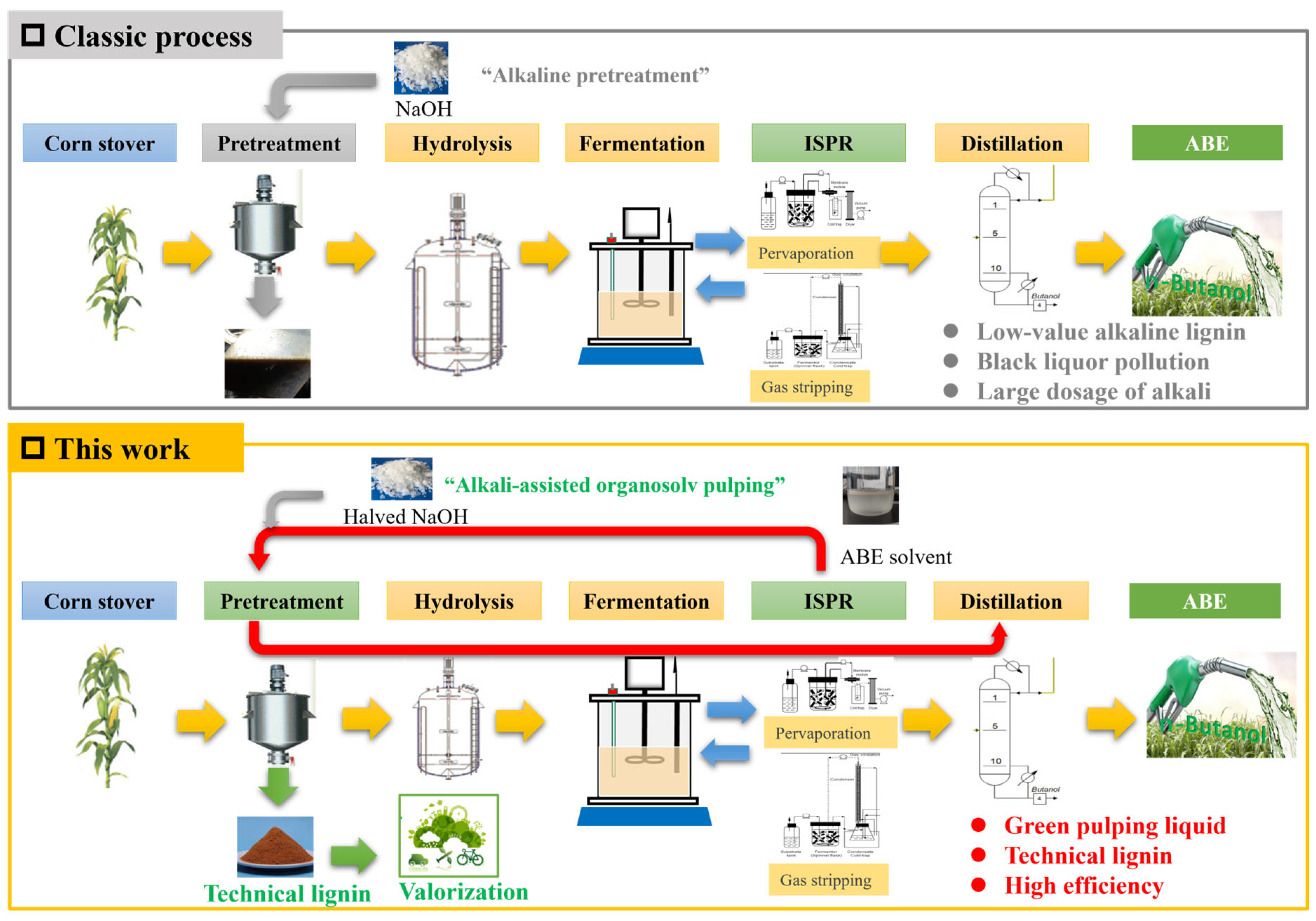
Circulating of In Situ Recovered Stream from Fermentation Broth as the Liquor for Lignocellulosic Biobutanol Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Organosolv Pretreatment
2.3. Enzymatic Hydrolysis
2.4. ABE Fermentation
2.5. Characterization and Analysis
3. Results and Discussions
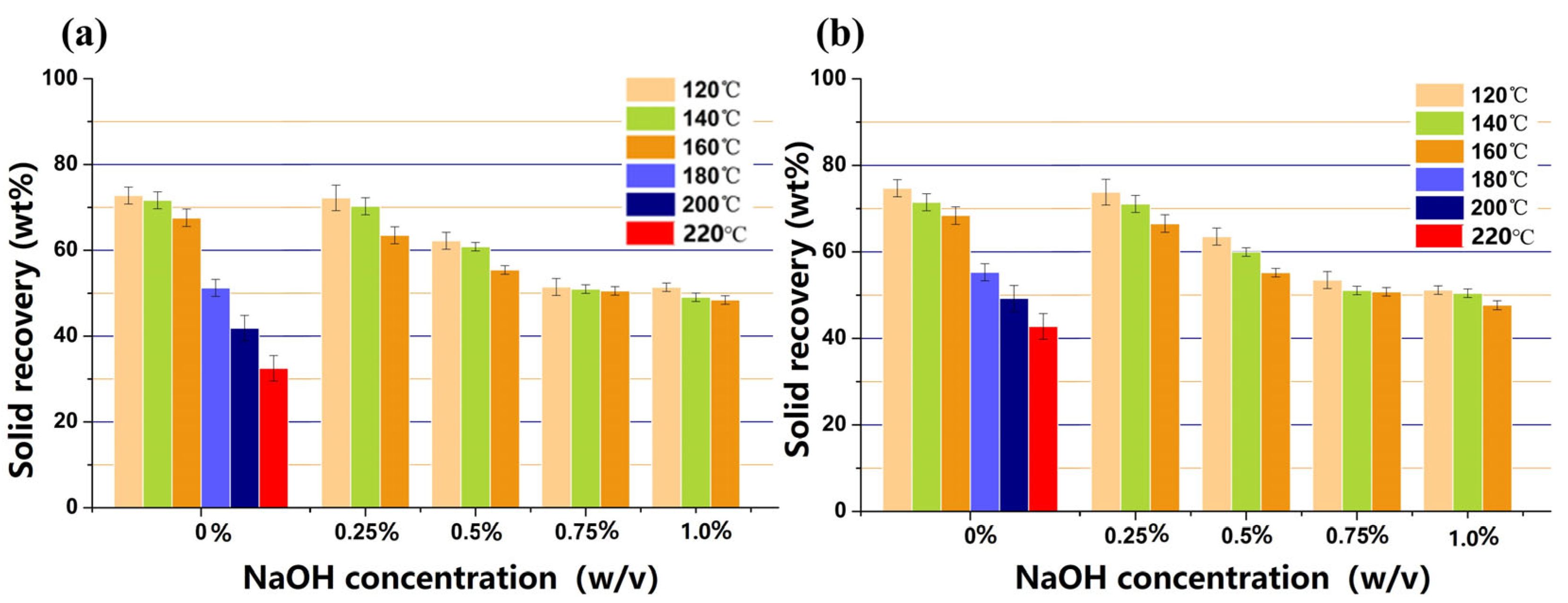
3.1. Acetone–Butanol–Ethanol Pretreatment Performances
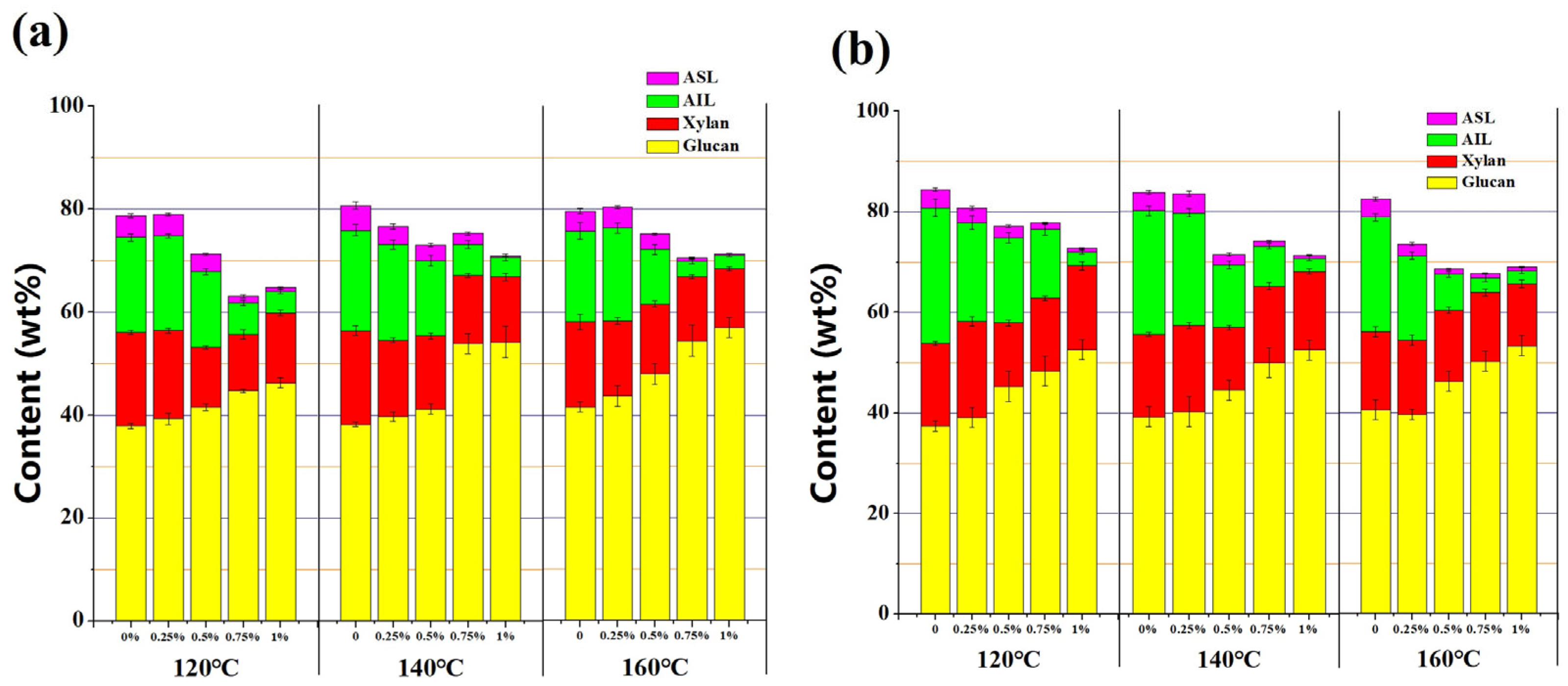
3.2. Structural Characterization of the Pretreated Solids
3.3. Enzymatic Hydrolysis of the Pretreated Solids
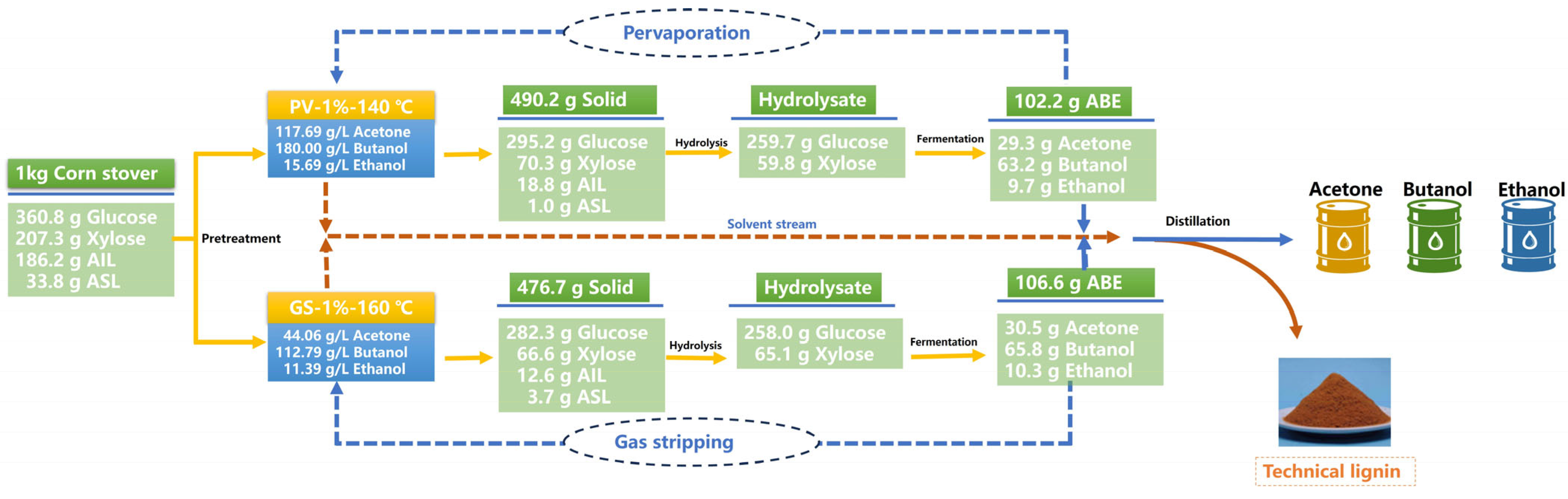
3.4. ABE Fermentation Using the Hydrolysates
3.5. Characterization of the Isolated Lignin
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamalesh, R.; Shaji, A.; Saravanan, A.; Vickram, A.; Yaashikaa, P. Advances in engineered microbes for sustainable biofuel production: Current research and future outlook on lignocellulose utilization. Ind. Crops Prod. 2024, 222, 119988. [Google Scholar] [CrossRef]
- Abolore, R.S.; Jaiswal, S.; Jaiswal, A.K. Green and sustainable pretreatment methods for cellulose extraction from lignocellulosic biomass and its applications: A review. Carbohydr. Polym. Technol. Appl. 2024, 7, 100396. [Google Scholar] [CrossRef]
- Chakraborty, P.; Kumar, R.; Chakrabortty, S.; Saha, S.; Chattaraj, S.; Roy, S.; Banerjee, A.; Tripathy, S.K.; Ghosh, A.K.; Jeon, B.-H. Technological advancements in the pretreatment of lignocellulosic biomass for effective valorization: A review of challenges and prospects. J. Ind. Eng. Chem. 2024, 137, 29–60. [Google Scholar] [CrossRef]
- Li, R.; Zheng, Y.; Zhao, X.; Yong, Q.; Meng, X.; Ragauskas, A.; Huang, C. Recent advances in biomass pretreatment using biphasic solvent systems. Green Chem. 2023, 25, 2505–2523. [Google Scholar] [CrossRef]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K. Pretreatment of lignocellulosic biomass: A review on recent advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef] [PubMed]
