Light and Temperature Effects on the Accumulation of Carotenoids in Rhodotorula spp. Yeasts
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strain Isolation and Media
2.2. Yeast Species Verification
2.3. Yeast Culturing Conditions
2.4. Carotenoids Extraction from Yeasts
2.5. Quantification of β-Carotene Equivalents
- ε = A/(c × l);
- ɛ—extinction coefficient (M−1·cm−1);
- A—light absorption (O.D.);
- l—cuvette width (cm);
- c—substance concentration (M).
- c = A × V × 104 × (m × ε);
- c—β-carotene (mg) / dry biomass (g);
- A—light absorption (O.D.);
- V—solvent volume (mL);
- m—yeast biomass (g);
- ɛ—extinction coefficient DMF (M−1·cm−1).
3. Results and Discussion
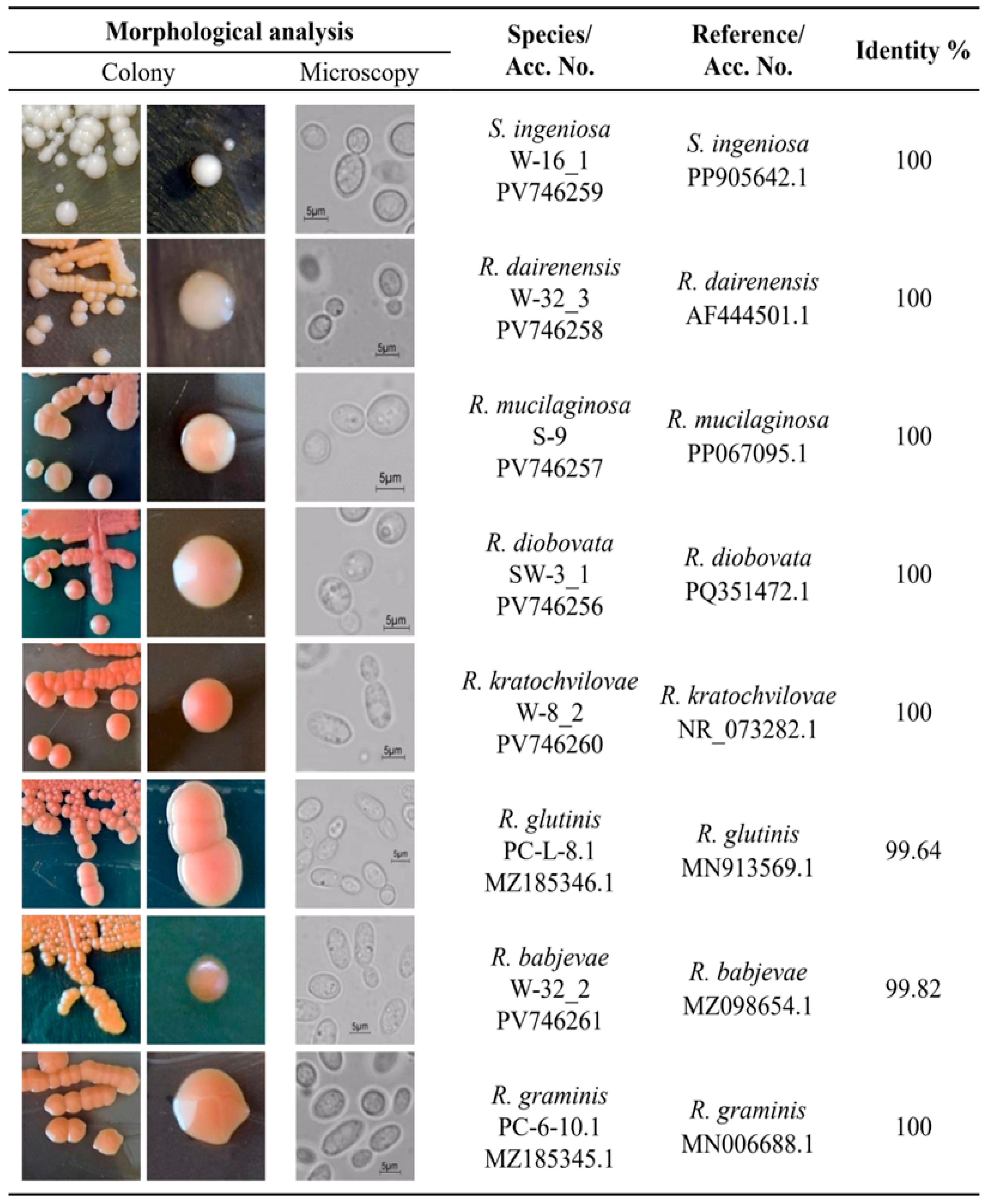
3.1. Isolation, Identification, and Phylogenetic Characterization of Red Yeast
3.2. Carotenoid Accumulation in Rhodotorula spp. Yeasts Cultured at a 26 °C Temperature and Differing Lighting Conditions
