Abstract
Microbial fermentation is a fundamental bioconversion mechanism widely used in diverse industrial sectors, notably in food processing and bioenergy production. Over the years, the wealth of information and scientific and technological advances in the field of fermentation have made considerable progress. Most recent research studies are currently devoted to the implementation of innovative technological processes in order to increase fermentation effectiveness while consuming less energy and processing time. The aim of the present review is to investigate the impact of innovative physical techniques (pulsed electric field, PEFs; cold atmospheric plasma, CAP; and magnetic fields, MFs) on fermentation processes. The bibliographic analysis will mainly focus on recent advances towards non-destructive methods (PEF, CAP, and MF) and their induced changes in fermentation dynamics, fermented product quality, metabolite synthesis, and microbial growth kinetics. Various databases, including PubMed, ScienceDirect, Google Scholar, ResearchGate, Scopus, and Web of Science, were used to collect pertinent scientific literature on the impact of innovative physical techniques on microorganisms and fermentation processes and to investigate the potential applications of these emerging technologies in the food and health sectors. According to the results, all techniques have the potential to optimize fermentation dynamics, boost metabolite synthesis, and enhance product quality. However, each technology displayed its own specific advantages and disadvantages.
1. Introduction
Microbial fermentation is an exothermic biochemical process in which microorganisms, including bacteria, yeasts, and fungi, metabolize organic substrates into simpler molecular products. In addition to its biological value, fermentation has also been of crucial importance for culture and nutrition throughout the history of mankind. For example, fermentation is used by Mediterranean civilizations for producing bread, wine, cheese, and fermented olives, among other basic foods and drinks. These practices became ingrained in culinary traditions that continue to thrive today in addition to being crucial for food preservation in hotter areas. The probiotic potential of fermented foods continues to be an essential element of the Mediterranean diet, which is widely recognized for its health advantages [1], such as improving gut microbiota function, lowering gastrointestinal inflammation, reducing the risk of cardiovascular disease, osteoporosis and atherosclerosis, enhancing cognitive function and the immune system, lowering of blood cholesterol levels, preventing cancer and allergic reactions, alleviating lactose malabsorption and gastrointestinal disorders, and preventing diabetes [2,3]. Lactic acid bacteria (LAB) are essential agents in fermentation processes due to their ability to synthesize a wide array of bioactive secondary metabolites. Among these metabolites, exopolysaccharides (EPSs) are produced at concentrations of about 10–15 g/L, contributing not only to the rheological properties but also to the probiotic efficacy of the fermented products [4]. Bacteriocins, which are antimicrobial peptides such as nisin and plantaricin, have been identified at nanomolar concentrations and demonstrate strong inhibitory activity against pathogenic microorganisms [5]. LAB also produce significant levels of organic acids, particularly lactic acid (up to 12 g/L), with heterofermentative strains generating additional compounds like acetic acid and ethanol in the range of 100–500 mg/L [6]. Moreover, their proteolytic activity releases peptides with confirmed antioxidant, antimicrobial, and antihypertensive effects [7]. Certain LAB strains are able to synthesize B-group vitamins, including folate (10–100 µg/L) and riboflavin (50–300 µg/L), thereby enhancing the nutritional quality of fermented foods [8,9]. The spectrum and quantity of these metabolites are highly strain-dependent and are influenced by the composition of the substrate and specific fermentation conditions.
Fermentation produces compounds that can be categorized as alcoholic, acetic, lactic, butyric, or propionic compounds. Besides its central role in food processing [10], microbial fermentation is also used to produce biofuels, enzymes, and pharmaceutical components [11,12]. This complex biological process involves specific metabolic pathways and symbiotic interactions between microorganisms that have a strong impact on biodiversity and ecological balance.
Understanding the fundamentals of microbial fermentation is, in our opinion, essential to improve food production methods, develop new sustainable technologies, and emphasize the central role of microbes in the global ecosystem. Traditional Mediterranean fermentation methods provide a useful paradigm for integrating traditional knowledge with cutting-edge research such as modern food technologies to develop solutions that are health-promoting, sustainable, and culturally relevant.
Nowadays, the application of physical treatments in microbial fermentation is a rapidly developing research topic, offering numerous options to activate microorganisms or to inactivate undesirable ones [3,13,14,15]. These physical methods are typically divided into two main groups: thermal and non-thermal. Non-thermal methods such as ultrasound [16], irradiation (microwave irradiation, gamma irradiation) [17], high pressure [18], pulsed electric fields [19], and cold atmospheric plasma [20] were used for chemical reaction acceleration, monitoring fermentation progress and pasteurization. According to Koubaa et al. [17], these methods are also used to shorten the fermentation process, increase microbial metabolism and enzyme production, inactivate undesirable bacteria, and extend food shelf life.
Notably, all the mentioned innovative processes are non-thermal, green, and inherently safe for application [21]. Unlike heat-treated foods, these non-thermal processes are often used at ambient temperature or below 40 °C, which preserves the integrity of heat-sensitive ingredients in processed goods [22]. The integration of optimized physical methods with microbial fermentation constitutes an emerging and dynamic field of research, offering significant potential for advancing the future of food and beverage manufacturing [23,24]. Accordingly, elucidating the impact of these physical interventions is crucial to validate their efficacy and ensure the quality and functional properties of the resulting fermented products. Ongoing research into these technologies sets the stage for significant advances in the microbial fermentation development and has the potential to benefit the food industry, biotechnology and related fields.
The current study compiles the latest and most relevant research findings (from 1993 to 2025) on the use of magnetic fields, pulsed electric fields, and cold atmospheric plasma in microbial fermentation. It further provides an in-depth analysis of the induced biological changes and explores the possible applications of these emerging technologies in the food and health sectors.
2. Effect of Pulsed Electric Fields (PEFs) on Fermentation and Food Processing
2.1. Fundamental Aspects of Pulsed Electric Fields
PEFs are an innovative and promising technology in the food processing and microbial fermentation fields. The biometric publication analysis we conducted on the Scopus database showed that about 24,500 documents were published on fundamental and application aspects of pulsed electric fields, including original articles (16,640), conference papers (5186), reviews (1389), book chapters (822), conference reviews (199), and other publication types. Most of the research work (about 43.5%) was published by laboratory teams from United States and China.
According to Marszałek et al. [23], the PEF-treated material characteristics also include cell size and shape, pH value, electrical conductivity, porosity, water content and activity, and structure. Gram-positive bacteria exhibit better resistance to electroporation compared to Gram-negative bacteria, primarily due to their thicker cell walls and higher peptidoglycan content. In occurrence, disulphide bonds can provide Gram-positive bacteria with protection, making them more resistant to PEF-induced electroporation [25]. According to Barba et al. [26], pulsed electric field (PEF) technology applies high-voltage pulses, generally between 20 and 80 kV/cm, to inactivate and eradicate microorganisms during fermentation, while lower to moderate voltages can effectively regulate microbial activity within these processes.
2.2. Effect of PEFs on Microbial Activity and Fermentation
2.2.1. Bacteria
The effect of PEFs on microbial activity and fermentation is based on its ability to induce electroporation and to initiate metabolic cascades. Moreover, the induced porosity of the cell membrane facilitates enhanced nutrient absorption as well as improved diffusion of ions and molecules [27]. PEFs have shown a number of beneficial effects on the growth rate and metabolism of bacteria. For instance, pulsed electric fields applied with an intensity of 2.5 to 7.5 kV/cm and a duration of 3 to 4.5 ms significantly enhanced cell membrane porosity in the Lactobacillus acidophilus strains BT 1088 and FTCC 0291, the Lactobacillus bulgaricus strains FTCC 0411 and FTDC 1311, and Lactobacillus casei BT 1268. Compared to the untreated control, the cells exposed to PEF electroporation showed a significantly enhanced growth rate. Additionally, Lye et al. [28] reported that electroporation triggered lipid peroxidation in the cell membrane, which in turn enhanced the cholesterol uptake from the external medium. Additionally, when exposed to 1 kV pulses for 0.5 s, Lb. acidophilus LA-K and Lb. bulgaricus LB-12 showed improved acid tolerance, exponential growth, and protease activity in comparison to the untreated strains [29].
Ohba et al. [30] proved that exposure of Lactococcus cremoris to pulsed electric fields significantly enhanced the production of exopolysaccharides (EPSs). The EPS yield was increased by 32% in response to a single treatment with 8 kV/cm and a pulse length of 1 s, applied 200 times. Conversely, a circular treatment over 4 h led to a 94% increase in EPS yield relative to the control. Following fermentation, the molecular weight of the EPS decreased while its chemical composition remained unchanged, a phenomenon that may be attributed to enhanced EPS synthesis coupled with increased cell membrane permeability. Other studies have highlighted the novel potential of pulsed electric field (PEF) technology to increase bacterial zinc levels. Interestingly, the application of PEFs to Lactobacillus rhamnosus B 442 with specific parameters (field intensity of 3 kV/cm, 20 µs pulses over 15 min) significantly increased the zinc content of the bacterial cells, reaching a concentration of 500 µg/mL. This increase was observed without affecting the entire bacterial population or its biomass. Góral et al. [31] emphasized that this technique offers valuable insights into improving microorganisms’ enrichment with zinc. Kanafusa et al. [32] activated the lactic acid metabolic pathway in Lactobacillus plantarum DSM 9843 during its logarithmic development phase by applying PEFs at 40–60 kV/cm with 100–600 pulses, a pulse width of 35 ns, and a frequency of 1–50 Hz. Relative to the control, the fermented watermelon juice contents in L-lactic acid, D-lactic acid, and acetic acid were increased by 19%, 6.8%, and 15%, respectively [32].
Recently, Mohamed et al. [33] demonstrated that PFE application at a frequency of 0.8 Hz for 60 min significantly inhibited the growth of Klebsiella pneumoniae by about 96.5% relative to the clinical and reference strains. The treatment increased the sensitivity of the bacteria to antibiotics targeting cell wall synthesis, protein function, β-lactamase activity, and DNA replication. In addition, PEF exposure reduced biofilm formation by 36.11–46.63%, suggesting disruption of microbial adhesion and colonization mechanisms. These results suggest an eventual synergic action of PEFs with current treatments in addressing antibiotic-resistant infections. In fact, the high-voltage pulsed electric fields applied under optimal conditions (9.6 kV/cm, 20 min, 1000 Hz, and 50% duty cycle) was an effective antimicrobial treatment reducing Alicyclobacillus spp. in apple juice from 1.89 to 4.76 log CFU/mL [34]. The authors found that the antimicrobial mechanism is related to a modification in cell membrane permeability and fatty acid composition, which resulted in cell deformation and shrinkage as well as leakage of intracellular proteins. It is noteworthy that the concentration of soluble solids, soluble sugars, organic acids, flavor components, titratable acidity, and the sensory analysis of the juice were unchanged by PEF treatment. Although the clinical application of PEF technology has shown promising results, further research is needed to elucidate the underlying cellular mechanisms and optimize safety parameters.