- Keshav, P.K.; Banoth, C.; Kethavath, S.N.; Bhukya, B. Lignocellulosic ethanol production from cotton stalk: An overview on pretreatment, saccharification and fermentation methods for improved bioconversion process. Biomass Convers. Biorefin. 2023, 13, 4477–4493. [Google Scholar] [CrossRef]
- Park, Y.C.; Kim, J.S. Comparison of various alkaline pretreatment methods of lignocellulosic biomass. Energy 2012, 47, 31–35. [Google Scholar] [CrossRef]
- Vaidya, A.A.; Murton, K.D.; Smith, D.A.; Dedual, G. A review on organosolv pretreatment of softwood with a focus on enzymatic hydrolysis of cellulose. Biomass Convers. Biorefin. 2022, 12, 5427–5442. [Google Scholar] [CrossRef]
- Veza, I.; Said, M.F.M.; Latiff, Z.A. Recent advances in butanol production by acetone-butanol-ethanol (ABE) fermentation. Biomass Bioenergy 2021, 144, 105919. [Google Scholar] [CrossRef]
- Amiri, H.; Karimi, K.; Zilouei, H. Organosolv pretreatment of rice straw for efficient acetone, butanol, and ethanol production. Bioresour. Technol. 2014, 152, 450–456. [Google Scholar] [CrossRef]
- Amiri, H.; Karimi, K. Improvement of acetone, butanol, and ethanol production from woody biomass using organosolv pretreatment. Bioprocess Biosyst. Eng. 2015, 38, 1959–1972. [Google Scholar] [CrossRef]
- Amiri, H.; Karimi, K. Integration of autohydrolysis and organosolv delignification for efficient acetone, butanol, and ethanol production and lignin recovery. Ind. Eng. Chem. Res. 2016, 55, 4836–4845. [Google Scholar] [CrossRef]
- Kujawska, A.; Kujawski, J.; Bryjak, M.; Kujawski, W. ABE fermentation products recovery methods—A review. Renew. Sustain. Energy Rev. 2015, 48, 648–661. [Google Scholar] [CrossRef]
- Sánchez-Ramírez, E.; Alcocer-García, H.; Quiroz-Ramírez, J.J.; Ramírez-Márquez, C.; Segovia-Hernández, J.G.; Hernández, S.; Errico, M.; Castro-Montoya, A.J. Control properties of hybrid distillation processes for the separation of biobutanol. J. Chem. Technol. Biotechnol. 2017, 92, 959–970. [Google Scholar] [CrossRef]
