Ethanol and Xylitol Co-Production by Clavispora lusitaniae Growing on Saccharified Sugar Cane Bagasse in Anaerobic/Microaerobic Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism and Inoculum Preparation
2.2. Pretreatment and Saccharification of Sugar Cane Bagasse (SCB)
2.3. Batch Reactor Fermentation
2.4. Sugars and Metabolites Determination
2.5. Crude-Cell Extract Obtention and Protein Determination
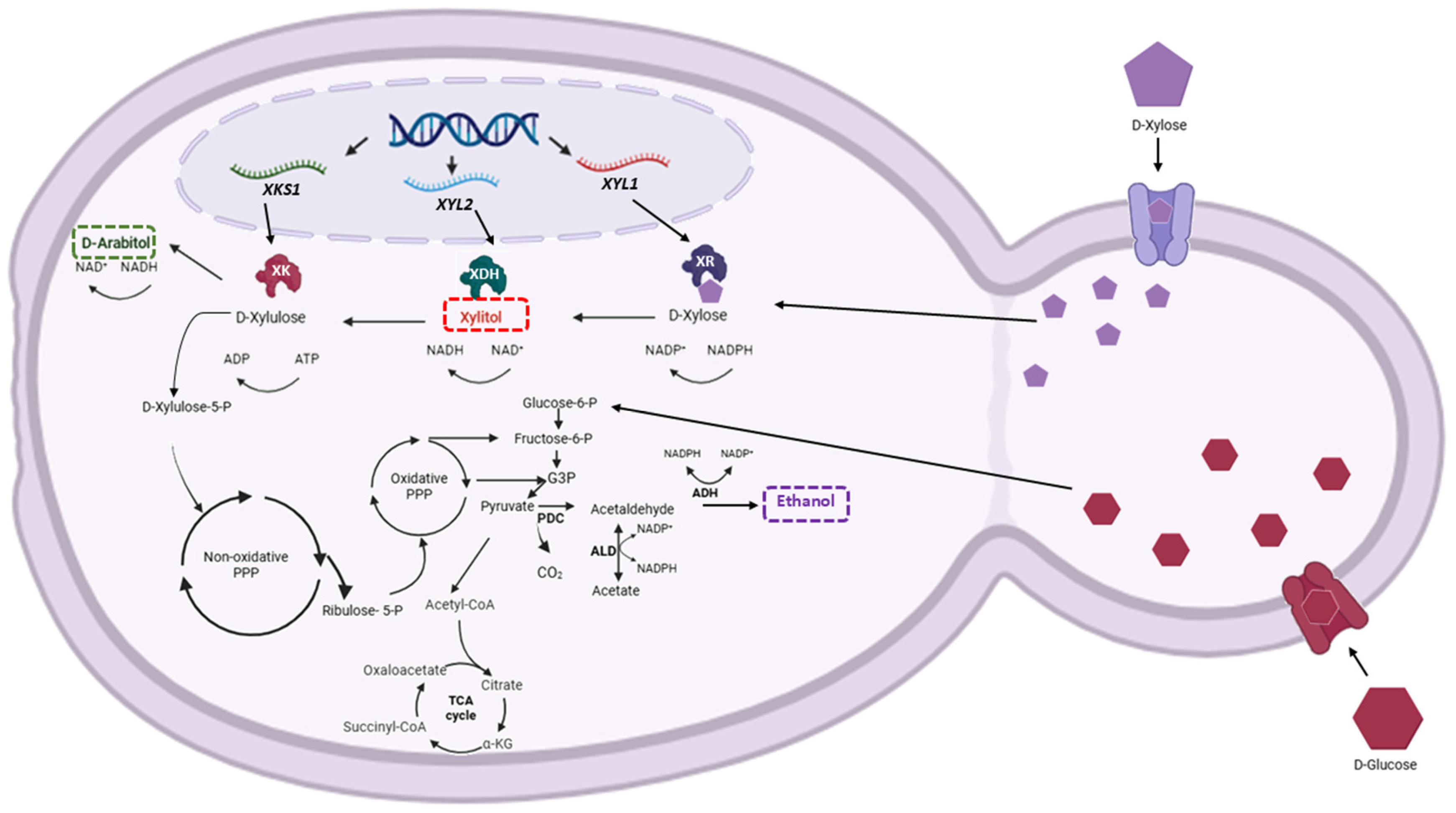
2.6. Xylose Reductase (XR) and Xylitol Dehydrogenase (XDH) Activity Assays
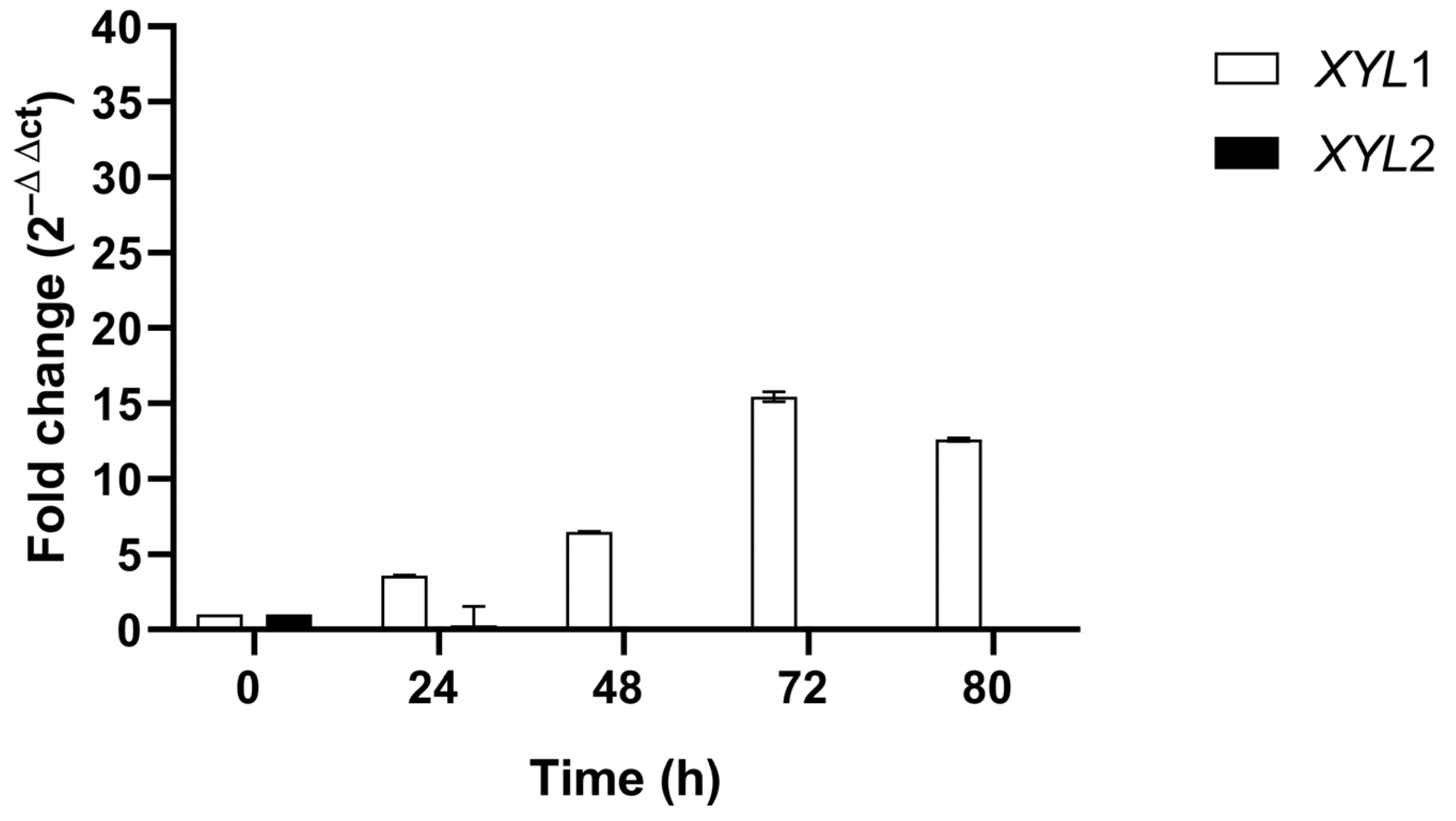
2.7. Expression Levels of XYL1 and XYL2 Genes of C. lusitaniae
2.8. Statistical Analyses
3. Results and Discussion
3.1. Co-Production of Ethanol and Xylitol Under Different Conditions
3.1.1. Microaerobic Condition (C1)
3.1.2. Anaerobic Condition (C2)
3.1.3. Sequential Anaerobic/Microaerobic Phases (C3)
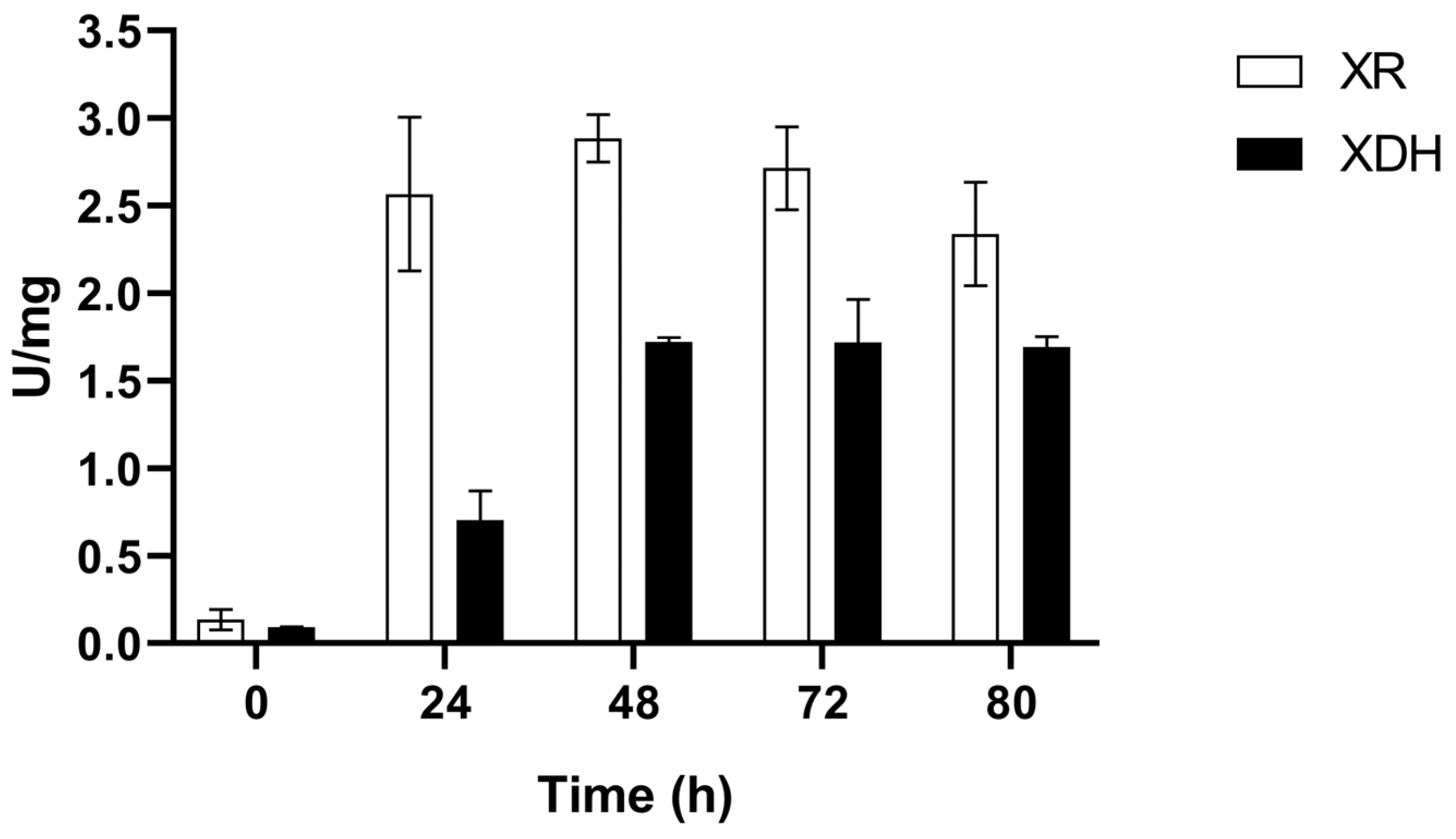
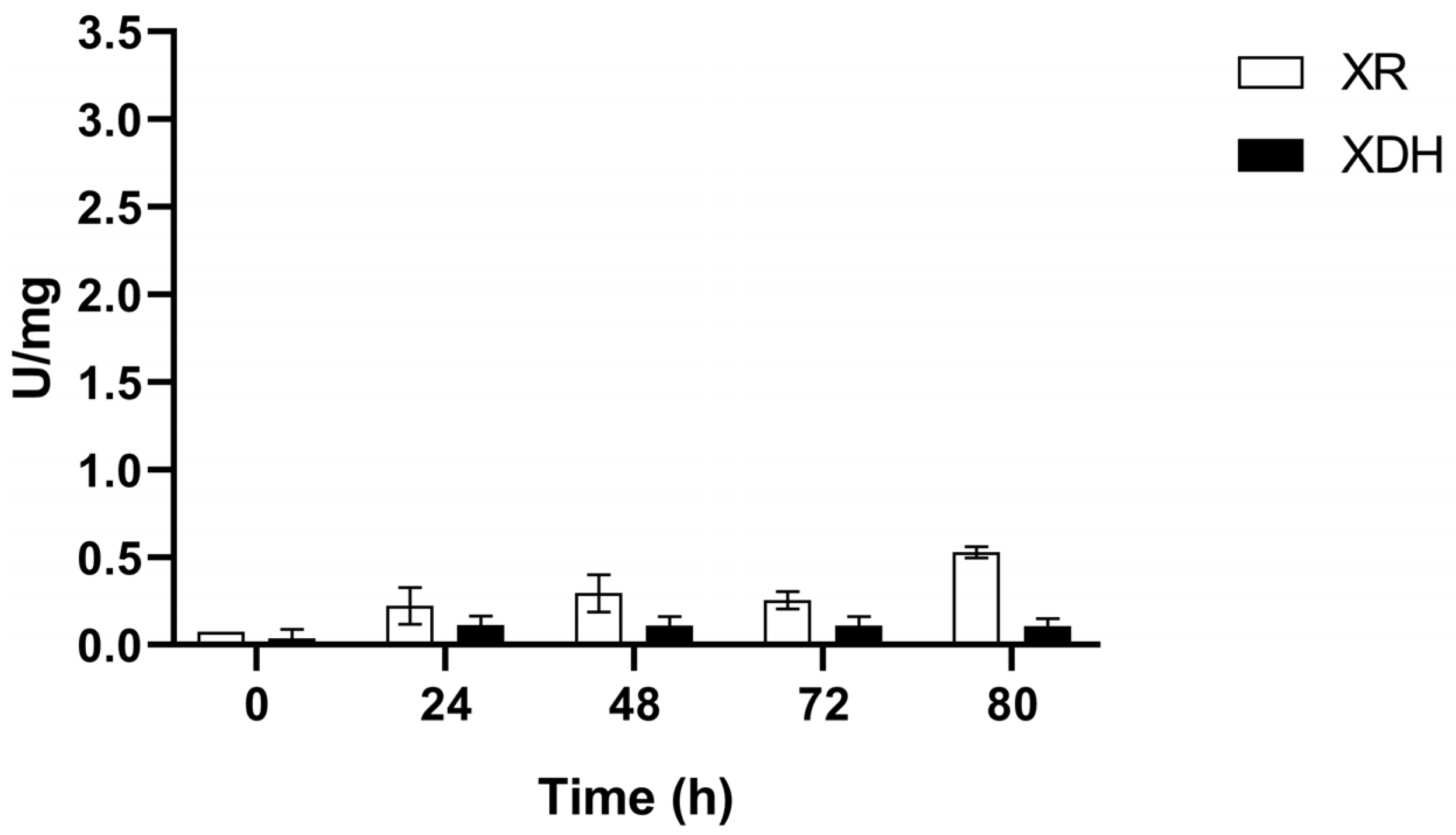
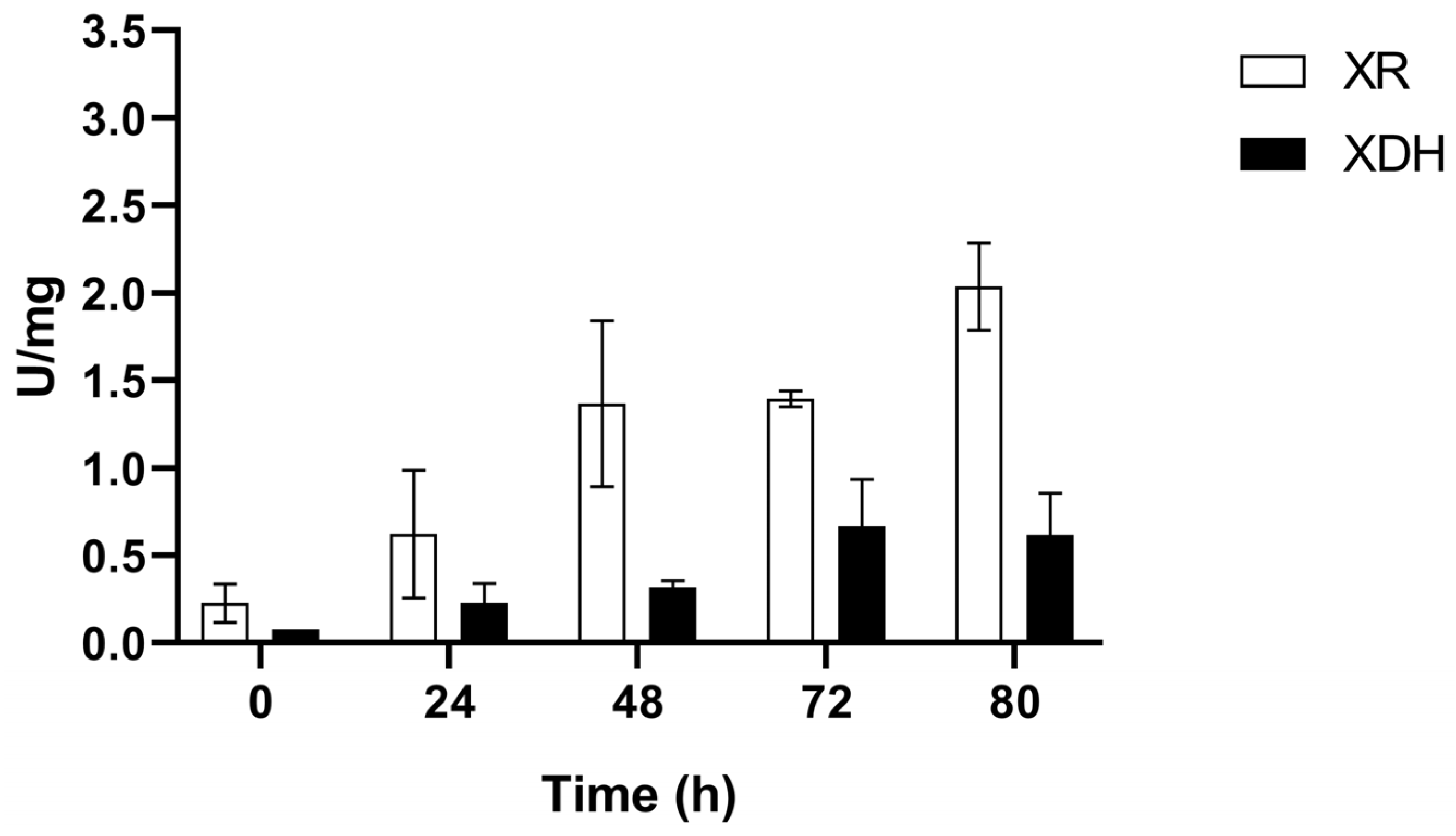
3.2. XR and XDH Enzymatic Activities and Expression Levels of XYL1 and XYL2 Genes
3.2.1. Microaerobic Condition (C1)
3.2.2. Anaerobic Condition (C2)
3.2.3. Sequential Anaerobic/Microaerobic Phases (C3)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thomas, S.; Hosur, M.; Pasquini, D.; Chirayil, C.J. (Eds.) Handbook of Biomass; Springer Nature: Singapore, 2024. [Google Scholar] [CrossRef]
- Anwar, Z.; Gulfraz, M.; Irshad, M. Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: A brief review. J. Radiat. Res. Appl. Sci. 2014, 7, 163–173. [Google Scholar] [CrossRef]
- Santos, A.C.D.; Ximenes, E.; Kim, Y.; Ladisch, M.R. Lignin–Enzyme Interactions in the Hydrolysis of Lignocellulosic Biomass. Trends Biotechnol. 2019, 37, 518–531. [Google Scholar] [CrossRef]
- Meneses, D.B.; De Oca-Vásquez, G.M.; Vega-Baudrit, J.R.; Rojas-Álvarez, M.; Corrales-Castillo, J.; Murillo-Araya, L.C. Pretreatment methods of lignocellulosic wastes into value-added products: Recent advances and possibilities. Biomass Convers. Biorefinery 2022, 12, 547–564. [Google Scholar] [CrossRef]
- Rangel-Basto, Y.A.; Ramos-Valdivia, A.C.; Rojas, C.M.C.-G.; Ponce-Noyola, T. Saccharified sugarcane bagasse as a substrate for astaxanthin production by Xanthophyllomyces dendrorhous. Biomass Convers. Biorefinery 2024, 14, 8071–8079. [Google Scholar] [CrossRef]
