Potential Prebiotic Effect of Caatinga Bee Honeys from the Pajeú Hinterland (Pernambuco, Brazil) on Synbiotic Alcoholic Beverages Fermented by Saccharomyces boulardii CNCM I-745
Abstract
1. Introduction
2. Materials and Methods
2.1. Origin of Bee Honeys
2.2. Physicochemical and Microbiological Analysis of Bee Honey
2.3. Probiotic Yeast and Cultivation Conditions
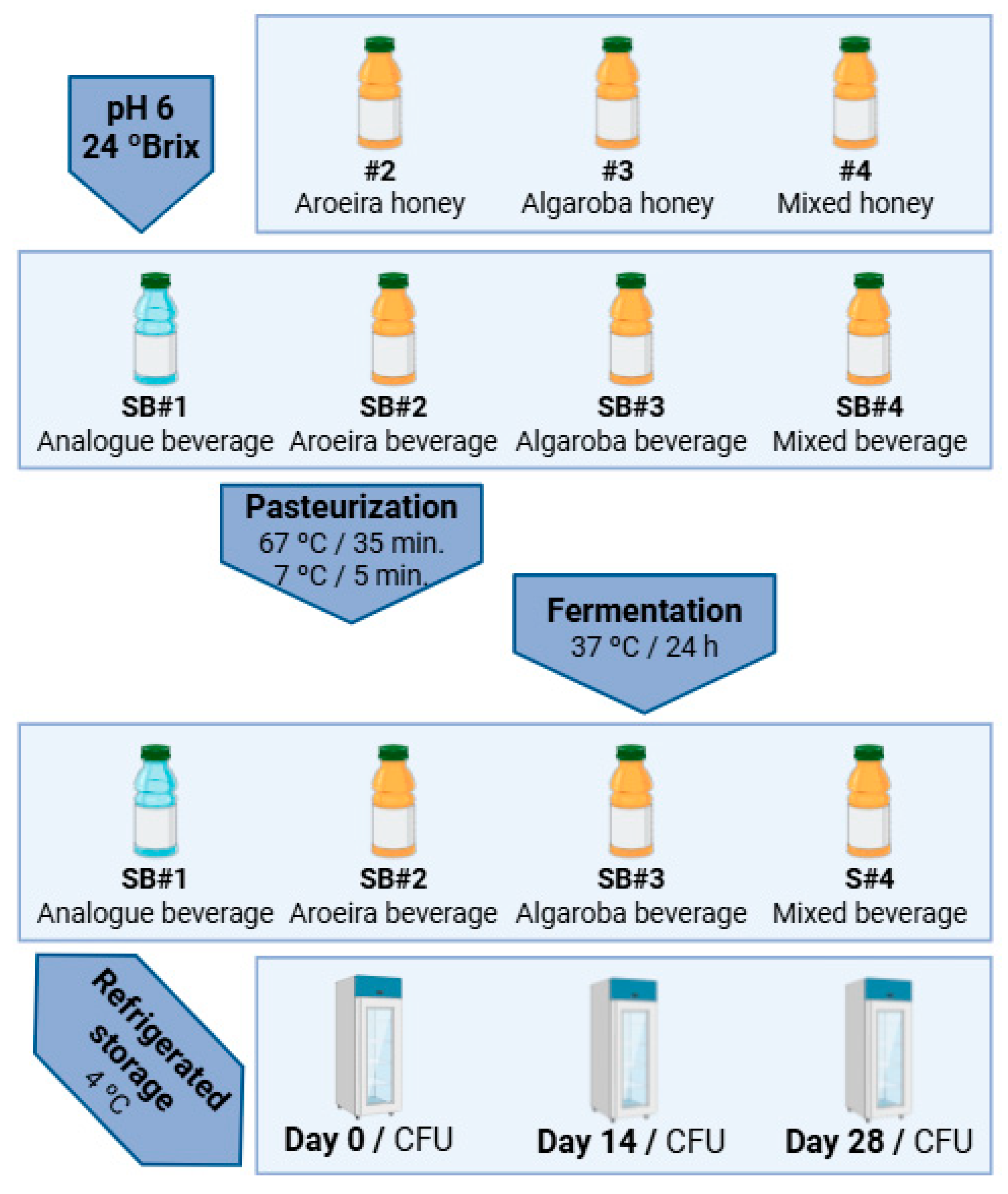
2.4. Formulation and Experimental Design of Bee Honey Beverages
2.5. Assessment of Probiotic Metabolic Activity
2.5.1. Carbohydrates, Glycerol, and Ethanol
2.5.2. Phenylethyl Alcohol Ester
2.6. In Vitro Simulation Tests of the GIT
2.6.1. Static Simulation of the GIT Under Refrigerated Storage
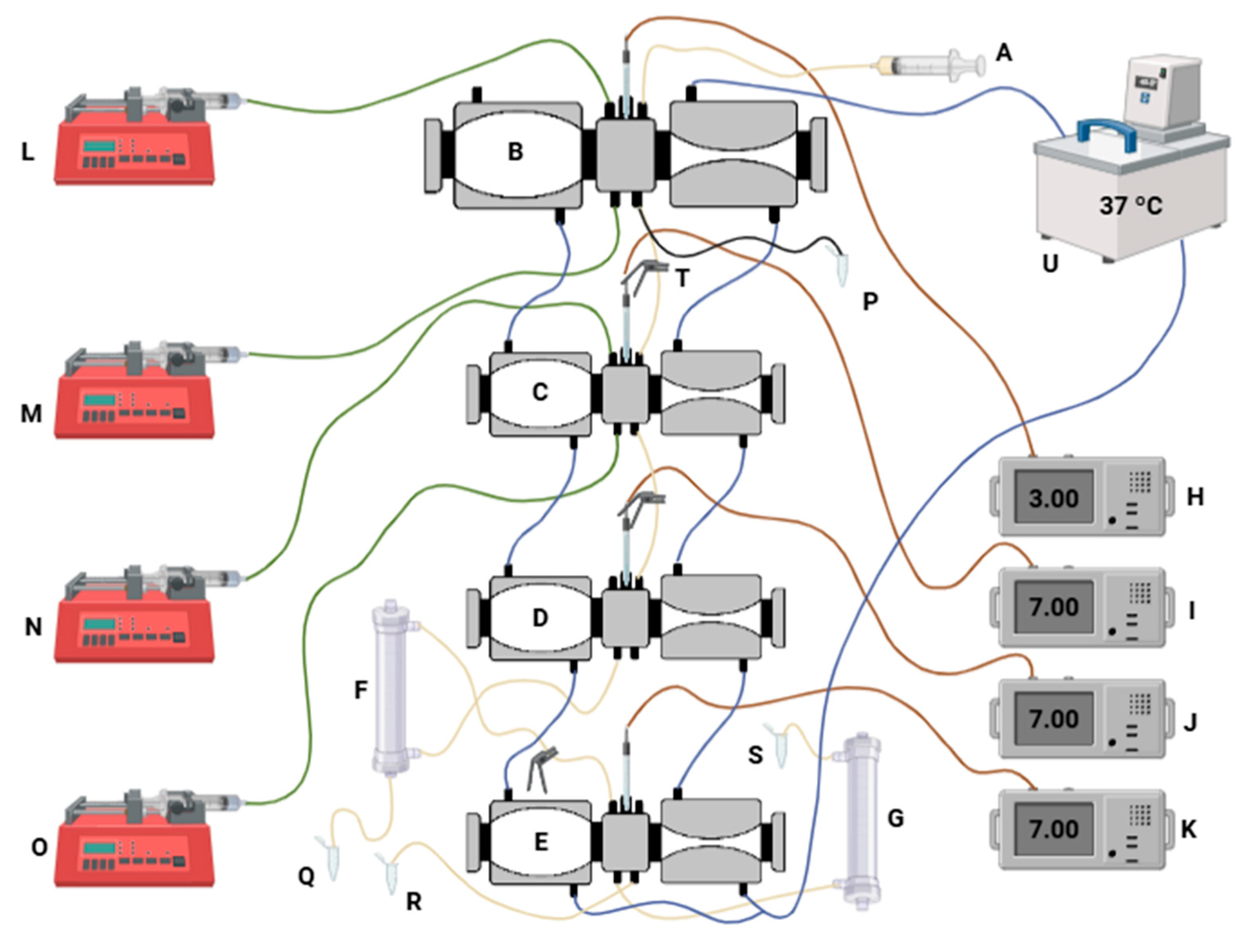
2.6.2. Dynamic Simulation of the GIT Under Refrigerated Storage
2.7. Assessment of Probiotic Viability and Survival
2.8. Assessment of Probiotic Cell Membrane Integrity
2.9. Statistical Analysis
3. Results and Discussion
3.1. Physicochemical and Microbiological Parameters of Bee Honeys
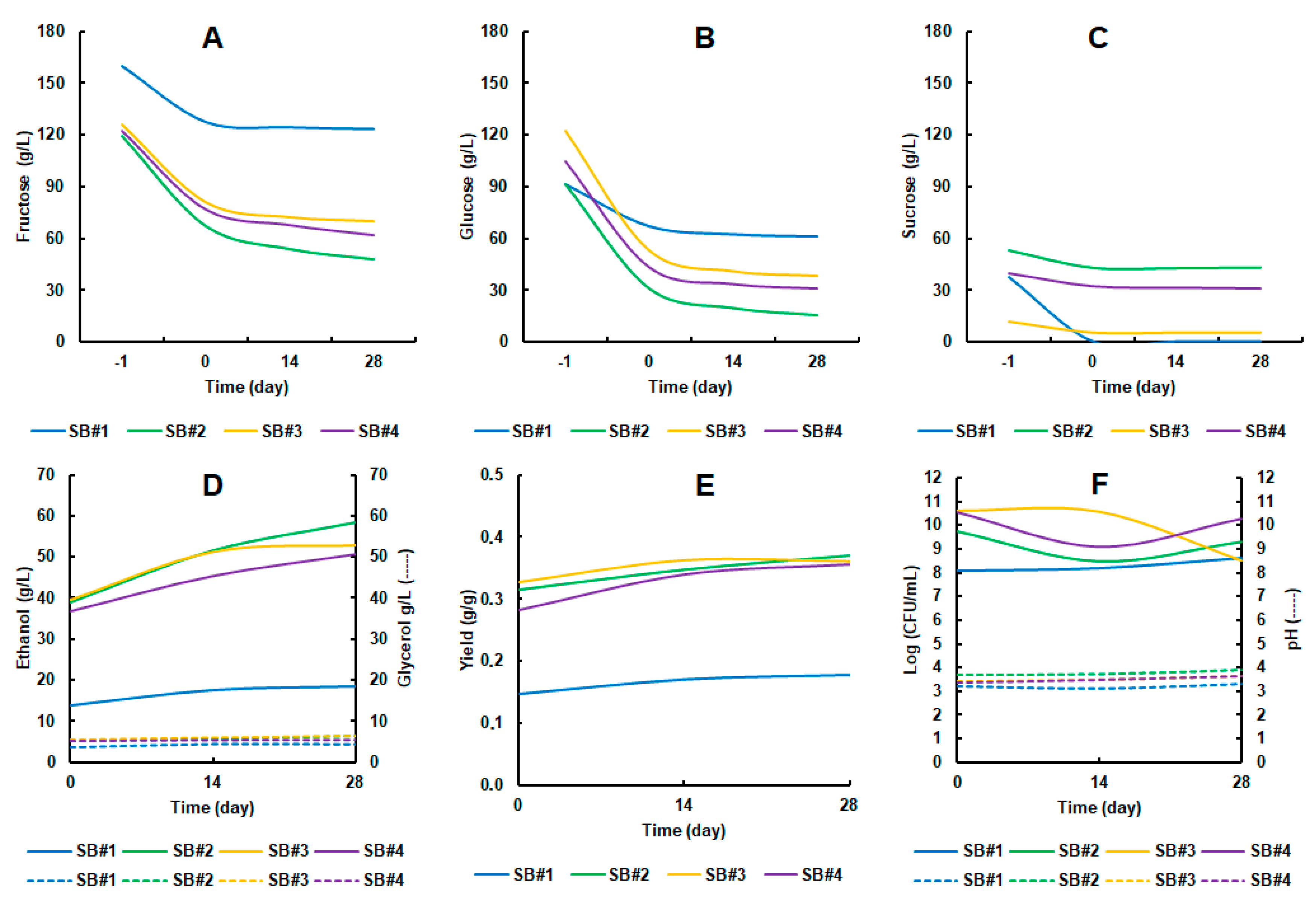
3.2. The Performance of Saccharomyces Boulardii CNCM I-745 During Fermentation and Storage Under Refrigeration
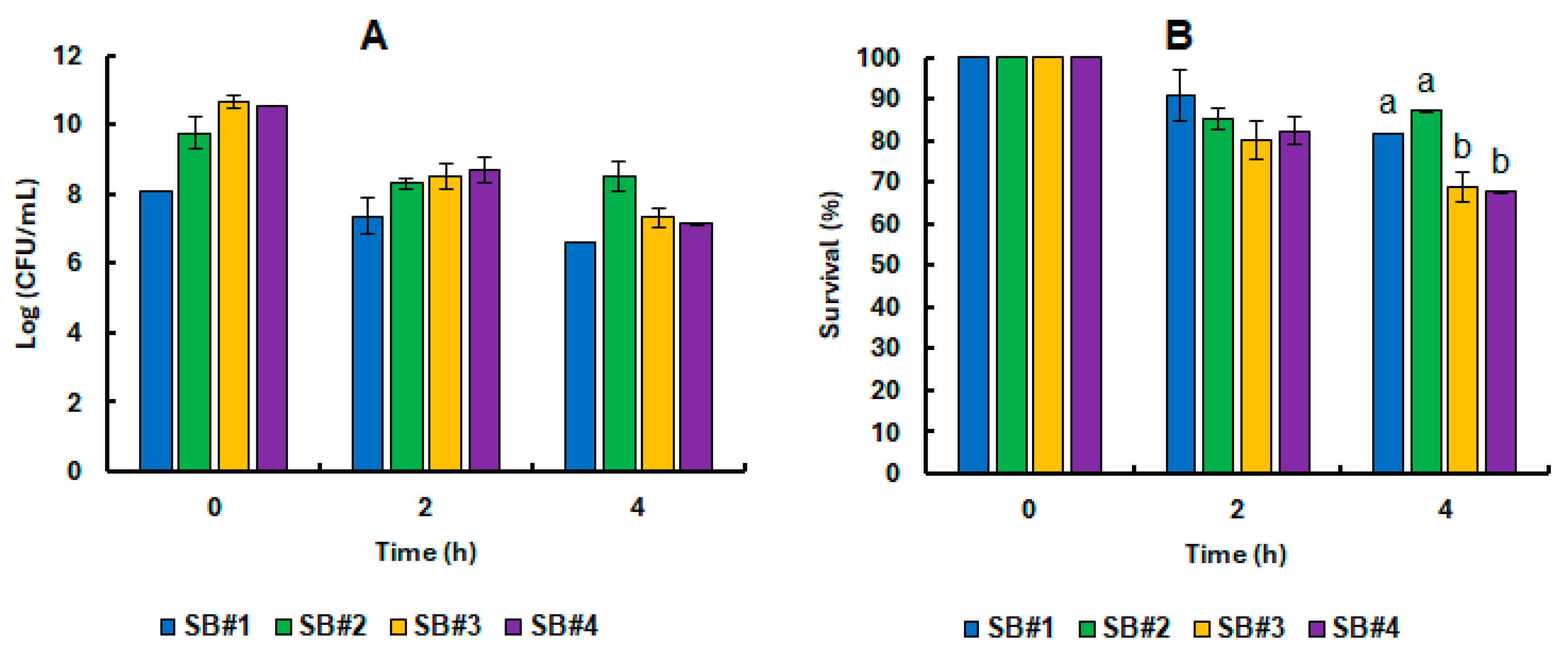
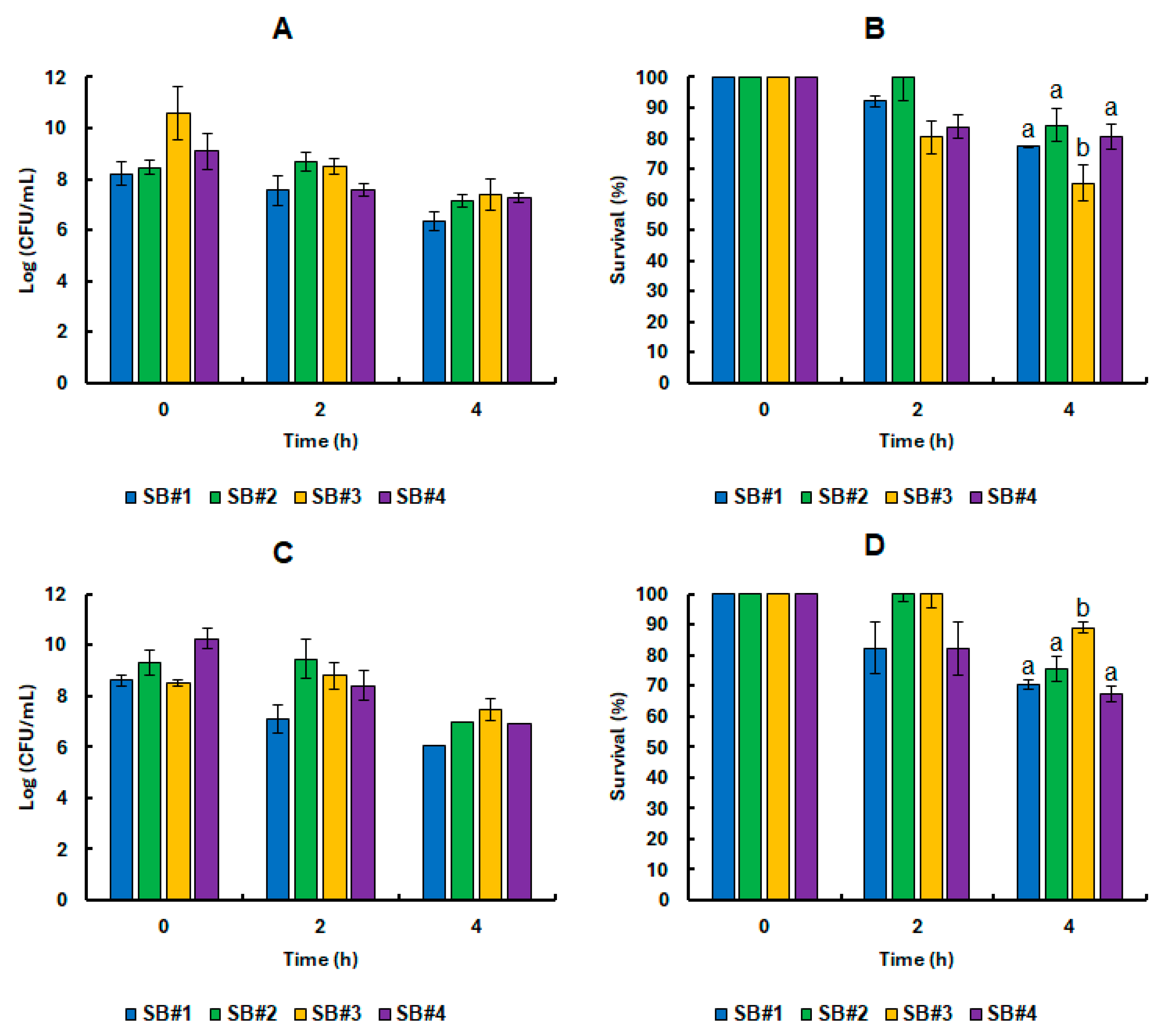
3.3. Survival of the Saccharomyces Boulardii CNCM I-745 Cell in Synbiotic Beverages Under GIT Static Simulation
3.4. Impact of Bee Honeys from Different Blooms on the Tolerance of Probiotic Yeast in Refrigerated Stock and Under GIT Static Simulation
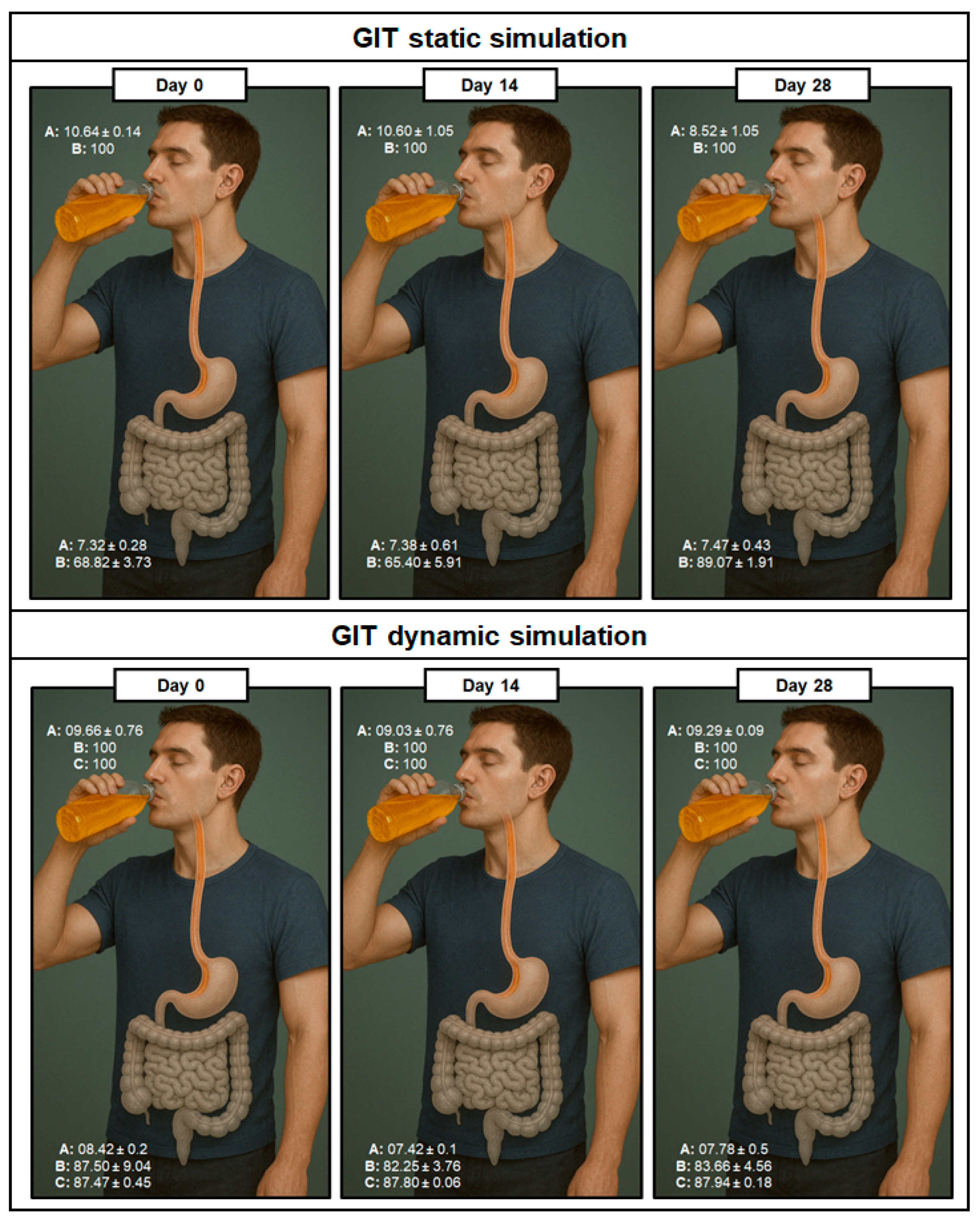
3.5. Honey Confers Resilience to Saccharomyces Boulardii CNCM I-745 Under More Realistic GIT Conditions: A Multimethodological Approach
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CXS 12-1981; FAO/WHO Standard for Honey. Codex Alimentarius: International Food Standards. United Nations Press: New York, NY, USA, 2019.
