkinson’s diseases [10]. Thus, inhibiting these enzymes may represent a promising therapeutic approach for managing neurodegenerative disorders.
Germination of cereals has been shown to enhance the levels of γ-aminobutyric acid (GABA), a non-protein amino acid. GABA is synthesized via two principal metabolic routes: the GABA biosynthetic pathway and the polyamine degradation pathway [11]. GABA functions as an inhibitory neurotransmitter in the central nervous system, playing a key role in regulating neuronal excitability. Therefore, GABA has been associated with several health benefits, including anti-anxiety, antihypertensive, and neuroprotective effects [12].
Rice is a major dietary source of GABA for more than half of the world’s population. Thus, the development of rice with high levels of GABA would not only meet diverse nutritional needs but would also increase the value of rice as a staple food.
Cian et al. [13] reported that germination of barley increased the neuroprotective properties of malt, mainly by increasing AChE, TYR, and POP inhibition capacity. Moreover, it was observed that LAB fermentation of germinated brown rice increased the production of bioactive compounds with anti-inflammatory properties [14]. Although the previous study demonstrated that germinated brown rice is suitable for the formulation of fermented products and that fermentation enhances the GABA content, the neuroprotective potential, evaluated through the inhibitory effects on AChE, TYR, and POP, of LAB-fermented products derived from germinated rice flour, has not yet been investigated. Moreover, neither the processing of germinated rice for fermentation nor the impact of gastrointestinal digestion on the neuroprotective compounds and properties of these fermented products has been previously evaluated. This work aims to evaluate the neuroprotective properties of fermented malted rice products obtained through two different processing methods, assessing their effects both before and after simulated gastrointestinal digestion, thereby addressing a critical gap in current knowledge.
2. Materials and Methods
2.1. Reagents
Amino acid standard solution, γ-amino butyric acid, diethyl ethoxymethylenemalonate, acetylcholinesterase (AChE, C3389), tyrosinase (TYR, T3824), acetylthiocholine iodide (01480), L-tyrosine (T4321), 3,4-dihydroxy-L-phenylalanine (D9628), Z-Gly-Pro-4-nitroanilide (96286), DL-Dithiothreitol (D0632), pepsin from porcine gastric mucosa (EC Number: 3.4.23.1; p-7000; 250 U mg−1 solid), pancreatin from porcine pancreas (EC Number: 232-468-9; p-1750; 4X USP), blue dextran (D4772), HPLC peptide standard mixture (H2016), o-phthaldialdehyde (P1378), caffeic acid (C0625), ferulic acid (W518301), p-coumaric acid (C9008), sinapic acid (D7927), gallic acid (67384), protocatechuic acid (08992), hydroxybenzoic acid (PHL83051), and vanillic acid (94770) were obtained from Sigma Chemical Co. (St Louis, MO, USA). Trifluoroacetic acid and acetonitrile of HPLC grade were purchased from Merck (Buenos Aires, Argentina). Analytical-grade reagents were obtained from Cicarelli Laboratorios (San Lorenzo, Santa Fe, Argentina). Prolyl oligopeptidase enzyme (POP) was extracted from the porcine brain according to Aquino et al. (2025) [8].
2.2. Raw Materials
Paddy rice (Oryza sativa L.) was obtained from a local market in Santa Fe (Argentina). Malted rice (R) was produced by soaking grains at 25 °C for 10 h, followed by germination at 35 °C for 72 h (>90% relative humidity). Then, germinated grains were dried at 50 °C for 24 h, dehulled, and milled to obtain rice flour (R). The protein, lipid, ash, total dietary fiber, and total starch of R were 6.7 ± 0.1, 2.2 ± 0.2 g 100 g−1, 1.4 ± 0.1, 2.1 ± 0.0, and 59.0 ± 0.1 g 100 g−1, respectively. Mashed malted rice (MR) flour was obtained according to [15].
2.3. Fermented Product Production and Characterization
Aliquots of 100 g of malted rice flour (R) and the mashed malted rice flour (MR) were inoculated at 0.2 g 100 g−1 with the starter Yoflex Premium 1.0 (CHR Hansen, Copenhagen, Denmark), then incubated at 43 ± 1 °C until a pH of 4.5 was reached. No aeration was provided during fermentation, and all experiments were performed in duplicate. Microbiological analyses were conducted before and immediately after fermentation, and after 14 days of storage at 4 °C. Fermented samples were subsequently stored at −20 °C until further analyses were performed.
The pH of samples was measured using a glass puncture electrode coupled to a digital pH meter (FC200B; Hanna Instruments, Pittsburgh, PA, USA). Titratable acidity was determined by titration with 0.1 mol L−1 NaOH until reaching a pH of 8.3. Results were expressed as grams of lactic acid per 100 g of product (g LA 100 g−1). At the same time, organic acids were quantified by HPLC using an Aminex HPX-87H column (Bio-Rad, Hercules, CA, USA). Sulfuric acid 0.08 N was employed as the mobile phase at a flow rate of 0.6 mL/min and a column temperature of 35 °C. Detection was performed at 210 nm using a DAD detector (SHIMADZU, Tokyo, Japan). Commercial standards of acetic, malic, and lactic acid (Bio-Rad) were used for identification. Calibration curves were constructed for acetic (0.41–3.30 mg/mL), malic (0.18–1.4 mg/mL), and lactic acid (0.25–0.50 mg/mL). Results were expressed as mg pe 100 g of dw.
Free amino groups were measured according to Nielsen et al. [16] using a calibration curve of L-Serine from 0 to 0.95 mEq L−1. The degree of protein hydrolysis (DH) of FR and FMR was calculated considering that the total number of peptide bonds in the protein substrate was 8.65 mEq g−1 of protein [16].
2.3.1. Microbiological Analysis
Lactic acid bacterial counts were performed at 37 °C on selective media according to [17]. MRS acidified and M17 broths, with incubation times of 72 h (anaerobically) and 48 h (aerobically), were used for L. bulgaricus and S. thermophilus, respectively. Total coliforms were analyzed by the method in [18]. Yeasts and molds were analyzed by FIL/IDF 94B:1990 [19]. Results were expressed as log CFU mL−1 of fermented product. Counts were taken at the time of inoculation, after fermentation, and after 14 days of cold storage.
2.3.2. Peptide Analysis by FPLC and HPLC
Fast peptide liquid chromatography (FPLC) of fermented products was performed following the methodology described by Aquino et al. [7] using an KNAUER AZURA® system (Berlin, Germany) equipped with a Superdex 10/300 GL column (GE Life Sciences, Piscataway, NJ, USA). The molecular mass of peptide fractions was estimated by comparison with molecular weight standards (Sigma Chemical Co., St Louis, MO, USA). Moreover, the ratio between peak areas and the total area of the chromatogram was calculated. The FPLC profile was determined in triplicate (n = 3).
