Orchards and Varietals Shape Apple and Cider Local Microbial Terroirs in the Hudson Valley of New York
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Sites and Sampling Processing
2.2. Fermentation Conditions
2.3. DNA Extraction and Sequencing
2.4. Quantifying Microbial Diversity
2.5. Microbial Community Analysis
3. Results
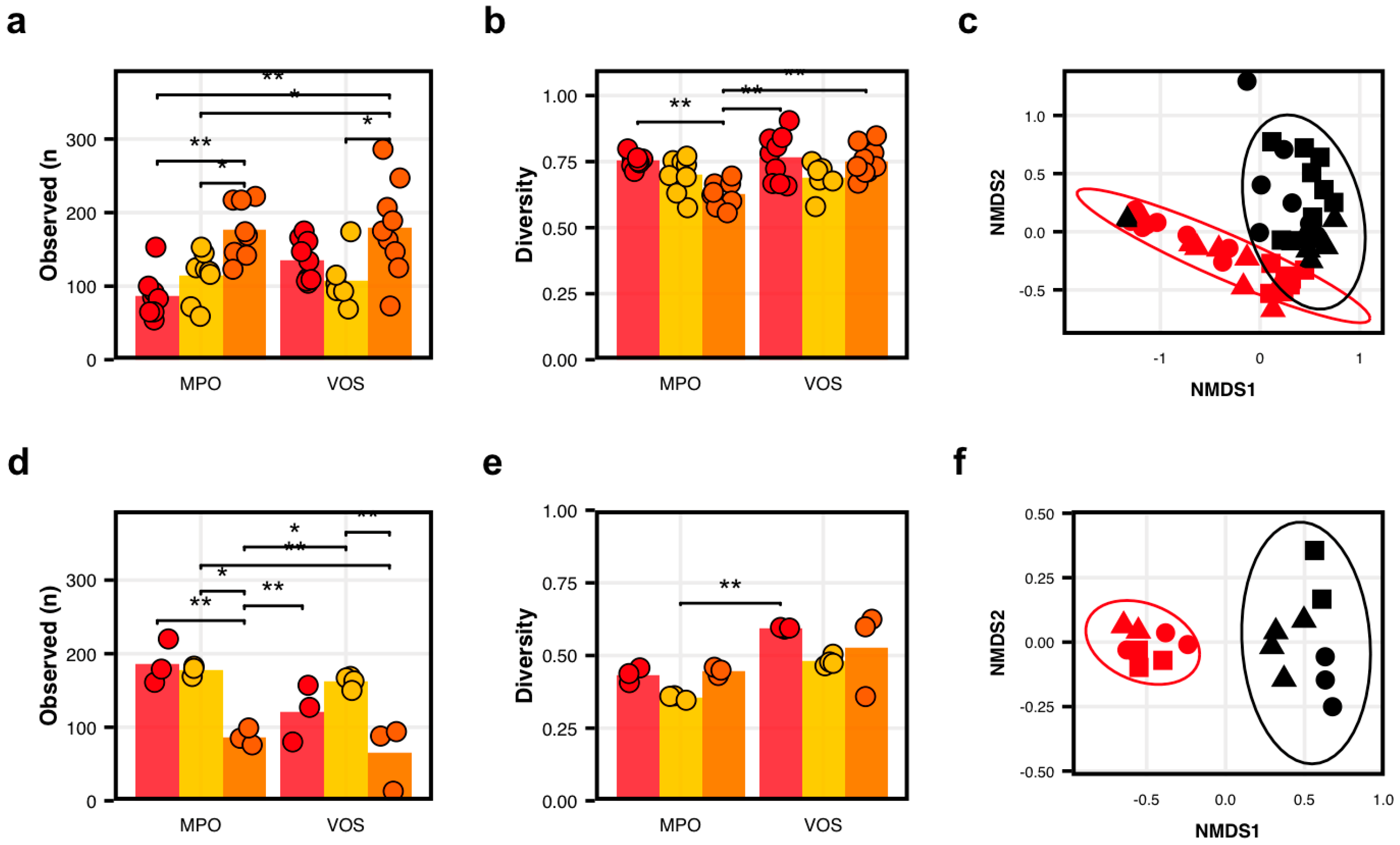
3.1. Bacterial Communities Differ Among Apple Varietals and Between Orchards
3.2. Fungal Communities Differ Among Apple Varietals and Between Orchards
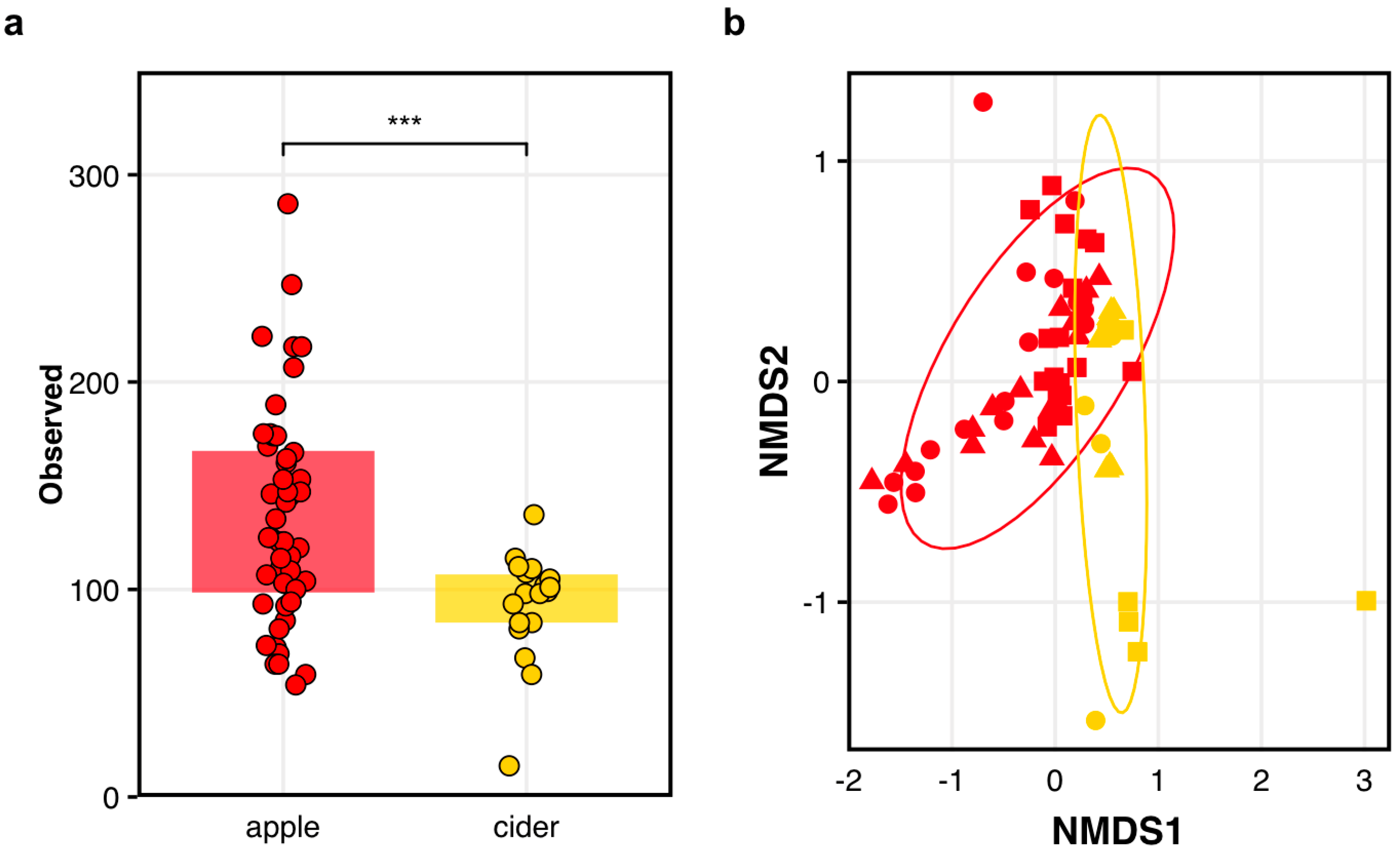
3.3. Bacterial Communities in Fermented Cider Differ Among Varietals and Between Orchards
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; van der Putten, W.H. Going Back to the Roots: The Microbial Ecology of the Rhizosphere. Nat. Rev. Microbiol. 2013, 11, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The Rhizosphere Microbiome and Plant Health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; van der Lelie, D.; Zarraonaindia, I. Microbial Terroir for Wine Grapes. Proc. Natl. Acad. Sci. USA 2014, 111, 5–6. [Google Scholar] [CrossRef]
- Mas, A.; Portillo, M.C. Strategies for Microbiological Control of the Alcoholic Fermentation in Wines by Exploiting the Microbial Terroir Complexity: A Mini-Review. Int. J. Food Microbiol. 2022, 367, 109592. [Google Scholar] [CrossRef]
- Franco, G.C.; Leiva, J.; Nand, S.; Lee, D.M.; Hajkowski, M.; Dick, K.; Withers, B.; Soto, L.; Mingoa, B.-R.; Acholonu, M.; et al. Soil Microbial Communities and Wine Terroir: Research Gaps and Data Needs. Foods 2024, 13, 2475. [Google Scholar] [CrossRef]
- Silva, V.; Brito, I.; Alexandre, A. The Vineyard Microbiome: How Climate and the Main Edaphic Factors Shape Microbial Communities. Microorganisms 2025, 13, 1092. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Seguin, G. The Concept of Terroir in Viticulture. J. Wine Res. 2006, 17, 1–10. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Collins, T.S.; Masarweh, C.; Allen, G.; Heymann, H.; Ebeler, S.E.; Mills, D.A. Associations among Wine Grape Microbiome, Metabolome, and Fermentation Behavior Suggest Microbial Contribution to Regional Wine Characteristics. mBio 2016, 7, 10–1128. [Google Scholar] [CrossRef]