- Khare, S.K.; Pandey, A.; Larroche, C. Current perspectives in enzymatic saccharification of lignocellulosic biomass. Biochem. Eng. J. 2015, 102, 38–44. [Google Scholar] [CrossRef]
- Shallom, D.; Shoham, Y. Microbial hemicellulases. Curr. Opin. Microbiol. 2003, 6, 219–228. [Google Scholar] [CrossRef]
- Mussatto, S.I. Application of Xylitol in Food Formulations and Benefits for Health. In D-Xylitol; Da Silva, S.S., Chandel, A.K., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 309–323. [Google Scholar] [CrossRef]
- Goli, J.K.; Panda, S.H.; Linga, V.R. Molecular Mechanism of d-Xylitol Production in Yeasts: Focus on Molecular Transportation, Catabolic Sensing and Stress Response. In D-Xylitol; Da Silva, S.S., Chandel, A.K., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 85–107. [Google Scholar] [CrossRef]
- Ochoa-Chacón, A.; Martinez, A.; Poggi-Varaldo, H.M.; Villa-Tanaca, L.; Ramos-Valdivia, A.C.; Ponce-Noyola, T. Xylose Metabolism in Bioethanol Production: Saccharomyces cerevisiae vs. Non-Saccharomyces Yeasts. BioEnergy Res. 2022, 15, 905–923. [Google Scholar] [CrossRef]
- Lara-Meléndez, A.; Guzmán-Hernández, D.; Montiel-Cruz, J.; Ponce-Noyola, T. Engineering of nonconventional yeasts for valuable products including bioethanol. In Advances in Yeast Biotechnology for Biofuels and Sustainability; Elsevier: Amsterdam, The Netherlands, 2023; pp. 99–116. [Google Scholar] [CrossRef]
- Barros, K.O.; Mader, M.; Krause, D.J.; Pangilinan, J.; Andreopoulos, B.; Lipzen, A.; Mondo, S.J.; Grigoriev, I.V.; Rosa, C.A.; Sato, T.K.; et al. Oxygenation influences xylose fermentation and gene expression in the yeast genera Spathaspora and Scheffersomyces. Biotechnol. Biofuels Bioprod. 2024, 17, 20. [Google Scholar] [CrossRef]
- Chen, Y. Development and application of co-culture for ethanol production by co-fermentation of glucose and xylose: A systematic review. J. Ind. Microbiol. Biotechnol. 2011, 38, 581–597. [Google Scholar] [CrossRef]
- Raj, K.; Krishnan, C. Improved co-production of ethanol and xylitol from low-temperature aqueous ammonia pretreated sugarcane bagasse using two-stage high solids enzymatic hydrolysis and Candida tropicalis. Renew. Energy 2020, 153, 392–403. [Google Scholar] [CrossRef]