Peptide analysis of fermented products was performed by RP-HPLC according to Aquino et al. [7]. A Shimadzu LC-20AT pump, with an SPD-M20A diode detector (SHIMADZU, Tokyo, Japan), and a Phenomenex column (Gemini 110A C18, 250 mm × 4.6 mm, 5 µm particle size) (Phenomenex Ltd., Zeppelinstr, Aschaffenburg, Germany) were used. Fractions with absorbance peaks at 280 and 220 nm were analyzed. The retention times and the areas of peptides in samples were registered. The hydrophobicity of the peptides was characterized by dividing the chromatogram into three sections: 0–20 min (low hydrophobicity), 20–40 min (intermediate hydrophobicity), and 40–60 min (high hydrophobicity) and calculating the percentage of each section with respect to the total area of the chromatogram. The analysis was performed in triplicate (n = 3).
2.3.3. Phenolic Acids
Free phenolic acids were extracted from samples according to Garzón et al. [20] with modifications. Briefly, 1 g of R and 3 g of FR and FMR were added to 5 mL of water, and the mixture was sonicated for 20 min (Arcano, Beijing, China). The mixture was then centrifuged at 5000× g (Cavour VT 3216, Buenos Aires, Argentina) for 20 min, and the resulting supernatants were collected int 15 mL centrifuge tubes. The pellet extractions were repeated using 5 mL of water. After centrifugation, both supernatants were combined and reserved for analysis. The resulting pellets were used for the extraction of bound phenolic acids. Pellets were treated with 5 mL of 2 mol L−1 NaOH for 3 h, acidified to pH 1 with 6 mol L−1 HCl, and extracted twice with ethyl acetate. Then, the solvent was evaporated, and the samples were resuspended in methanol and reserved for analysis. All extractions were performed in triplicate. HPLC analysis was conducted as reported in [20] on a 100 mm × 3.0 mm, 2.7 μm particle size Poroshell 120 column (Agilent, CA, USA). Peak identification was performed by comparing retention times and spectral characteristics with standard compounds. Quantification was carried out through external standard calibration curves of chlorogenic (5–70 mg L−1), caffeic, p-coumaric, ferulic, and sinapic acids (0.2–10 mg L−1). Results were expressed as mg phenolic compound per 100 g dry weight (dw).
2.3.4. Determination of γ-Aminobutyric Acid (GABA)
γ-aminobutyric acid (GABA) of fermented products was determined according to Garzón et al. [20] using an LC-20AT (Shimadzu Co., Kyoto, Japan) equipped with a 300 × 3.9 mm i.d. reverse-phase column Novapack C18 4 m (Waters®, Milford, MA, USA). Shimadzu LC solution software (Ver. 5.118) was used for processing data. The GABA content was calculated using a calibration curve from 0 to 325 mmol GABA L−1, and results were expressed as mg 100 g−1 dw. The analysis was carried out in triplicate (n = 3).
2.4. Neuroprotective Properties of Fermented Products
Neuroprotective properties of fermented products were evaluated as AChE, TYR, and POP enzyme inhibitory activity. The AChE inhibitory activity of malted rice flour and fermented products was measured according to Spontón et al. (2016) [21]. Moreover, the evaluation of the TYR inhibitory activity of R, FR, and FMR was performed according to Oyama et al. [22] using L-tyrosine as a monophenolase substrate and L-DOPA as a diphenolase substrate. The POP inhibition assay was performed according to Chanajon et al. [23]. Enzyme inhibition rates were calculated according to Aquino et al. (2025) [7]. All determinations were performed in triplicate (n = 3).
The concentration of the sample needed to inhibit 50% of enzyme activity was defined as the IC50 value. To determine the IC50 value of AChE, TYR, and POP, serial dilutions of samples from 0 to 15 mg mL−1 of solids were prepared. The experimental data were fitted according to Aquino et al. [7] using Origin software version 7.5 (OriginLab®, Northampton, MA, USA). In the same way, the IC50 value of AChE, TYR, and POP was determined for fermented products. All determinations were performed in triplicate (n = 3).
To characterize the neuroprotective properties of peptides from fermented products, anion exchange chromatography was carried out according to Aquino et al. [7]. For this, an AmberChrom™ 1 × 4 Ion Exchange Resin chloride form (100–200 mesh) (Merck, Buenos Aires, Argentina) was used. Elution was performed at pH 7.8 with 0 and 0.6 mol L−1 NaCl, which allowed for obtaining two fractions (F0 and F0.6, respectively). Free amino groups and neuroprotective properties of fractions at a 0.5 mol L−1 peptide concentration were measured as mentioned above.
The in vitro kinetic analysis of the most active fractions (F0.6) against AChE and TYR was performed according to Aquino et al. (2025) [7]. For this, the in vitro kinetic analysis of enzymes was performed using the Michaelis–Menten equation. For AChE, different substrate concentrations (0.01–0.12 mmol L−1 acetylthiocholine iodide) were incubated with the enzyme solution with and without the F0.6 peptide fraction. The same occurred for TYR, with substrate concentrations of 0.1–0.5 mmol L−1 L-tyrosine. Then, the experimental data were fitted with GraphPad Prism software version 6.07 (GraphPad Software, La Jolla, San Diego, CA, USA) using the Michaelis–Menten equation. Vmax and Km parameters were provided with the same software, considering all the experimental plots. All determinations were performed in triplicate (n = 3).
Peptide identification from the most neuroprotective fraction (F0.6) was performed by MS/MS analysis according to Aquino et al. [7]. The MS/MS analysis was performed on AXIMA-iD Plus (Shimadzu) equipment. The determinations were carried out in the reflectron mode. The MS/MS data were submitted to the MASCOT server for database searching. The searches were performed against the NCBI protein database. The probability of random hits (p) was set to <0.05, meaning 95% confidence in correct peptide identification. The peptide mass and the fragment mass tolerance were set at 1.2 Da. A maximum of two missing cleavages was allowed. Also, methionine oxidation was set as the variable modification. Peptide identifications were accepted if they were statistically significant (p < 0.05).
The GFD and AADQP peptides identified from FR0.6 and FMR0.6 fractions were used for molecular docking assays. Flexible enzyme–peptide docking was performed with the HPEPDOCK server (http://huanglab.phys.hust.edu.cn/hpepdock/, Access: 23 July 2025). The enzyme structures were selected from https://www.rcsb.org/ in PDB format (PDB entry codes: 1EVE and 2Y9X for AChE and TYR, respectively). The best model of each enzyme–peptide interaction was selected according to the most negative docking score. The structures obtained were used for the second stage of refinement to perform high-resolution modeling of the enzyme–peptide interactions using the FlexPepDock server (https://r2.graylab.jhu.edu/apps/submit/flexpepdock, Access: 25 July 2025). Visual analyses to obtain the interaction points were performed using the PyMol 4.60 software.
2.5. Bioaccessibility of Bioactive Compounds from Fermented Products
Simulated gastrointestinal digestion of fermented products with gastric and intestinal phases was performed according to Garzón et al. [20]. To simulate the intestinal absorption, dialysis bags (cut-off: 6–8 kDa) were used. After the digestive process, bag contents corresponding to dialysates of the in vitro intestinal phase were transferred to flasks, weighed, and stored at −20 °C until analysis. The dialysates of FR and FMR were named FR-D and FMR-D, respectively. The entire procedure was performed in triplicate (n = 3).