3.3. Effect of Low Temperature on Carotenoid Content in Rhodotorula spp.
3.4. Comparative Analysis of Temperature and Lighting Impact on Carotenoid Content in Rhodotorula spp.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mata-Gómez, L.C.; Montañez, J.C.; Méndez-Zavala, A.; Aguilar, C.N. Biotechnological production of carotenoids by yeasts: An overview. Microb. Cell Factories 2014, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, A.; Guzhova, I.; Bernetti, L.; Buzzini, P.; Kieliszek, M.; Kot, A.M. Carotenoids and some other pigments from fungi and yeast. Metabolites 2021, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Kirti, K.; Amita, S.; Priti, S.; Kumar, A.M.; Jyoti, S. Colorful world of microbes: Carotenoids and their applications. Adv. Biol. 2014, 2014, 837891. [Google Scholar] [CrossRef]
- Bogacz-Radomska, L.; Harasym, J. β-Carotene—Properties and production methods. Food Qual. Saf. 2018, 2, 69–74. [Google Scholar] [CrossRef]
- Toma, M.A.; Rahman, M.H.; Rahman, M.S.; Arif, M.; Nazir, K.H.M.N.H.; Dufossé, L. Fungal pigments: Carotenoids, riboflavin, and polyketides with diverse applications. J. Fungi 2023, 9, 454. [Google Scholar] [CrossRef] [PubMed]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Tazeddinova, D.; Aljoumaa, K.; Kazhmukhanbetkyzy, Z.A.; Orazov, A.; Toshev, A.D. Carotenoids: Therapeutic Strategy in the Battle against Viral Emerging Diseases, COVID-19: An Overview. Prev. Nutr. Food Sci. 2021, 26, 241–261. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Kumari, P.K.; Siddiqui, N. Yeast Carotenoids: Cost-Effective Fermentation Strategies for Health Care Applications. Fermentation 2023, 9, 147. [Google Scholar] [CrossRef]
- Pham, K.D.; Shida, Y.; Miyata, A.; Takamizawa, T.; Suzuki, Y.; Ara, S.; Yamazaki, H.; Masaki, K.; Mori, K.; Aburatani, S.; et al. Effect of light on carotenoid and lipid production in the oleaginous yeast Rhodosporidium toruloides. Biosci. Biotechnol. Biochem. 2020, 84, 1501–1512. [Google Scholar] [CrossRef] [PubMed]
- Kreusch, M.; Duarte, R.T.D. Photoprotective compounds and radioresistance in pigmented and non-pigmented yeasts. Appl. Microbiol. Biotechnol. 2021, 105, 3521–3532. [Google Scholar] [CrossRef] [PubMed]
- Limbo, S.; Torri, L.; Piergiovanni, L. Light-induced changes in an aqueous β-carotene system stored under halogen and fluorescent lamps, affected by two oxygen partial pressures. J. Agric. Food Chem. 2007, 55, 5238–5245. [Google Scholar] [CrossRef] [PubMed]
- Ochoa-Viñals, N.; Alonso-Estrada, D.; Pacios-Michelena, S.; García-Cruz, A.; Ramos-González, R.; Faife-Pérez, E.; Michelena-Álvarez, L.G.; Martínez-Hernández, J.L.; Iliná, A. Current Advances in Carotenoid Production by Rhodotorula sp. Fermentation 2024, 10, 190. [Google Scholar] [CrossRef]
- Kot, A.M.; Błażejak, S.; Kieliszek, M.; Gientka, I.; Piwowarek, K.; Brzezińska, R. Production of lipids and carotenoids by Rhodotorula gracilis ATCC 10788 yeast in a bioreactor using low-cost wastes. Biocatal. Agric. Biotechnol. 2020, 26, 101634. [Google Scholar] [CrossRef]
- Naz, T.; Ullah, S.; Nazir, Y.; Li, S.; Iqbal, B.; Liu, Q.; Mohamed, H.; Song, Y. Industrially Important Fungal Carotenoids: Advancements in Biotechnological Production and Extraction. J. Fungi 2023, 9, 578. [Google Scholar] [CrossRef] [PubMed]
- Moliné, M.; Libkind, D.; Van Broock, M. Production of torularhodin, torulene, and β-carotene by Rhodotorula yeasts. Methods Mol. Biol. 2012, 898, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Naisi, S.; Bayat, M.; Salehi, Z.T.; Zarif, R.B.; Yahyaraeyat, R. Antimicrobial and anti-biofilm effects of carotenoid pigment extracted from Rhodotorula glutinis strain on food-borne bacteria. Iran J. Microbiol. 2023, 15, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Di Canito, A.; Mateo-Vargas, M.A.; Mazzieri, M.; Cantoral, J.; Foschino, R.; Cordero-Bueso, G.; Vigentini, I. The Role of Yeasts as Biocontrol Agents for Pathogenic Fungi on Postharvest Grapes: A Review. Foods 2021, 10, 1650. [Google Scholar] [CrossRef] [PubMed]
