Impact of the Thermovinification Practice Combined with the Use of Autochthonous Yeasts on the Fermentation Kinetics of Red Wines
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Material
2.2. Yeasts
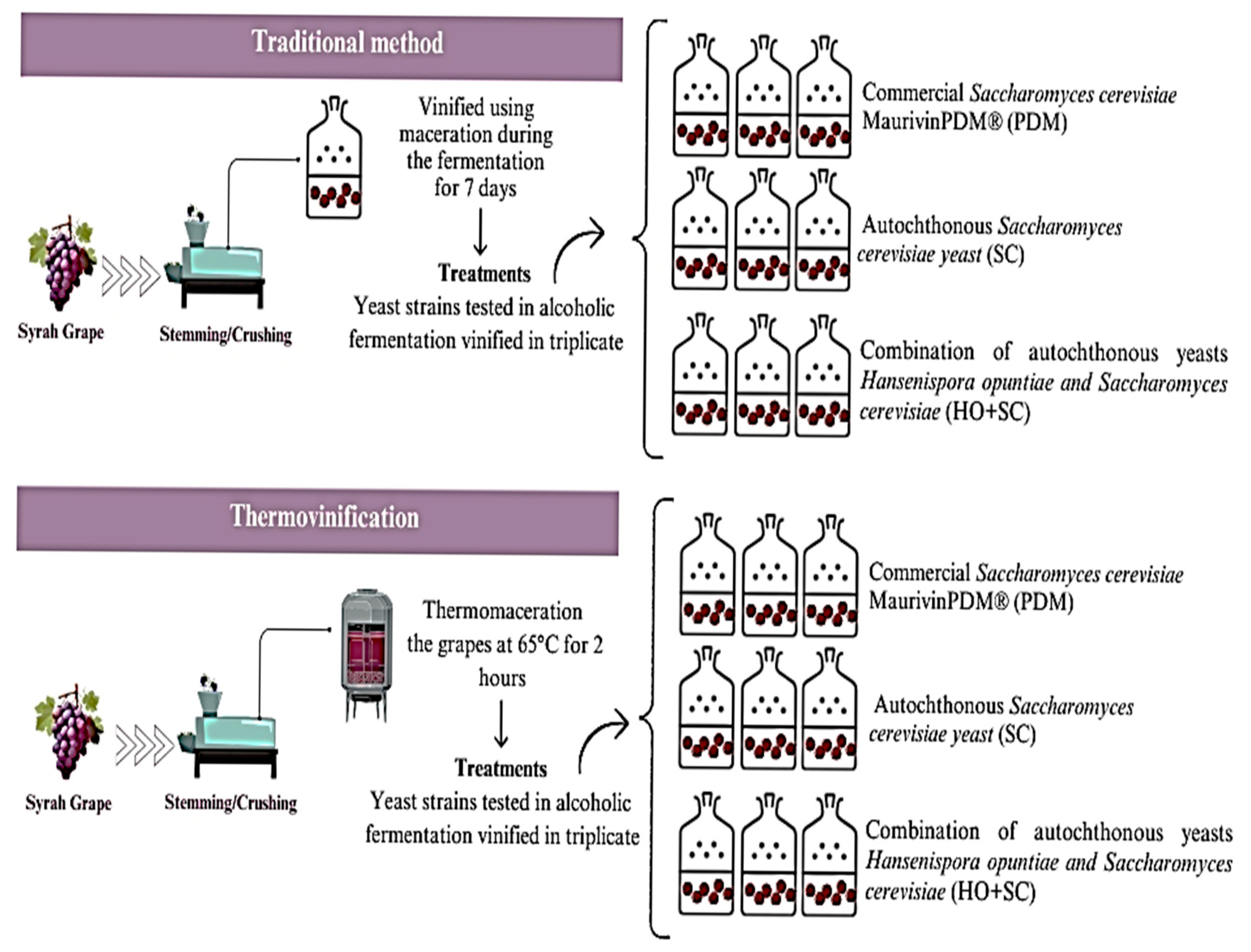
2.3. Winemaking
2.4. Fermentation Kinetics
2.4.1. Simultaneous Determination of the Sugars and Alcohols
2.4.2. Monitoring of the Yeast Cell Growth
2.4.3. Kinetic Parameters, Productivity, and Efficiency of the Fermentation Process
2.5. Physicochemical Composition of the Wines
2.6. Statistical Analysis
3. Results and Discussion
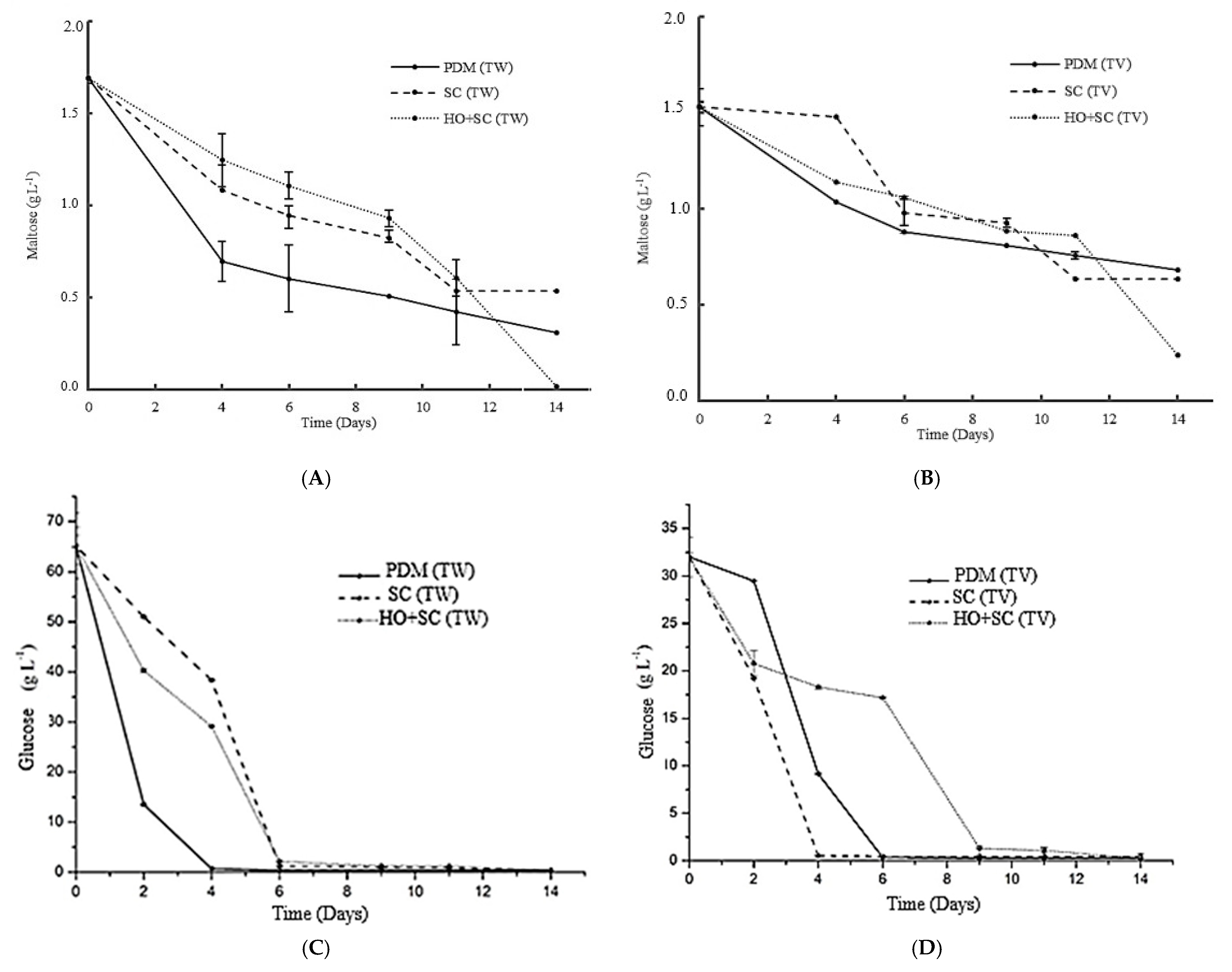
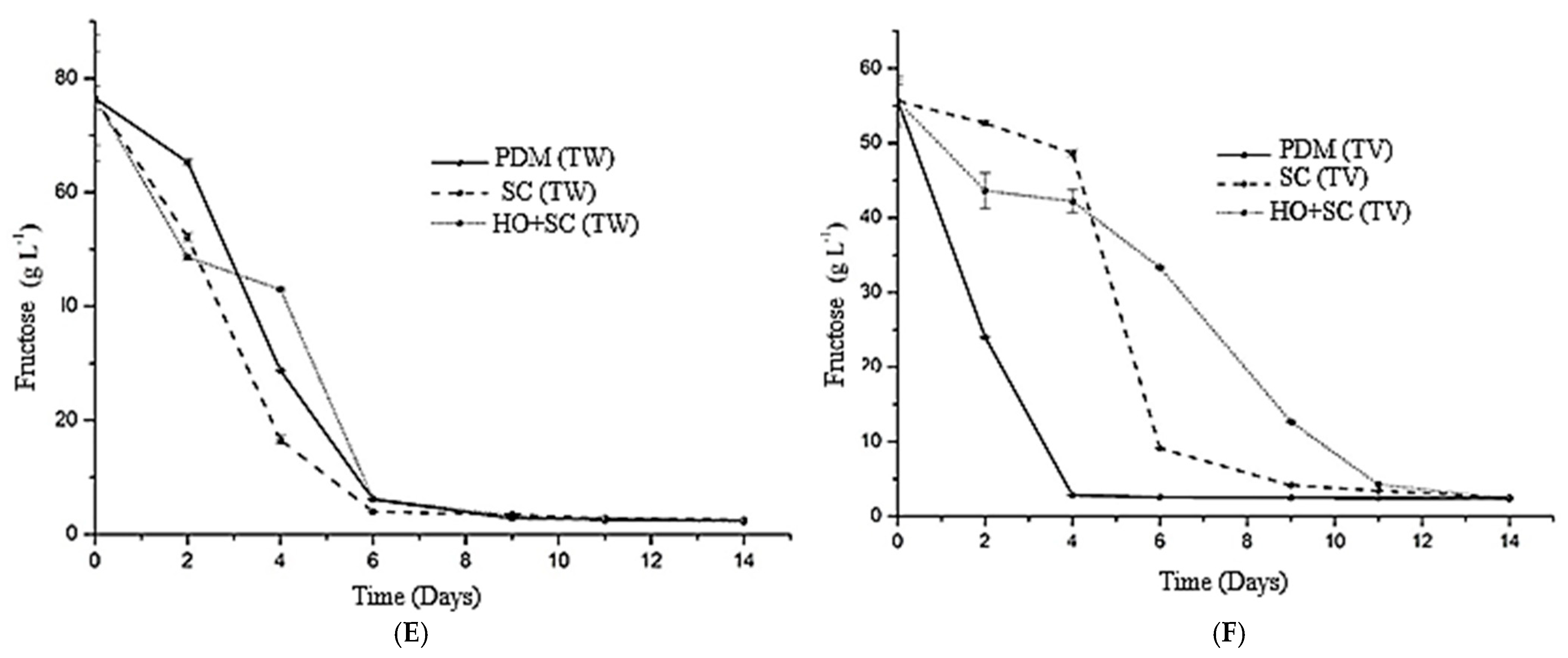
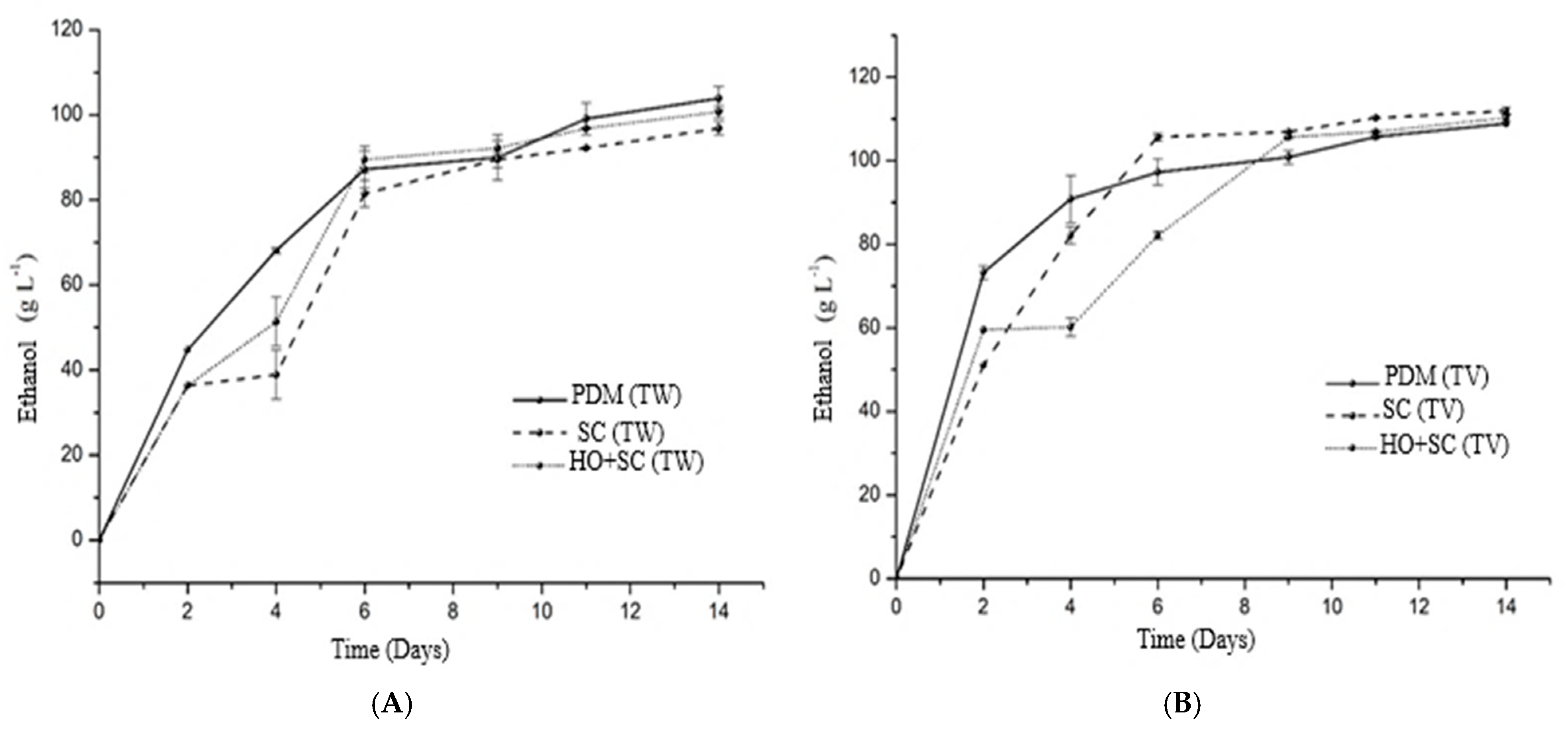
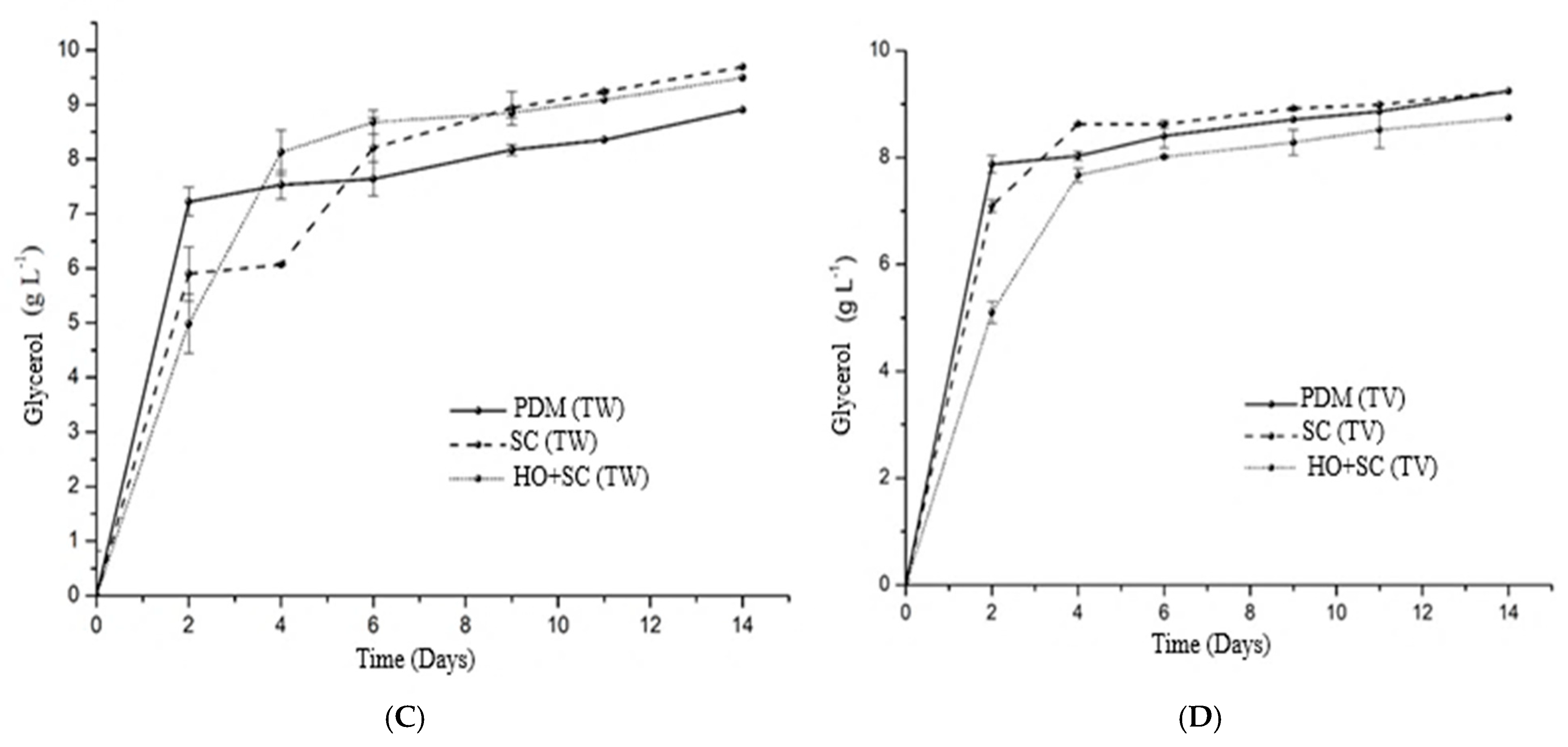
3.1. Evolution of Sugars and Alcohols Throughout the Fermentation
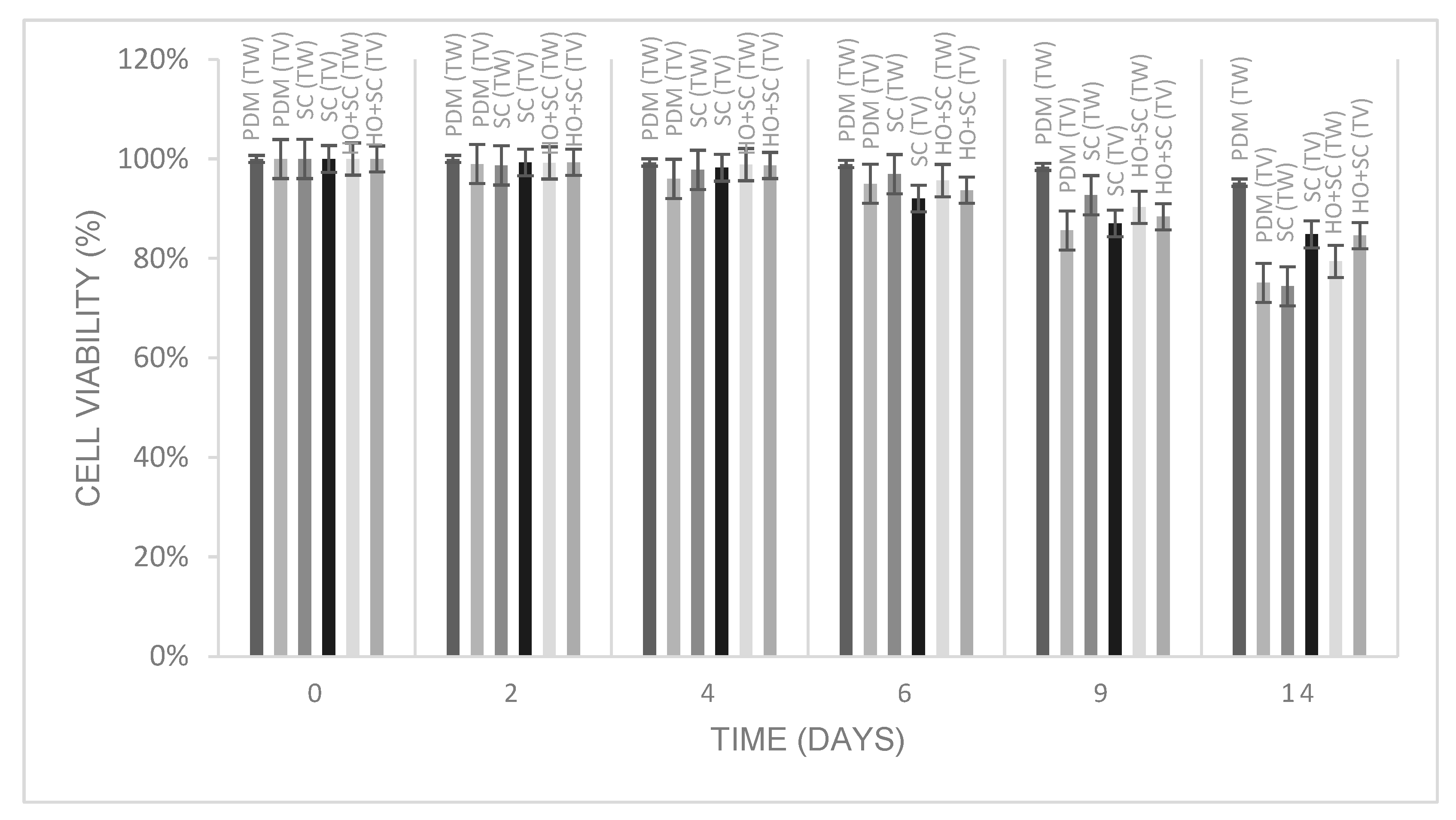
3.2. Cell Viability of Yeasts During the Fermentation Process
3.3. Kinetic Parameters of the Fermentation Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Giovannini, E.; Manfroi, V. Viticultura e Enologia: Elaboração de Grandes Vinhos nos Terroirs Brasileiros; IFRS: São Paulo, Brazil, 2009. [Google Scholar]
- Geffroy, O.; Lopez, R.; Feilhes, C.; Violleau, F.; Kleiber, D.; Favarel, J.L.; Ferreira, V. Modulating analytical characteristics of thermovinified Carignan musts and the volatile composition of the resulting wines through the heating temperature. Food Chem. 2018, 257, 7–14. [Google Scholar] [CrossRef]
- Orbanić, F.; Rossi, S.; Bestulić, E.; Budić-Leto, I.; Kovačević Ganić, K.; Horvat, I.; Plavša, T.; Bubola, M.; Lukić, I.; Jeromel, A.; et al. Applying different vinification techniques in Teran red wine production: Impact on bioactive compounds and sensory attributes. Foods 2023, 12, 3838. [Google Scholar] [CrossRef]
