Interactive Effects of Sulfide Addition and Heat Pretreatment on Hydrogen Production via Dark Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Inoculum and Substrate Preparation with Thermal Pretreatment
2.2. Experimental Setup
2.3. Analytical Methods
2.4. Statistical Analysis
3. Results and Discussion
3.1. Impact of Sulfide and Heat Pretreatment on Biohydrogen Production
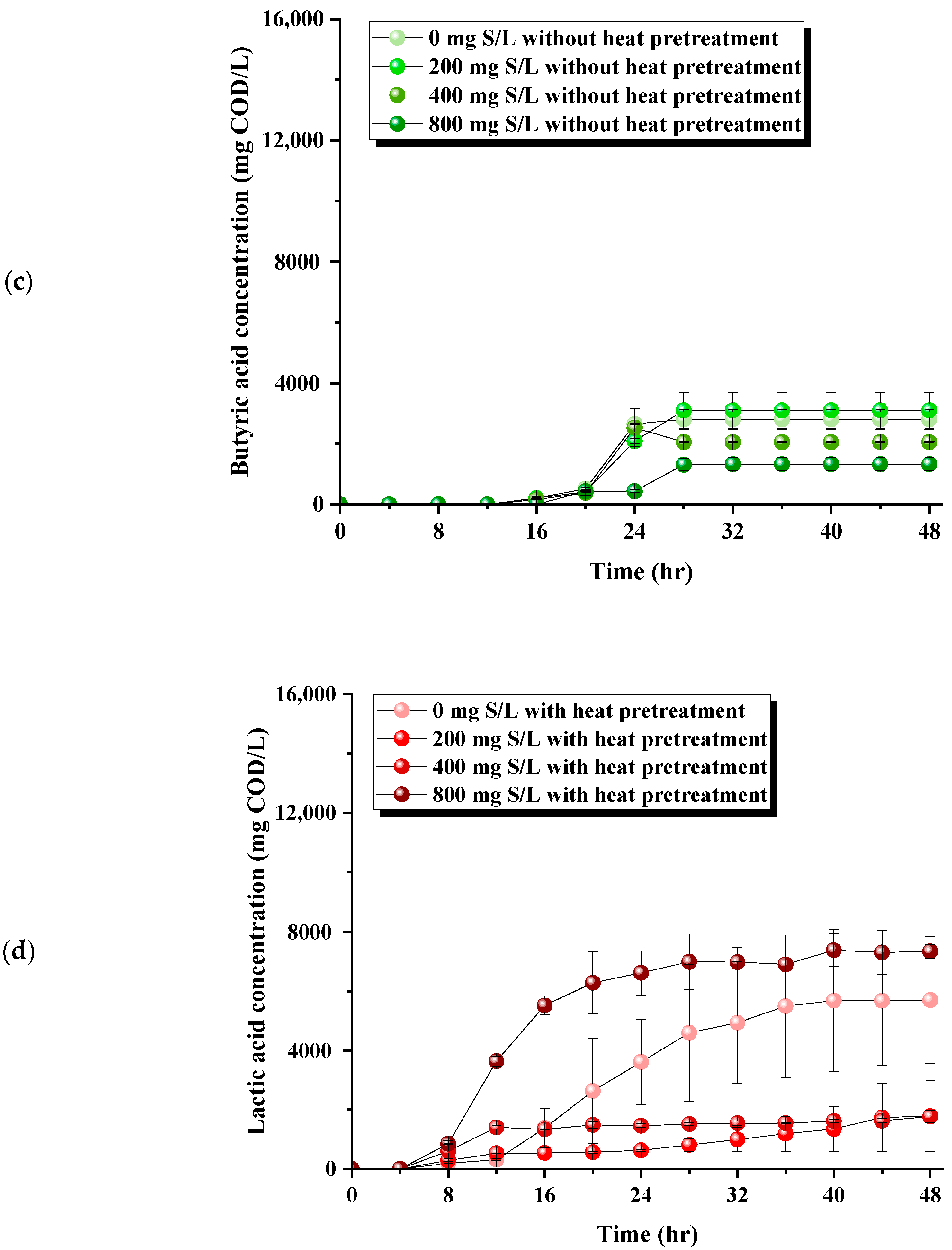
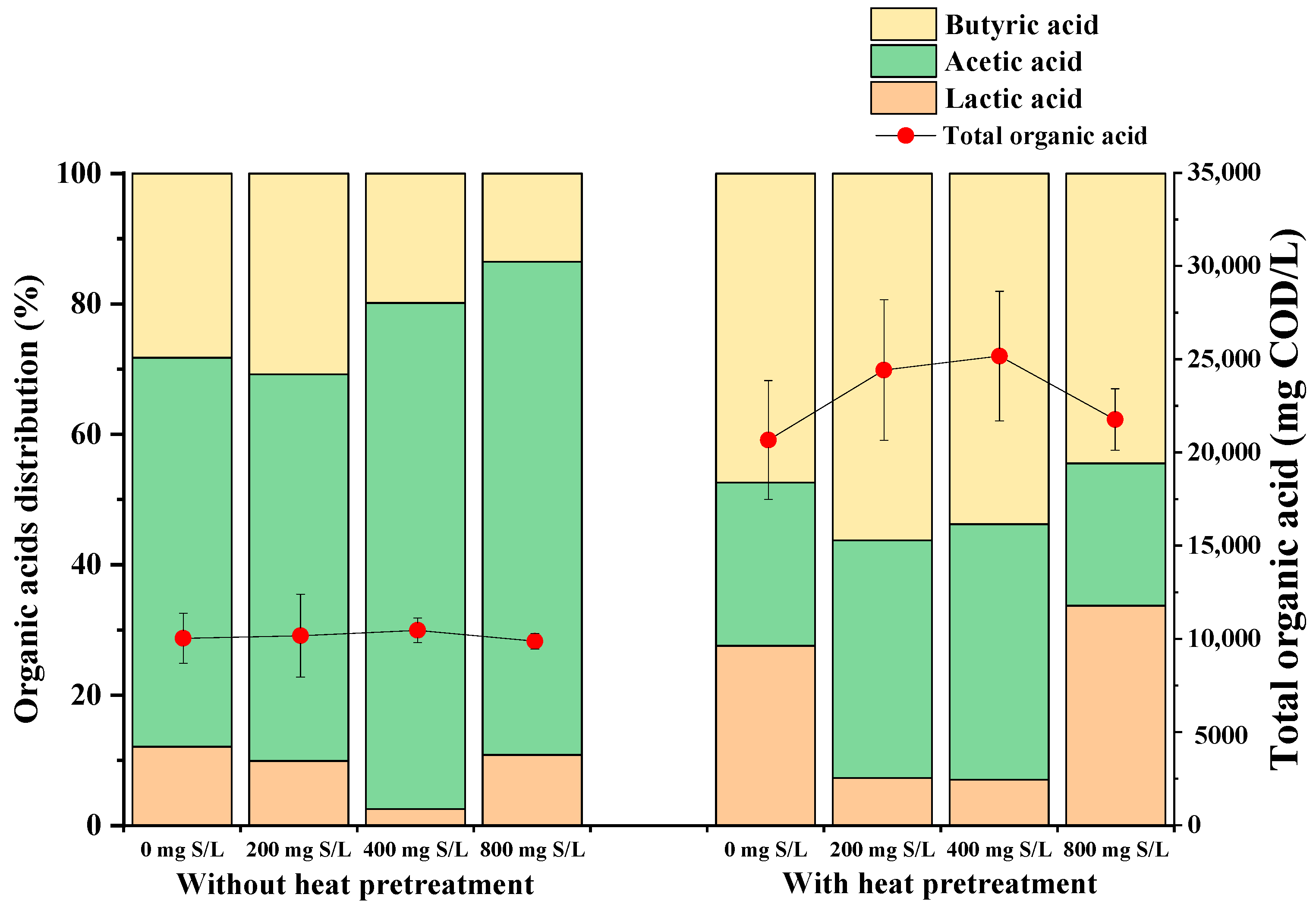
3.2. Modulation of Organic Acid Pathways by Sulfide Levels and Pretreatment in Dark Fermentation
3.3. Conditional Microbial Responses and Mechanistic Interpretation Under Sulfide Stress in Dark Fermentation
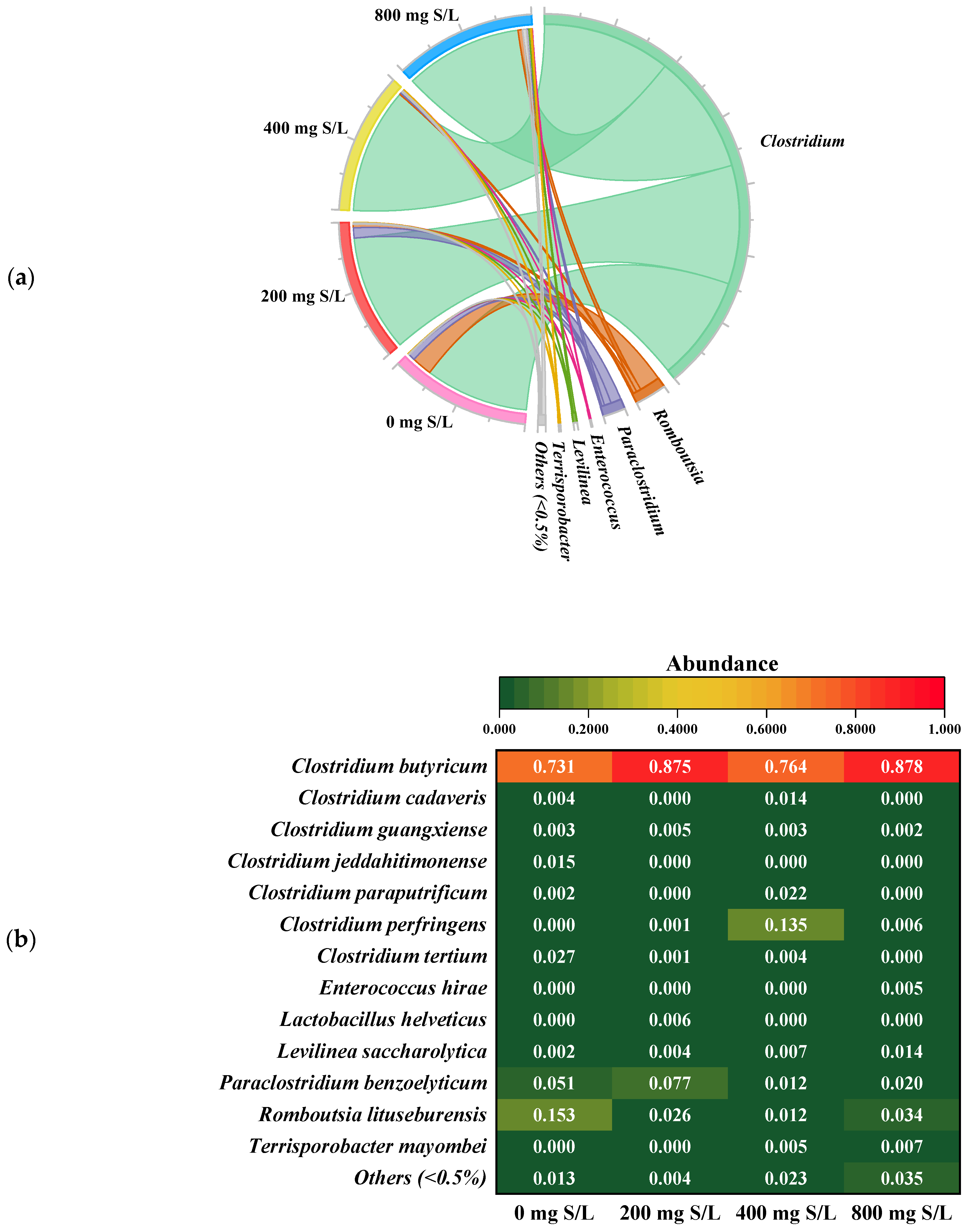
3.3.1. Microbial Community Dynamics Under Sulfide and Heat Pretreatment
3.3.2. Mechanistic Insights into Sulfide Toxicity and Conditional Microbial Responses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, M.; Fu, Q.; Huang, J.; Xu, Q.; Wang, D.; Liu, X.; Yang, J.; Wu, Y.; He, D.; Ni, B.-J. Effect of sodium dodecylbenzene sulfonate on hydrogen production from dark fermentation of waste activated sludge. Sci. Total Environ. 2021, 799, 149383. [Google Scholar] [CrossRef]
- Roslan, E.; Mohamed, H.; Hassan, S.H.A.; Carrere, H.; Trably, E. Coupling lactic acid fermentation of food waste at various concentrations as storage strategy with dark fermentation for biohydrogen production. Int. J. Hydrogen Energy 2024, 88, 358–368. [Google Scholar] [CrossRef]
- Delavar, M.A.; Wang, J. Numerical investigation of pH control on dark fermentation and hydrogen production in a microbioreactor. Fuel 2021, 292, 120355. [Google Scholar] [CrossRef]
- Wang, D.; Duan, Y.; Yang, Q.; Liu, Y.; Ni, B.-J.; Wang, Q.; Zeng, G.; Li, X.; Yuan, Z. Free ammonia enhances dark fermentative hydrogen production from waste activated sludge. Water Res. 2018, 133, 272–281. [Google Scholar] [CrossRef]