- Mussagy, C.U.; Guimarães, A.C.; Rocha, L.V.F.; Winterburn, J.; De Carvalho Santos-Ebinuma, V.; Pereira, J.F.B. Improvement of carotenoids production from Rhodotorula glutinis CCT-2186. Biochem. Eng. J. 2021, 165, 107827. [Google Scholar] [CrossRef]
- Guo, R.; Liu, T.; Guo, C.; Chen, G.; Fan, J.; Zhang, Q. Carotenoid biosynthesis is associated with low-temperature adaptation in Rhodosporidium kratochvilovae. BMC Microbiol. 2022, 22, 319. [Google Scholar] [CrossRef] [PubMed]
- Sereti, F.; Papadaki, A.; Alexandri, M.; Kachrimanidou, V.; Kopsahelis, N. Exploring the potential of novel R. kratochvilovae red yeasts towards the sustainable synthesis of natural carotenoids. Sustain. Chem. Pharm. 2023, 31, 100927. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. PCR—Protocols and Applications; Chapter 38; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. Molecular Evolutionary Genetics Analysis Version 12 for adaptive and green computing. Mol. Biol. Evol. 2024, 411, msae263. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K. Estimation of the number of nucleotide substitutions when there are strong transition-transversion and G + C-content biases. Mol. Biol. Evol. 1992, 9, 678–687. [Google Scholar] [CrossRef] [PubMed]
- Lopes, N.A.; Remedi, R.D.; Sá, C.D.S.; Burkert, C.V.; De Medeiros Burkert, J. Different cell disruption methods for obtaining carotenoids by Sporodiobolus. Food Sci. Biotechnol. 2017, 26, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Guo, L.; Xia, Y.; Zhuang, X.; Chu, W. Isolation, Identification of Carotenoid-Producing Rhodotorula sp. from Marine Environment and Optimization for Carotenoid Production. Mar. Drugs 2019, 17, 161. [Google Scholar] [CrossRef] [PubMed]
- Allahkarami, S.; Sepahi, A.A.; Hosseini, H.; Razavi, M.R. Isolation and identification of carotenoid-producing Rhodotorula sp. from Pinaceae forest ecosystems and optimization of in vitro carotenoid production. Biotechnol. Rep. 2021, 32, e00687. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yurkov, A.; Göker, M.; Lumbsch, H.T.; Leavitt, S.D.; Groenewald, M.; Theelen, B.; Liu, X.; Boekhout, T.; Bai, F. Phylogenetic classification of yeasts and related taxa within Pucciniomycotina. Stud. Mycol. 2015, 81, 149–189. [Google Scholar] [CrossRef] [PubMed]
- Kot, A.M.; Sęk, W.; Kieliszek, M.; Błażejak, S.; Pobiega, K.; Brzezińska, R. Diversity of Red Yeasts in Various Regions and Environments of Poland and Biotechnological Potential of the Isolated Strains. Appl. Biochem. Biotechnol. 2024, 196, 3274–3316. [Google Scholar] [CrossRef] [PubMed]
- Maldonade, I.; Adilma, R.P.; Scamparini, A.R.P.; Rodriguez-Amaya, D.B. Selection and characterization of carotenoid-producing yeasts from Campinas region, Brazil. Braz. J. Microbiol. 2007, 38, 65–70. [Google Scholar] [CrossRef]
- Díaz-Ruiz, E.; Balbino, T.R.; Dos Santos, J.C.; Kumar, V.; da Silva, S.S.; Chandel, A.K. Fermentative Production of β-Carotene from Sugarcane Bagasse Hydrolysate by Rhodotorula glutinis CCT-2186. Appl. Biochem. Biotechnol. 2024, 196, 4188–4204. [Google Scholar] [CrossRef] [PubMed]
- Dias Rodrigues, T.V.; Amore, T.D.; Teixeira, E.C.; de Medeiros Burkert, J.F. Carotenoid Production by Rhodotorula mucilaginosa in Batch and Fed-Batch Fermentation Using Agroindustrial Byproducts. Food Technol. Biotechnol. 2019, 57, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, P. Environmental and cultural stimulants in the production of carotenoids from microorganisms. Appl. Microbiol. Biotechnol. 2004, 63, 351–361. [Google Scholar] [CrossRef] [PubMed]