- Silva, I.S.; Barros, A.P.A.; Correa, L.C.; de Souza, C.O.; Biasoto, A.C.T. Effect of Thermovinification Temperature on Phenolic Compounds and Colour of Syrah Wine. Beverages 2024, 10, 117. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. (Eds.) Handbook of Enology, Volume 1: The Microbiology of Wine and Vinifications; John Wiley & Sons: Hoboken, NJ, USA, 2006; Volume 1. [Google Scholar]
- De Andrade Neves, N.; de Araújo Pantoja, L.; Dos Santos, A.S. Thermovinification of grapes from the Cabernet Sauvignon and Pinot Noir varieties using immobilized yeasts. Eur. Food Res. Technol. 2014, 238, 79–84. [Google Scholar] [CrossRef]
- Padilla, B.; Gil, J.V.; Manzanares, P. Past and future of non-Saccharomyces yeasts: From spoilage microorganisms to biotechnological tools for improving wine aroma complexity. Front. Microbiol. 2016, 7, 411. [Google Scholar] [CrossRef] [PubMed]
- Chalvantzi, I.; Banilas, G.; Tassou, C.; Nisiotou, A. Biogeographical regionalization of wine yeast communities in Greece and environmental drivers of species distribution at a local scale. Front. Microbiol. 2021, 12, 705001. [Google Scholar] [CrossRef]
- Drumonde-Neves, J.; Franco-Duarte, R.; Lima, T.; Schuller, D.; Pais, C. Association between grape yeast communities and the vineyard ecosystems. PLoS ONE 2017, 12, e0169883. [Google Scholar] [CrossRef]
- Sgouros, G.; Chalvantzi, I.; Mallouchos, A.; Paraskevopoulos, Y.; Banilas, G.; Nisiotou, A. Biodiversity and enological potential of non-Saccharomyces yeasts from Nemean vineyards. Fermentation 2018, 4, 32. [Google Scholar] [CrossRef]
- Esteves, M.; Barbosa, C.; Vasconcelos, I.; Tavares, M.J.; Mendes-Faia, A.; Pereira Mira, N.; Mendes-Ferreira, A. Characterizing the potential of the non-conventional yeast Saccharomycodes ludwigii UTAD17 in winemaking. Microorganisms 2019, 7, 478. [Google Scholar] [CrossRef]