- Hor, S.; Kongkeitkajorn, M.B.; Reungsang, A. Sugarcane Bagasse-Based Ethanol Production and Utilization of Its Vinasse for Xylitol Production as an Approach in Integrated Biorefinery. Fermentation 2022, 8, 340. [Google Scholar] [CrossRef]
- Ahuja, V.; Chinnam, S.; Bhatt, A.K. Yeast based biorefinery for xylitol and ethanol production from sugarcane bagasse. Process Saf. Environ. Prot. 2024, 191, 676–684. [Google Scholar] [CrossRef]
- de la Torre, M. SCP production from cellulosic wastes. Conser. Recycl. 1982, 5, 41–45. [Google Scholar] [CrossRef]
- De La Torre, M.; Campillo, C.C. Isolation and characterization of a symbiotic cellulolytic mixed bacterial culture. Appl. Microbiol. Biotechnol. 1984, 19, 430–434. [Google Scholar] [CrossRef]
- Ponce-Noyola, T.; De La Torre, M. Isolation of a high-specific-growth-rate mutant of Cellulomonas flavigena on sugar cane bagasse. Appl. Microbiol. Biotechnol. 1995, 42, 709–712. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, W.; Tan, X.; Chen, X.; Guo, Y.; Yu, Q.; Yuan, Z.; Zhuang, X. Low-temperature sodium hydroxide pretreatment for ethanol production from sugarcane bagasse without washing process. Bioresour. Technol. 2019, 291, 121844. [Google Scholar] [CrossRef]
- Rojas-Rejón, Ó.A.; Poggi-Varaldo, H.M.; Ramos-Valdivia, A.C.; Ponce-Noyola, T.; Cristiani-Urbina, E.; Martínez, A.; de la Torre, M. Enzymatic saccharification of sugar cane bagasse by continuous xylanase and cellulase production from Cellulomonas flavigena PR-22. Biotechnol. Prog. 2016, 32, 321–326. [Google Scholar] [CrossRef]
- Breus, N.A.; Ryazanova, L.P.; Dmitriev, V.V.; Kulakovskaya, T.V.; Kulaev, I.S. Accumulation of phosphate and polyphosphate by Cryptococcus humicola and Saccharomyces cerevisiae in the absence of nitrogen. FEMS Yeast Res. 2012, 12, 617–624. [Google Scholar] [CrossRef]
- Lowry, O.; Rosebrough, N.; Farr, A.L.; Randall, R. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Cocotle-Ronzon, Y.; Zendejas-Zaldo, M.; Castillo-Lozano, M.L.D.; Aguilar-Uscanga, M. Preliminary Characterization of Xylose Reductase Partially Purified by Reversed Micelles from Candida tropicalist IEC5-ITV, an Indigenous Xylitol-Producing Strain. Adv. Chem. Eng. Sci. 2012, 2, 9–14. [Google Scholar] [CrossRef][Green Version]