- Leff, J.W.; Fierer, N.; Angelo, T. Apple Orchard Microbiomes: Composition, Diversity, and Management. Appl. Environ. Microbiol. 2013, 79, 2632–2640. [Google Scholar]
- Lfattah, A.; Wisniewski, M.; Droby, S.; Schena, L. Spatial and Compositional Variation in the Fungal Communities of Organic and Conventionally Grown Apple Fruit at the Consumer Point-of-Purchase. Hortic. Res. 2016, 3, 16047. [Google Scholar]
- Knight, S.; Goddard, M.R. Quantifying Separation and Similarity in a Saccharomyces Cerevisiae Metapopulation. ISME J. 2015, 9, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Lea, A.G.H.; Drilleau, J.F. Cider-Making. In Fermented Beverage Production; Springer: Berlin/Heidelberg, Germany, 2003; pp. 59–87. [Google Scholar]
- Merwin, I.A.; Valois, S.; Padilla-Zakour, O.I. Cider Apples and Cider-Making Techniques in Europe and North America. In Horticultural Reviews; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2008; pp. 365–415. ISBN 9780470380147. [Google Scholar]
- Empire. Available online: https://www.applesfromny.com/varieties/empire/ (accessed on 5 May 2025).
- Golden Delicious. Available online: https://www.applesfromny.com/varieties/golden-delicious/ (accessed on 5 May 2025).
- Idared. Available online: https://www.applesfromny.com/varieties/idared/ (accessed on 5 May 2025).
- Lax, S.; Hampton-Marcell, J.T.; Gibbons, S.M.; Colares, G.B.; Smith, D.; Eisen, J.A.; Gilbert, J.A. Forensic Analysis of the Microbiome of Phones and Shoes. Microbiome 2015, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Walters, W.; Hyde, E.R.; Berg-Lyons, D.; Ackermann, G.; Humphrey, G.; Parada, A.; Gilbert, J.A.; Jansson, J.K.; Caporaso, J.G.; Fuhrman, J.A.; et al. Improved Bacterial 16S RRNA Gene (V4 and V4-5) and Fungal Internal Transcribed Spacer Marker Gene Primers for Microbial Community Surveys. mSystems 2016, 1, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Bokulich, N.A.; Mills, D.A. Improved Selection of Internal Transcribed Spacer-Specific Primers Enables Quantitative, Ultra-High-Throughput Profiling of Fungal Communities. Appl. Environ. Microbiol. 