- Lepe, J.A.S.; Leal, B.I. Microbiología Enológica. Fundamentos de Vinificación; Mundi-Prensa Libros: Madrid, Spain, 2004. [Google Scholar]
- Tonietto, J.; Carbonneau, A. A multicriteria climatic classification system for grape-growing regions worldwide. Agric. For. Meteorol. 2004, 124, 81–97. [Google Scholar] [CrossRef]
- Agustini, B.C.; Silva, L.P.; Bloch Jr, C.; Bonfim, T.M.; da Silva, G.A. Evaluation of MALDI-TOF mass spectrometry for identification of environmental yeasts and development of supplementary database. Appl. Microbiol. Biotechnol. 2014, 98, 5645–5654. [Google Scholar] [CrossRef]
- Duarte, F.L.; Alves, A.C.; Alemao, M.F.; Baleiras-Couto, M.M. Influence of red wine fermentation oenological additives on inoculated strain implantation. World J. Microbiol. Biotechnol. 2013, 29, 1139–1144. [Google Scholar] [CrossRef] [PubMed]
- Maurivin. Active Dried Yeast: Wine. Available online: https://www.maurivin.com/perch/resources/maurivn-pdm-pis-april-2023-web-repro-pt.pdf (accessed on 7 October 2024).
- Ball, S.; Lloyd, L. Analysis of typical components of alcoholic beverages. In Analysis of Carbohydrates, Alcohols, and Organic Acids by Ion-Exchange Chromatography; Ball, S., Bullock, S., Lloyd, L., Mapp, K., Ewen, A., Eds.; Agilent Technologies Inc.: Santa Clara, CA, USA, 2011; pp. 1–2. Available online: https://www.agilent.com/cs/library/applications/5990-8801EN%20Hi-Plex%20Compendium.pdf (accessed on 12 February 2024).
- Viana, A.C.; Pimentel, T.C.; do Vale, R.B.; Clementino, L.S.; Ferreira, E.T.J.; Magnani, M.; dos Santos Lima, M. American pale Ale craft beer: Influence of brewer’s yeast strains on the chemical composition and antioxidant capacity. LWT 2021, 152, 112317. [Google Scholar] [CrossRef]
- Gilliland, R.B. Determination of yeast viability. J. Inst. Brew. 1959, 65, 424–429. [Google Scholar] [CrossRef]