The protein content of dialysates was measured according to Lowry et al. [24], and free amino groups and GABA contents were determined as above. The phenolic acid profile of dialysates was determined as mentioned before, and the phenolic dialyzability was calculated as reported in [20]. In addition, fast peptide liquid chromatography (FPLC) of dialysates was performed according to Aquino et al. [7]. The DH of fermented products after the simulated gastrointestinal digestion was measured as mentioned before. GABA dialyzability was calculated according to Garzón et al. [20]. The neuroprotective properties of dialysates were measured as described earlier.
2.6. Statistical Analysis
Results were presented as mean ± standard deviation. Statistical analysis was conducted utilizing one-way analysis of variance (ANOVA) with STATGRAPHICS Centurion XV 15.2.06 software (Statpoint Technologies, Inc., Warrenton, VA, USA). The significant disparities between samples were assessed through the Tukey HSD.
3. Results and Discussions
3.1. Characterization of Fermented Products and Bioactive Compounds (GABA and Phenolic Acid Contents)
The composition of fermented products from malted (FR) and mashed malted rice (FMR) did not differ significantly. The final pH value of 4.5 was reached after 4.5 and 6 h of incubation for FR and FMR, respectively. Titratable acidity was higher for FMR (0.16 ± 0.01 and 0.25 ± 0.02 g lactic acid 100 g−1 for FR and FMR, respectively). In agreement, the contents of lactic and acetic acids, analyzed by HPLC, were significatively higher for FMR (1.24 ± 0.01 and 0.83 ± 0.01 g 100 g−1, respectively) than for FR (1.13 ± 0.02 and 0.83 ± 0.30 ± 0.01 g 100 g−1, respectively). Conversely, the malic acid content was higher in FR (0.93 ± 0.01 g 100 g−1) than in FMR (0.60 ± 0.02 g 100 g−1).
Microbiological results are presented in Table 1. Fermentation resulted in a slight increase in Streptococcus thermophilus counts in both FR and FMR products, with levels remaining stable after 14 days of cold storage, comparable to those observed at inoculation. Although Lactobacillus delbrueckii subsp. bulgaricus counts decreased by approximately 1 log in both fermented products compared to initial inoculation levels, viable cells were maintained during 14 days of storage at 4 °C. Other authors have reported LAB count increments or maintenance after fermentation and during cold storage in germinated rice [14] and rice extracts [25].

Table 1.
Microbiological analysis of fermented malted rice (FR) and fermented mashed malted rice (FMR) before and after fermentation and after 14 days of cold storage.
L. delbrueckii subsp. bulgaricus and S. thermophilus are key lactic acid bacteria commonly employed as starter cultures in fermented dairy products. Although these strains do not consistently fulfill all probiotic criteria, their administration at viable cell counts of ≥107 CFU/g is critical to ensure survival through the gastric environment and subsequent delivery to the intestine in sufficient numbers to exert beneficial physiological effects. At these levels, they contribute to enhanced lactose hydrolysis, synthesis of bioactive metabolites, and modulation of the gut microbiota ecosystem [26]. Despite their transient colonization, their presence at adequate concentrations supports intestinal homeostasis and may facilitate the proliferation of beneficial indigenous microorganisms. Therefore, maintaining viable populations above the threshold of 107 CFU/g in fermented products is essential to optimize their functional impact. Hence, germinated rice may serve as a suitable matrix for delivering LAB in fermented foods, supporting the viability of Streptococcus thermophilus at levels ≥ 107 CFU/g for at least 14 days of storage at 4 °C.
In addition, yogurt starter has been successfully used for the fermentation of cereal matrix [27]. Cereals and pseudocereals are generally considered suitable substrates for microbial fermentation, as they are rich in polysaccharides that serve as carbon and energy sources for microorganisms. In addition, they provide minerals, vitamins, sterols, and other essential growth factors [28].
Regarding the GABA content, malted rice (R) presented a content of 69.4 ± 1.3 mg 100 g−1 dw. After fermentation, the product made with mashed malted rice (FMR) reduced the GABA level by 20% (54.6 ± 1.7 mg 100 g−1 dw), and the product made with malted rice (FR) showed a 35% lower GABA concentration (45.0 ± 1.3 mg 100 g−1 dw). Malted or germinated rice is known to have higher GABA levels than other malted cereals such as sorghum (20–60 mg 100 g−1) or barley (30–60 mg 100 g−1) [20]. It is known that the increase in GABA content is primarily attributed to the germination process, which activates several enzymes, most notably glutamate decarboxylase (GAD), responsible for converting L-glutamic acid into GABA [11,12]. Other factors contributing to GABA accumulation during germination include protein synthesis, the breakdown of the precursor compounds, and the hydrolysis of storage proteins into amides, which serve as nitrogen sources for embryonic development [29]. The impact of technological processes such as mashing and fermentation on GABA levels is variable and may result in an increase, retention, or decrease in its content, depending on both the processing parameters and the specific cultivar employed [14]. The decrease in GABA content observed after fermentation may be attributed to several microbiological and physicochemical factors. Certain lactic acid bacteria, including some strains of Streptococcus thermophilus, are capable of metabolizing GABA as carbon or nitrogen sources under specific conditions, leading to its degradation during fermentation [30]. Moreover, the acidification of the medium due to organic acid production (mainly lactic and acetic acids) can alter the stability of GABA and other bioactive compounds. Changes in pH, redox potential, and enzymatic activity during fermentation may further contribute to the degradation or metabolization of GABA. Therefore, the reduction in GABA levels is likely the result of microbial catabolism and environmental shifts that may affect the chemical integrity of this compound.
Table 2 shows the profile of phenolic acids analyzed in R, FR, and FMR. Five phenolic acids (chlorogenic, caffeic, p-coumaric, ferulic, and p-sinapic) were detected in all samples. These compounds were present in both free and bound forms, except for chlorogenic acid, which was not detected in its bound form. In cereals, phenolic acids are present in both free and bound forms; however, the majority are covalently linked to cell wall components such as arabinoxylans, cellulose, and lignin. Alkaline hydrolysis is required to break these bonds before analysis [31].

Table 2.
Phenolic acid profile of malted rice (R), fermented malted rice (FR), and fermented mashed malted rice (FMR).
Ferulic acid showed the highest concentration among the rest of the phenolic acids, in agreement with data reported for brown rice [32].
FMR had the lowest total free phenolic compounds, accounting for 15% of the values registered for R and FR. This result may be attributed either to extraction technical limitations, as discussed below, or to degradation of phenolics by LAB fermentation in FMR. Lactobacilli can utilize phenolic compounds as a prebiotic substrate to support their growth [33], and it seems that free phenolic acids in FMR were more highly available for bacterial metabolization than in the rest of the products (R and FR). The mashing applied to R before fermentation to obtain FMR induced changes in the matrix, leaving free phenolic acids more prone to being metabolized by LAB.