- Ezaka, E.; Nchedo, O.; Ugbo, E.N.; Adediran, A.B.; Ayanda, O.E. Effects of Environmental Factors on the Growth and Proliferation of Yeasts. Nig. J. Biotech. 2021, 38, 166–178. [Google Scholar] [CrossRef]
- Gosalawit, C.; Imsoonthornruksa, S.; Gilroyed, B.H.; Mcnea, L.; Boontawan, A.; Ketudat-Cairns, M. The potential of the oleaginous yeast Rhodotorula paludigena CM33 to produce biolipids. J. Biotechnol. 2021, 329, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Xu Zhang, X.; Tan, T. Lipid and carotenoid production by Rhodotorula glutinis under irradiation/high-temperature and dark/low-temperature cultivation. Bioresour. Technol. 2014, 157, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Zeng, N.; Wang, D.; Li, B.; Yu, G.; Li, C. Exploring the Influence Mechanism of Low/High Temperatures on Carotenoid Production in Sporobolomyces pararoseus: Insights from Physiological and Transcriptomic Analyses. Biotechnol. Bioeng. 2025, 122, 1615–1626. [Google Scholar] [CrossRef] [PubMed]
- Bhosale, P.; Gadre, R.V. Manipulation of temperature and illumination conditions for enhanced beta-carotene production by mutant 32 of Rhodotorula glutinis. Lett. Appl. Microbiol. 2002, 34, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Kot, A.M.; Błażejak, S.; Kieliszek, M.; Gientka, I.; Bryś, J.; Reczek, L.; Pobiega, K. Effect of exogenous stress factors on the biosynthesis of carotenoids and lipids by Rhodotorula yeast strains in media containing agro-industrial waste. World J. Microbiol. Biotechnol. 2019, 35, 157. [Google Scholar] [CrossRef] [PubMed]
- Camponeschi, I.; Montanari, A.; Mazzoni, C.; Bianchi, M.M. Light Stress in Yeast: Signaling and response in creatures of the night. Int. J. Mol. Sci. 2023, 24, 6929. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.B.; Davis, C.R.; Johnson, C.H. Visible light alters yeast metabolic rhythms by inhibiting respiration. Proc. Natl. Acad. Sci. USA 2013, 110, 21130–21135. [Google Scholar] [CrossRef] [PubMed]
- Saubenova, M.; Rapoport, A.; Venkatachalam, M.; Dufossé, L.; Yermekbay, Z.; Oleinikova, Y. Production of Carotenoids by Microorganisms. Fermentation 2024, 10, 502. [Google Scholar] [CrossRef]



| Conditions | Settings | Temperature (°C) | Days | Temperature (°C) | Days |
|---|---|---|---|---|---|
| Dark | I | 26 | 2, 3, 5, 7, 14 | ||
| II | 26 | 2 | 4 | 1, 3, 5, 12 | |
| Light | III | 26 | 2, 3, 5, 7, 14 | ||
| IV | 26 | 2 | 4 | 1, 3, 5, 12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Losinska-Sičiūnienė, R.; Strazdaitė-Žielienė, Ž.; Pranckevičiūtė, S.; Servienė, E. Light and Temperature Effects on the Accumulation of Carotenoids in Rhodotorula spp. Yeasts. Fermentation 2025, 11, 412. https://doi.org/10.3390/fermentation11070412
Losinska-Sičiūnienė R, Strazdaitė-Žielienė Ž, Pranckevičiūtė S, Servienė E. Light and Temperature Effects on the Accumulation of Carotenoids in Rhodotorula spp. Yeasts. Fermentation. 2025; 11(7):412. https://doi.org/10.3390/fermentation11070412
Chicago/Turabian StyleLosinska-Sičiūnienė, Regina, Živilė Strazdaitė-Žielienė, Saulė Pranckevičiūtė, and Elena Servienė. 2025. "Light and Temperature Effects on the Accumulation of Carotenoids in Rhodotorula spp. Yeasts" Fermentation 11, no. 7: 412. https://doi.org/10.3390/fermentation11070412
APA StyleLosinska-Sičiūnienė, R., Strazdaitė-Žielienė, Ž., Pranckevičiūtė, S., & Servienė, E. (2025). Light and Temperature Effects on the Accumulation of Carotenoids in Rhodotorula spp. Yeasts. Fermentation, 11(7), 412. https://doi.org/10.3390/fermentation11070412