- Damaurai, J.; Preechakun, T.; Raita, M.; Champreda, V.; Laosiripojana, N. Investigation of alkaline hydrogen peroxide in aqueous organic solvent to enhance enzymatic hydrolysis of rice straw. BioEnergy Res. 2021, 14, 122–134. [Google Scholar] [CrossRef]
- Yang, M.; Guo, X.; Liu, G.; Nan, Y.; Zhang, J.; Noyazzesh, H.; Kuittinen, S.; Vepsäläinen, J.; Pappinen, A. Effect of solvent mixture pretreatment on sugar release from short-rotation coppice Salix schwerinii for biobutanol production. Bioresour. Technol. 2022, 344, 126262. [Google Scholar] [CrossRef]
- Jiménez-Bonilla, P.; Wang, Y. In situ biobutanol recovery from clostridial fermentations: A critical review. Crit. Rev. Biotechnol. 2018, 38, 469–482. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, X.; Li, K.; Nan, Y.; Wang, J.; Zhang, J.; Dou, S.; Li, L.; Liu, G.; Yang, M. Comparison of a solvent mixture assisted dilute acid and alkali pretreatment in sugar production from hybrid Pennisetum. Ind. Crops Prod. 2019, 141, 111806. [Google Scholar] [CrossRef]
- Outram, V.; Lalander, C.A.; Lee, J.G.; Davies, E.T.; Harvey, A.P. Applied in situ product recovery in ABE fermentation. Biotechnol. Prog. 2017, 33, 563–579. [Google Scholar] [CrossRef]
- Cai, D.; Chen, H.; Chen, C.; Hu, S.; Wang, Y.; Chang, Z.; Miao, Q.; Qin, P.; Wang, Z.; Wang, J. Gas stripping–pervaporation hybrid process for energy-saving product recovery from acetone–butanol–ethanol (ABE) fermentation broth. Chem. Eng. J. 2016, 287, 1–10. [Google Scholar] [CrossRef]
- Zhao, P.; Lin, X.; Chen, H.; Chang, Z.; Yang, M.; Su, C.; Gao, Y.; Zhang, C.; Cai, D.; Hou, X. Towards the effective distillation sequences for the purification of acetone-butanol-ethanol from the condensate of gas stripping-vapor permeation system. J. Taiwan Inst. Chem. Eng. 2023, 151, 105102. [Google Scholar] [CrossRef]