- Pinto-Neto, W.d.P.; Silva, R.K.; Lima, B.d.S.; Acioli, G.F.d.S.; Paixão, G.A.d.; Muniz, B.C.; Silva, P.K.N.d.; Costa, R.M.P.B.; Silva, F.S.B.d.; Melo, H.F.d.; et al. Bee Honey of the Pajeú Hinterland, Pernambuco, Brazil: Physicochemical Characterization and Biological Activity. Food Biosci. 2024, 60, 104289. [Google Scholar] [CrossRef]
- Sabóia Guerra Diógenes, É.; Da Silva, A.L.C.; Chagas Neto, F.C.D.; Silveira, E.R.; Leal, L.K.A.M.; Nicolete, R.; De Araújo, T.G. Evaluation of the Skin Whitening and Antioxidant Activity of Myracrodruon urundeuva Extract (Aroeira-Do-Sertão). Nat. Prod. Res. 2024, 38, 3663–3668. [Google Scholar] [CrossRef]
- Aguirre-Loredo, R.Y. Mesquite Tree (Prosopis spp.): A Native Resource for the Potential for Human Consumption and Healthcare. Plant Foods Hum. Nutr. Dordr. Neth. 2024, 80, 5. [Google Scholar] [CrossRef]
- Falcão, M.P.M.M.; Oliveira, T.K.B.; do Ó, N.P.R.; Sarmento, D.A.; Gadelha, N.C. Schinus terebinthifolius Raddi (Aroeira) e suas propriedades na Medicina Popular. Rev. Verde Agroecol. E Desenvolv. Sustentável 2015, 10, 23–27. [Google Scholar] [CrossRef][Green Version]
- Ogrodowczyk, A.M.; Markiewicz, L.; Szmatowicz, B.; Koźniewski, B.; Wróblewska, B. Improved Quality, Sensory Properties and Nutraceutical Potential of the Fermented Beverages Fortified with Freeze-Dried Berries and Acacia Honey. Food Chem. 2025, 486, 144469. [Google Scholar] [CrossRef]
- FAO. WHO Guidelines for the Evaluation of Probiotics in Food; FAO: London, UK, 2002. [Google Scholar]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Mendonça, A.A.; Pinto-Neto, W.d.P.; da Paixão, G.A.; Santos, D.d.S.; De Morais, M.A., Jr.; De Souza, R.B. Journey of the Probiotic Bacteria: Survival of the Fittest. Microorganisms 2023, 11, 95. [Google Scholar] [CrossRef]
- Czerucka, D.; Rampal, P. Diversity of Saccharomyces Boulardii CNCM I-745 Mechanisms of Action against Intestinal Infections. World J. Gastroenterol. 2019, 25, 2188–2203. [Google Scholar] [CrossRef]