- MacRae, E. Extraction of Plant RNA. In Protocols for Nucleic Acid Analysis by Nonradioactive Probes; Humana Press: Totowa, NJ, USA, 2007; Volume 353, pp. 15–24. [Google Scholar] [CrossRef]
- Shakeel, M.; Rodriguez, A.; Tahir, U.B.; Jin, F. Gene expression studies of reference genes for quantitative real-time PCR: An overview in insects. Biotechnol. Lett. 2018, 40, 227–236. [Google Scholar] [CrossRef]
- Cortivo, P.R.D.; Hickert, L.R.; Hector, R.; Ayub, M.A.Z. Fermentation of oat and soybean hull hydrolysates into ethanol and xylitol by recombinant industrial strains of Saccharomyces cerevisiae under diverse oxygen environments. Ind. Crops Prod. 2018, 113, 10–18. [Google Scholar] [CrossRef]
- Unrean, P.; Ketsub, N. Integrated lignocellulosic bioprocess for co-production of ethanol and xylitol from sugarcane bagasse. Ind. Crops Prod. 2018, 123, 238–246. [Google Scholar] [CrossRef]
- Jain, V.; Awasthi, A.; Santhagunam, A.; Ghosh, S. Sequential fermentation with in situ distillation for bioethanol and xylitol production from mixed sugars: A novel approach for lignocellulosic material-based biorefineries. Biomass Convers. Biorefinery 2024, 14, 24529–24537. [Google Scholar] [CrossRef]
- Du, C.; Li, Y.; Zhao, X.; Pei, X.; Yuan, W.; Bai, F.; Jiang, Y. The production of ethanol from lignocellulosic biomass by Kluyveromyces marxianus CICC 1727-5 and Spathaspora passalidarum ATCC MYA-4345. Appl. Microbiol. Biotechnol. 2019, 103, 2845–2855. [Google Scholar] [CrossRef]
- Chen, C.; Tang, X.; Xiao, Z.; Zhou, Y.; Jiang, Y.; Fu, S. Ethanol fermentation kinetics in a continuous and closed-circulating fermentation system with a pervaporation membrane bioreactor. Bioresour. Technol. 2012, 114, 707–710. [Google Scholar] [CrossRef]
- Fan, S.; Xiao, Z.; Li, M. Energy efficient of ethanol recovery in pervaporation membrane bioreactor with mechanical vapor compression eliminating the cold traps. Bioresour. Technol. 2016, 211, 24–30. [Google Scholar] [CrossRef]
- Santos, J.C.; Converti, A.; De Carvalho, W.; Mussatto, S.I.; Da Silva, S.S. Influence of aeration rate and carrier concentration on xylitol production from sugarcane bagasse hydrolyzate in immobilized-cell fluidized bed reactor. Process Biochem. 2005, 40, 113–118. [Google Scholar] [CrossRef]