2.2.2. Yeast and Mold
The yeast Saccharomyces cerevisiae has established itself as the excellent model organism in the field of pulsed electric field (PEF)-assisted microbial fermentation. This strain, widely used in biotechnology, has attracted the interest of researchers and has been the subject of studies aimed at determining how fermentation is affected by PEF. Fologea et al. [35] showed that yeast growth was 2-fold higher upon exposure to a 0.85 kV/cm pulse. Mattar et al. [36] reported the positive effects of electrical treatment on wine yeast (S. cerevisiae). The PEF treatment (0.1 to 6 kV/cm, 1000 pulses of 100 μs) enhanced growth rate and sugar consumption, especially fructose. Treatment with 6 kV/cm resulted in a 30% decrease in the fermentation time compared to the control samples, which required an additional 20 h. Nevertheless, the advantages of electrotherapy are restricted in the final fermentation phase by many factors, including nutrient depletion and the accumulation of inhibitory by-products. Moreover, PEF application to Saccharomyces cerevisiae at 3 kV/cm using pulses of 10 μs at a frequency of 1 Hz for 10 min resulted in a 65% and 100% increase in the accumulation of selenium and zinc ions in yeast cells, respectively [37].
One of the most notable effects of reversible electroporation on Saccharomyces cerevisiae is the reduction in protein co-extraction and the extraction of glutathione, a potent antioxidant. To achieve this, the yeast cells were treated with PEFs at a field strength of 12 kV/cm and a pulse width of 150 µs. They were then incubated at various pH values and temperatures. After an incubation time of one hour, more than 60% of the total glutathione was released from the PEF-treated cells, regardless of pH or temperature [38]. In the same context, other yeast and mold species were also investigated, including Aspergillus niger [39] and Hanseniaspora sp. [40] (Table 1).
Furthermore, electrical stimulation of Aspergillus niger spores prior to inoculation with electric fields of 2.85 kV/cm at a frequency of 1 Hz and a pulse width of 1 ms increased the efficacy of citric acid by 1.4-fold compared to untreated samples [39]. Due to its special properties, citric acid is used in a variety of industries. For instance, in the food industry, citric acid is a flavor enhancer, pH regulator, and preservative. In the pharmaceutical industry, it stabilizes formulations and masks unpleasant odors, while in cosmetics it acts as a gentle, natural exfoliant that balances pH levels [41]. Citric acid is also used in biology as a buffer and chelating agent [42].

Table 1.
Summary of studies reporting the use of pulsed electric fields for microbial fermentation.
Table 1.
Summary of studies reporting the use of pulsed electric fields for microbial fermentation.
| Microorganisms | Experimental Conditions | Main Result | Reference | |
|---|---|---|---|---|
| Bacteria | Lactobacillus casei BT 1268, Lactobacillus bulgaricus FTCC 0411, Lactobacillus acidophilus BT 1088, Lactobacillus acidophilus FTCC 0291, and Lactobacillus bulgaricus FTDC 1311 | A capacitance of 25 μF, resistance of 200 Ω, constant cooling at 4 °C, 2.5–7.5 kV/cm, exposure time of 3–4.5 ms | PEF treatment enhanced cell membrane permeability, insuring more efficient transport of cholesterol from the fermentation medium into the cytoplasm | [28] |
| Lactobacillus acidophilus and Lactobacillus delbrueckii ssp. Bulgaricus | 20 μs, 60 mL/min flow rate, 1 kV/cm, and PEF treatment temperature of 40.5 °C; 3 μs is the positive square unipolar pulse width | When exposed to mild PEF conditions, Lb. acidophilus LA-K and Lb. bulgaricus LB-12 exhibited notably improved acid resistance, enhanced exponential phase growth, and increased protease activity relative to the untreated control | [29] | |
| Lactococcus cremori chemically defined medium (CDM) with lactose 1% | Incubation at 25 °C, 200 pulses at 8 kV/cm for 1 s and for 4 h; pulse width at 1 μs | The application of PEF resulted in a 32% increase in exopolysaccharide (EPS) yield with a single treatment for 1 s and a 94% increase with circular treatment for 4 h compared to the control | [30] | |
| L. plantarum in MRS medium | E: 40–60 kV/cm, number of pulses: 100–600, Pulse width: 35 ns, frequency: 1–50 Hz, applied during the log growth phase of the bacteria | The nsPEF treatment positively enhanced the metabolism of lactic acid bacteria. A 19% rise in L-lactic acid, a 6.8% increase in D-lactic acid, and a 15% increase in acetic acid were observed compared to the control | [32] | |
| Klebsiella pneumoniae in MacConkey agar | Aerobic incubation at 37 °C, power supply of 9V-DC, resonant frequency of 0.8 Hz, exposure time 60 min | PEF inhibited the growth of Klebsiella pneumoniae, increased the sensitivity of bacteria to antibiotics targeting cell wall synthesis, protein function, β-lactamase activity, and DNA replication | [33] | |
| Alicyclobacillus spp. | Culture of samples and bacterial growth on AAM at 45 °C for 48 h, 9.6 kV/cm, exposure time 20 min, 1000 Hz, 50% duty cycle | PEF reduced Alicyclobacillus spp. in apple juice by 1.89 to 4.76 log CFU/mL | [34] | |
| Yeast and mold | Trichoderma reesei | 1.5 KV/cm | Cellulase activity and secretion were increased by increasing membrane permeability | [43] |
| Kluyveromyces marxianus IMB3 | Culture of samples on malt extract agar at 45 °C, 0.625–3.750 kV/cm 10 ms | Ethanol production from cellulose was enhanced by 40% through the application of PEF, ethanol production was boosted by an increased electric field, although the enhancement was not as significant as when using a specific intensity of 0.625 kV/cm | [44] | |
| S. cerevisiae in YEPG medium | YEPG medium and kept for 2 h at 30 °C 0.5–1.5 kV/cm, bipolar square pulses of 20 μs, total length of pulse: 8 ms | Cell growth doubled with a field strength of 0.85 kV/cm | [35] | |
| Aspergillus niger in Basal Medium | MCILVAINE buffer (pH 7.0) 0.57–2.85 kV/cm 1–20 ms pulse duration 0.1–10 Hz frequency | The output of the citric acid synthesis process remained constant over a range of pulse durations from 1 to 20 ms. With an electric field strength of 2.854 kV/cm, the rose had the strongest electric field. The peak value was at a frequency of 1 Hz, which is 1.4 times higher than the control. | [39] | |
| S. cerevisiae suspension in water | The fermentation was run for 150 h in an incubator (30 °C) with synchronic agitation; 0.1 and 6 kV/cm monopolar pulses 1000 pulses, 100 μs pulse duration, 100 ms pulse repetition time, 18 μs/cm conductivity | PEFs enhanced the efficiency of the fermentation process and promoted greater sugar utilization. Following fermentation, samples treated with PEF showed a 30% greater mass reduction compared to untreated ones, which required an additional 20 h to reach a similar level of reduction. | [36] | |
| S. cerevisiae | Optimized parameters 3 kV/cm, 10 μs pulse width, 1 Hz, total exposure time 10 min, 20 h culture | PEF boosted the accumulation of selenium and zinc within yeast cells. | [37] | |
| Hanseniaspora sp. yeast in YPD medium | Culture on a Yeast Extract Peptone Dextrose (YPD) medium, intensity in the range 0.072–0.285 kV/cm during the fermentation (lag, exponential, and log phases) | The yeast Hanseniaspora sp. is stimulated by moderate PEFs, which shorten the fermentation time and increase biomass production. When 285 V/cm was administered during the lag and early exponential stage as well as the log phase, the growth rate of the yeast reached its peak. | [40] | |
| Microalgae | Arthrospira platensis | Culture on diluted seawater nitrogen (DSN) medium at 25 °C, E: 10.5–19.97 kV/cm, number of pulses: 1.83–15.88, pulse width: 25–100 ns, f: 3–20 Hz, treatment time: 0.61 s, energy input: 217–507 J/Kg | The highest biomass output was obtained with the longest pulse width of 100 ns. Through their effects on intracellular and plasma membrane dynamics, nsPEF treatments stimulate cell growth. | [45] |
| Arthrospira platensis | Culture on in a modified Zarrouk medium at 25 ± 0.2 °C, 70% relative humidity, 150 rpm, ambient CO2, and continuous illumination, pulses of 100 ns, energy input of 256 J/kg | The exponential phase (36 h) was correlated with the rising influence of biomass growth. | [46] |
2.2.3. Microalgae
In addition to yeast, the potential use of short pulsed electric fields (nsPEFs) has also been investigated in other microorganisms, such as different strains of microalgae. Indeed, this technique has improved the performance of microalgae-based biorefineries by leading to better biomass yields of Chlorella vulgaris (10 kV/cm, 100 ns pulses, 5 Hz) [45]. The observed PEF-induced changes seem to be the result of simultaneous effects at the intracellular and membrane levels, although the exact processes are not yet fully understood. Internal compartments such as the endoplasmic reticulum appear to release calcium (Ca2+) more readily when nsPEFs are applied [46,47]. Intracellular Ca2+ is important for metabolic pathway activation, such as nitrate signaling, which is involved in cellular metabolism and nitrogen assimilation [48].
In addition, the use of long pulses (less than 100 ns) generated a transmembrane potential sufficient to induce the formation of pores in the membrane facilitating the calcium ions and nutrients uptake from the outside [47]. The nsPEF may also modulate photosynthetic activity, perhaps by altering the size of antennae or electronic flux rather than by increasing pigment content [49]. Even if the proportion of pigments such as chlorophyll or carotenoids did not change [50], photosynthetic efficiency could still be increased. These synergistic effects lead to an increase in biomass, mainly due to the accumulation of cellulose [46], making nsPEFs an interesting choice for the optimization of industrial microalgae production.
The effects of PEF application (100 ns pulses, energy input of 256 kJ/kg) were also assessed in different stages of cell development of the microalgae Arthrospira platensis. Cell proliferation was quantified 12, 36, and 60 h after inoculation. The obtained results showed that the exponential phase (36 h) was associated with an increasing influence on biomass growth. These results suggest that microalgae could be a valuable resource in photoautotrophic biorefineries after treatment [46].
2.3. Use of Pulsed Electric Fields for Fermented Foods
The potential of PEF technology has been demonstrated in many areas of fermented beverage development. PEFs with high intensity (>15 kV/cm) have been used primarily for preservation and have successfully inactivated pathogens and broken-down bacteria. For example, Rios-Corripio et al. [51] found that the microbial load of Brettanomyces ssp. was significantly reduced in a PEF-treated beverage relative to thermally pasteurized equivalents. A fermented pomegranate beverage was treated with a PEF (bipolar pulses of 6 ms at 18 kV/cm and 200 Hz). In addition, the PEF-treated beverages showed improved sensory acceptability and increased levels of antioxidant compounds after storage. In comparison, a high-intensity PEF (37–53 kV/cm) was investigated as a potential preservation technique for kombucha beverages, preserving the organoleptic properties as well as the amount of beneficial components present [52] (see Table 2).
The PEF technique is commonly used in winemaking for microbial decontamination while preserving the wine quality [38]. The use of high-intensity PEFs offers the advantages of shorter processing times, the avoidance of temperature increases, and irreversible electroporation. This ensures the inactivation of microbial cells while preserving thermolabile compounds such as antioxidants and volatiles, resulting in the production of safe, storable, and high-quality products [53].

Table 2.
Summary of studies reporting the use of pulsed electric fields for fermented foods.
Table 2.