- Hiss, H.; Gombert, A.K. Cinética de processos fermentativos. In Biotecnologia Industrial: Engenharia Bioquímica; Editora Blucher: São Paulo, Brazil, 2001; Volume 2. [Google Scholar]
- Nascimento, R.P.D.; Ribeiro, B.D.; Pereira, K.S.; Coelho, M.A.Z. Microbiologia Industrial: Bioprocessos, 1st ed.; Elsevier: Rio de Janeiro, Brazil, 2018; p. 674. [Google Scholar]
- Becchetti, R.; Sanvito, M. Metodi di Analisi del Vini e delle Bevante Spiritose, 6th ed.; Gibertini: Novate Milanese, Italy, 1999. [Google Scholar]
- OIV—Organização Mundial da Uva e do Vinho. Compendium of International Methods of Wine and Must Analysis; OIV: Dijon, France, 2024. [Google Scholar]
- Barbará, J.A.; Silva, É.A.S.; Biasoto, A.C.T.; Gomes, A.A.; Correa, L.C.; Leão, P.C.S.; Zini, C.A. Maturation and maceration effects on tropical red wines assessed by chromatography and analysis of variance—Principal component analysis. J. Braz. Chem. Soc. 2019, 30, 1357–1377. [Google Scholar] [CrossRef]
- Fraige, K.; González-Fernández, R.; Carrilho, E.; Jorrín-Novo, J.V. Metabolite and proteome changes during the ripening of Syrah and Cabernet Sauvignon grape varieties cultured in a nontraditional wine region in Brazil. J. Proteom. 2015, 113, 206–225. [Google Scholar] [CrossRef]
- Morvai, M.; Molnár-Perl, I. Simultaneous gas chromatographic quantitation of sugars and acids in citrus fruits, pears, bananas, grapes, apples and tomatoes. Chromatographia 1992, 34, 502–504. [Google Scholar] [CrossRef]
- Apolinar-Valiente, R.; Romero-Cascales, I.; Williams, P.; Gómez-Plaza, E.; López-Roca, J.M.; Ros-García, J.M.; Doco, T. Oligosaccharides of cabernet sauvignon, syrah and monastrell red wines. Food Chem. 2015, 179, 311–317. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Duboudieu, D. Handbook of Enology Volume 2: The Chemistry of Wine, Stabilization and Treatments, 2nd ed.; Wiley: London, UK, 2002; Volume 2. [Google Scholar]