- Kaźmierczak-Siedlecka, K.; Ruszkowski, J.; Fic, M.; Folwarski, M.; Makarewicz, W. Saccharomyces Boulardii CNCM I-745: A Non-Bacterial Microorganism Used as Probiotic Agent in Supporting Treatment of Selected Diseases. Curr. Microbiol. 2020, 77, 1987–1996. [Google Scholar] [CrossRef]
- Gopalan, S.; Ganapathy, S.; Mitra, M.; Neha; Kumar Joshi, D.; Veligandla, K.C.; Rathod, R.; Kotak, B.P. Unique Properties of Yeast Probiotic Saccharomyces Boulardii CNCM I-745: A Narrative Review. Cureus 2023, 15, e46314. [Google Scholar] [CrossRef]
- Moré, M.I.; Swidsinski, A. Saccharomyces Boulardii CNCM I-745 Supports Regeneration of the Intestinal Microbiota after Diarrheic Dysbiosis—A Review. Clin. Exp. Gastroenterol. 2015, 8, 237–255. [Google Scholar] [CrossRef]
- Neut, C.; Mahieux, S.; Dubreuil, L.J. Antibiotic Susceptibility of Probiotic Strains: Is It Reasonable to Combine Probiotics with Antibiotics? Médecine Mal. Infect. 2017, 47, 477–483. [Google Scholar] [CrossRef]
- Tamang, J.P.; Lama, S. Probiotic Properties of Yeasts in Traditional Fermented Foods and Beverages. J. Appl. Microbiol. 2022, 132, 3533–3542. [Google Scholar] [CrossRef]
- Instituto Adolfo Lutz (Ed.) Métodos Físico-Químicos Para Análise de Alimentos, 1st digital ed.; Instituto Adolfo Lutz: São Paulo, Brazil, 2008. [Google Scholar]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of Protein Using Bicinchoninic Acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Orujei, Y.; Shabani, L.; Sharifi-Tehrani, M. Induction of Glycyrrhizin and Total Phenolic Compound Production in Licorice by Using Arbuscular Mycorrhizal Fungi. Russ. J. Plant Physiol. 2013, 60, 855–860. [Google Scholar] [CrossRef]
- de Sousa Araújo, T.A.; Alencar, N.L.; de Amorim, E.L.C.; de Albuquerque, U.P. A New Approach to Study Medicinal Plants with Tannins and Flavonoids Contents from the Local Knowledge. J. Ethnopharmacol. 2008, 120, 72–80. [Google Scholar] [CrossRef]