- Cai, D.; Hu, S.; Miao, Q.; Chen, C.; Chen, H.; Zhang, C.; Li, P.; Qin, P.; Tan, T. Two-stage pervaporation process for effective in situ removal acetone-butanol-ethanol from fermentation broth. Bioresour. Technol. 2017, 224, 380–388. [Google Scholar] [CrossRef] [PubMed]
- Cai, D.; Chang, Z.; Gao, L.; Chen, C.; Niu, Y.; Qin, P.; Wang, Z.; Tan, T. Acetone-butanol-ethanol (ABE) fermentation integrated with simplified gas stripping using sweet sorghum bagasse as immobilized carrier. Chem. Eng. J. 2015, 277, 176–185. [Google Scholar] [CrossRef]
- Xu, F.; Sun, J.; Liu, C.; Sun, R. Comparative study of alkali-and acidic organic solvent-soluble hemicellulosic polysaccharides from sugarcane bagasse. Carbohydr. Res. 2006, 341, 253–261. [Google Scholar] [CrossRef]
- Sluiter, J.B.; Ruiz, R.O.; Scarlata, C.J.; Sluiter, A.D.; Templeton, D.W. Compositional analysis of lignocellulosic feedstocks. 1. Review and description of methods. J. Agric. Food Chem. 2010, 58, 9043–9053. [Google Scholar] [CrossRef]
- Liu, G.; Si, Z.; Chen, B.; Chen, C.; Cheng, S.; Ouyang, J.; Chen, H.; Cai, D.; Qin, P.; Wang, J. Selection of eco-efficient downstream distillation sequences for acetone-butanol-ethanol (ABE) purification from in situ product recovery system. Renew. Energy 2022, 185, 17–31. [Google Scholar] [CrossRef]
- Cai, D.; Li, P.; Luo, Z.; Qin, P.; Chen, C.; Wang, Y.; Wang, Z.; Tan, T. Effect of dilute alkaline pretreatment on the conversion of different parts of corn stalk to fermentable sugars and its application in acetone-butanol-ethanol fermentation. Bioresour. Technol. 2016, 211, 117–124. [Google Scholar] [CrossRef]
- Li, P.; Cai, D.; Luo, Z.; Qin, P.; Chen, C.; Wang, Y.; Zhang, C.; Wang, Z.; Tan, T. Effect of acid pretreatment on different parts of corn stalk for second generation ethanol production. Bioresour. Technol. 2016, 206, 86–92. [Google Scholar] [CrossRef]