On the other hand, FMR and FR exhibited 1.3 times higher levels of bound phenolic acids than R. Among the individual compounds, bound p-coumaric and ferulic acids were notably elevated in FMR and FR, reaching levels up to 1.7 times higher than in R, and contributing substantially to the increased total bound phenolic acid content of the fermented products (Table 2). Lactobacilli can de-esterify, decarboxylate, and demethylate dietary phenolic compounds bound to matrix constituents such as fiber and proteins, thereby enhancing their extractability and improving their bioaccessibility [34]. As shown, FR and FMR exhibited higher levels of total bound phenolic acids compared to R, likely due to increased extractability following fermentation. However, these differences were not reflected in the free phenolic acid fraction, possibly due to the limitations of water used as the extraction solvent. Phenolic compounds are soluble in polar solvents such as water and protic organic solvents like ethanol and methanol [35]. However, the higher viscosity and lower diffusivity of water may reduce the efficiency of phenolic compound release from the matrix [36]. Although mixtures of organic solvents and water are commonly recommended for the extraction of phenolic compounds, the use of methanol or ethanol in starch-rich matrices, such as rice-based products, may lead to starch precipitation, which can reduce extraction efficiency and interfere with subsequent analytical procedures. Therefore, water was chosen as the extraction solvent for this study. Thus, it is possible that a portion of the free phenolic acids released during fermentation in FR and FRM did not transfer into the aqueous phase and instead contributed to the bound phenolic fraction.
3.2. Neuroprotective Properties of Fermented Products
As shown in Figure 1, malted rice flour (R) showed AChE, TYR, and POP inhibitory properties. This effect may be attributed to the release of bioactive peptides and phenolic compounds during the germination process. In this regard, Cian et al. [13] reported that barley germination produced a high proportion of low-molecular-weight hydrophobic peptides and phenolic acids that act as strong inhibitors against these enzymes. Moreover, the AChE, TYR, and POP inhibitory activity of fermented products was higher than that obtained for R (Figure 1). Thus, the metabolic activity of LAB during fermentation exerted a positive effect on the neuroprotective properties. As is known, during LAB fermentation, microbial proteolytic systems hydrolyze protein substrates, releasing bioactive peptides [20]. In addition, the LAB proteolytic system is composed of proteases with different specificities, which contribute to the generation of low molecular weight and high charge and/or hydrophobic peptides [20]. In this regard, the FPLC profiles of fermented rice (FR) and fermented malted rice (FMR) revealed a predominance of low-molecular-weight peptides, ranging from 159 to approximately 860 Da (Figure 2a,b). It is important to note that the FPLC profile of each fermented product was similar, but with a difference in the proportion of very-low-molecular-weight peptides. In this sense, FR showed a higher proportion of 250 Da peptides than FMR, while FMR had a higher content of 159 Da peptides than FR (Figure 3). The differences in the peptide profile between the two fermented products could be due to the proteins/peptides used as substrates by LAB during fermentation. It should be noted that the mashing process promotes additional protein hydrolysis [37] favoring the generation of new peptides from the germinated flour. Thus, in the case of FR, the LAB consumed the proteins and peptides generated during germination, while for FMR, the LAB could also have used as substrates the additional peptides produced during the mashing process, which modifies the low-MW peptide profile.
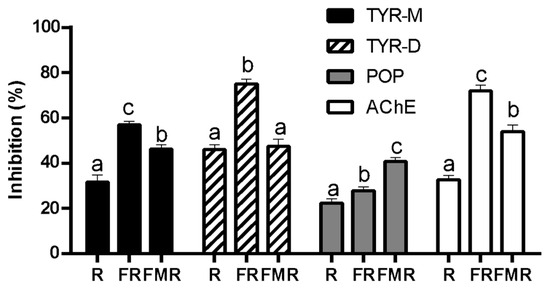
Figure 1.
Acetylcholinesterase (AChE), tyrosinase (TYR), and prolyl oligopeptidase (POP) inhibitory activity of malted rice flour (R), fermented product made with malted rice flour (FR), and fermented product made with mashed malted rice flour (FMR). Different letters in a column for the same enzyme mean significant differences between samples (p < 0.05). TYR-M: monophenolase activity. TYR-D: diphenolase activity.
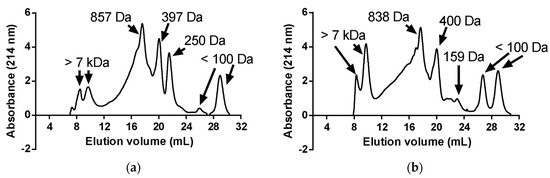
Figure 2.
FPLC gel filtration profile of fermented products made with malted rice flour (FR) (a) and with mashed malted rice flour (FMR) (b).

Figure 3.
Proportion of molecular weight species with respect to total area obtained from the FPLC gel filtration profile of FR and FMR. Different letters in a column mean significant differences between samples (p < 0.05). ND: not detected.
On the other hand, FR showed higher AChE, TYR, and POP inhibitory activity than that obtained for FMR (Figure 1). This may be due to the nature of the peptides produced from R during LAB fermentation. As shown in Figure 4a, the FR profile showed hydrophobic peptides that were not present in the RT-HPLC profile of FMR (Figure 4b). Furthermore, the proportion of hydrophobic peptides was higher in FR compared to FMR (Figure 4c). Hydrophobic peptides elute at higher retention times in the RT-HPLC peptide profile (40–60 min) [7]. In this context, hydrophobic peptides with low molecular weight have been reported to exhibit strong inhibitory activity against AChE, TYR, and POP enzymes [6,10,13]. Therefore, the pronounced neuroprotective effects observed in FR may be linked to the presence of these highly hydrophobic low-MW peptides (250 Da). Cian et al. [13] found that peptides from malted barley with a molecular weight lower than 1 kDa and high hydrophobicity exerted the highest AChE, TYR, and POP inhibitory properties.
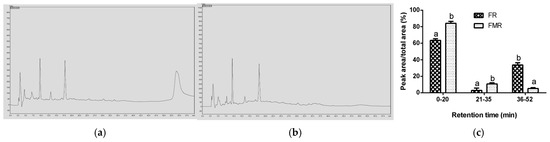
Figure 4.
RT-HPLC profile of FR (a) and FMR (b), and proportion of peptide area with respect to total area obtained from RT-HPLC profile of FR and FMR (c). Different letters in a column mean significant differences between samples (p < 0.05).
Regarding the neuroprotective properties of phenolic compounds, it was reported that phenolic acids can inhibit the AChE enzyme [38]. Moreover, it was observed that phenolic acids can inhibit the TYR enzyme competitively by mimicking its substrate [39]. In this regard, FR had a higher free phenolic acid content than FMR (1.23 ± 0.35 vs. 0.18 ± 0.05 mg 100 g−1 dw, respectively). Thus, the high neuroprotective properties of FR can also be attributed to free phenolic acids.
On the other hand, it was observed that the peptide charge influences the AChE and TYR inhibitory activity. In this regard, negatively charged peptides effectively inhibit the TYR enzyme [40], while positively charged peptides strongly inhibit the AChE enzyme through a competitive mode [6,41]. To evaluate the effect of peptide charge on AChE, TYR, and POP inhibitory activity, fermented products were fractioned by anion exchange chromatography, obtaining two fractions: F0 (uncharged or positively charged peptides at pH 7.8) and F0.6 (negatively charged peptides at pH 7.8). As shown in Figure 5a,b, F0.6 showed the highest TYR inhibitory activity for both fermented products. In this regard, it was observed that negatively charged amino acid residues bind to the copper located at the active site of TYR, blocking the catalytic center. Moreover, aromatic residues have been shown to establish π-metallic coordination bonds with Cu2+, contributing to TYR inhibition. Additionally, small peptides have better access to the TYR active site, promoting its inhibition [40]. Therefore, peptides from fermented products with highly negatively charged and hydrophobic amino acid residues could be primarily responsible for TYR inhibitory activity. Additionally, the kinetic analysis of TYR inhibition showed that F0.6 from both fermented products inhibited this enzyme in a competitive mode (Figure 5c). As presented in Table 3, no significant differences were observed in Vmaxapp in the absence of inhibitors or the presence of FR0.6 or FRM0.6 (p > 0.05). However, the Kmapp value for TYR activity was increased with the addition of these peptide fractions (p < 0.05), indicating that the FR0.6 or FRM0.6 inhibited this enzyme through a competitive mechanism (Figure 5c).