- Woisky, R.G.; and Salatino, A. Analysis of Propolis: Some Parameters and Procedures for Chemical Quality Control. J. Apic. Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- Bencherif, K.; Djaballah, Z.; Brahimi, F.; Boutekrabt, A.; Dalpè, Y.; Lounès-Hadj Sahraoui, A. Arbuscular Mycorrhizal Fungi Affect Total Phenolic Content and Antimicrobial Activity of Tamarix Gallica in Natural Semi-Arid Algerian Areas. S. Afr. J. Bot. 2019, 125, 39–45. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International, 17th ed.; AOAC International: Gaithersburg, MD, USA, 2002. [Google Scholar]
- AOAC International. Official Methods of Analysis of AOAC International, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Vidal, E.E.; de Billerbeck, G.M.; Simões, D.A.; Schuler, A.; François, J.M.; de Morais, M.A. Influence of Nitrogen Supply on the Production of Higher Alcohols/Esters and Expression of Flavour-Related Genes in Cachaça Fermentation. Food Chem. 2013, 138, 701–708. [Google Scholar] [CrossRef]
- Espinosa Vidal, E.; de Morais Jr, M.A.; François, J.M.; de Billerbeck, G.M. Biosynthesis of Higher Alcohol Flavour Compounds by the Yeast Saccharomyces Cerevisiae: Impact of Oxygen Availability and Responses to Glucose Pulse in Minimal Growth Medium with Leucine as Sole Nitrogen Source. Yeast 2015, 32, 47–56. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A Standardised Static in Vitro Digestion Method Suitable for Food—An International Consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Mulet-Cabero, A.-I.; Egger, L.; Portmann, R.; Ménard, O.; Marze, S.; Minekus, M.; Feunteun, S.L.; Sarkar, A.; Grundy, M.M.-L.; Carrière, F.; et al. A Standardised Semi-Dynamic in Vitro Digestion Method Suitable for Food—An International Consensus. Food Funct. 2020, 11, 1702–1720. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Coimbra, M.A.; Vicente, A.A. In Vitro Behaviour of Curcumin Nanoemulsions Stabilized by Biopolymer Emulsifiers—Effect of Interfacial Composition. Food Hydrocoll. 2016, 52, 460–467. [Google Scholar] [CrossRef]