- Cai, D.; Li, P.; Chen, C.; Wang, Y.; Hu, S.; Cui, C.; Qin, P.; Tan, T. Effect of chemical pretreatments on corn stalk bagasse as immobilizing carrier of Clostridium acetobutylicum in the performance of a fermentation-pervaporation coupled system. Bioresour. Technol. 2016, 220, 68–75. [Google Scholar] [CrossRef]
- Cai, D.; Deng, L.; Wu, J.; Su, C.; Wu, Y.; Bi, H.; Wang, Y.; Wang, B.; Zhang, C.; Qin, P. Alkali pretreated corn stalk combined with enzyme cocktail at low cellulase dosage for the high-titer L-lactic acid production. Ind. Crops Prod. 2025, 224, 120332. [Google Scholar] [CrossRef]
- Tsegaye, B.; Balomajumder, C.; Roy, P. Optimization of microwave and NaOH pretreatments of wheat straw for enhancing biofuel yield. Energy Convers. Manag. 2019, 186, 82–92. [Google Scholar] [CrossRef]
- Cheng, Y.-S.; Zheng, Y.; Yu, C.W.; Dooley, T.M.; Jenkins, B.M.; VanderGheynst, J.S. Evaluation of high solids alkaline pretreatment of rice straw. Appl. Biochem. Biotechnol. 2010, 162, 1768–1784. [Google Scholar] [CrossRef]
- Beukes, N.; Pletschke, B.I. Effect of alkaline pre-treatment on enzyme synergy for efficient hemicellulose hydrolysis in sugarcane bagasse. Bioresour. Technol. 2011, 102, 5207–5213. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Chen, S.; Zhang, X.; Xu, F. Exploring crystalline-structural variations of cellulose during alkaline pretreatment for enhanced enzymatic hydrolysis. Bioresour. Technol. 2017, 224, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Karp, E.M.; Resch, M.G.; Donohoe, B.S.; Ciesielski, P.N.; O’Brien, M.H.; Nill, J.E.; Mittal, A.; Biddy, M.J.; Beckham, G.T. Alkaline pretreatment of switchgrass. ACS Sustain. Chem. Eng. 2015, 3, 1479–1491. [Google Scholar] [CrossRef]
- Akimkulova, A.; Zhou, Y.; Zhao, X.; Liu, D. Improving the enzymatic hydrolysis of dilute acid pretreated wheat straw by metal ion blocking of non-productive cellulase adsorption on lignin. Bioresour. Technol. 2016, 208, 110–116. [Google Scholar] [CrossRef]