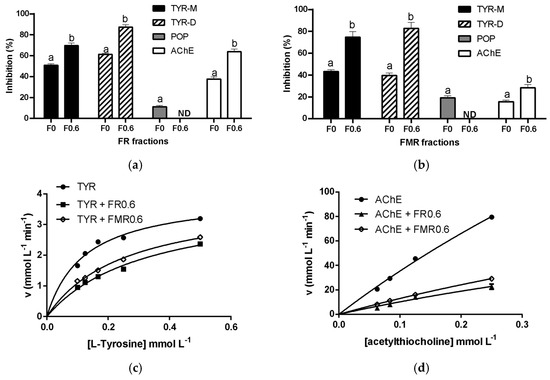
Figure 5.
Acetylcholinesterase (AChE), tyrosinase (TYR), and prolyl oligopeptidase (POP) inhibitory activity of fractions obtained after anion exchange chromatography from FR (a) and FMR (b) evaluated at 0.5 mol peptide L−1, Michaelis–Menten plots of TYR enzyme in the absence (●) or presence of FMR0.6 (◇) or FR0.6 (■) (c), and Michaelis–Menten plots of AChE enzyme in the absence (●) or presence of FMR0.6 (◇) or FR0.6 (■) (d). Different letters in a column mean significant differences between samples (p < 0.05).

Table 3.
Enzyme kinetic analysis of TYR and AChE enzymes inhibited by F0.6 fractions.
Regarding AChE inhibition, F0.6 had higher enzyme inhibitory activity than that obtained for F0. Surprisingly, negatively charged peptides were primarily responsible for the inhibitory effect of FR and FMR. As mentioned, the AChE active sites are negatively charged. Thus, the positively charged peptides are the primary inhibitors by electrostatic interactions [41]. Therefore, the F0.6 peptides likely inhibited the enzyme allosterically. This inhibition mode was confirmed by the kinetic analysis of AChE. As presented in Table 3, there was no significant difference in Kmapp in the absence of inhibitors or the presence of FR0.6 or FRM0.6 (p > 0.05). However, the Vmaxapp value for AChE activity was reduced with the addition of these peptide fractions (p < 0.05), indicating that FR0.6 or FRM0.6 inhibited this enzyme through a non-competitive mechanism (Figure 5c). As is known, a non-competitive inhibitor binds to allosteric sites of the enzyme located in a place other than the active site, causing conformational changes that reduce enzymatic activity [7]. In this sense, Bondžić et al. [41] reported that negatively charged molecules bind to the allosteric site of AChE (β-AS), producing enzyme inhibition. For POP, the F0 showed the highest inhibitory activity, indicating that positive and/or neutral peptides were the main POP inhibitors.
As mentioned, hydrophobic peptides showed high AChE, TYR, and POP inhibitory activity [6,10,13]. However, there was no significant difference in the proportion of hydrophobic peptides to the total area between FR0.6 and FMR0.6, with values being 76.3 ± 1.2 and 76.4 ± 1.2%, respectively. Thus, the charge of peptides played an important role in the neuroprotective properties of fermented products. To characterize the molecular mass and amino acid sequences of FR0.6 and FMR0.6, tandem mass spectrometry analysis was performed. An MS/MS fragment search identified sixteen peptides from FR0.6 and fourteen from FMR0.6. The identified peptides were statistically significant (p < 0.05), confirming their identity with 95% confidence. The molecular weights of the identified FR0.6 peptides ranged from 310.8 to 1370.2 Da, while those of FMR0.6 ranged from 490.8 to 1649.7 Da. The peptide with the highest intensity of those identified in FR0.6 was GFD (337.13 Da), while in the case of FMR0.6, it was AADQP (500.2 Da). It is appropriate to add that both peptides have a negative charge at pH 7.0. Therefore, these peptides could be strongly involved in the inhibition of AChE and TYR enzymes. In this regard, the probable model of interaction of GFD and AADQP peptides with AChE and TYR was studied by in silico analysis using the HPEPDOCK server. The docking energy score obtained from HPEPDOCK allows for weighing the relative binding affinity and stability of the peptide–enzyme complexes. The affinity and stability of the complex increase as the obtained value becomes more negative [6]. The docking score for GFD-AChE was −109.7, indicating a favorable interaction between the peptide and the enzyme. Moreover, the Rosetta score of GFD was −1525.7, while the interface energy was −15.4 kcal mol−1. These results indicate that interaction between GFD and AChE was possible and favorable. As shown in Figure 6a, the GFD peptide (red color) interacted with amino acid residues of the enzyme (blue color), other than those in the active site (orange color). In addition, there were no interactions smaller than 3.0 Å with the enzyme active site (orange color), indicating that this peptide could inhibit the AChE enzyme through a non-competitive mechanism. For the AADQP–AChE complex, the docking score, Rosetta score, and interface energies were −120.1, −1536.7 kcal mol−1, and −25.1 kcal mol−1, respectively. In this case, the AADQP peptide (red color) interacted with the enzyme residues (blue color) close to the active site (orange color). However, there are no interactions below 3.0 Å with the active site (Figure 6b), indicating that this peptide could inhibit the AChE enzyme through a non-competitive mechanism.
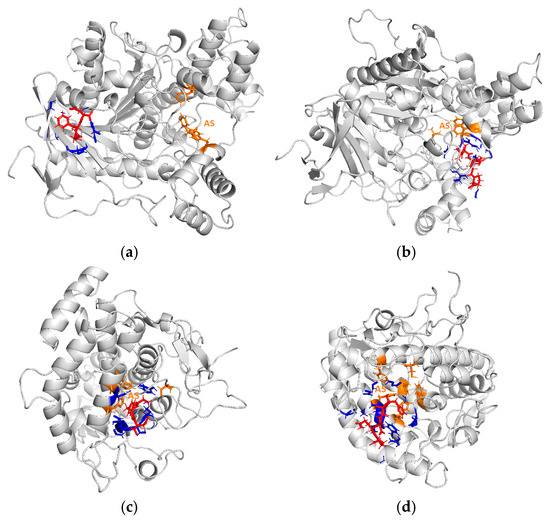
Figure 6.
Interaction between GFD peptide and AChE enzyme (a), interaction between AADQP peptide and AChE enzyme (b), interaction between GFD peptide and TYR enzyme (c), and interaction between AADQP peptide and TYR enzyme (d). Red color: GFD or AADQP peptide. Blue color: enzyme interaction zone with peptides. Orange color: active site of AChE and TYR enzymes. AS: active site.