Summary of studies reporting the use of pulsed electric fields for fermented foods.
| Fermented Product | Experimental Conditions | Observation | Reference |
|---|---|---|---|
| Kombucha analogues | Fermentation time 7 days, sugar 10%, starting culture 10%, and inoculum 2.5%, at 25 °C. PEF processing time 445.3–1979.2 μs, output temperature 18.31 ± 0.98 °C, input energy 21.2–136.5 KJ/L, feed flow 102.85 L/h, 30 width pulse, 200 Hz, 60 V | Deactivation of acetic acid bacteria within kombucha consortium | [52] |
| Wine | 5 kV/cm, effective time of PEF treatment: 1 ms, frequency of 0.5 Hz. Electric field strengths of 5 and 10 kV/cm, frequency of 1 Hz at 30 °C. Monopolar square wave pulses of different field strengths (0.5–1.5 kV/cm), energy inputs (1–50 kJ/kg), a frequency of 1 Hz, and a pulse width of 10 μs; 17, 24, and 31 kV/cm at 10, 20, and 30 °C, flow rate 40 mL/min, pulse duration of 3 μs, and frequency of 500 pps | Heightened flavanols and phenolic compounds Increment of the color intensity, anthocyanin content, and total polyphenolic index Significantly higher release of polyphenols (+20%) and anthocyanins (+75%), thus improving the color intensity (+20%) and the antioxidant activity of the wine (+20%) Better extraction of phenolics and color retention, more aroma compounds and inactivation of Escherichia coli O157:H7, Lactobacillus delbrueckii ssp. bulgaricus, Candida lipolytica, Saccharomyces cerevisiae, and Hansenula anomala | [54,55,56,57] |
| Fermented pomegranate beverage | Incubation at 25 ± 1 °C, 6 ms of bipolar PEFs at 18 kV/cm with 200 Hz repetition frequency | Reduction in the microbial load by approximately 4 log cycles, with a final concentration of <10 CFU/mL. (Initial mesophilic aerobic bacteria, molds and yeasts in the beverage.) | [51] |
| Natural drinkable yogurt | Yogurt starter inoculum suspended in milk (2.8% fat) treated at 1 kV/cm for 1600 µs | Low fermentation time (5.1 ± 0.16 h) | [58] |
Alternatively, it has been investigated whether PEFs at low intensity can improve the color quality and polyphenol profile of red wines, leading to a significant reduction in maceration time [59]. Nevertheless, further investigations are needed to comprehend the fundamental mechanisms of the observed induced effects and to assess the risk of electrochemical contamination by PEF chamber electrodes.
PEF treatment is a promising physical method for accelerating the aging of vinegar and wine [60]. Sun et al. [61] showed that PEF-treated orange vinegar (25 kV/cm, 300 μs) had an increased content of important flavor compounds (ethyl acetate, isoamyl acetate, 3-methyl-1-butanol, acetoin, and phenethyl alcohol), similar to naturally aged vinegar. Liu et al. [62] also found that PEFs (5–20 kV/cm) improved the total sum of esters, acids, and phenylethyl alcohols in red wine while reducing fuel oils. Moreover, a greater color intensity and higher phenolic content were observed in young red wine treated with 14–22 kV/cm for 6 μs [63]. Application of PEF treatment of Hanseniaspora sp. before or during apple juice fermentation with voltages of 72 to 285 V/cm and ten 100 μs pulses resulted in a significant decrease in ethanol production with a simultaneous biomass accumulation [40].
Recent studies have shown that the application of low-intensity pulsed electric fields can accelerate fermentation and stimulate microbial activity in the production of fermented milk products. For instance, as reported by Chanos et al. [64], the use of pulsed electric fields (PEFs) improved yogurt fermentation by inducing cellular stress and increasing the metabolic activity of lactic acid bacteria (a mixture of Streptococcus thermophilus and Lactobacillus delbrueckii ssp. bulgaricus) and acidification potential in a reconstituted skimmed milk medium. In a study conducted by Yeo and Liong [65], the effects of PEFs (2.5–7.5 kV/cm for 3–4 ms) were evaluated prior to initiating fermentation in biotin- and mannitol-enriched soya milk. They observed that bioconversion was improved at higher PEF intensity (4 or 7.5 kV/cm), which can be attributed to changes in beta-glucosidase activity [65].
Miranda-Mejía et al. [66] investigated the impact of low-intensity PEFs on a starter culture mixture containing Streptococcus thermophilus, Lactobacillus bulgaricus, and partially skimmed milk prior to the fermentation phase of natural yogurt production. Their findings indicated that applying low-intensity PEF treatments (from 1 to 3 kV/cm) led to a reduction in fermentation time of approximately 0.31 to 0.52 h compared to conventional fermentation approaches. It is noteworthy that the PEF treatment had no negative impact on the quality and organoleptic parameters of the yogurt. Joo et al. [67] previously observed that higher PEF intensities were associated with lower levels of lactic acid and ethanol, while acetic acid production increased in Weissella cibaria SKkimchi and mixed kimchi fermentations. It was shown that PEF intensities (1.5 and 2.0 kV/cm) accelerated the ripening process and extended the shelf life of fermented radish kimchi by increasing the permeability of the cell membranes while maintaining the integrity of the cells [68]. The pre-treatment restricted the availability of reducing sugars and suppressed the proliferation of lactic acid bacteria, thereby slowing the fermentation process and preventing premature over-ripening. In addition, PEF improved the overall quality of fermentation, reduced the undesirable green flavor and aroma, and shortened the ripening time by up to 70%, while maintaining product texture and antioxidant activity. By controlling the fermentation rate and extending the shelf life of fermented products, PEFs are a potential method for optimizing the fermentation of salty vegetables, such as kimchi.
In summary, most of the published studies indicated that reversible electroporation can be induced by low-to-moderate PEF intensity, which shortens the processing time and ensures the quality of the final product by accelerating microbial activity and improving the extraction of bioactive chemicals. It is hypothesized that PEF induced-changes in cytoplasmic membranes, enzymatic activity, and genetic expression are responsible for the increased growth and fermentation rate. However, further studies are required to fully understand the mechanisms underlying the increased production of specific metabolites.
2.4. Impact of PEFs on Metabolic Pathways and Enzyme Activity
Cell exposure to pulsed electric fields (PEFs) leads to the formation of transient pores in cellular membranes that severely impair the homeostasis of metabolites. Ions such as sodium, potassium, and calcium as well as tiny molecules can flow uncontrollably through these induced pores. Moreover, the loss of membrane integrity triggers stress signals that activate metabolic pathways that generate reactive oxygen species (ROS), which are responsible for oxidative stress [60]. The ROS can alter the expression of genes related to metabolism and damage proteins, lipids, and DNA. In addition, mitochondrial function can be impaired by oxidative stress and ionic disturbances, which can reduce ATP synthesis and occasionally force the cell to switch from oxidative to anaerobic metabolism, which is less energy-efficient [69]. PEFs can induce apoptosis, especially via the mitochondrial pathway, when the damage is irreparable. Oxidation triggers enzymes and irreversibly disrupts energy metabolism [70]. As the cell attempts to compensate for the imbalance and mitigate the stress, these effects lead to a reduction in the production of macromolecules, a shift in enzyme regulation, and a reorganization of metabolic pathways [71]. Various studies have shown that pulsed electric fields (PEFs) can alter the activity of enzymes involved in metabolic pathways by either inactivating them or altering their activity, depending on the treatment setting. For example, Qian et al. [72] used PEFs to deactivate brown rice lipase, an enzyme important for lipid metabolism, with an optimal deactivation rate of about 60%. Lipoxygenase (LOX), which contributes to the oxidation of fatty acids and the formation of off-flavors in legumes, has also been shown to be sensitive to PEFs [73]. Although the aim of PEF treatments is to limit changes in food quality, they do not always have the desired effect [74]. The use of hurdle technology has been investigated to improve the inactivation of enzymes. For example, Tian et al. [75]. found that PEFs and radiofrequency (RF) treatment greatly improved the inactivation of polyphenol oxidase (PPO) in apple tissue. Curiously, moderate PEF treatments can have a beneficial effect on certain enzymes. Increased pectinase activity was observed upon PEF treatment [76]. Both inactivation and activation effects have been associated with structural changes. For example, pepsin was found to lose its β-sheets [60], but improved thermostability was demonstrated for α-amylase [77]. Although further research is needed to fully understand the underlying mechanisms of PEF effects of enzyme activity in metabolic pathways.
3. Effect of Magnetic Fields on Fermentation
3.1. Fundamental Aspects of Magnetic Fields
A magnetic field is a vector field generated by direct or alternating electric currents (AC/DC) flowing through conductors or permanent magnets [78]. It is characterized by the presence of magnetic forces that influence moving charges, such as electrons, and is responsible for phenomena such as magnetism and electromagnetic induction. Magnetic fields are usually represented by lines known as magnetic field lines, which indicate the direction and intensity of the magnetic field. The strength of a magnetic field is expressed in units of Tesla (T) or Gauss (G). Based on their temporal behavior, magnetic fields can be divided into static magnetic fields (SMFs), oscillating magnetic fields (OMFs), or pulsed magnetic fields (PMFs). A magnetic field is considered homogeneous when the gradient at the location of sample exposure equals zero, indicating a uniform spatial distribution. In other cases, it can be characterized as heterogeneous. In terms of magnetic flux density, the categories super-weak (100 to 0.5 mT) and weak (<1 mT), moderate (1 to 1 T), strong (1 to 5 T), and ultra-strong (>5 T) are used to distinguish SMFs [79].
3.2. Application of Magnetic Fields in Microbial Fermentation
Recent studies have shown that MFs can affect microbial growth, metabolism, and fermentation depending on microbial species and field characteristics [15]. Previous studies have primarily focused on the effects of parameters such as magnetic field intensity, frequency, and exposure time on fermentation [80,81]. However, it is important to note that the relationship between the magnetic effects and these parameters may not always be proportional, a phenomenon known as the “window effect”, suggesting non-linear effects of MFs on microbial fermentation and that the specific combination of field properties can lead to different outcomes.
3.2.1. Impact of Magnetic Fields on Bacteria
The impact of MFs on microbial fermentation is a topic that has been extensively studied. The findings of this research suggest that exposure to MFs can influence microbial growth, metabolism, and fermentation processes. However, the observed effects depend on the specific characteristics of the applied magnetic field and the microbial species under consideration.
It has been shown that the use of MFs can influence metabolic pathways and facilitate the synthesis of specific products. For example, it has been shown that exposure to a static magnetic field increases the ability of Rhodobacter sphaeroides to produce porphyrin [82]. The yield of the antimicrobial compound nisin produced by Lactococcus lactis was increased threefold when exposed to a magnetic field of 5 T for four hours [83]. However, it is critical to take into account the specific bacterial strain and its characteristics when evaluating the effects of MFs. Interestingly, identical MF conditions can have different or reverse effects on different bacterial strains and the synthesis of disparate products [84]. This highlights the complex relationship between MFs and microbial responses. It also emphasizes the need for a deeper understanding of the underlying mechanisms.