- Bundhoo, M.Z.; Mohee, R.; Hassan, M.A. Effects of pre-treatment technologies on dark fermentative biohydrogen production: A review. J. Environ. Manag. 2015, 157, 20–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, G.; Zhu, D.; Pan, G. Enrichment of the hydrogen-producing microbial community from marine intertidal sludge by different pretreatment methods. Int. J. Hydrogen Energy 2009, 34, 9696–9701. [Google Scholar] [CrossRef]
- Kim, M.-S.; Oh, Y.-K.; Yun, Y.-S.; Lee, D.-Y. Fermentative hydrogen production from anaerobic bacteria using a membrane bioreactor. In Proceedings of the 16th World Hydrogen Energy Conference, Lyon, France, 13–16 June 2006. [Google Scholar]
- Karthikeyan, O.P.; Trably, E.; Mehariya, S.; Bernet, N.; Wong, J.W.; Carrere, H. Pretreatment of food waste for methane and hydrogen recovery: A review. Bioresour. Technol. 2018, 249, 1025–1039. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-H.; Song, D.; Jeon, Y.-J.; Hwang, O.; Nam, J.-Y.; Yun, Y.-M. Enhanced production of biohydrogen and biomethane through a two-stage anaerobic fermentation of food waste mixed with conductive additives. Chem. Eng. J. 2023, 476, 146520. [Google Scholar] [CrossRef]
- Shu, W.; Du, B.; Wu, G. Deciphering microbial responses to H2S inhibition of typical functional microorganisms in anaerobic digestion ecosystems. Chem. Eng. J. 2025, 513, 162766. [Google Scholar] [CrossRef]
- Hao, T.-w.; Xiang, P.-y.; Mackey, H.R.; Chi, K.; Lu, H.; Chui, H.-k.; van Loosdrecht, M.C.; Chen, G.-H. A review of biological sulfate conversions in wastewater treatment. Water Res. 2014, 65, 1–21. [Google Scholar] [CrossRef]
- Hwang, J.-H.; Choi, J.-A.; Abou-Shanab, R.; Bhatnagar, A.; Min, B.; Song, H.; Kumar, E.; Choi, J.; Lee, E.S.; Kim, Y.J. Effect of pH and sulfate concentration on hydrogen production using anaerobic mixed microflora. Int. J. Hydrogen Energy 2009, 34, 9702–9710. [Google Scholar] [CrossRef]