- Peynaud, E.; Ribereau-Gayon, G.P. The grape. In The Biochemistry of Fruits and Their Products; Hulme, A.C., Ed.; Academic Press: London, UK, 1971. [Google Scholar]
- Geffroy, O.; Lopez, R.; Serrano, E.; Dufourcq, T.; Gracia-Moreno, E.; Cacho, J.; Ferreira, V. Changes in analytical and volatile compositions of red wines induced by pre-fermentation heat treatment of grapes. Food Chem. 2015, 187, 243–253. [Google Scholar] [CrossRef]
- Hornsey, I.S. The Chemistry and Biology of Winemaking; The Royal Society of Chemistry: Cambridge, UK, 2007; p. 444. [Google Scholar]
- Clarke, R.J.; Bakker, J. Wine Flavour Chemistry; Blackwell: Oxford, UK, 2008; p. 318. [Google Scholar]
- Canonico, L.; Galli, E.; Ciani, E.; Comitini, F.; Ciani, M. Exploitation of three non-conventional yeast species in the brewing process. Microorganisms 2019, 7, 11. [Google Scholar] [CrossRef]
- Aquarone, E.; Schmidell, W.; de Almeida Lima, U.; Borzani, W. Biotecnologia Industrial-Vol. 4: Biotecnologia na Produção de Alimentos; Editora Blucher: São Paulo, Brazil, 2001; Volume 3. [Google Scholar]
- Jackson, R.S. Wine Tasting, 2nd ed.; Academic Press: San Diego, CA, USA, 2009; 512p. [Google Scholar]
- Bokulich, N.A.; Thorngate, J.H.; Richardson, P.M.; Mills, D.A. Microbial biogeography of wine grapes is conditioned by cultivar, vintage, and climate. Proc. Natl. Acad. Sci. USA 2024, 111, E139–E148. [Google Scholar] [CrossRef] [PubMed]
- Stanley, D.; Bandara, A.; Fraser, S.; Chambers, P.J.; Stanley, G.A. The ethanol stress response and ethanol tolerance of Saccharomyces cerevisiae. J. Appl. Microbiol. 2010, 109, 13–24. [Google Scholar] [CrossRef]