Regarding the TYR enzyme, the docking score, Rosetta score, and interface energies obtained for GFD-TYR complex were −112.1, −1062.6, and −37.7 kcal mol−1, respectively. For the AADQP-TYR complex, the docking score, Rosetta score, and interface energies were −196.2, −2002.3, and −30.2 kcal mol−1, respectively. These results confirm that the interactions between GFD or AADQP and the TYR enzyme were possible and favorable. As shown in Figure 6c,d, both peptides (red color) interacted with TYR at the enzyme active site (orange color), indicating competitive inhibition. In this regard, the GFD peptide interacted with His259 and His263 residues of the TYR active site, while the AADQP peptide interacted with the His61 residue of the TYR active site. Moreover, there were interactions between the peptides and other amino acid residues of TYR (blue color).
3.3. Neuroprotective Properties of Fermented Products After Gastrointestinal Digestion
It is well established that numerous physiological functions are regulated by endogenous peptides, such as hormones, neurotransmitters, etc. [42]. Throughout the digestive process, proteases can cleave dietary peptides, as well as release new bioactive peptides from ingested proteins [43], which can share structural similarities with endogenous peptides and exert a biological effect by interacting with the same receptors in the body [42]. As shown in Figure 7, dialysates from fermented products presented neuroprotective properties. Thus, the neuroprotective peptides from FR and FMR were bioaccessible. Furthermore, new peptides with neuroprotective properties may have been released from the proteins in the fermented products. As is known, many bioactive peptides may lose their activity following gastrointestinal digestion because of proteolytic degradation; however, in some cases, digestion can enhance bioactivity by generating more potent peptide fragments [7]. Note that the DH of FR and FMR were 11.7 ± 1.2 and 10.0 ± 1.3%, respectively, while in FR-D and FMR-D, they were 50.4 ± 1.2 and 47.0 ± 0.6%, respectively. Thus, in vitro digestion produced effective proteolysis, releasing new peptide species. In this way, the AChE, TYR, and POP-IC50 values obtained for FR-D and FMR-D were lower than those obtained for FR or FMR (Figure 7), indicating that digestive proteolysis increased the neuroprotective properties of fermented products. Thus, the new bioaccessible peptides released from FR or FMR after digestion were more active than those from the fermented products. In this regard, the FPLC peptide profile of FR-D and FMR-D showed effective degradation of the >7 kDa peak (Figure 8a,b), indicating that the digestive proteases degraded the oligopeptides and proteins from the fermented products (Figure 2a,b), which contributed to the generation of new peptides. This was evidenced in the ~ 800 Da peptide proportions of FR-D and FMR-D, with values being 71.4 ± 1.3 and 75.1 ± 2.3% of the total area, respectively. Note that the proportions of ~800 Da in FR and FMR were around 50%. However, there were no differences between the proportions of 400 Da peptides in the dialysates and the fermented products (Figure 3 and Figure 8), which would indicate that some peptides might not be degraded by digestive proteases, dialyzing intact.
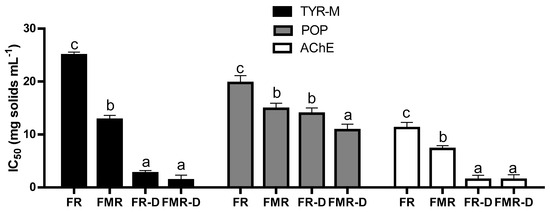
Figure 7.
IC50 values of acetylcholinesterase (AChE), tyrosinase (TYR), and prolyl oligopeptidase (POP) inhibitory activity of the fermented products (FR and FMR) and their dialysates (FR-D and FMR-D) obtained after digestion. Different letters in a column for the same enzyme mean significant differences among samples (p < 0.05). TYR-M: monophenolase activity.
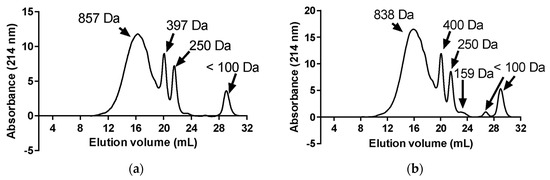
Figure 8.
FPLC gel filtration profile of dialysates obtained from FR (a) and FMR (b) after gastrointestinal digestion.
On the other hand, the ferulic and p-sinapic acid dialyzability of FR-D were 1.03 and 6.03% respectively, while in FMR, they were 1.07 and 8.02%, respectively (Table 4). Thus, the neuroprotective properties of dialysates obtained from fermented and digested products can also be due to phenolic acids that are dialyzed during the simulated digestive process. Moreover, the total free phenolic acid content recovered from FR-D and FMR-D was around 0.30 mg 100g −1 dw (Table 4). This value was higher than that obtained for FMR (Table 2), indicating that the in vitro digestion promoted the release of phenolic acids from this product. As mentioned, the AChE, TYR, and POP-IC50 values obtained for FMR-D were lower than those obtained for FMR (Figure 7). In this regard, it was observed that phenolic acids such as ferulic and p-sinapic acids inhibited both acetylcholinesterase and tyrosinase activities, contributing to neuroprotective effects in models of cognitive decline [44].

Table 4.
Free phenolic acid profile of dialysates and intestinal bioaccessibility (IB) from fermented malted rice (FR) and fermented mashed malted rice (FMR).
Regarding GABA dialyzability, significant differences were observed between the fermented products (p ≤ 0.05). The FR exhibited a higher dialyzability than FMR (50.7 ± 3.2% and 44.7 ± 0.3%, respectively). These results indicate that both fermented samples would enhance their neuroprotective properties through the supply of bioaccessible GABA (~23 mg GABA per 100 g d.w).
4. Conclusions
Both fermented malted rice products exhibited inhibitory effects against tyrosinase, acetylcholinesterase, and prolyl oligopeptidase, with FR demonstrating the highest inhibitory activity. The neuroprotective properties increased after digestion and could potentially be attributed to bioactive peptides generated by germination, fermentation, and digestion, as well as free phenolic acids. Germination followed by fermentation allows for obtaining products that can increase the value of rice as a staple food. This approach holds promise for public health by offering foods that provide health benefits beyond basic nutrition, positioning rice as an effective vehicle for delivering bioactive compounds. Fermented malted rice products with elevated GABA levels and neuroprotective properties could be considered as functional food. Moreover, fermented malted rice possesses promising biofunctional properties and may serve as a suitable dietary option for individuals with gluten and lactose intolerance, offering additional neuroprotective benefits. This contribution not only supports the value of whole grains but also offers a novel strategy to improve the nutritional and functional quality of rice-based products.
Despite the in vitro neuroprotective effects observed in rice-based fermented products, in vivo studies are required to validate these findings.