- Nicholls, P.; Marshall, D.C.; Cooper, C.E.; Wilson, M.T. Sulfide inhibition of and metabolism by cytochrome c oxidase. Biochem. Soc. Trans. 2013, 41, 1312–1316. [Google Scholar] [CrossRef]
- Chen, Y.; Yin, Y.; Wang, J. Recent advance in inhibition of dark fermentative hydrogen production. Int. J. Hydrogen Energy 2021, 46, 5053–5073. [Google Scholar] [CrossRef]
- Dhar, B.R.; Elbeshbishy, E.; Nakhla, G. Influence of iron on sulfide inhibition in dark biohydrogen fermentation. Bioresour. Technol. 2012, 126, 123–130. [Google Scholar] [CrossRef]
- Im, W.-T.; Kim, D.-H.; Kim, K.-H.; Kim, M.-S. Bacterial community analyses by pyrosequencing in dark fermentative H2-producing reactor using organic wastes as a feedstock. Int. J. Hydrogen Energy 2012, 37, 8330–8337. [Google Scholar] [CrossRef]
- Kim, D.-H.; Kim, S.-H.; Shin, H.-S. Hydrogen fermentation of food waste without inoculum addition. Enzym. Microb. Technol. 2009, 45, 181–187. [Google Scholar] [CrossRef]
- American Public Health Association; American Water Works Association; Water Environment Federation. Standard Methods for the Examination of Water and Wastewater; Baird, R.B., Eaton, A.D., Rice, E.W., Eds.; American Public Health Association: Washington, DC, USA; American Water Works Association: Denver, CO, USA; Water Environment Federation: Alexandria, VA, USA, 2017; ISBN 9788578110796. [Google Scholar]
- Schloss, P.D.; Westcott, S.L.; Ryabin, T.; Hall, J.R.; Hartmann, M.; Hollister, E.B.; Lesniewski, R.A.; Oakley, B.B.; Parks, D.H.; Robinson, C.J. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009, 75, 7537–7541. [Google Scholar] [CrossRef] [PubMed]
- Nath, K.; Das, D. Modeling and optimization of fermentative hydrogen production. Bioresour. Technol. 2011, 102, 8569–8581. [Google Scholar] [CrossRef]