A number of studies have shown that MFs can have a bactericidal effect on bacteria. For example, exposure of Gram-negative Escherichia coli to an MF with a frequency of 50 Hz, an intensity between 2.7 and 10 mT, and an exposure duration ranging between 0 and 12 min was shown to reduce the ability of these bacteria to form colonies. Furthermore, the effect was found to be proportional to the increase in magnetic field intensity and exposure duration [85]. Similarly, when sulphate-reducing bacteria (SRB) on the surface of X80 steel were exposed to a perpendicular magnetic field, a decrease in bacterial population was observed with increasing field intensity. This method could prove advantageous for the protection of underground pipelines against stress corrosion cracking (SCC), as it curbs microbial activity and facilitates the development of a protective layer [86].
3.2.2. Effect of Magnetic Fields on Yeasts and Fungus
Magnetic field (MF) application has demonstrated significant promise in enhancing the fermentation efficiency of Saccharomyces cerevisiae. For example, Sincak et al. [87] demonstrated that exposure of cells to a moderate magnetic field with flux densities of 10 and 15 mT leads to 1–2 h earlier uptake of glucose, oxygen, and nitrogen as well as an increase in biomass yield [87] and a general increase in metabolic activity. Nevertheless, this led to a reduction in total ethanol production in the range of 7–28%. In addition, Bubanja et al. [88] observed that a treatment of 33 mT magnetic field with frequencies between 10 and 50 Hz enhanced the anaerobic metabolism of Saccharomyces cerevisiae. The observed changes were reflected in a decrease in oxygen consumption and an increase in carbon dioxide production, indicating a transition to a more efficient anaerobic fermentation. According to another study by Deutmeyer et al. [89] on the fermentation kinetics of Saccharomyces cerevisiae, a homogeneous MF had no significant effect on yeast cell growth, while a non-homogeneous static MF led to an increase in peak ethanol concentration. Conversely, Kobayashi et al. [90] reported that exposure of two Saccharomyces cerevisiae strains to a static magnetic field of 8 T significantly inhibited their growth, resulting in reductions of 19% and 12% after 24 h of cultivation. When a field of about 4 T was applied to a third strain, its growth was reduced by about 10%. Magnetic field (MF) application has exhibited substantial promise in enhancing the biosynthetic capacity of fungi for the production of various bioproducts within the food industry. For example, exposure of the filamentous fungus Aspergillus oryzae to a radiofrequency electromagnetic field (2 GHz, 10 min) was shown to increase total protein concentration and alpha-amylase activity by 1.5- to 3-fold compared to control samples. In addition, exposure to a 0.4 mT magnetic field increased the synthesis of yellow and red pigments in Monascus purpureus, with maximum yields 65.4% and 59.2% higher than in the control group [91]. Furthermore, David et al. [92] reported a modest 6% increase in cell proliferation and a 30% stimulation in lipase production in Yarrowia lipolytica when subjected to a magnetic field intensity of 9.0 kA/m.
Beyond these specific examples, MF technology proves to be a promising approach to improve the fermentation process of rare edible fungi, such as Grifola frondosa, Hericium erinaceus, Phellinus igniarius, Cordyceps militaris, Ganoderma lucidum, and Antrodia cinnamomea. The ability of MFs to enhance mycelial production and synthesis of beneficial products makes this technology a valuable tool for optimizing fermentation processes involving these valuable fungal species [15]. For instance, Guo et al. [93] found that the application of a low-intensity oscillating magnetic field (35G) during submerged fermentation of Grifola frondosa led to an increase in mycelium biomass and polysaccharide yield.
3.2.3. Effect of Magnetic Fields on Microalgae
Enhanced Biomass Concentration and Product Yield
Deamici et al. [94] demonstrated that applying magnetic fields in microalgae cultures can significantly increase the accumulation of biomass and the yield of product output. A magnetic field of 5 Gauss (500 µT) applied to Chlorella vulgaris showed remarkable effects, increasing overall growth with a moderate but significant impact on protein and beta-carotene production [95]. In line with these findings, Bauer et al. [96] observed that a magnetic field with an intensity of 60 mT applied daily for one hour increased the biomass of Chlorella kessleri by 83.2% while promoting lipid, chlorophyll, and carotenoid synthesis. In addition, magnetic field exposure has been shown to enhance the efficiency of oxygen production by microalgae and accelerate bacterial growth and pollutant degradation in symbiotic algae–bacteria systems [97].
Similarly, a study by Li et al. [98] showed that magnetic field interventions can affect intracellular carbon allocation during protein synthesis in Chlorella pyrenoidosa, resulting in a 23.4% decrease in lipid synthesis, a 19.7% decrease in carbohydrate synthesis, and a 44.3% increase in protein production.
Variable Effects Based on Growth Environment
The effects of MF on the growth and metabolism of microalgae depend on environmental conditions and culture method [81]. The effects of temperature and cultivation techniques (batch versus semi-continuous) on biomass production and nutrient removal in starch wastewater were investigated by Huo et al. [99] using Tribonema sp. and a low-intensity magnetic field (MF). The application of the magnetic field resulted in a remarkable algal growth improvement, particularly in the final stages of the logarithmic phase in batch cultures, with the effects being more pronounced at lower temperatures. At 30° C, the biomass of the batch culture reached 4.44 g/L, an increase of 15.0% compared to the control. It was also found that the application of a magnetic field (MF) increased the oil content of Tribonema sp. especially in batch cultures.
In a semi-continuous culture under MF, the biomass of Tribonema sp. reached 18.45 g/L after 25 days. Reducing the hydraulic retention time to one day resulted in improved nutrient removal efficiency, with an average reduction of more than 90% for key parameters such as ammonium nitrogen, total nitrogen, COD, and total phosphorus. The findings of Huo et al. [99] emphasized the potential of Tribonema sp. in semi-continuous culture for wastewater treatment and biomass production and highlighted the potential use of magnetic field (MF) technology to improve productivity under controlled conditions. A weak magnetic field (MF) in outdoor semi-continuous cultivation of filamentous algae Tribonema sp. effectively increased biomass yield and oil production, except under winter conditions [81]. The biomass concentration and oil yield of Tribonema sp. at 130 mT increased during summer by 9.8% and 35.8%, respectively.
4. Effect of Cold Atmospheric Plasma on Fermentation
4.1. Fundamental Aspects of Cold Atmospheric Plasma (CAP)
The partially ionized gas state, known as CAP or non-thermal plasma, consists of ions, electrons, and electrically neutral particles such as atoms, molecules and radicals. According to Bai et al. [100], it is characterized by its low temperature, which is typically below 40° C and almost room temperature. In the laboratory, CAP is produced using a variety of techniques, such as corona discharge, plasma jet, and dielectric barrier discharge (DBD). CAP can be generated using a diversity of gases, including argon, helium, oxygen, nitrogen, and air, or their combinations.
These gases are exposed to various forms of energy, e.g., electrical or magnetic fields, to produce a plasma enriched in ions as well as in reactive oxygen species (ROS) and reactive nitrogen species (RNS) such as O3, HO−, O2, NO, and N2O. Due to its versatility, CAP is used in various industries, including the chemical process, electronic technology, medical, and food industries [22].
4.2. Cold Atmospheric Plasma Combined with Fermentation
The interdisciplinary approach of combining CAP with fermentation has numerous potential uses in a variety of industries, such as food technology, biotechnology, and medicine. When CAP is used in fermentation, a biological process in which organic compounds are transformed by microorganisms such as bacteria, yeasts, or fungi, a number of effects can be observed.
Firstly, CAP can be used to eliminate fermentation inhibitors and thus improve the quality of the fermentation process. For example, Lin et al. [101] successfully demonstrated the removal of toxic compounds (45% acetic acid, 31% formic acid, 80% hydroxymethylfurfural and 100% furfural) from acidic sugarcane bagasse hydrolysate using CAP. The detoxified hydrolysate was then used as a nutrient source for the production of bioethanol with Kluyveromyces marxianus. After the introduction of optimal CAP conditions (200 W power for 25 min), a notable bioethanol productivity increase from 0.25 to 0.65 g/L/h was observed.
An additional advantage of CAP is the improved tolerance to aldehyde inhibitors and the fermentability of cellulose-derived bioethanol for the Zymomonas mobilis ZM4 chassis. Compared to the control, a 51.31% increase in bioethanol accumulation was observed after 24 h in Z. mobilis pretreated with cold plasma (20 s, 140 W, and 165 Pa) [20]. In addition, CAP enables the inactivation and decontamination of microbial cells by generating reactive species and UV photons that can degrade microbial cells and deactivate microorganisms [102]. This can be beneficial for fermentation processes as it enables the control of undesirable microbial proliferation and improves the quality of the final product.
Recently, CAP has been used to preserve red wine instead of traditional preservation methods [103]. The combined method (cold plasma with 30 mg/L potassium metabisulphite) showed less pronounced color changes and better preservation of phenolic compounds and antioxidant activity compared to methods using potassium metabisulphite or a helium–nitrogen mixture [104]. Regarding biological purity, the samples treated with CAP showed a decrease in the number of microorganisms. After a period of three months, the total content of microorganisms in the samples treated with the combined method was significantly lower than in the non-preserved ones [103]. In addition, CAP and related technologies are being investigated for the purpose of microbial and enzymatic inactivation in food and offer a novel approach for the degradation of aflatoxins in food matrices [105,106]. For example, Zhao et al. [107] have proved the effectiveness of dielectric barrier discharge (DBD) plasma technology in inactivating Pichia manshurica, a yeast responsible for spoilage of fermented foods, especially fermented vegetables. DBD with a voltage of 80 kV, a frequency of 50 Hz, and an exposure time of 3 min reduced biofilm formation by 39% and yeast survival by 35% compared to other alternative treatments. Transcriptome profiling showed that DBD primarily affects pathways related to DNA synthesis and metabolism, disrupts cell adhesion, and stimulates the oxidative stress response of P. manshurica, ultimately leading to biofilm degradation. The presence of acids, alcohols, and reactive radical species has been shown to exacerbate oxidative stress in yeast, leading to an enhanced redox reaction [108]. This reaction is likely triggered by reactive oxygen species (ROS) and reactive nitrogen species (RNS) generated by the cold plasma of DBD [109,110]. The observed transcriptional changes suggest that P. manshurica actively deploys defense mechanisms in response to DBD-induced stress. Although this technology has achieved a 5 log CFU/mL reduction in P. manshurica viability [86], it has often proven insufficient to completely inhibit biofilm formation. According to Mahmoud et al. [111], longer exposure of samples to CAP enhanced the antimicrobial activity. The produced reactive radicals resulted in lipid peroxidation, enzyme inactivation, and DNA degradation, which ultimately inactivated the microorganisms [112]. Different mechanisms of microbial inactivation by cold plasma are suggested (Figure 1).
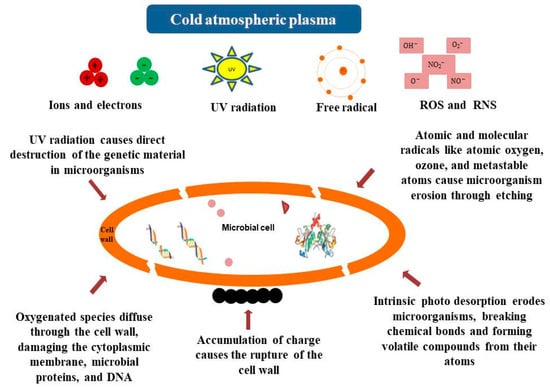
Figure 1.