Author Contributions
Conceptualization, R.E.C. and S.R.D.; data curation, R.E.C., F.V.d.V. and M.A.; formal analysis, R.E.C., F.V.d.V. and M.A.; funding acquisition, M.A. and S.R.D.; investigation and methodology, R.E.C., F.V.d.V. and M.A.; R.E.C. and S.R.D.; project administration, M.A. and S.R.D.; resources M.A. and S.R.D.; supervision, R.E.C. and S.R.D.; writing—original draft, R.E.C., F.V.d.V. and M.A.; writing—review and editing, R.E.C. and S.R.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by ANPCyT—Argentina (Project PICT-2020-3116) and Consejo Nacional de Promociones Científicas y Técnicas (CONICET)—Argentina (PIBAA number 28720210101147CO).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Acknowledgments
The authors thank ANPCyT—Argentina and the National Council for Scientific and Technical Promotion (CONICET)—Argentina for their financial support.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| FR | Fermented malted rice |
| FMR | Fermented mashed malted rice |
| LAB | Lactic acid bacteria |
| GABA | γ-aminobutyric acid |
| CFU | Colony-forming units |
| AChE | Acetylcholinesterase |
| TYR | Tyrosinase |
| POP | Prolyl oligopeptidase |
| R | Malted rice flour |
| MR | Mashed malted rice flour |
| DH | Degree of protein hydrolysis |
| FR-D | Dialysates of FR |
| FMR-D | Dialysates of FMR |
References
- Abdul Hakim, B.N.; Xuan, N.J.; Oslan, S.N.H. A Comprehensive Review of Bioactive Compounds from Lactic Acid Bacteria: Potential Functions as Functional Food in Dietetics and the Food Industry. Foods 2023, 12, 2850. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Chen, Z.; Shao, J.; Wang, C.; Zhan, C. Effect of Fermentation on the Peptide Content, Phenolics and Antioxidant Activity of Defatted Wheat Germ. Food Biosci. 2017, 20, 141–148. [Google Scholar] [CrossRef]
- Porras-García, E.; Fernández-Espada Calderón, I.; Gavala-González, J.; Fernández-García, J.C. Potential Neuroprotective Effects of Fermented Foods and Beverages in Old Age: A Systematic Review. Front. Nutr. 2023, 10, 1170841. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-H.; Yin, X.; He, H.; Lu, L.W.; Wang, M.; Liu, B.; Cheng, K.-W. Potential Neuroprotective Benefits of Plant-Based Fermented Foods in Alzheimer’s Disease: An Update on Preclinical Evidence. Food Funct. 2024, 15, 3920–3938. [Google Scholar] [CrossRef]
- Castro-Salomón, M.; Beltrán-Barrientos, L.M.; González-Córdova, A.F.; Hernández-Mendoza, A.; Torres-Llanez, M.J.; Vallejo-Cordoba, B. Screening of Fermented Milks with Lactococcus and Lactobacillus Strains Isolated from Artisanal Mexican Cheeses by the Evaluation of the in Vitro Inhibition of Enzymes Associated to Neurodegeneration. Int. J. Food Sci. Technol. 2024, 12, 9310–9325. [Google Scholar] [CrossRef]
- Asen, N.D.; Aluko, R.E. Acetylcholinesterase and Butyrylcholinesterase Inhibitory Activities of Antioxidant Peptides Obtained from Enzymatic Pea Protein Hydrolysates and Their Ultrafiltration Peptide Fractions. J Food Biochem. 2022, 46, e14289. [Google Scholar] [CrossRef]
- Aquino, M.E.; Drago, S.R.; Schierloh, L.P.; Cian, R.E. Identification of Bioaccessible Glycosylated Neuroprotective Peptides from Brewer’s Spent Yeast Mannoproteins by in Vitro and in Silico Studies. Food Res. Int. 2025, 209, 116188. [Google Scholar] [CrossRef]
- Iannitelli, A.F.; Hassenein, L.; Mulvey, B.; Blankenship, H.E.; Liles, L.C.; Sharpe, A.L.; Pare, J.-F.; Segal, A.; Sloan, S.A.; Martinowich, K.; et al. Tyrosinase-Induced Neuromelanin Accumulation Triggers Rapid Dysregulation and Degeneration of the Mouse Locus Coeruleus. bioRxiv 2024. [Google Scholar] [CrossRef]
- Nagatsu, T.; Nakashima, A.; Watanabe, H.; Ito, S.; Wakamatsu, K. Neuromelanin in Parkinson’s Disease: Tyrosine Hydroxylase and Tyrosinase. Int. J. Mol. Sci. 2022, 23, 4176. [Google Scholar] [CrossRef] [PubMed]
- Taraszkiewicz, A.; Sinkiewicz, I.; Sommer, A.; Staroszczyk, H. The Biological Role of Prolyl Oligopeptidase and the Procognitive Potential of Its Peptidic Inhibitors from Food Proteins. Crit. Rev. Food Sci. Nutr. 2024, 64, 6567–6580. [Google Scholar] [CrossRef]
- Shen, L.; Yang, Y.; Liu, X.; Zhao, H.; Zhang, Y.; Shen, L.; Zhu, L.; Hu, J.; Ren, D.; Zhang, Q.; et al. Strategies to Improve γ-Aminobutyric Acid Biosynthesis in Rice via Optimal Conditions. Plants 2025, 14, 1290. [Google Scholar] [CrossRef]
- Sun, Y.; Mehmood, A.; Battino, M.; Xiao, J.; Chen, X. Enrichment of Gamma-Aminobutyric Acid in Foods: From Conventional Methods to Innovative Technologies. Food Res. Int. 2022, 162, 111801. [Google Scholar] [CrossRef]
- Cian, R.E.; Garzón, A.G.; Albarracín, M.; Drago, S.R. Production of Neuroprotective Compounds from Barley (Hordeum vulgare L.) Germinated under Different Conditions during the Malting Process. Int. J. Food Sci. Technol. 2025, 60, vvae033. [Google Scholar] [CrossRef]
- Pino, A.; Nicosia, F.D.; Agolino, G.; Timpanaro, N.; Barbagallo, I.; Ronsisvalle, S.; Caggia, C.; Randazzo, C.L. Formulation of Germinated Brown Rice Fermented Products Functionalized by Probiotics. Innov. Food Sci. Emerg. Technol. 2022, 80, 103076. [Google Scholar] [CrossRef]
- Drago, S.; Garzon, A. Formulaciones Alimenticias a Base de Sorgo Fermentado y Su Proceso de Elaboración. Master’s Thesis, Autonomous University of the State of Hidalgo, Pachuca, Mexico, 2023. [Google Scholar]
- Nielsen, P.M.; Petersen, D.; Dambmann, C. Improved Method for Determining Food Protein Degree of Hydrolysis. J. Food Sci. 2001, 66, 642–646. [Google Scholar] [CrossRef]
- ISO 7889/IDF 117A; Milk and Milk Products—Enumeration of Lactic Acid Bacteria—Colony-Count Technique at 30 °C. International Organization for Standardization/International Dairy Federation: Geneva, Switzerland, 1988.
- FIL/IDF 73A; Milk and Milk Products—Enumeration of Microorganisms—Colony-Count Technique at 30 °C. International Dairy Federation: Geneva, Switzerland, 1985.
- FIL/IDF 94B; Milk and Milk Products—Enumeration of Coliforms—Colony-Count Technique at 30 °C. International Organization for Standardization/International Dairy Federation: Geneva, Switzerland, 1990.