- Ge, H.; Zhang, L.; Batstone, D.J.; Keller, J.; Yuan, Z. Impact of iron salt dosage to sewers on downstream anaerobic sludge digesters: Sulfide control and methane production. J. Environ. Eng. 2013, 139, 594–601. [Google Scholar] [CrossRef]
- Wang, H.-Z.; Li, J.; Yi, Y.; Nobu, M.K.; Narihiro, T.; Tang, Y.-Q. Response to inhibitory conditions of acetate-degrading methanogenic microbial community. J. Biosci. Bioeng. 2020, 129, 476–485. [Google Scholar] [CrossRef]
- Zhao, H.; Lu, Y.; Wang, L.; Zhang, C.; Yang, C.; Xing, X. Disruption of lactate dehydrogenase and alcohol dehydrogenase for increased hydrogen production and its effect on metabolic flux in Enterobacter aerogenes. Bioresour. Technol. 2015, 194, 99–107. [Google Scholar] [CrossRef]
- Cui, P.; Wang, D.; Wang, S.; Su, H.; Wang, Y. Regulatory mechanism of antioxidant enzymes on microbial metabolism and NADH in anaerobic fermentation of food waste for hydrogen production. J. Clean. Prod. 2024, 474, 143607. [Google Scholar] [CrossRef]
- Yang, G.; Wang, J. Changes in microbial community structure during dark fermentative hydrogen production. Int. J. Hydrogen Energy 2019, 44, 25542–25550. [Google Scholar] [CrossRef]
- Wang, J.; Yin, Y. Clostridium species for fermentative hydrogen production: An overview. Int. J. Hydrogen Energy 2021, 46, 34599–34625. [Google Scholar] [CrossRef]
- Khanal, S.K.; Chen, W.-H.; Li, L.; Sung, S. Biological hydrogen production: Effects of pH and intermediate products. Int. J. Hydrogen Energy 2004, 29, 1123–1131. [Google Scholar] [CrossRef]
- Suzuki, H.; Fujiwara, Y.; Thongbhubate, K.; Maeda, M.; Kanaori, K. Spore-forming lactic acid-producing bacterium Bacillus coagulans synthesizes and excretes spermidine into the extracellular space. J. Agric. Food Chem. 2023, 71, 9868–9876. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Wang, R.; Bi, T.; Sun, W.; Zhou, Z. Blocking the butyrate-formation pathway impairs hydrogen production in Clostridium perfringens. Acta. Biochim. Biophys. Sin. 2013, 45, 408–415. [Google Scholar] [CrossRef]