- Dasgupta, D.; Ghosh, D.; Bandhu, S.; Adhikari, D.K. Lignocellulosic sugar management for xylitol and ethanol fermentation with multiple cell recycling by Kluyveromyces marxianus IIPE453. Microbiol. Res. 2017, 200, 64–72. [Google Scholar] [CrossRef]
- Rech, F.R.; Fontana, R.C.; Rosa, C.A.; Camassola, M.; Ayub, M.A.Z.; Dillon, A.J.P. Fermentation of hexoses and pentoses from sugarcane bagasse hydrolysates into ethanol by Spathaspora hagerdaliae. Bioprocess Biosyst. Eng. 2019, 42, 83–92. [Google Scholar] [CrossRef]
- Da Silveira, F.A.; Fernandes, T.A.R.; Bragança, C.R.S.; Balbino, T.R.; Diniz, R.H.S.; Passos, F.M.L.; da Silveira, W.B. Isolation of xylose-assimilating yeasts and optimization of xylitol production by a new Meyerozyma guilliermondii strain. Int. Microbiol. 2020, 23, 325–334. [Google Scholar] [CrossRef]
- Bonan, C.I.D.G.; Biazi, L.E.; Dionísio, S.R.; Soares, L.B.; Tramontina, R.; Sousa, A.S.; de Oliveira Filho, C.A.; Costa, A.C.; Ienczak, J.L. Redox potential as a key parameter for monitoring and optimization of xylose fermentation with yeast Spathaspora passalidarum under limited-oxygen conditions. Bioprocess Biosyst. Eng. 2020, 43, 1509–1519. [Google Scholar] [CrossRef]
- Prawphan, Y.; Mallika, B. Ethanol Production Capability of Candida shehatae in Mixed Sugars and Rice Straw Hydrolysate. Sains Malaysiana 2016, 45, 581–587. [Google Scholar]
- Yu, X.; Zheng, Y.; Xiong, X.; Chen, S. Co-utilization of glucose, xylose and cellobiose by the oleaginous yeast Cryptococcus curvatus. Biomass Bioenergy 2014, 71, 340–349. [Google Scholar] [CrossRef]
- Zhang, G.; Lin, Y.; He, P.; Li, L.; Wang, Q.; Ma, Y. Characterization of the sugar alcohol-producing yeast Pichia anomala. J. Ind. Microbiol. Biotechnol. 2014, 41, 41–48. [Google Scholar] [CrossRef]
- Ravikumar, Y.; Razack, S.A.; Ponpandian, L.N.; Zhang, G.; Yun, J.; Huang, J.; Lee, D.; Li, X.; Dou, Y.; Qi, X. Microbial hosts for production of D-arabitol: Current state-of-art and future prospects. Trends Food Sci. Technol. 2022, 120, 100–110. [Google Scholar] [CrossRef]
- Da Silva, D.D.V.; De Almeida Felipe, M.D.G. Effect of glucose:xylose ratio on xylose reductase and xylitol dehydrogenase activities from Candida guilliermondii in sugarcane bagasse hydrolysate. J. Chem. Technol. Biotechnol. 2006, 81, 1294–1300. [Google Scholar] [CrossRef]