- Alves, J.G.L.F. Estudo da Influência da Temperatura na Cinética de Crescimento Anaerobio de Sccharomyces cerevisiae. Ph.D. Thesis, Universidade Estadual de Campinas, Campinas, Brazil, 1996. [Google Scholar]
- Atala, D.I.P. Montagem, Instrumentação, Controle e Desenvolvimento Experimental de um Processo Fermentativo Extrativo de Produção de Etanol; Universidade Estadual de Campinas-UNICAMP: Campinas, Brazil, 2004. [Google Scholar]
- Bai, F.W.; Anderson, W.A.; Moo-Young, M. Ethanol fermentation technologies from sugar and starch feedstocks. Biotechnol. Adv. 2008, 26, 89–105. [Google Scholar] [CrossRef]
- Benito, Á.; Calderón, F.; Benito, S. The influence of non-Saccharomyces species on wine fermentation quality parameters. Fermentation 2019, 5, 54. [Google Scholar] [CrossRef]
- Alberico, G.; Capece, A.; Mauriello, G.; Pietrafesa, R.; Siesto, G.; Garde-Cerdán, T.; Maresca, D.; Romano, R.; Romano, P. Influence of microencapsulation on fermentative behavior of Hanseniaspora osmophila in wine mixed starter fermentation. Fermentation 2021, 7, 112. [Google Scholar] [CrossRef]
- Hisson, L.F.; Karpel, J.E. Genética da levedura impactando a qualidade do vinho. Ann. Rev. Food Sci. Technol. 2010, 1, 139–162. [Google Scholar]
- Tartian, A.C.; Cotea, V.V.; Niculaua, M.; Zamfir, C.I.; Colibaba, C.L.; Moroşanu, A.M. The influence of the different techniques of maceration on the aromatic and phenolic profile of the Busuioacă de Bohotin wine. Bio Web Conf. 2017, 9, 02032. [Google Scholar] [CrossRef]
- Casassa, L.F.; Sari, S.E.; Bolcato, E.A.; Fanzone, M.L. Microwave-assisted extraction applied to Merlot grapes with contrasting maturity levels: Effects on phenolic chemistry and wine color. Fermentation 2019, 5, 15. [Google Scholar] [CrossRef]
- Moldovan, A.; Mudura, E.; Coldea, T.; Rotar, A.; Pop, C. Effect of maceration conditions on chemical composition and colour characteristics of Merlot wines. Bull. UASVM Food Sci. Technol. 2015, 72, 104–108. [Google Scholar] [CrossRef]