- Li, X.; Højberg, O.; Canibe, N.; Jensen, B.B. Phylogenetic diversity of cultivable butyrate-producing bacteria from pig gut content and feces. J. Anim. Sci. 2016, 94, 377–381. [Google Scholar] [CrossRef]
- Hu, Y.; Shen, Y.; Wang, J. Pretreatment of antibiotic fermentation residues by combined ultrasound and alkali for enhancing biohydrogen production. J. Clean. Prod. 2020, 268, 122190. [Google Scholar] [CrossRef]
- Fuchs, A.-R.; Bonde, G. The availability of sulphur for Clostridium perfringens and an examination of hydrogen sulphide production. Microbiology 1957, 16, 330–340. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Sekiguchi, Y.; Hanada, S.; Imachi, H.; Ohashi, A.; Harada, H.; Kamagata, Y. Anaerolinea thermolimosa sp. nov., Levilinea saccharolytica gen. nov., sp. nov. and Leptolinea tardivitalis gen. nov., sp. nov., novel filamentous anaerobes, and description of the new classes Anaerolineae classis nov. and Caldilineae classis nov. in the bacterial phylum Chloroflexi. Int. J. Syst. Evol. Microbiol. 2006, 56, 1331–1340. [Google Scholar] [CrossRef] [PubMed]
- Montoya, L.; Celis, L.B.; Gallegos-García, M.; Razo-Flores, E.; Alpuche-Solís, Á.G. Consortium diversity of a sulfate-reducing biofilm developed at acidic pH influent conditions in a down-flow fluidized bed reactor. Eng. Life Sci. 2013, 13, 302–311. [Google Scholar] [CrossRef]
- Kimura, H. Production and physiological effects of hydrogen sulfide. Antioxid. Redox Signal. 2014, 20, 783–793. [Google Scholar] [CrossRef]
- Borisov, V.B.; Forte, E. Terminal oxidase cytochrome bd protects bacteria against hydrogen sulfide toxicity. Biochemistry 2021, 86, 22–32. [Google Scholar] [CrossRef]