- Tamburini, E.; Bianchini, E.; Bruni, A.; Forlani, G. Cosubstrate effect on xylose reductase and xylitol dehydrogenase activity levels, and its consequence on xylitol production by Candida tropicalis. Enzyme Microb. Technol. 2010, 46, 352–359. [Google Scholar] [CrossRef]
- Ribeiro, L.E.; Albuini, F.M.; Castro, A.G.; Campos, V.J.; de Souza, G.B.; Mendonça, J.G.; Rosa, C.A.; Mendes, T.A.; Santana, M.F.; da Silveira, W.B.; et al. Influence of glucose on xylose metabolization by Spathaspora passalidarum. Fungal Genet. Biol. 2021, 157, 103624. [Google Scholar] [CrossRef]
- Pal, S.; Choudhary, V.; Kumar, A.; Biswas, D.; Mondal, A.K.; Sahoo, D.K. Studies on xylitol production by metabolic pathway engineered Debaryomyces hansenii. Bioresour. Technol. 2013, 147, 449–455. [Google Scholar] [CrossRef]
- Atzmüller, D.; Ullmann, N.; Zwirzitz, A. Identification of genes involved in xylose metabolism of Meyerozyma guilliermondii and their genetic engineering for increased xylitol production. AMB Express 2020, 10, 78. [Google Scholar] [CrossRef]
- Kim, S.R.; Ha, S.-J.; Kong, I.I.; Jin, Y.-S. High expression of XYL2 coding for xylitol dehydrogenase is necessary for efficient xylose fermentation by engineered Saccharomyces cerevisiae. Metab. Eng. 2012, 14, 336–343. [Google Scholar] [CrossRef]
- Bolzico, B.C.; Racca, S.; Khawam, J.N.; Leonardi, R.J.; Tomassi, A.H.; Benzzo, M.T.; Comelli, R.N. Exploring xylose metabolism in non-conventional yeasts: Kinetic characterization and product accumulation under different aeration conditions. J. Ind. Microbiol. Biotechnol. 2024, 51, kuae023. [Google Scholar] [CrossRef]
- Kumar, K.; Singh, E.; Shrivastava, S. Microbial xylitol production. Appl. Microbiol. Biotechnol. 2022, 106, 971–979. [Google Scholar] [CrossRef]
- Zou, Y.; Qi, K.; Chen, X.; Miao, X.; Zhong, J.-J. Favorable effect of very low initial KLa value on xylitol production from xylose by a self-isolated strain of Pichia guilliermondii. J. Biosci. Bioeng. 2010, 109, 149–152. [Google Scholar] [CrossRef]