| Yeasts | ||||
|---|---|---|---|---|
| Winemaking Technique | PDM | SC | HO + SC | |
| VP (Ethanol) g/L∙h 1 | TW | 0.03 ± 0.01 Aa | 0.04 ± 0.01 Aa | 0.04 ± 0.01 Aa |
| TV | 0.05 ± 0.01 Aa | 0.05 ± 0.02 Aa | 0.06 ± 0.01 Aa | |
| VS (Sugars) g/L∙h 2 | TW | 0.51 ± 0.11 Ba | 0.81 ± 0.13 Aa | 0.77 ± 0.17 Ba |
| TV | 1.02 ± 0.16 Aa | 0.91 ± 0.06 Aa | 1.09 ± 0.01 Aa | |
| YPS (Ethanol/Sugar) g/L∙h 3 | TW | 0.06 ± 0.01 Aa | 0.05 ± 0.01 Aa | 0.05 ± 0.02 Aa |
| TV | 0.05 ± 0.01 Aa | 0.05 ± 0.02 Aa | 0.05 ± 0.01 Aa | |
| Productivity (g/L∙h) | TW | 0.03 ± 0.00 Ba | 0.04 ± 0.00 Ba | 0.04 ± 0.00 Ba |
| TV | 0.05 ± 0.00 Aa | 0.05 ± 0.00 Aa | 0.06 ± 0.01 Aa | |
| Efficiency (%) | TW | 94.67 ± 0.33 Aa | 83.97 ± 1.03 Bb | 82.75 ± 2.75 Ab |
| TV | 91.40 ± 1.4 Ba | 89.65 ± 0.35 Aa | 88.34 ± 3.33 Aa | |
| Traditional Winemaking 1,2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| pH | Total Acidity (g L−1) | Volatile Acidity (g L−1) | Density (g mL−1) | Alcohol Content (% v/v) | Sugars (g L−1) | Dry Extract (g L−1) | Free SO2 (mg L−1) | Total SO2 (mg L−1) | |
| PDM (TW) | 3.60 ± 0.0 a | 5.52 ± 0.02 c | 0.58 ± 0.01 b | 0.995 ± 0.00 c | 12.4.2 ± 0.06 a | 2.15 ± 0.02 c | 29.19 ± 0.22 b | 42.24 ± 1.39 a | 91.26 ± 3.36 b |
| SC (TW) | 3.44 ± 0.0 c | 6.08 ± 0.04 a | 0.67 ± 0.01 a | 0.997 ± 0.00 b | 12.20 ± 0.05 b | 2.56 ± 0.03 a | 33.93 ± 0.23 a | 43.99 ± 1.11 a | 97.11 ± 4.6 ab |
| HO + SC (TW) | 3.46 ± 0.0 b | 5.89 ± 0.05 b | 0.67 ± 0.04 a | 0.997 ± 0.00 a | 11.78 ± 0.14 c | 2.33 ± 0.02 b | 32.66 ± 0.78 a | 30.91 ± 0.42 b | 113.07 ± 6.1 a |
| Thermovinification 1,3 | |||||||||
| pH | Total Acidity (g L−1) | Volatile Acidity (g L−1) | Density (g mL−1) | Alcohol Content (% v/v) | Sugars (g L−1) | Dry Extract (g L−1) | Free SO2 (mg L−1) | Total SO2 (mg L−1) | |