- Pinheiro, A.C.; Gonçalves, R.F.; Madalena, D.A.; Vicente, A.A. Towards the Understanding of the Behavior of Bio-Based Nanostructures during in vitro Digestion. Curr. Opin. Food Sci. 2017, 15, 79–86. [Google Scholar] [CrossRef]
- Fernandes, J.-M.; Madalena, D.A.; Vicente, A.A.; Pinheiro, A.C. Influence of the Addition of Different Ingredients on the Bioaccessibility of Glucose Released from Rice during Dynamic in Vitro Gastrointestinal Digestion. Int. J. Food Sci. Nutr. 2021, 72, 45–56. [Google Scholar] [CrossRef]
- Günerken, E.; D’Hondt, E.; Eppink, M.; Elst, K.; Wijffels, R. Flow Cytometry to Estimate the Cell Disruption Yield and Biomass Release of Chlorella Sp. during Bead Milling. Algal Res. 2017, 25, 25–31. [Google Scholar] [CrossRef]
- Loureiro, L.; Machado, L.; Geada, P.; Vasconcelos, V.; Vicente, A.A. Evaluation of Efficiency of Disruption Methods for Coelastrella Sp. in Order to Obtain High Yields of Biochemical Compounds Release. Algal Res. 2023, 73, 103158. [Google Scholar] [CrossRef]
- Azeredo, L.d.C.; Azeredo, M.A.A.; de Souza, S.R.; Dutra, V.M.L. Protein Contents and Physicochemical Properties in Honey Samples of Apis Mellifera of Different Floral Origins. Food Chem. 2003, 80, 249–254. [Google Scholar] [CrossRef]
- Kadri, S.M.; Zaluski, R.; Pereira Lima, G.P.; Mazzafera, P.; de Oliveira Orsi, R. Characterization of Coffea Arabica Monofloral Honey from Espírito Santo, Brazil. Food Chem. 2016, 203, 252–257. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, C.; Li, C.; Huang, Z.Y.; Miao, X. Pathway of 5-Hydroxymethyl-2-Furaldehyde Formation in Honey. J. Food Sci. Technol. 2019, 56, 2417–2425. [Google Scholar] [CrossRef]
- Ministério da Agricultura e Abastecimento. Regulamento Técnico de Identidade e Qualidade Do Mel; Ministério da Agricultura e Abastecimento: Brasilia, Brazil, 2000. [Google Scholar]
- European Community (EC) Council Directive 2001/110/EC of 20 December 2001 Relating to Honey; European Union: Brussels, Belgium, 2002.
- Codex Alimentarius Revised Codex Standard for Honey; United Nations Press: New York, NY, USA, 2022.