- Kim, O.C.; Suwannarangsee, S.; Oh, D.B.; Kim, S.; Seo, J.W.; Kim, C.H.; Kang, H.A.; Kim, J.Y.; Kwon, O. Transcriptome analysis of xylose metabolism in the thermotolerant methylotrophic yeast Hansenula polymorpha. Bioprocess Biosyst. Eng. 2013, 36, 1509–1518. [Google Scholar] [CrossRef]
- Manjarres-Pinzón, K.; Mendoza-Meza, D.; Arias-Zabala, M.; Correa-Londoño, G.; Rodriguez-Sandoval, E. Effects of agitation rate and dissolved oxygen on xylose reductase activity during xylitol production at bioreactor scale. Food Sci. Technol. 2022, 42, e04221. [Google Scholar] [CrossRef]
- Cadete, R.M.; de Las Heras, A.M.; Sandström, A.G.; Ferreira, C.; Gírio, F.; Gorwa-Grauslund, M.F.; Rosa, C.A.; Fonseca, C. Exploring xylose metabolism in Spathaspora species: XYL1.2 from Spathaspora passalidarum as the key for efficient anaerobic xylose fermentation in metabolic engineered Saccharomyces cerevisiae. Biotechnol. Biofuels 2016, 9, 167. [Google Scholar] [CrossRef]





| SCB Treatment | Lignin (%) | Cellulose (%) | Hemicellulose (%) |
|---|---|---|---|
| Raw material | 23.27 ± 0.39 | 47.4 ± 1.54 | 17.98 ± 1.27 |
| NaOH (2%) | 15.37 ± 0.66 | 65.83 ± 3.64 | 12.52 ± 1.52 |
| Primer | Sequence (5′3′) | Amplicon Length (pb) | Match Side in the Gene (pb) |
|---|---|---|---|
| Fw-XYL1 | CGGTTACAGATTGTTCGACGGT | 480 | 111 |
| Rv-XYL1 | TGGTTGTTGCAAGTATGGGTG | 591 | |
| Fw-XYL2 | GGTATCTGTGGTTCCGATATCCA | 219 | 926 |
| Rv-XYL2 | CATGTGAGGACACAAGTTGTAGT | 707 | |
| Fw-ACT1 | TCTACAACGAATTGAGAGTTGC | 269 | 245 |
| Rv-ACT1 | GACAAGATCTTCATCAAGTAGTC | 514 |
| Strain | Bioprocess and Substrate | Ethanol (g/L) 24 h | Xylitol (g/L) 80 h | Arabitol (g/L) 80 h | Glucose Consumption (%) 24 h | Xylose Consumption (%) 80 h | Y EtOH/Glu 24 h | Y XOH/Xyl 80 h | Ethanol Productivity (g/L/h) 24 h | Xylitol Productivity (g/L/h) 80 h | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| C. lusitaniae (C1) | Batch SSCB | 18.1 ± 0.957 | 14.30 ± 0.334 | 0.74 ± 0.090 | 100 ± 0.028 | 100 ± 0.000 | 0.28 ± 0.014 | 0.58 ± 0.003 | 0.75 ± 0.040 | 0.17 ± 0.004 | This work |
| C. lusitaniae (C2) | Batch SSCB | 31.80 ± 0.910 | 4.10 ± 0.001 | 0.81 ± 0.017 | 100 ± 0.000 | 27.97 ± 0.078 | 0.49 ± 0.005 | 0.51 ± 0.015 | 1.32 ± 0.038 | 0.05 ± 0.001 | This work |
| C. lusitaniae (C3) | Batch SSCB | 30.90 ± 0.566 | 11.99 ± 0.002 | 2.33 ± 0.715 | 100 ± 0.255 | 94.74 ± 0.018 | 0.47 ± 0.009 | 0.53 ± 0.062 | 1.28 ± 0.025 | 0.14 ± 0.001 | This work |