- Buret, A.G.; Allain, T.; Motta, J.-P.; Wallace, J.L. Effects of hydrogen sulfide on the microbiome: From toxicity to therapy. Antioxid. Redox Signal. 2022, 36, 211–219. [Google Scholar] [CrossRef] [PubMed]



| Sulfide (mg S/L) | Cumulative Hydrogen Production (mL) | Hydrogen Yield (mL/g COD) | Hydrogen Production Rate (mL/h) | Lag Phase (h) | |
|---|---|---|---|---|---|
| Without heat pretreatment | 0 | 1649 ± 34 | 83 ± 2 | 575 ± 40 | 23 ± 0 |
| 200 | 737 ± 12 | 37 ± 1 | 347 ± 24 | 24 ± 0 | |
| 400 | 578 ± 17 | 29 ± 1 | 251 ± 28 | 24 ± 0 | |
| 800 | 2 ± 0 | 0 ± 0 | 1 ± 0 | 21 ± 0 | |
| With heat pretreatment | 0 | 3199 ± 36 | 160 ± 2 | 549 ± 22 | 17 ± 0 |
| 200 | 4628 ± 17 | 231 ± 1 | 1144 ± 22 | 8 ± 0 | |
| 400 | 4463 ± 31 | 223 ± 2 | 1462 ± 64 | 10 ± 0 | |
| 800 | 3430 ± 40 | 172 ± 2 | 303 ± 6 | 14 ± 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, T.-H.; Jeon, Y.-J.; Park, S.; Ahn, J.-H.; Park, J.; Yun, Y.-M. Interactive Effects of Sulfide Addition and Heat Pretreatment on Hydrogen Production via Dark Fermentation. Fermentation 2025, 11, 418. https://doi.org/10.3390/fermentation11070418
Kim T-H, Jeon Y-J, Park S, Ahn J-H, Park J, Yun Y-M. Interactive Effects of Sulfide Addition and Heat Pretreatment on Hydrogen Production via Dark Fermentation. Fermentation. 2025; 11(7):418. https://doi.org/10.3390/fermentation11070418
Chicago/Turabian StyleKim, Tae-Hoon, Yun-Ju Jeon, Sungjin Park, Ji-Hye Ahn, Junsu Park, and Yeo-Myeong Yun. 2025. "Interactive Effects of Sulfide Addition and Heat Pretreatment on Hydrogen Production via Dark Fermentation" Fermentation 11, no. 7: 418. https://doi.org/10.3390/fermentation11070418
APA StyleKim, T.-H., Jeon, Y.-J., Park, S., Ahn, J.-H., Park, J., & Yun, Y.-M. (2025). Interactive Effects of Sulfide Addition and Heat Pretreatment on Hydrogen Production via Dark Fermentation. Fermentation, 11(7), 418. https://doi.org/10.3390/fermentation11070418