| PDM (TV) | 3.89 ± 0.25 a | 5.88 ± 0.04 b | 0.47 ± 0.01 b | 0.996 ± 0.0 a | 13.71 ± 0.05 b | 2.18 ± 0.02 c | 34.66 ± 0.29 c | 45.87 ± 0.57 a | 78.40 ± 0.8 ab |
| SC (TV) | 3.63 ± 0.02 a | 6.30 ± 0.19 a | 0.47 ± 0.04 b | 0.996 ± 0.0 a | 13.87 ± 0.02 a | 2.25 ± 0.04 b | 36.53 ± 0.15 b | 45.23 ± 1.3 a | 74.00 ± 1.89 b |
| HO + SC (TV) | 3.54 ± 0.0 a | 5.88 ± 0.01 b | 0.56 ± 0.0 a | 0.997 ± 0.0 a | 13.67 ± 0.01 b | 2.34 ± 0.03 a | 37.38 ± 0.06 a | 47.45 ± 0.56 a | 80.56 ± 1.1 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, I.S.; Barros, A.P.A.; Lima, M.d.S.; Agustini, B.C.; Souza, C.O.d.; Biasoto, A.C.T. Impact of the Thermovinification Practice Combined with the Use of Autochthonous Yeasts on the Fermentation Kinetics of Red Wines. Fermentation 2025, 11, 436. https://doi.org/10.3390/fermentation11080436
Silva IS, Barros APA, Lima MdS, Agustini BC, Souza COd, Biasoto ACT. Impact of the Thermovinification Practice Combined with the Use of Autochthonous Yeasts on the Fermentation Kinetics of Red Wines. Fermentation. 2025; 11(8):436. https://doi.org/10.3390/fermentation11080436
Chicago/Turabian StyleSilva, Islaine Santos, Ana Paula André Barros, Marcos dos Santos Lima, Bruna Carla Agustini, Carolina Oliveira de Souza, and Aline Camarão Telles Biasoto. 2025. "Impact of the Thermovinification Practice Combined with the Use of Autochthonous Yeasts on the Fermentation Kinetics of Red Wines" Fermentation 11, no. 8: 436. https://doi.org/10.3390/fermentation11080436
APA StyleSilva, I. S., Barros, A. P. A., Lima, M. d. S., Agustini, B. C., Souza, C. O. d., & Biasoto, A. C. T. (2025). Impact of the Thermovinification Practice Combined with the Use of Autochthonous Yeasts on the Fermentation Kinetics of Red Wines. Fermentation, 11(8), 436. https://doi.org/10.3390/fermentation11080436