Basic mechanism of microbial inactivation by cold plasma treatment. Adopted from [113,114].
It remains to be supposed that the effect of CAP on microbiological development closely depends on the growing conditions. Sometimes, the activity of beneficial microbes can be promoted by CAP, resulting in higher production or better quality of products during fermentation. Depending on the plasma conditions, different modes of action are assumed in the literature (Table 3). It was shown that plasma agitation in Saccharomyces cerevisiae can rapidly lead to positive metabolic changes after a short duration treatment [115]. These induced changes increased metabolic activity, which in turn enabled faster and more effective conversion of glucose to ethanol and higher yields of secondary metabolites (ethanol, acetic acid, and glycerol). Another study published by Zhou et al. [116] showed that CAP (1.7 MHz, 2–6 kV) acts as a multifunctional agent to induce environmental stress on Saccharomyces cerevisiae cells by generating reactive species.

Table 3.
An overview of research on the application of cold atmospheric plasma to microbial fermentation.
These reactive species enhance the cellular uptake of the Prussian blue analogue nanoparticles FeCo-PBA. At the same time, the FeCo-PBA nanoparticles protect the cells from oxidative stress caused by both the plasma and the fermentation process. The obtained synergy between the plasma and the nanoparticles leads to an enhancement in the yield of secondary metabolism, particularly in the production of energy and ethanol.
Likewise, Dong et al. [120] successfully increased the ethanol yield in the fermentation of Saccharomyces cerevisiae through optimized CAP treatment. Based on the response surface method, they determined the optimal conditions by monitoring three key parameters: the volume of the sample cell, the electrical supply rate, and the duration of plasma exposure. The model with the three optimized parameters (one-minute exposure time, 26 V electric feed rate, and 9 mL volume of the sample cell) predicted a maximum theoretical ethanol yield of 0.49 g/g, which was in close agreement with the experimental yield of 0.48 g/g. This model can be used to validate the actual ethanol fermentation process and as a reference for modifying the experimental parameters of CAP to increase the ethanol yield. Another study was conducted by Dong et al. [117], investigating the possible mechanism of the effects of CAP on the cofactor metabolism of Saccharomyces cerevisiae, and revealing that the increase in plasma membrane potential is the primary mechanism by which the plasma induces changes in cofactor content. This in turn leads to an increase in cytosolic concentrations of free Ca2+ in the cells, thereby increasing microbial productivity.
Dong et al. [118] provided the first evidence of the long-term mechanism of CAP-generated reactive oxygen and nitrogen species, leading to an increase in the ability of Saccharomyces cerevisiae to produce ethanol. During the treatment course, CAP was able to activate the Yvc1p channel openings in the vacuolar membrane and Cch1p/Mid1p channels in the plasma membrane, thereby increasing the concentration of cytoplasmic calcium for Saccharomyces cerevisiae. In addition, this activation led to an increase in the expression of H+-ATPase, which facilitated the degradation of ATP and the production of NADH. Compared to the control group, the increase in ATP hydrolysis led to a five-fold increase in NADH. As a result, both biomass production and ethanol yield increased; in particular, bioethanol yield increased by 34.2% compared to the control group. These results illustrate the potential of CAP as a bioprocess intensification technology that can increase the yield of target products in microbial systems [118].
An innovative mutation method for Streptomyces avermitilis was performed using CAP [119]. The main objective of the study was to determine whether the use of CAP can increase the efficiency of avermectin fermentation. The results showed that plasma had a pronounced mutagenic effect on S. avermitilis, resulting in mutation rates of over 30% and a positive mutation rate of about 21%. It was recorded that plasma treatment resulted in remarkable lethality rates, leading to high mutation frequencies and a spectrum of mutants with different morphologies and productivities compared to the wild-type S. avermitilis strain. The mutant strain G1-1 produced almost 2-fold higher B1 avermectin contents relative to the wild type.
Moreover, a novel technique using dielectric barrier plasma (DBD) was also used to increase the production of 1,3-propanediol (1,3-PD) in Klebsiella pneumoniae [118]. The DBD plasma enabled the generation of a stable K. pneumoniae strain, called Kp-M2, with increased 1,3-PD production. The Kp-M2 showed superior performance relative to the wild-type strain in both batch fermentation and feed fermentation, reaching a final concentration of 1,3-PD. In addition, the possible DBD effects on the anaerobic fermentation of cyanobacteria were investigated in order to increase short-chain fatty acid (SCFA) production and regulate microcystin content [14]. The results showed that the application of DBD (voltage = 15 kV, frequency = 16 kHz, duration = 30 min) led to an increased proportion of acetic acid during anaerobic fermentation of cyanobacteria, accompanied by an increased yield of saturated fatty acids. In particular, the maximum accumulation of acetic acid and SCFAs was observed in the DBD-treated group. The concentrations were 1.49 and 3.30 times higher than in the control group. It is likely that the hydrolysis and acidification process was favored by an increase in the abundance of Bacteroidetes, Firmicutes and Chloroflexi and a decrease in the abundance of Proteobacteria in the DBD-treated group. In addition, the increased level of acetic acid could be due to the decrease in Methanosaeta sp. and Methanosarcina sp. in the DBD-treated group. In addition, DBD pre-treatment showed increased degradation of microcystin-LR, which may involve superoxide, hydroxyl, and microbial entities. In conclusion, DBD treatment is an effective method to remove carbon resources from cyanobacteria.
5. Conclusions
In this review, we have analyzed the impact of three innovative, non-destructive physical methods—pulsed electric fields (PEFs), magnetic fields (MFs), and cold atmospheric plasma (CAP)—on microbial fermentation processes. Each technique has unique advantages and limitations and offers different opportunities to optimize fermentation dynamics, enhance metabolite synthesis, and improve product quality. PEFs are characterized by their ability to accelerate microbial growth and metabolic activity while maintaining product integrity, but require significant energy input and precise calibration. MFs are promising when it comes to modulating microbial kinetics without thermal damage, although their applications are less explored compared to PEFs. CAP is characterized by antimicrobial efficacy and environmental sustainability, making it ideal for pathogen control, but its complexity and potential impact on sensitive substances require further investigation. Taken together, these techniques showed significant potential to improve fermentation efficiency and product quality. Future research should focus on overcoming the barriers to scaling up these methods for wider industrial applications to ensure their practical applicability in the microbial industry.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The doctoral training abroad was supported by scholarships from the Ministry of Higher Education and Scientific Research of Tunisia and the Erasmus+ KA171 Tunisia/2020-1-FR01-KA107-079644.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| PEFs | Pulsed electric fields |
| MFs | Magnetic fields |
| CAP | Cold atmospheric plasma |
References
- Tamang, J.P.; Watanabe, K.; Holzapfel, W.H. Diversity of Microorganisms in Global Fermented Foods and Beverages. Front. Microbiol. 2020, 11, 1443. [Google Scholar] [CrossRef]
- Adebiyi, J.A.; Obadina, A.O.; Adebo, O.A.; Kayitesi, E. Fermented and Malted Millet Products in Africa: Expedition from Traditional/Ethnic Foods to Industrial Value-Added Products. Crit. Rev. Food Sci. Nutr. 2016, 58, 463–474. [Google Scholar] [CrossRef]
- Adebo, O.A.; Njobeh, P.B.; Adeboye, A.S.; Adebiyi, J.A.; Sobowale, S.S.; Ogundele, O.M.; Kayitesi, E. Advances in Fermentation Technology for Novel Food Products. In Innovations in Technologies for Fermented Food and Beverage Industries; Panda, S.K., Shetty, P.H., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 71–87. [Google Scholar] [CrossRef]
- Torino, M.I.; Font de Valdez, G.; Mozzi, F. Biopolymers from lactic acid bacteria. Food Technol. Biotechnol. 2015, 53, 145–158. [Google Scholar] [CrossRef]
- Yang, S.-C.; Lin, C.-H.; Sung, C.T.; Fang, J.-Y. Antibacterial activities of bacteriocins: Application in foods and pharmaceuticals. Front. Microbiol. 2014, 5, 241. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, L. Lactic acid bacteria: Classification and physiology. In Lactic Acid Bacteria: Microbiological and Functional Aspects, 3rd ed.; Salminen, S., von Wright, A., Ouwehand, A., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 1–66. [Google Scholar]