- Terrab, A.; Recamales, A.F.; Hernanz, D.; Heredia, F.J. Characterisation of Spanish Thyme Honeys by Their Physicochemical Characteristics and Mineral Contents. Food Chem. 2004, 88, 537–542. [Google Scholar] [CrossRef]
- Dekeba Tafa, K.; Sundramurthy, V.P.; Subramanian, N. Rheological and Thermal Properties of Honey Produced in Algeria and Ethiopia: A Review. Int. J. Food Prop. 2021, 24, 1117–1131. [Google Scholar] [CrossRef]
- Almeida, A.M.M.d.; Oliveira, M.B.S.; Costa, J.G.d.; Valentim, I.B.; Goulart, M.O.F. Antioxidant Capacity, Physicochemical and Floral Characterization of Honeys from the Northeast of Brazil. Rev. Virtual Química 2016, 8, 57–77. [Google Scholar] [CrossRef]
- Di Marco, G.; Manfredini, A.; Leonardi, D.; Canuti, L.; Impei, S.; Gismondi, A.; Canini, A. Geographical, Botanical and Chemical Profile of Monofloral Italian Honeys As Food Quality Guarantee and Territory Brand. Plant Biosyst. - Int. J. Deal. Asp. Plant Biol. 2016, 151, 450–463. [Google Scholar] [CrossRef]
- Alugoju, P.; Janardhanshetty, S.S.; Subaramanian, S.; Periyasamy, L.; Dyavaiah, M. Quercetin Protects Yeast Saccharomyces Cerevisiae Pep4 Mutant from Oxidative and Apoptotic Stress and Extends Chronological Lifespan. Curr. Microbiol. 2018, 75, 519–530. [Google Scholar] [CrossRef]
- Alevia, M.; Rasines, S.; Cantero, L.; Sancho, M.T.; Fernández-Muiño, M.A.; Osés, S.M. Chemical Extraction and Gastrointestinal Digestion of Honey: Influence on Its Antioxidant, Antimicrobial and Anti-Inflammatory Activities. Foods 2021, 10, 1412. [Google Scholar] [CrossRef]
- Mongi, R.J.; Ruhembe, C.C. Sugar Profile and Sensory Properties of Honey from Different Geographical Zones and Botanical Origins in Tanzania. Heliyon 2024, 10, e38094. [Google Scholar] [CrossRef]
- Anklam, E. A Review of the Analytical Methods to Determine the Geographical and Botanical Origin of Honey. Food Chem. 1998, 63, 549–562. [Google Scholar] [CrossRef]
- Silva, J.; Lima, C.; Miranda, R.; Seraglio, S.; Barbosa, E.; Souza, A.; Cardoso, D. Sensorial Quality of Sugarcane Juice with the Addition of Fruits Pulp from the Semi-Arid. Res. Soc. Dev. 2020, 9, 200973745. [Google Scholar] [CrossRef]
- Pinto Neto, W.d.P.; Costa de Lucena, T.M.; Alves da Paixão, G.; Shinohara, N.K.S.; Pinheiro, A.C.; Vicente, A.A.; de Souza, R.B.; de Morais Junior, M.A. Symbiotic Honey Beverages: A Matrix Which Tells a Story of Survival and Protection of Human Health from a Gastronomic and Industrial Perspective. Int. J. Gastron. Food Sci. 2025, 40, 101183. [Google Scholar] [CrossRef]
- Hinojosa-Avila, C.R.; García-Gamboa, R.; Chedraui-Urrea, J.J.T.; García-Cayuela, T. Exploring the Potential of Probiotic-Enriched Beer: Microorganisms, Fermentation Strategies, Sensory Attributes, and Health Implications. Food Res. Int. 2024, 175, 113717. [Google Scholar] [CrossRef]
- Zhao, X.; Procopio, S.; Becker, T. Flavor Impacts of Glycerol in the Processing of Yeast Fermented Beverages: A Review. J. Food Sci. Technol. 2015, 52, 7588–7598. [Google Scholar] [CrossRef]
- Rasika, D.M.; Vidanarachchi, J.K.; Rocha, R.S.; Balthazar, C.F.; Cruz, A.G.; Sant’Ana, A.S.; Ranadheera, C.S. Plant-Based Milk Substitutes as Emerging Probiotic Carriers. Curr. Opin. Food Sci. 2021, 38, 8–20. [Google Scholar] [CrossRef]
- Andrade, R.; Santos, E.; Azoubel, P.; Ribeiro, E. Increased Survival of Lactobacillus Rhamnosus ATCC 7469 in Guava Juices with Simulated Gastrointestinal Conditions during Refrigerated Storage. Food Biosci. 2019, 32, 100470. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; Medeiros, A.B.P.; Camara, M.C.; Magalhães Júnior, A.I.; de Carvalho Neto, D.P.; Bier, M.C.J.; Soccol, C.R. Chapter 11—Production and Recovery of Bioaromas Synthesized by Microorganisms. In The Role of Alternative and Innovative Food Ingredients and Products in Consumer Wellness; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 315–338. ISBN 978-0-12-816453-2. [Google Scholar]
- Mahmoud, M.A.A.; Kılıç-Büyükkurt, Ö.; Aboul Fotouh, M.M.; Selli, S. Aroma Active Compounds of Honey: Analysis with GC-MS, GC-O, and Molecular Sensory Techniques. J. Food Compos. Anal. 2024, 134, 106545. [Google Scholar] [CrossRef]
- Camilleri, M. Integrated Upper Gastrointestinal Response to Food Intake. Gastroenterology 2006, 131, 640–658. [Google Scholar] [CrossRef]
- Orij, R.; Brul, S.; Smits, G.J. Intracellular pH Is a Tightly Controlled Signal in Yeast. Biochim. Biophys. Acta BBA - Gen. Subj. 2011, 1810, 933–944. [Google Scholar] [CrossRef]
- Guan, N.; Liu, L. Microbial Response to Acid Stress: Mechanisms and Applications. Appl. Microbiol. Biotechnol. 2020, 104, 51–65. [Google Scholar] [CrossRef]
- De Melo, H.F.; Bonini, B.M.; Thevelein, J.; Simões, D.A.; Morais, M.A., Jr. Physiological and Molecular Analysis of the Stress Response of Saccharomyces Cerevisiae Imposed by Strong Inorganic Acid with Implication to Industrial Fermentations. J. Appl. Microbiol. 2010, 109, 116–127. [Google Scholar] [CrossRef]