- Garzón, A.G.; Van de Velde, F.; Drago, S.R. Gastrointestinal and Colonic in Vitro Bioaccessibility of γ-Aminobutiric Acid (GABA) and Phenolic Compounds from Novel Fermented Sorghum Food. LWT 2020, 130, 109664. [Google Scholar] [CrossRef]
- Spontón, P.G.; Spinelli, R.; Drago, S.R.; Tonarelli, G.G.; Simonetta, A.C. Acetylcholinesterase-Inhibitor Hydrolysates Obtained from “in Vitro” Enzymatic Hydrolysis of Mannoproteins Extracted from Different Strains of Yeasts. Int. J. Food Sci. Technol. 2016, 51, 300–308. [Google Scholar] [CrossRef]
- Oyama, T.; Takahashi, S.; Yoshimori, A.; Yamamoto, T.; Sato, A.; Kamiya, T.; Abe, H.; Abe, T.; Tanuma, S.I. Discovery of a New Type of Scaffold for the Creation of Novel Tyrosinase Inhibitors. Bioorg. Med. Chem. 2016, 24, 4509–4515. [Google Scholar] [CrossRef] [PubMed]
- Chanajon, P.; Noisa, P.; Yongsawatdigul, J. Prolyl Oligopeptidase Inhibition and Cellular Antioxidant Activities of a Corn Gluten Meal Hydrolysate. Cereal Chem. 2022, 99, 1183–1195. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- De Mendonça Brandão, H.C.A.D.N.T.; De Mendonça Brandão, W.A.P.L.N.T.; De Mendonça, S.N.T.G.; Felsner, M.L. Probiotic Fermented Rice Extract Beverage: An Alternative Food for Lactose Intolerants and People Allergic to Bovine Milk and Soy Protein. Braz. J. Food Technol. 2021, 24, e2020119. [Google Scholar] [CrossRef]
- Ayed, L.; M’hir, S.; Nuzzolese, D.; Di Cagno, R.; Filannino, P. Harnessing the Health and Techno-Functional Potential of Lactic Acid Bacteria: A Comprehensive Review. Foods 2024, 13, 1538. [Google Scholar] [CrossRef] [PubMed]
- Garzón, A.G.; Veras, F.F.; Brandelli, A.; Drago, S.R. Bio-Functional and Prebiotics Properties of Products Based on Whole Grain Sorghum Fermented with Lactic Acid Bacteria. J. Sci. Food Agric. 2024, 104, 2971–2979. [Google Scholar] [CrossRef]
- Rollán, G.C. Fermentación Láctica de Cereales y Granos Ancestrales Andinos. In Alimentos Fermentados: Microbiología, Nutrición, Salud y Cultura; Ferrari, A., Vinderola, G., Weill, R., Eds.; Instituto Danone: Buenos Aires, Argentina, 2020. [Google Scholar]
- Duka, F.S.; Rahman, A.N.F. Germinated Rice: An Overview of Gaba, Phenolic Components and Antioxidant Activity. In Proceedings of the IOP Conference Series: Earth and Environmental Science; Institute of Physics: Philadelphia, PA, USA, 2023; Volume 1230. [Google Scholar]
- Sahab, N.R.M.; Subroto, E.; Balia, R.L.; Utama, G.L. γ-Aminobutyric Acid Found in Fermented Foods and Beverages: Current Trends. Heliyon 2020, 6, e05526. [Google Scholar] [CrossRef]
- Horvat, D.; Šimić, G.; Drezner, G.; Lalić, A.; Ledenčan, T.; Tucak, M.; Plavšić, H.; Andrić, L.; Zdunić, Z. Phenolic Acid Profiles and Antioxidant Activity of Major Cereal Crops. Antioxidants 2020, 9, 527. [Google Scholar] [CrossRef]
- Li, S.; Xu, H.; Sui, Y.; Mei, X.; Shi, J.; Cai, S.; Xiong, T.; Carrillo, C.; Castagnini, J.M.; Zhu, Z.; et al. Comparing the LC-MS Phenolic Acids Profiles of Seven Different Varieties of Brown Rice (Oryza sativa L.). Foods 2022, 11, 1552. [Google Scholar] [CrossRef]
- Abdi, R.; Joye, I.J. Prebiotic Potential of Cereal Components. Foods 2021, 10, 2338. [Google Scholar] [CrossRef]
- Polia, F.; Pastor-Belda, M.; Martínez-Blázquez, A.; Horcajada, M.N.; Tomás-Barberán, F.A.; García-Villalba, R. Technological and Biotechnological Processes to Enhance the Bioavailability of Dietary (Poly)Phenols in Humans. J. Agric. Food Chem. 2022, 70, 2092–2107. [Google Scholar] [CrossRef]
- Xu, C.C.; Wang, B.; Pu, Y.Q.; Tao, J.S.; Zhang, T. Advances in Extraction and Analysis of Phenolic Compounds from Plant Materials. Chin. J. Nat. Med. 2017, 15, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Şahin, S.; Şamli, R. Optimization of Olive Leaf Extract Obtained by Ultrasound-Assisted Extraction with Response Surface Methodology. Ultrason. Sonochemistry 2013, 20, 595–602. [Google Scholar] [CrossRef]
- Garzón, A.G.; Albarracín, M.; Drago, S.R. Bioactive Properties of Sorghum-Based Beverages from Whole or Refined Grains. Recent Prog. Nutr. 2023, 3, 1–15. [Google Scholar] [CrossRef]
- Oluwole, O.; Fernando, W.B.; Lumanlan, J.; Ademuyiwa, O.; Jayasena, V. Role of Phenolic Acid, Tannins, Stilbenes, Lignans and Flavonoids in Human Health—A Review. Int. J. Food Sci. Technol. 2022, 57, 6326–6335. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A Comprehensive Review on Tyrosinase Inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef] [PubMed]
- Putri, S.A.; Maharani, R.; Maksum, I.P.; Siahaan, T.J. Peptide Design for Enhanced Anti-Melanogenesis: Optimizing Molecular Weight, Polarity, and Cyclization. Drug Des. Dev. Ther. 2025, 19, 645–670. [Google Scholar] [CrossRef] [PubMed]
- Bondžić, A.M.; Lazarević-Pašti, T.D.; Leskovac, A.R.; Petrović, S.; Čolović, M.B.; Parac-Vogt, T.N.; Janjić, G.V. A New Acetylcholinesterase Allosteric Site Responsible for Binding Voluminous Negatively Charged Molecules—The Role in the Mechanism of AChE Inhibition. Eur. J. Pharm. Sci. 2020, 151, 105376. [Google Scholar] [CrossRef]
- Fernández-Tomé, S.; Hernández-Ledesma, B. Gastrointestinal Digestion of Food Proteins under the Effects of Released Bioactive Peptides on Digestive Health. Mol. Nutr. Food Res. 2020, 64, 2000401. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.S.; Pereira, J.O.; Ferreira, C.; Faustino, M.; Durão, J.; Pintado, M.E.; Carvalho, A.P. Peptide-Rich Extracts from Spent Yeast Waste Streams as a Source of Bioactive Compounds for the Nutraceutical Market. Innov. Food Sci. Emerg. Technol. 2022, 81, 103148. [Google Scholar] [CrossRef]
- Tavan, M.; Hanachi, P.; de la Luz Cádiz-Gurrea, M.; Segura Carretero, A.; Mirjalili, M.H. Natural Phenolic Compounds with Neuroprotective Effects. Neurochem. Res. 2024, 49, 306–326. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).