- Baral, N.R.; Shah, A. Microbial inhibitors: Formation and effects on acetone-butanol-ethanol fermentation of lignocellulosic biomass. Appl. Microbiol. Biotechnol. 2014, 98, 9151–9172. [Google Scholar] [CrossRef]
- Sun, S.; Huang, Y.; Sun, R.; Tu, M. The strong association of condensed phenolic moieties in isolated lignins with their inhibition of enzymatic hydrolysis. Green Chem. 2016, 18, 4276–4286. [Google Scholar] [CrossRef]
- Shuai, L.; Luterbacher, J. Organic solvent effects in biomass conversion reactions. ChemSusChem 2016, 9, 133–155. [Google Scholar] [CrossRef]
- Sacia, E.R.; Balakrishnan, M.; Deaner, M.H.; Goulas, K.A.; Toste, F.D.; Bell, A.T. Highly selective condensation of biomass-derived methyl ketones as a source of aviation fuel. ChemSusChem 2015, 8, 1726–1736. [Google Scholar] [CrossRef]
- Wen, J.-L.; Yuan, T.-Q.; Sun, S.-L.; Xu, F.; Sun, R.-C. Understanding the chemical transformations of lignin during ionic liquid pretreatment. Green Chem. 2014, 16, 181–190. [Google Scholar] [CrossRef]
- Provost, V.; Dumarcay, S.; Ziegler-Devin, I.; Boltoeva, M.; Trébouet, D.; Villain-Gambier, M. Deep eutectic solvent pretreatment of biomass: Influence of hydrogen bond donor and temperature on lignin extraction with high β-O-4 content. Bioresour. Technol. 2022, 349, 126837. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.-Y.; Xiao, L.-P.; Shi, Z.-J.; Sun, R.-C. Structural variation of bamboo lignin before and after ethanol organosolv pretreatment. Int. J. Mol. Sci. 2013, 14, 21394–21413. [Google Scholar] [CrossRef] [PubMed]
- Dutta, T.; Isern, N.G.; Sun, J.; Wang, E.; Hull, S.; Cort, J.R.; Simmons, B.A.; Singh, S. Survey of lignin-structure changes and depolymerization during ionic liquid pretreatment. ACS Sustain. Chem. Eng. 2017, 5, 10116–10127. [Google Scholar] [CrossRef]
- Bykov, I. Characterization of Natural and Technical Lignins Using FTIR Spectroscopy. Master’s Thesis, Luleå University of Technology, Norrbotten, Sweden, 2008. [Google Scholar]