- Korhonen, H.; Pihlanto, A. Bioactive peptides: Production and functionality. Int. Dairy J. 2006, 16, 945–960. [Google Scholar] [CrossRef]
- Sybesma, W.; Starrenburg, M.; Tijsseling, L.; Hoefnagel, M.H.N.; Hugenholtz, J. Controlled modulation of folate production in Lactococcus lactis. Appl. Environ. Microbiol. 2003, 69, 3069–3076. [Google Scholar] [CrossRef]
- Capozzi, V.; Russo, P.; Dueñas, M.T.; Spano, G. Lactic acid bacteria producing B-group vitamins: A great potential for functional cereals products. Appl. Microbiol. Biotechnol. 2012, 96, 1383–1394. [Google Scholar] [CrossRef]
- Al-Maqtari, Q.A.; AL-Ansi, W.; Mahdi, A.A. Microbial Enzymes Produced by Fermentation and Their Applications in the Food Industry—A Review. Int. J. Agric. Innov. Res. 2019, 8, 62–82. [Google Scholar]
- Banu, J.R.; Kumar, G.; Chattopadhyay, I. Management of Microbial Enzymes for Biofuels and Biogas Production by Using Metagenomic and Genome Editing Approaches. 3 Biotech 2021, 11, 429. [Google Scholar] [CrossRef]
- Pérez-Rivero, C.; López-Gómez, J.P. Unlocking the Potential of Fermentation in Cosmetics: A Review. Fermentation 2023, 9, 463. [Google Scholar] [CrossRef]
- Gavahian, M.; Mathad, G.N.; Oliveira, C.A.F.; Mousavi Khaneghah, A. Combinations of Emerging Technologies with Fermentation: Interaction Effects for Detoxification of Mycotoxins? Food Res. Int. 2021, 141, 110104. [Google Scholar] [CrossRef]
- Wang, J.; Xu, J.; Lu, M.; Shangguan, Y.; Liu, X. Mechanism of Dielectric Barrier Plasma Technology to Improve the Quantity and Quality of Short Chain Fatty Acids in Anaerobic Fermentation of Cyanobacteria. Waste Manag. 2023, 155, 65–76. [Google Scholar] [CrossRef]
- Chen, S.; Jin, Y.; Yang, N.; Wei, L.; Xu, D.; Xu, X. Improving Microbial Production of Value-Added Products through the Intervention of Magnetic Fields. Bioresour. Technol. 2024, 393, 130087. [Google Scholar] [CrossRef]
- Ojha, K.S.; Mason, T.J.; O’Donnell, C.P.; Kerry, J.P.; Tiwari, B.K. Ultrasound Technology for Food Fermentation Applications. Ultrason. Sonochem. 2017, 34, 410–417. [Google Scholar] [CrossRef]
- Koubaa, M.; Barba-Orellana, S.; Roselló-Soto, E.; Barba, F.J. Gamma Irradiation and Fermentation. In Novel Food Fermentation Technologies; Ojha, K.S., Tiwari, B.K., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 143–153. [Google Scholar] [CrossRef]
- George, J.M.; Rastogi, N.K. High Pressure Processing for Food Fermentation. In Novel Food Fermentation Technologies; Ojha, K.S., Tiwari, B.K., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 57–83. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Arias, M.; Martín-Belloso, O.; Ancín-Azpilicueta, C. Pulsed Electric Field and Fermentation. In Novel Food Fermentation Technologies; Ojha, K.S., Tiwari, B.K., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 85–123. [Google Scholar] [CrossRef]
- Yi, X.; Yang, D.; Xu, X.; Wang, Y.; Guo, Y.; Zhang, M.; Wang, Y.; He, Y.; Zhu, J. Review of Cold Plasma Pretreatment Reinforces the Lignocellulose-Derived Aldehyde Inhibitors Tolerance and Bioethanol Fermentability for Zymomonas mobilis. Biotechnol. Biofuels Bioprod. 2023, 16, 102. [Google Scholar] [CrossRef]
- Mukhtar, K.; Nabi, B.G.; Arshad, R.N.; Roobab, U.; Yaseen, B.; Ranjha, M.M.A.N.; Aadil, R.M.; Ibrahim, S.A. Potential Impact of Ultrasound, Pulsed Electric Field, High-Pressure Processing and Microfludization against Thermal Treatments Preservation Regarding Sugarcane Juice (Saccharum officinarum). Ultrason. Sonochem. 2022, 90, 106194. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, H.B.; Annapure, U.S.; Deshmukh, R.R. Non-Thermal Technologies for Food Processing. Front. Nutr. 2021, 8, 657090. [Google Scholar] [CrossRef] [PubMed]
- Marszałek, K.; Woźniak, Ł.; Wiktor, A.; Szczepańska, J.; Skąpska, S.; Witrowa-Rajchert, D.; Saraiva, J.A.; Lorenzo, J.M.; Barba, F.J. Emerging Technologies and Their Mechanism of Action on Fermentation. In Fermentation Processes; Koubaa, M., Barba, F.J., Roohinejad, S., Eds.; Wiley: Hoboken, NJ, USA, 2021; pp. 117–144. [Google Scholar] [CrossRef]
- Morales-de La Peña, M.; Miranda-Mejía, G.A.; Martín-Belloso, O. Recent Trends in Fermented Beverages Processing: The Use of Emerging Technologies. Beverages 2023, 9, 51. [Google Scholar] [CrossRef]
- Galván-D’Alessandro, L.; Carciochi, R. Fermentation Assisted by Pulsed Electric Field and Ultrasound: A Review. Fermentation 2018, 4, 1. [Google Scholar] [CrossRef]
- Barba, F.J.; Parniakov, O.; Pereira, S.A.; Wiktor, A.; Grimi, N.; Boussetta, N.; Saraiva, J.A.; Raso, J.; Martin-Belloso, O.; Witrowa-Rajchert, D.; et al. Current Applications and New Opportunities for the Use of Pulsed Electric Fields in Food Science and Industry. Food Res. Int. 2015, 77, 773–798. [Google Scholar] [CrossRef]
- Mota, M.J.; Lopes, R.P.; Koubaa, M.; Roohinejad, S.; Barba, F.J.; Delgadillo, I.; Saraiva, J.A. Fermentation at Non-Conventional Conditions in Food- and Bio-Sciences by the Application of Advanced Processing Technologies. Crit. Rev. Biotechnol. 2018, 38, 122–140. [Google Scholar] [CrossRef]
- Lye, H.S.; Karim, A.A.; Rusul, G.; Liong, M.T. Electroporation Enhances the Ability of Lactobacilli to Remove Cholesterol. J. Dairy Sci. 2011, 94, 4820–4830. [Google Scholar] [CrossRef]
- Najim, N.; Aryana, K.J. A Mild Pulsed Electric Field Condition That Improves Acid Tolerance, Growth, and Protease Activity of Lactobacillus acidophilus LA-K and Lactobacillus delbrueckii Subspecies bulgaricus LB-12. J. Dairy Sci. 2013, 96, 3424–3434. [Google Scholar] [CrossRef]
- Ohba, T.; Uemura, K.; Nabetani, H. Moderate Pulsed Electric Field Treatment Enhances Exopolysaccharide Production by Lactococcus lactis Subspecies cremoris. Process Biochem. 2016, 51, 1120–1128. [Google Scholar] [CrossRef]
- Góral, M.; Pankiewicz, U.; Sujka, M.; Kowalski, R. Bioaccumulation of Zinc Ions in Lactobacillus rhamnosus B 442 Cells under Treatment of the Culture with Pulsed Electric Field. Eur. Food Res. Technol. 2019, 245, 817–824. [Google Scholar] [CrossRef]
- Kanafusa, S.; Uhlig, E.; Uemura, K.; Gómez Galindo, F.; Håkansson, Å. The Effect of Nanosecond Pulsed Electric Field on the Production of Metabolites from Lactic Acid Bacteria in Fermented Watermelon Juice. Innov. Food Sci. Emerg. Technol. 2021, 72, 102749. [Google Scholar] [CrossRef]
- Mohamed, D.H.; Mohammed, H.; El-Gebaly, R.H.; Adam, M.; Ali, F.M. Pulsed Electric Field at Resonance Frequency Combat Klebsiella pneumoniae Biofilms. Appl. Microbiol. Biotechnol. 2024, 108, 505. [Google Scholar] [CrossRef]
- Cai, R.; Jing, Z.; Li, Y.; Zhong, X.; Sheng, Q.; Yue, T.; Wang, Z.; Yuan, Y. Inactivation Activity and Mechanism of High-Voltage Pulsed Electric Fields Combined with Antibacterial Agents against Alicyclobacillus spp. in Apple Juice. Int. J. Food Microbiol. 2025, 431, 111079. [Google Scholar] [CrossRef]
- Fologea, D.; Vassu-Dimov, T.; Stoica, I.; Csutak, O.; Radu, M. Increase of Saccharomyces cerevisiae Plating Efficiency after Treatment with Bipolar Electric Pulses. Bioelectrochem. Bioenerg. 1998, 46, 285–287. [Google Scholar] [CrossRef]
- Mattar, J.R.; Turk, M.F.; Nonus, M.; Lebovka, N.I.; Zakhem, H.E.; Vorobiev, E. S. cerevisiae Fermentation Activity after Moderate Pulsed Electric Field Pre-Treatments. Bioelectrochemistry 2015, 103, 92–97. [Google Scholar] [CrossRef]
- Pankiewicz, U.; Sujka, M.; Kowalski, R.; Mazurek, A.; Włodarczyk-Stasiak, M.; Jamroz, J. Effect of Pulsed Electric Fields (PEF) on Accumulation of Selenium and Zinc Ions in Saccharomyces cerevisiae Cells. Food Chem. 2017, 221, 1361–1370. [Google Scholar] [CrossRef]
- Delso, C.; Berzosa, A.; Sanz, J.; Álvarez, I.; Raso, J. Pulsed Electric Field Processing as an Alternative to Sulfites (SO2) for Controlling Saccharomyces cerevisiae Involved in the Fermentation of Chardonnay White Wine. Food Res. Int. 2023, 165, 112525. [Google Scholar] [CrossRef] [PubMed]
- Fiedurek, J. Influence of a Pulsed Electric Field on the Spores and Oxygen Consumption of Aspergillus niger and Its Citric Acid Production. Acta Biotechnol. 1999, 19, 179–186. [Google Scholar] [CrossRef]
- Al Daccache, M.; Koubaa, M.; Maroun, R.G.; Salameh, D.; Louka, N.; Vorobiev, E. Pulsed Electric Field-Assisted Fermentation of Hanseniaspora sp. Yeast Isolated from Lebanese Apples. Food Res. Int. 2020, 129, 108840. [Google Scholar] [CrossRef] [PubMed]
- Latif, A.; Hassan, N.; Ali, H.; Niazi, M.B.K.; Jahan, Z.; Ghuman, I.L.; Hassan, F.; Saqib, A. An Overview of Key Industrial Product Citric Acid Production by Aspergillus niger and Its Application. J. Ind. Microbiol. Biotechnol. 2024, 52, kuaf007. [Google Scholar] [CrossRef]
- Rentsch, K.M.; Khanna, N.; Halbeisen, D.; Osthoff, M. Enhancing Stability and Investigating Target Attainment of Benzylpenicillin in Outpatient Parenteral Antimicrobial Therapy: Insights from In Vitro and In Vivo Evaluations. Antibiotics 2024, 13, 970. [Google Scholar] [CrossRef]
- Kerns, G.; Bauer, E.; Berg, H. Electrostimulation of Cellulase Fermentation by Pulsatile Electromagnetically Induced Currents. Bioelectrochem. Bioenerg. 1993, 32, 89–94. [Google Scholar] [CrossRef]
- McCabe, A.; Barron, N.; McHale, L.; McHale, A.P. Increased Efficiency of Substrate Utilization by Exposure of the Thermotolerant Yeast Strain, Kluyveromyces marxianus IMB3 to Electric-Field Stimulation. Biotechnol. Technol. 1995, 9, 133–136. [Google Scholar] [CrossRef]