- de Lucena, R.M.; Elsztein, C.; de Barros Pita, W.; de Souza, R.B.; de Sá Leitão Paiva Júnior, S.; de Morais Junior, M.A. Transcriptomic Response of Saccharomyces Cerevisiae for Its Adaptation to Sulphuric Acid-Induced Stress. Antonie Van Leeuwenhoek 2015, 108, 1147–1160. [Google Scholar] [CrossRef]
- de Lucena, R.M.; Elsztein, C.; Simões, D.A.; de Morais, M.A. Participation of CWI, HOG and Calcineurin Pathways in the Tolerance of Saccharomyces Cerevisiae to Low pH by Inorganic Acid. J. Appl. Microbiol. 2012, 113, 629–640. [Google Scholar] [CrossRef]
- de Lucena, R.M.; Elsztein, C.; Barros de Souza, R.; de Barros Pita, W.; Paiva, S.d.S.L.; de Morais, M.A. Genetic Interaction between HOG1 and SLT2 Genes in Signalling the Cellular Stress Caused by Sulphuric Acid in Saccharomyces Cerevisiae. J. Mol. Microbiol. Biotechnol. 2015, 25, 423–427. [Google Scholar] [CrossRef]
- Lucena, R.M.; Dolz-Edo, L.; Brul, S.; de Morais, M.A.; Smits, G. Extreme Low Cytosolic pH Is a Signal for Cell Survival in Acid Stressed Yeast. Genes 2020, 11, 656. [Google Scholar] [CrossRef]
- Alkalbani, N.S.; Osaili, T.M.; Al-Nabulsi, A.A.; Olaimat, A.N.; Liu, S.-Q.; Shah, N.P.; Apostolopoulos, V.; Ayyash, M.M. Assessment of Yeasts as Potential Probiotics: A Review of Gastrointestinal Tract Conditions and Investigation Methods. J. Fungi 2022, 8, 365. [Google Scholar] [CrossRef]
- Pais, P.; Almeida, V.; Yılmaz, M.; Teixeira, M.C. Saccharomyces Boulardii: What Makes It Tick as Successful Probiotic? J. Fungi 2020, 6, 78. [Google Scholar] [CrossRef]
- Aguilar-Ballester, M.; Herrero-Cervera, A.; Vinué, Á.; Martínez-Hervás, S.; González-Navarro, H. Impact of Cholesterol Metabolism in Immune Cell Function and Atherosclerosis. Nutrients 2020, 12, 2021. [Google Scholar] [CrossRef]
- Casamayor, A.; Serrano, R.; Platara, M.; Casado, C.; Ruiz, A.; Ariño, J. The Role of the Snf1 Kinase in the Adaptive Response of Saccharomyces Cerevisiae to Alkaline pH Stress. Biochem. J. 2012, 444, 39–49. [Google Scholar] [CrossRef]
- Serra-Cardona, A.; Petrezsélyová, S.; Canadell, D.; Ramos, J.; Ariño, J. Coregulated Expression of the Na+/Phosphate Pho89 Transporter and Ena1 Na+-ATPase Allows Their Functional Coupling under High-pH Stress. Mol. Cell. Biol. 2014, 34, 4420–4435. [Google Scholar] [CrossRef]
- Higuchi, Y.; Mori, H.; Kubota, T.; Takegawa, K. Analysis of Ambient pH Stress Response Mediated by Iron and Copper Intake in Schizosaccharomyces pombe. J. Biosci. Bioeng. 2018, 125, 92–96. [Google Scholar] [CrossRef]
- Dżugan, M.; Tomczyk, M.; Sowa, P.; Grabek-Lejko, D. Antioxidant Activity as Biomarker of Honey Variety. Molecules 2018, 23, 2069. [Google Scholar] [CrossRef]
- Becerril-Sánchez, A.L.; Quintero-Salazar, B.; Dublán-García, O.; Escalona-Buendía, H.B. Phenolic Compounds in Honey and Their Relationship with Antioxidant Activity, Botanical Origin, and Color. Antioxidants 2021, 10, 1700. [Google Scholar] [CrossRef]
- Schell, K.R.; Fernandes, K.E.; Shanahan, E.; Wilson, I.; Blair, S.E.; Carter, D.A.; Cokcetin, N.N. The Potential of Honey as a Prebiotic Food to Re-Engineer the Gut Microbiome Toward a Healthy State. Front. Nutr. 2022, 9, 957932. [Google Scholar] [CrossRef]
- Dimitriu, L.; Constantinescu-Aruxandei, D.; Preda, D.; Moraru, I.; Băbeanu, N.E.; Oancea, F. The Antioxidant and Prebiotic Activities of Mixtures Honey/Biomimetic NaDES and Polyphenols Show Differences between Honeysuckle and Raspberry Extracts. Antioxidants 2023, 12, 1678. [Google Scholar] [CrossRef]
- Agarbati, A.; Canonico, L.; Marini, E.; Zannini, E.; Ciani, M.; Comitini, F. Potential Probiotic Yeasts Sourced from Natural Environmental and Spontaneous Processed Foods. Foods 2020, 9, 287. [Google Scholar] [CrossRef]
- da Silva Filho, E.A.; de Melo, H.F.; Antunes, D.F.; dos Santos, S.K.B.; do Monte Resende, A.; Simões, D.A.; de Morais, M.A. Isolation by Genetic and Physiological Characteristics of a Fuel-Ethanol Fermentative Saccharomyces Cerevisiae Strain with Potential for Genetic Manipulation. J. Ind. Microbiol. Biotechnol. 2005, 32, 481–486. [Google Scholar] [CrossRef]
- Chiron, C.; Tompkins, T.A.; Burguière, P. Flow Cytometry: A Versatile Technology for Specific Quantification and Viability Assessment of Micro-Organisms in Multistrain Probiotic Products. J. Appl. Microbiol. 2018, 124, 572–584. [Google Scholar] [CrossRef]
| SSF *1 (pH 7) | SGF *2 (pH 7) | SIF *3 (pH 7) | ||||||
|---|---|---|---|---|---|---|---|---|
| Stock Concentrations | mL of Stock Added to Prepare 0.4 L (1.25×) | Final Salt Conc. in SSF | mL of Stock Added to Prepare 0.4 L (1.25×) | Final Salt Conc. in SGF | mL of Stock Added to Prepare 0.4 L (1.25×) | Final Salt Conc. in SIF | ||
| Salts | g/L | mol/L | mL | mmol/L | mL | mmol/L | mL | mmol/L |
| KCl | 37.3 | 0.5 | 15.1 | 15.1 | 6.9 | 6.9 | 6.8 | 6.8 |