- Yang, H.; Yoo, C.G.; Meng, X.; Pu, Y.; Muchero, W.; Tuskan, G.A.; Tschaplinski, T.J.; Ragauskas, A.J.; Yao, L. Structural changes of lignins in natural Populus variants during different pretreatments. Bioresour. Technol. 2020, 295, 122240. [Google Scholar] [CrossRef]
- Samuel, R.; Pu, Y.; Raman, B.; Ragauskas, A.J. Structural characterization and comparison of switchgrass ball-milled lignin before and after dilute acid pretreatment. Appl. Biochem. Biotechnol. 2010, 162, 62–74. [Google Scholar] [CrossRef]
- Dong, C.; Meng, X.; Yeung, C.S.; Ho-Yin, T.; Ragauskas, A.J.; Leu, S.-Y. Diol pretreatment to fractionate a reactive lignin in lignocellulosic biomass biorefineries. Green Chem. 2019, 21, 2788–2800. [Google Scholar] [CrossRef]
- Sun, R.C. Lignin source and structural characterization. ChemSusChem 2020, 13, 4385–4393. [Google Scholar] [CrossRef]


| Conditions | I002 | Iam | CrI (%) |
|---|---|---|---|
| PV-1%-120 °C | 3.81 × 103 | 2.23 × 103 | 41.5 |
| PV-1%-140 °C | 3.46 × 103 | 2.16 × 103 | 37.6 |
| PV-1%-160 °C | 3.77 × 103 | 2.50 × 103 | 33.7 |
| PV-0.25%-140 °C | 3.59 × 103 | 2.17 × 103 | 39.6 |
| PV-0.50%-140 °C | 3.59 × 103 | 2.11 × 103 | 41.2 |
| PV-0.75%-140 °C | 3.60 × 103 | 2.25 × 103 | 37.5 |
| GS-1%-120 °C | 2.29 × 103 | 1.61 × 103 | 29.7 |
| GS-1%-140 °C | 2.23 × 103 | 1.49 × 103 | 33.2 |
| GS-1%-160 °C | 2.74 × 103 | 1.71 × 103 | 37.6 |
| GS-0.25%-160 °C | 2.77 × 103 | 1.88 × 103 | 32.1 |
| GS-0.5%-160 °C | 2.77 × 103 | 1.82 × 103 | 34.3 |
| GS-0.75%-160 °C | 2.73 × 103 | 1.75 × 103 | 35.9 |
| Raw straw | 2.36 × 103 | 1.34 × 103 | 43.2 |
| Alkaline Dosage (%, w/v) | PV–ABE Pretreated Solid (%) | GS–ABE Pretreated Solid (%) | ||||
|---|---|---|---|---|---|---|
| 120 °C | 140 °C | 160 °C | 120 °C | 140 °C | 160 °C | |
| 0.00 | 28.8 | 29.3 | 34.8 | 20.2 | 20.8 | 34.3 |
| 0.25 | 35.4 | 42.5 | 39.7 | 24.4 | 24.6 | 42.8 |
| 0.50 | 56.2 | 52.4 | 45.6 | 47.9 | 63.9 | 66.6 |
| 0.75 | 68.4 | 64.1 | 82.2 | 54.8 | 69.4 | 87.8 |
| 1.00 | 88.7 | 87.4 | 82.7 | 58.6 | 74.0 | 92.6 |