| S. hagerdaliae UFMG-CM-Y303 | Batch Hydrolysates of soyabean and oat | 9.5 ± 0.9 | 4.5 ± 0.3 | - | - | - | 0.38 ± 0.02 | 0.20 ± 0.01 | 0.04 ± 0.01 | 0.02 ± 0.00 | [27] |
| S. cerevisiae S288C and C. tropicalis | SSF SCB | 56.1 ± 1.7 | 24 ± 0.9 | - | 100 ± 0.9 | 100 ± 0.8 | 0.44 ± 0.01 | 0.50 ± 0.01 | 0.58 ± 0.03 | 0.25 ± 0.01 | [28] |
| Z. mobilis C. tropicalis | Fed-batch Glucose and xylose | 29.74 ± 0.2 | 27.14 ± 0.16 | - | 100 ± 0.01 | 100 ± 0.4 | 0.49 ± 0.005 | 0.54 ± 0.002 | 2.47 ± 0.01 | 0.76 ± 0.004 | [29] |
| K. marxianus CICC 1727-5 | SSCF Corn cob | 14.13 NR | 8.26 NR | - | 100 NR | 100 NR | 0.41 NR | 0.54 NR | - | - | [30] |
| S. passalidarum | SSCF Corn cob | 9.85 NR | 6.17 NR | - | 100 NR | 100 NR | 0.27 NR | 0.52 NR | - | - | [30] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzmán-Hernández, D.; Ramos-Valdivia, A.C.; Poggi-Varaldo, H.M.; Barrera-Cortés, J.; Cristiani-Urbina, E.; Ponce-Noyola, T. Ethanol and Xylitol Co-Production by Clavispora lusitaniae Growing on Saccharified Sugar Cane Bagasse in Anaerobic/Microaerobic Conditions. Fermentation 2025, 11, 344. https://doi.org/10.3390/fermentation11060344
Guzmán-Hernández D, Ramos-Valdivia AC, Poggi-Varaldo HM, Barrera-Cortés J, Cristiani-Urbina E, Ponce-Noyola T. Ethanol and Xylitol Co-Production by Clavispora lusitaniae Growing on Saccharified Sugar Cane Bagasse in Anaerobic/Microaerobic Conditions. Fermentation. 2025; 11(6):344. https://doi.org/10.3390/fermentation11060344
Chicago/Turabian StyleGuzmán-Hernández, David, Ana C. Ramos-Valdivia, Héctor Mario Poggi-Varaldo, Josefina Barrera-Cortés, Eliseo Cristiani-Urbina, and Teresa Ponce-Noyola. 2025. "Ethanol and Xylitol Co-Production by Clavispora lusitaniae Growing on Saccharified Sugar Cane Bagasse in Anaerobic/Microaerobic Conditions" Fermentation 11, no. 6: 344. https://doi.org/10.3390/fermentation11060344
APA StyleGuzmán-Hernández, D., Ramos-Valdivia, A. C., Poggi-Varaldo, H. M., Barrera-Cortés, J., Cristiani-Urbina, E., & Ponce-Noyola, T. (2025). Ethanol and Xylitol Co-Production by Clavispora lusitaniae Growing on Saccharified Sugar Cane Bagasse in Anaerobic/Microaerobic Conditions. Fermentation, 11(6), 344. https://doi.org/10.3390/fermentation11060344