| KH2PO4 | 68.0 | 0.5 | 3.7 | 3.7 | 0.9 | 0.9 | 0.8 | 0.8 |
| NaHCO3 | 84.0 | 1.0 | 6.8 | 13.6 | 12.5 | 25.0 | 42.5 | 85.0 |
| NaCl | 117.0 | 2.0 | - | - | 11.8 | 47.2 | 9.6 | 38.4 |
| MgCl2(H2O)6 | 30.5 | 0.15 | 0.5 | 0.15 | 0.4 | 0.12 | 1.1 | 0.33 |
| (NH4)2CO3 | 48.0 | 0.5 | 0.06 | 0.06 | 0.5 | 0.5 | - | - |
| CaCl2(H2O)2 | 44.1 | 0.3 | - | 1.5 | - | 0.15 | - | 0.6 |
| HCl ** | - | 6 | 0.09 | 1.1 | 1.3 | 15.6 | 0.7 | 8.4 |
| Parameters | Mastic (Aroeira) | Mesquite (Algaroba) | Mixed |
|---|---|---|---|
| Colour (Pfund) | Light Amber | Dark amber | Dark amber |
| Water activity (Aw) | 0.61 ± 0.00 | 0.56 ± 0.02 | 0.59 ± 0.01 |
| Humidity (%) | 14.01 ± 0.80 | 19.56 ± 0.52 | 19.71 ± 0.15 |
| Soluble solids (°Brix) | 85.00 ± 0.00 | 83.00 ± 0.00 | 81.50 ± 0.00 |
| Density (g/cm3) | 1.42 ± 0.06 | 1.42 ± 0.00 | 1.42 ± 0.00 |
| pH | 3.92 ± 0.05 | 3.61 ± 0.07 | 3.78 ± 0.00 |
| Free acidity (mEq/kg of honey) | 56.00 ± 0.01 | 34.50 ± 0.10 | 22.50 ± 0.06 |
| Lactonic acidity (mEq/kg of honey) | 11.00 ± 0.02 | 08.50 ± 0.00 | 25.00 ± 0.20 |
| Total acidity (mEq/kg of honey) | 67.00 ± 0.04 | 43.00 ± 0.02 | 47.50 ± 0.00 |
| Electric conductivity (µS/cm) | 436.25 ± 2.06 | 997.50 ± 0.71 | 270.50 ± 0.71 |
| Ashes (%) | 0.21 ± 0.02 | 0.57 ± 0.02 | 0.09 ± 0.01 |
| Firmness (g) | 57.74 ± 2.75 | 51.97 ± 0.13 | 66.95 ± 1.42 |
| Consistency (g/s) | 646.61 ± 27.09 | 585.50 ± 3.18 | 753.78 ± 17.15 |
| Cohesiveness (g) | −29.52 ± 2.98 | −26.34 ± 0.59 | −40.50 ± 2.28 |
| Work of cohesion (g/s) | −238.99 ± 25.55 | −204.36 ± 7.19 | −396.23 ± 17.79 |
| Viscosity (Pa/s) | 14.67 ± 0.37 | 18.46 ± 0.64 | 17.66 ± 0.50 |
| Hydroxymethyl furfural (mg/kg of honey) | 3.04 ± 0.09 | 9.45 ± 0.01 | 8.28 ± 0.11 |
| Diastase activity (Gothe units/g of honey) | 19.17 ± 0.73 | 4.10 ± 0.40 | 3.50 ± 0.20 |
| Glucose (g/100 g of honey) | 26.32 ± 0.23 | 29.83 ± 0.75 | 31.54 ± 0.16 |
| Fructose (g/100 g of honey) | 41.42 ± 0.56 | 32.41 ± 1.04 | 32.92 ± 0.65 |
| Apparent sucrose (g/100 g of honey) | 9.48 ± 0.68 | 9.82 ± 0.33 | 10.33 ± 0.11 |
| Total sugars (g/100 g of honey) | 85.90 ± 0.51 | 72.05 ± 1.45 | 74.79 ± 0.69 |
| Total proteins (g/100 g of honey) | 0.29 ± 0.03 | 0.22 ± 0.01 | 0.22 ± 0.02 |
| Antioxidant activity (FRAP method) (µM FeSO4/mL) | 403.92 ± 0.00 | 145.43 ± 1.62 | 301.38 ± 1.21 |
| Antioxidant activity (DPPH method) (%) | 52.7 ± 3.05 | 44.18 ± 2.32 | 39.32 ± 2.27 |
| Flavonoids (mg Rutin/100 g of honey) | 74.3 ± 0.00 | 102.6 ± 0.00 | 69.4 ± 0.00 |
| Flavonols (mg Quercetin/100 g of honey) | 54.3 ± 0.00 | 75.0 ± 0.00 | 59.5 ± 0.00 |
| Total phenolic (mg Tannic Acid/100 g of honey) | 185.72 ± 2.9 | 256.40 ± 0.64 | 191.31 ± 2.16 |
| Lund reaction | Positive | Positive | Positive |
| Lugol reaction | Negative | Negative | Negative |
| Coliforms (CFU/g) | <10 | <10 | <10 |
| Escherichia coli (CFU/g) | <10 | <10 | <10 |
| Salmonella (in 25 g of honey) | Negative | Negative | Negative |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto-Neto, W.d.P.; Loureiro, L.; Gonçalves, R.F.S.; Marques, M.C.T.; Rodrigues, R.M.M.; Abrunhosa, L.; de Barros, A.M.; Shinohara, N.K.S.; Pinheiro, A.C.; Vicente, A.A.; et al. Potential Prebiotic Effect of Caatinga Bee Honeys from the Pajeú Hinterland (Pernambuco, Brazil) on Synbiotic Alcoholic Beverages Fermented by Saccharomyces boulardii CNCM I-745. Fermentation 2025, 11, 405. https://doi.org/10.3390/fermentation11070405
Pinto-Neto WdP, Loureiro L, Gonçalves RFS, Marques MCT, Rodrigues RMM, Abrunhosa L, de Barros AM, Shinohara NKS, Pinheiro AC, Vicente AA, et al. Potential Prebiotic Effect of Caatinga Bee Honeys from the Pajeú Hinterland (Pernambuco, Brazil) on Synbiotic Alcoholic Beverages Fermented by Saccharomyces boulardii CNCM I-745. Fermentation. 2025; 11(7):405. https://doi.org/10.3390/fermentation11070405
Chicago/Turabian StylePinto-Neto, Walter de Paula, Luis Loureiro, Raquel F. S. Gonçalves, Márcia Cristina Teixeira Marques, Rui Miguel Martins Rodrigues, Luís Abrunhosa, Aline Magalhães de Barros, Neide Kazue Sakugawa Shinohara, Ana Cristina Pinheiro, Antonio Augusto Vicente, and et al. 2025. "Potential Prebiotic Effect of Caatinga Bee Honeys from the Pajeú Hinterland (Pernambuco, Brazil) on Synbiotic Alcoholic Beverages Fermented by Saccharomyces boulardii CNCM I-745" Fermentation 11, no. 7: 405. https://doi.org/10.3390/fermentation11070405
APA StylePinto-Neto, W. d. P., Loureiro, L., Gonçalves, R. F. S., Marques, M. C. T., Rodrigues, R. M. M., Abrunhosa, L., de Barros, A. M., Shinohara, N. K. S., Pinheiro, A. C., Vicente, A. A., Souza, R. B. d., & Junior, M. A. d. M. (2025). Potential Prebiotic Effect of Caatinga Bee Honeys from the Pajeú Hinterland (Pernambuco, Brazil) on Synbiotic Alcoholic Beverages Fermented by Saccharomyces boulardii CNCM I-745. Fermentation, 11(7), 405. https://doi.org/10.3390/fermentation11070405