- Haberkorn, I. Continuous Nanosecond Pulsed Electric Field Treatments Foster the Upstream Performance of Chlorella vulgaris-Based Biorefinery Concepts. Bioresour. Technol. 2019, 293, 122029. [Google Scholar] [CrossRef]
- Buchmann, L.; Frey, W.; Gusbeth, C.; Ravaynia, P.S.; Mathys, A. Effect of Nanosecond Pulsed Electric Field Treatment on Cell Proliferation of Microalgae. Bioresour. Technol. 2019, 271, 402–408. [Google Scholar] [CrossRef]
- Batista Napotnik, T.; Reberšek, M.; Vernier, P.T.; Mali, B.; Miklavčič, D. Effects of High Voltage Nanosecond Electric Pulses on Eukaryotic Cells (In Vitro): A Systematic Review. Bioelectrochemistry 2016, 110, 1–12. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, F.; Crawford, N.M.; Wang, Y. Molecular Regulation of Nitrate Responses in Plants. Int. J. Mol. Sci. 2018, 19, 2039. [Google Scholar] [CrossRef]
- Perrine, Z.; Negi, S.; Sayre, R.T. Optimization of Photosynthetic Light Energy Utilization by Microalgae. Algal Res. 2012, 1, 134–142. [Google Scholar] [CrossRef]
- Sun, Z.; Li, T.; Zhou, Z.; Jiang, Y. Microalgae as a Source of Lutein: Chemistry, Biosynthesis, and Carotenogenesis. In Microalgae Biotechnology; Posten, C., Feng Chen, S., Eds.; Advances in Biochemical Engineering/Biotechnology; Springer International Publishing: Cham, Switzerland, 2015; Volume 153, pp. 37–58. ISBN 978-3-319-23807-4. [Google Scholar]
- Rios-Corripio, G.; La Peña, M.M.; Welti-Chanes, J.; Guerrero-Beltrán, J.Á. Pulsed Electric Field Processing of a Pomegranate (Punica granatum L.) Fermented Beverage. Innov. Food Sci. Emerg. Technol. 2022, 79, 103045. [Google Scholar] [CrossRef]
- Vazquez-Cabral, D.; Valdez-Fragoso, A.; Rocha-Guzman, N.E.; Moreno-Jimenez, M.R.; Gonzalez-Laredo, R.F.; Morales-Martinez, P.S.; Rojas-Contreras, J.A.; Mujica-Paz, H.; Gallegos-Infante, J.A. Effect of Pulsed Electric Field (PEF)-Treated Kombucha Analogues from Quercus obtusata Infusions on Bioactives and Microorganisms. Innov. Food Sci. Emerg. Technol. 2016, 34, 171–179. [Google Scholar] [CrossRef]
- Knirsch, M.C.; Alves Dos Santos, C.; Martins De Oliveira Soares Vicente, A.A.; Vessoni Penna, T.C. Ohmic Heating—A Review. Trends Food Sci. Technol. 2010, 21, 436–441. [Google Scholar] [CrossRef]
- El Darra, N.; Turk, M.F.; Ducasse, M.-A.; Grimi, N.; Maroun, R.G.; Louka, N.; Vorobiev, E. Changes in Polyphenol Profiles and Color Composition of Freshly Fermented Model Wine due to Pulsed Electric Field, Enzymes and Thermovinification PreTreatments. Food Chem. 2016, 194, 944–950. [Google Scholar] [CrossRef]
- López, N.; Puértolas, E.; Condón, S.; Álvarez, I.; Raso, J. Effects of Pulsed Electric Fields on the Extraction of Phenolic Compounds during the Fermentation of Must of Tempranillo Grapes. Innov. Food Sci. Emerg. Technol. 2008, 9, 477–482. [Google Scholar] [CrossRef]
- Donsì, F.; Ferrari, G.; Fruilo, M.; Pataro, G. Pulsed Electric Field-Assisted Vinification of Aglianico and Piedirosso Grapes. J. Agric. Food Chem. 2010, 58, 11606–11615. [Google Scholar] [CrossRef]
- Puértolas, E.; Barba, F.J. Electrotechnologies Applied to Valorization of By-Products from Food Industry: Main Findings, Energy and Economic Cost of Their Industrialization. Food Bioprod. Process. 2016, 100, 172–184. [Google Scholar] [CrossRef]
- Miranda-Mejía, G.A.; Cardador-Martínez, A.; Tejada-Ortigoza, V.; Morales-de la Peña, M.; Martín-Belloso, O. Modulating Yogurt Fermentation Through Pulsed Electric Fields and Influence of Milk Fat Content. Foods 2025, 14, 1927. [Google Scholar] [CrossRef] [PubMed]
- Saldaña, G.; Cebrián, G.; Abenoza, M.; Sánchez-Gimeno, C.; Álvarez, I.; Raso, J. Assessing the Efficacy of PEF Treatments for Improving Polyphenol Extraction during Red Wine Vinifications. Innov. Food Sci. Emerg. Technol. 2017, 39, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Niu, D.; Zeng, X.A.; Ren, E.-F.; Xu, F.-Y.; Li, J.; Wang, M.-S.; Wang, R. Review of the Application of Pulsed Electric Fields (PEF) Technology for Food Processing in China. Food Res. Int. 2020, 137, 109715. [Google Scholar] [CrossRef]
- Sun, Q.Y.; Yang, R.J.; Wu, L.; Zhao, W. Effects of Pulsed Electric Field on the Sterilization and Aging of Fermented Orange Vinegar. Sci. Technol. Food Ind. 2015, 36, 133–137. [Google Scholar] [CrossRef]
- Liu, X.J.; Yin, Y.G.; Fan, S.M.; Zhu, C.; Luo, G.C. GC-MS Analysis of Aroma Component Variation of Wine Treated by High Voltage Pulse Electric Field. Food Sci. 2006, 27, 654–657. [Google Scholar]
- Chen, J.; Zhang, R.B.; Wang, X.Q.; Luo, W.; Mo, M.B.; Wang, L.M.; Guan, Z.C. Effects of Pulsed Electric Fields on Phenols and Colour in Young Red Wine. Spectrosc. Spect. Anal. 2010, 30, 206–209. [Google Scholar] [CrossRef]
- Chanos, P.; Warncke, M.C.; Ehrmann, M.A.; Hertel, C. Application of Mild Pulsed Electric Fields on Starter Culture Accelerates Yogurt Fermentation. Eur. Food Res. Technol. 2020, 246, 621–630. [Google Scholar] [CrossRef]
- Yeo, S.; Liong, M. Effect of Electroporation on Viability and Bioconversion of Isoflavones in Mannitol-Soymilk Fermented by Lactobacilli and Bifidobacteria. J. Sci. Food Agric. 2013, 93, 396–409. [Google Scholar] [CrossRef]
- Miranda-Mejía, G.A.; Del Campo-Barba, S.T.M.; Arredondo-Ochoa, T.; Tejada-Ortigoza, V.; La Peña, M.M. Low-Intensity Pulsed Electric Fields Pre-Treatment on Yogurt Starter Culture: Effects on Fermentation Time and Quality Attributes. Innov. Food Sci. Emerg. Technol. 2024, 95, 103708. [Google Scholar] [CrossRef]
- Joo, D.H.; Jeon, B.Y.; Park, D.H. Effects of an Electric Pulse on Variation of Bacterial Community and Metabolite Production in Kimchi-Making Culture. Biotechnol. Bioprocess Eng. 2013, 18, 909–917. [Google Scholar] [CrossRef]
- Kim, S.-Y.; Gu, H.L.; Ju, H.; Jeon, J.; Jeong, S.-H.; Lee, D.-U. Effects of Pulsed Electric Fields on Controlling Fermentation Rate of Brined Raphanus sativus. Innov. Food Sci. Emerg. Technol. 2024, 92, 103553. [Google Scholar] [CrossRef]
- Zhang, Q.; Barbosa-Cánovas, G.V.; Swanson, B.G. Engineering Aspects of Pulsed Electric Field Pasteurization. J. Food Eng. 1995, 25, 261–281. [Google Scholar] [CrossRef]
- Weaver, J.C. Electroporation: A General Phenomenon for Manipulating Cells and Tissues. J. Cell. Biochem. 1993, 51, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Toepfl, S.; Mathys, A.; Heinz, V.; Knorr, D. Review: Potential of High Hydrostatic Pressure and Pulsed Electric Fields for Energy Efficient and Environmentally Friendly Food Processing. Food Rev. Int. 2006, 22, 405–423. [Google Scholar] [CrossRef]
- Qian, J.-Y.; Gu, Y.-P.; Jiang, W.; Chen, W. Inactivating Effect of Pulsed Electric Field on Lipase in Brown Rice. Innov. Food Sci. Emerg. Technol. 2014, 22, 89–94. [Google Scholar] [CrossRef]
- Zhao, W.; Tang, Y.; Lu, L.; Chen, X.; Li, C. Review: Pulsed Electric Fields Processing of Protein-Based Foods. Food Bioprocess Technol. 2014, 7, 114–125. [Google Scholar] [CrossRef]
- Zhao, W.; Yang, R.; Zhang, H.Q. Recent Advances in the Action of Pulsed Electric Fields on Enzymes and Food Component Proteins. Trends Food Sci. Technol. 2012, 27, 83–96. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, S.; Yan, W.; Tang, Y.; Yang, R.; Zhao, W. Inactivation of Apple (Malus Domestica Borkh) Polyphenol Oxidases by Radio Frequency Combined with Pulsed Electric Field Treatment. Int. J. Food Sci. Technol. 2018, 53, 2054–2063. [Google Scholar] [CrossRef]
- Zhang, Z.-H.; Han, Z.; Zeng, X.-A.; Wang, M.-S. The Preparation of Fe-Glycine Complexes by a Novel Method (Pulsed Electric Fields). Food Chem. 2017, 219, 468–476. [Google Scholar] [CrossRef]
- Tian, M.; Fang, T.; Du, M.; Zhang, F. Effects of Pulsed Electric Field (PEF) Treatment on Enhancing Activity and Conformation of α-Amylase. Protein J. 2016, 35, 154–162. [Google Scholar] [CrossRef]
- Miñano, H.L.A.; Silva, A.C.D.S.; Souto, S.; Costa, E.J.X. Magnetic Fields in Food Processing Perspectives, Applications and Action Models. Processes 2020, 8, 814. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, F.; Hu, H.; Liu, S.; Han, J. Experimental Study on Freezing of Liquids under Static Magnetic Field. Chin. J. Chem. Eng. 2017, 25, 1288–1293. [Google Scholar] [CrossRef]
- Otero, L.; Rodríguez, A.C.; Pérez-Mateos, M.; Sanz, P.D. Effects of Magnetic Fields on Freezing: Application to Biological Products. Compr. Rev. Food Sci. Food Saf. 2016, 15, 646–667. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Jin, Y.; Hong, T.; Yang, N.; Cui, B.; Xu, X.; Jin, Z. Effect of Static Magnetic Field on the Quality of Frozen Bread Dough. LWT 2022, 154, 112670. [Google Scholar] [CrossRef]
- Utsunomiya, T.; Yamane, Y.-I.; Watanabe, M.; Sasaki, K. Stimulation of Porphyrin Production by Application of an External Magnetic Field to a Photosynthetic Bacterium, Rhodobacter sphaeroides. J. Biosci. Bioeng. 2003, 95, 401–404. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, D.C.; Pérez, V.H.; Justo, O.R.; Alegre, R.M. Effect of the Extremely Low Frequency Magnetic Field on Nisin Production by Lactococcus lactis Subsp. Lactis Using Cheese Whey Permeate. Process Biochem. 2006, 41, 1967–1973. [Google Scholar] [CrossRef]
- Tessaro, L.W.E.; Murugan, N.J.; Persinger, M.A. Bacterial Growth Rates are Influenced by Cellular Characteristics of Individual Species When Immersed in Electromagnetic Fields. Microbiol. Res. 2015, 172, 26–33. [Google Scholar] [CrossRef]