| Conditions | Mw (g/mol) | Mn (g/mol) | PDI |
|---|---|---|---|
| PV-1%-120 °C | 1.54 × 103 | 1.29 × 103 | 1.19 |
| PV-1%-140 °C | 1.50 × 103 | 1.26 × 103 | 1.19 |
| PV-1%-160 °C | 1.38 × 103 | 1.22 × 103 | 1.13 |
| PV-0.25%-140 °C | 1.70 × 103 | 1.40 × 103 | 1.21 |
| PV-0.50%-140 °C | 1.65 × 103 | 1.36 × 103 | 1.21 |
| PV-0.75%-140 °C | 1.51 × 103 | 1.28 × 103 | 1.18 |
| GS-1%-140 °C | 1.20 × 103 | 1.11 × 103 | 1.08 |
| GS-1%-160 °C | 1.35 × 103 | 1.19 × 103 | 1.13 |
| GS-1%-180 °C | 1.45 × 103 | 1.24 × 103 | 1.17 |
| GS-0.25%-160 °C | 1.71 × 103 | 1.40 × 103 | 1.22 |
| GS-0.50%-160 °C | 1.71 × 103 | 1.38 × 103 | 1.24 |
| GS-0.75%-160 °C | 1.30 × 103 | 1.16 × 103 | 1.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, C.; Gao, Y.; Zhang, G.; Zhang, X.; Li, Y.; Zhang, H.; Wen, H.; Ren, W.; Zhang, C.; Cai, D. Circulating of In Situ Recovered Stream from Fermentation Broth as the Liquor for Lignocellulosic Biobutanol Production. Fermentation 2025, 11, 453. https://doi.org/10.3390/fermentation11080453
Su C, Gao Y, Zhang G, Zhang X, Li Y, Zhang H, Wen H, Ren W, Zhang C, Cai D. Circulating of In Situ Recovered Stream from Fermentation Broth as the Liquor for Lignocellulosic Biobutanol Production. Fermentation. 2025; 11(8):453. https://doi.org/10.3390/fermentation11080453
Chicago/Turabian StyleSu, Changsheng, Yunxing Gao, Gege Zhang, Xinyue Zhang, Yating Li, Hongjia Zhang, Hao Wen, Wenqiang Ren, Changwei Zhang, and Di Cai. 2025. "Circulating of In Situ Recovered Stream from Fermentation Broth as the Liquor for Lignocellulosic Biobutanol Production" Fermentation 11, no. 8: 453. https://doi.org/10.3390/fermentation11080453
APA StyleSu, C., Gao, Y., Zhang, G., Zhang, X., Li, Y., Zhang, H., Wen, H., Ren, W., Zhang, C., & Cai, D. (2025). Circulating of In Situ Recovered Stream from Fermentation Broth as the Liquor for Lignocellulosic Biobutanol Production. Fermentation, 11(8), 453. https://doi.org/10.3390/fermentation11080453