- Strašák, L.; Vetterl, V.; Šmarda, J. Effects of Low-Frequency Magnetic Fields on Bacteria Escherichia coli. Bioelectrochemistry 2002, 55, 161–164. [Google Scholar] [CrossRef]
- Hou, X.; Wang, J.; Mei, Y.; Ge, L.; Qian, J.; Huang, Y.; Yang, M.; Li, H.; Wang, Y.; Yan, Z.; et al. Antibiofilm Mechanism of Dielectric Barrier Discharge Cold Plasma against Pichia manshurica. Innov. Food Sci. Emerg. Technol. 2023, 85, 103340. [Google Scholar] [CrossRef]
- Sincak, M.; Turker, M.; Derman, Ü.C.; Alkan, G.; Budak, M.; Çakıroğulları, B. Exploring the Impact of Magnetic Fields on Biomass Production Efficiency under Aerobic and Anaerobic Batch Fermentation of Saccharomyces cerevisiae. Sci. Rep. 2024, 14, 12869. [Google Scholar] [CrossRef]
- Bubanja, I.N.; Lončarević, B.; Lješević, M.; Beškoski, V.; Gojgić-Cvijović, G.; Velikić, Z.; Stanisavljev, D. The Influence of Low-Frequency Magnetic Field Regions on the Saccharomyces cerevisiae Respiration and Growth. Chem. Eng. Process. Process Intensif. 2019, 143, 107593. [Google Scholar] [CrossRef]
- Deutmeyer, A.; Raman, R.; Murphy, P.; Pandey, S. Effect of Magnetic Field on the Fermentation Kinetics of Saccharomyces cerevisiae. Adv. Biosci. Biotechnol. 2011, 2, 207–213. [Google Scholar] [CrossRef][Green Version]
- Kobayashi, R.; Mitsui, Y.; Yoshizaki, Y.; Takahashi, K.; Takamine, K.; Koyama, K. Controlling the Growth of Yeast by Culturing in High Magnetic Fields. J. Magn. Magn. Mater. 2023, 586, 171193. [Google Scholar] [CrossRef]
- Veerana, M.; Yu, N.-N.; Bae, S.-J.; Kim, I.; Kim, E.-S.; Ketya, W.; Lee, H.-Y.; Kim, N.-Y.; Park, G. Enhancement of Fungal Enzyme Production by Radio-Frequency Electromagnetic Fields. J. Fungi 2022, 8, 1187. [Google Scholar] [CrossRef] [PubMed]
- David, G.F.; Perez, V.H.; Justo, O.R.; Cubides, D.C.; Cardona, C.A.; Hristov, J. Glycerol Bioconversion in Unconventional Magnetically Assisted Bioreactor Seeking Whole Cell Biocatalyst (Intracellular lipase) Production. Chem. Eng. Res. Des. 2016, 111, 243–252. [Google Scholar] [CrossRef]
- Guo, L.; Li, X.; Zhang, X.; Ma, H. Effect of Low-Intensity Magnetic Field on the Growth and Metabolite of Grifola frondosa in Submerged Fermentation and Its Possible Mechanisms. Food Res. Int. 2022, 159, 111537. [Google Scholar] [CrossRef]
- Deamici, K.M.; Cardias, B.B.; Costa, J.A.V.; Santos, L.O. Static Magnetic Fields in Culture of Chlorella fusca: Bioeffects on Growth and Biomass Composition. Process Biochem. 2016, 51, 912–916. [Google Scholar] [CrossRef]
- Asundi, S.; Rout, S.; Stephen, S.; Khandual, S.; Dutta, S.; Kumar, S. Parametric Study of the Effect of Increased Magnetic Field Exposure on Microalgae Chlorella vulgaris Growth and Bioactive Compound Production. Phycology 2024, 4, 314–329. [Google Scholar] [CrossRef]
- Bauer, L.M.; Costa, J.A.V.; Da Rosa, A.P.C.; Santos, L.O. Growth Stimulation and Synthesis of Lipids, Pigments and Antioxidants with Magnetic Fields in Chlorella kessleri Cultivations. Bioresour. Technol. 2017, 244, 1425–1432. [Google Scholar] [CrossRef]
- Tu, R.; Jin, W.; Xi, T.; Yang, Q.; Han, S.-F.; Abomohra, A.E.-F. Effect of Static Magnetic Field on the Oxygen Production of Scenedesmus obliquus Cultivated in Municipal Wastewater. Water Res. 2015, 86, 132–138. [Google Scholar] [CrossRef]
- Li, C.; Hu, Z.; Gao, Y.; Ma, Y.; Pan, X.; Li, X.; Liu, S.; Chu, B. Bioeffects of Static Magnetic Fields on the Growth and Metabolites of C. pyrenoidosa and T. obliquus. J. Biotechnol. 2022, 351, 1–8. [Google Scholar] [CrossRef]
- Huo, S.; Chen, X.; Zhu, F.; Zhang, W.; Chen, D.; Jin, N.; Cobb, K.; Cheng, Y.; Wang, L.; Ruan, R. Magnetic Field Intervention on Growth of the Filamentous Microalgae Tribonema sp. in Starch Wastewater for Algal Biomass Production and Nutrients Removal: Influence of Ambient Temperature and Operational Strategy. Bioresour. Technol. 2020, 303, 122884. [Google Scholar] [CrossRef]
- Bai, F.; Ran, Y.; Zhai, S.; Xia, Y. Cold Atmospheric Plasma: A Promising and Safe Therapeutic Strategy for Atopic Dermatitis. Int. Arch. Allergy Immunol. 2023, 184, 1184–1197. [Google Scholar] [CrossRef]
- Lin, S.-P.; Kuo, T.-C.; Wang, H.-T.; Ting, Y.; Hsieh, C.-W.; Chen, Y.-K.; Hsu, H.-Y.; Cheng, K.-C. Enhanced Bioethanol Production Using Atmospheric Cold Plasma-Assisted Detoxification of Sugarcane Bagasse Hydrolysate. Bioresour. Technol. 2020, 313, 123704. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Gajula, V.P.; Mohapatra, S.; Singh, G.; Kar, S. Role of Cold Atmospheric Plasma in Microbial Inactivation and the Factors Affecting Its Efficacy. Health Sci. Rev. 2022, 4, 100037. [Google Scholar] [CrossRef]
- Niedźwiedź, I.; Płotka-Wasylka, J.; Kapusta, I.; Simeonov, V.; Stój, A.; Waśko, A.; Pawłat, J.; Polak-Berecka, M. The Impact of Cold Plasma on the Phenolic Composition and Biogenic Amine Content of Red Wine. Food Chem. 2022, 381, 132257. [Google Scholar] [CrossRef] [PubMed]
- Niedźwiedź, I.; Simeonov, V.; Waśko, A.; Polak-Berecka, M. Comparison of the Effect of Cold Plasma with Conventional Preservation Methods on Red Wine Quality Using Chemometrics Analysis. Molecules 2022, 27, 7048. [Google Scholar] [CrossRef]
- Misra, N.N.; Koubaa, M.; Roohinejad, S.; Juliano, P.; Alpas, H.; Inácio, R.S.; Saraiva, J.A.; Barba, F.J. Landmarks in the Historical Development of Twenty First Century Food Processing Technologies. Food Res. Int. 2017, 97, 318–339. [Google Scholar] [CrossRef]
- Nwabor, O.F.; Onyeaka, H.; Miri, T.; Obileke, K.; Anumudu, C.; Hart, A. A Cold Plasma Technology for Ensuring the Microbiological Safety and Quality of Foods. Food Eng. Rev. 2022, 14, 535–554. [Google Scholar] [CrossRef]
- Zhao, N.; Mei, Y.; Hou, X.; Yang, M.; Li, H.; Liao, Q.; Zhao, J.; Ge, L. Comparative Transcriptomic Insight into Orchestrating Mode of Dielectric Barrier Discharge Cold Plasma and Lactate in Synergistic Inactivation and Biofilm-Suppression of Pichia manshurica. Food Res. Int. 2024, 198, 115323. [Google Scholar] [CrossRef]
- Geng, K.; Lin, Y.; Zheng, X.; Li, C.; Chen, S.; Ling, H.; Yang, J.; Zhu, X.; Liang, S. Enhanced Expression of Alcohol Dehydrogenase I in Pichia pastoris Reduces the Content of Acetaldehyde in Wines. Microorganisms 2023, 12, 38. [Google Scholar] [CrossRef]
- Cheng, J.-H.; Lv, X.; Pan, Y.; Sun, D.-W. Foodborne Bacterial Stress Responses to Exogenous Reactive Oxygen Species (ROS) Induced by Cold Plasma Treatments. Trends Food Sci. Technol. 2020, 103, 239–247. [Google Scholar] [CrossRef]
- Ruas, F.A.D.; Barboza, N.R.; Castro-Borges, W.; Guerra-Sá, R. Manganese Alters Expression of Proteins Involved in the Oxidative Stress of Meyerozyma guilliermondii. J. Proteomics 2019, 196, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, Y.A.-G.; Elkaliny, N.E.; Darwish, O.A.; Ashraf, Y.; Ebrahim, R.A.; Das, S.P.; Yahya, G. Comprehensive Review for Aflatoxin Detoxification with Special Attention to Cold Plasma Treatment. Mycotoxin Res. 2025, 41, 277–300. [Google Scholar] [CrossRef] [PubMed]
- Yarabbi, H.; Soltani, K.; Sangatash, M.M.; Yavarmanesh, M.; Shafafi Zenoozian, M. Reduction of Microbial Population of Fresh Vegetables (Carrot, White Radish) and Dried Fruits (Dried Fig, Dried Peach) Using Atmospheric Cold Plasma and Its Effect on Physicochemical Properties. J. Agric. Food Res. 2023, 14, 100789. [Google Scholar] [CrossRef]
- Moisan, M.; Barbeau, J.; Crevier, M.-C.; Pelletier, J.; Philip, N.; Saoudi, B. Plasma Sterilization. Methods and Mechanisms. Pure Appl. Chem. 2002, 74, 349–358. [Google Scholar] [CrossRef]
- Wiegand, C.; Beier, O.; Horn, K.; Pfuch, A.; Tölke, T.; Schimanski, A. Antimicrobial Impact of Cold Atmospheric Pressure Plasma on Medical Critical Yeasts and and Bacteria Cultures. Skin Pharmacol. Physiol. 2013, 27, 25–35. [Google Scholar] [CrossRef]
- Recek, N.; Zhou, R.; Teo, V.S.J.; Speight, R.E.; Mozetič, M.; Vesel, A.; Cvelbar, U.; Bazaka, K.; Ostrikov, K. Improved Fermentation Efficiency of S. cerevisiae by Changing Glycolytic Metabolic Pathways with Plasma Agitation. Sci. Rep. 2018, 8, 8252. [Google Scholar] [CrossRef]
- Zhou, R.; Wang, P.; Guo, Y.; Dai, X.; Xiao, S.; Fang, Z.; Speight, R.; Thompson, R.; Cullen, P.; Ostrikov, K. Prussian Blue Analogue Nanoenzymes Mitigate Oxidative Stress and Boost Bio-Fermentation. Nanoscale 2019, 11, 19497–19505. [Google Scholar] [CrossRef]
- Dong, X.; Liu, T.; Xiong, Y. A Novel Approach to Regulate Cell Membrane Permeability for ATP and NADH Formation in Saccharomyces cerevisiae Induced by Air Cold Plasma. Plasma Sci. Technol. 2017, 19, 024001. [Google Scholar] [CrossRef]
- Dong, X.-Y.; Xiu, Z.-L.; Li, S.; Hou, Y.-M.; Zhang, D.-J.; Ren, C.-S. Dielectric Barrier Discharge Plasma as a Novel Approach for Improving 1,3-Propanediol Production in Klebsiella pneumoniae. Biotechnol. Lett. 2010, 32, 1245–1250. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-Y.; Huang, Z.-L.; Li, G.; Zhao, H.-X.; Xing, X.-H.; Sun, W.-T.; Li, H.-P.; Gou, Z.-X.; Bao, C.-Y. Novel Mutation Breeding Method for Streptomyces avermitilis Using an Atmospheric Pressure Glow Discharge Plasma. J. Appl. Microbiol. 2010, 108, 851–858. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Yuan, Y.; Tang, Q.; Dou, S.; Di, L.; Zhang, X. Parameter Optimization for Enhancement of Ethanol Yield by Atmospheric Pressure DBD-Treated Saccharomyces cerevisiae. Plasma Sci. Technol. 2014, 16, 73–76. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).