2013, 79, 2519–2526. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Glöckner, F.O.; Yilmaz, P.; Quast, C.; Gerken, J.; Beccati, A.; Ciuprina, A.; Bruns, G.; Yarza, P.; Peplies, J.; Westram, R.; et al. 25 Years of Serving the Community with Ribosomal RNA Gene Reference Databases and Tools. J. Biotechnol. 2017, 261, 169–176. [Google Scholar] [CrossRef]
- Abarenkov, K.; Nilsson, R.H.; Larsson, K.-H.; Taylor, A.F.S.; May, T.W.; Frøslev, T.G.; Pawlowska, J.; Lindahl, B.; Põldmaa, K.; Truong, C.; et al. The UNITE database for molecular identification and taxonomic communication of fungi and other eukaryotes: Sequences, taxa and classifications reconsidered. Nucleic Acids Res. 2024, 52, D791–D797. [Google Scholar] [CrossRef]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef]
- Pielou, E.C. The Measurement of Diversity in Different Types of Biological Collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A New Phylogenetic Method for Comparing Microbial Communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef]
- Oksanen, J.; Simpson, G.L.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package. In CRAN: Contributed Packages; CRAN: Windhoek, Namibia, 2001. [Google Scholar]
- Martinez Arbizu, P. PairwiseAdonis: Pairwise Multilevel Comparison Using Adonis. 2020. Available online: https://github.com/pmartinezarbizu/pairwiseAdonis (accessed on 5 May 2025).
- Liaw, A.; Wiener, M. Classification and Regression by RandomForest. Available online: https://journal.r-project.org/articles/RN-2002-022/RN-2002-022.pdf (accessed on 5 May 2025).
- Wickham, H.; Chang, W.; Henry, L.; Pedersen, T.L.; Takahashi, K.; Wilke, C.; Woo, K.; Yutani, H.; Dunnington, D.; van den Brand, T. Ggplot2: Create Elegant Data Visualisations Using the Grammar of Graphics. In CRAN: Contributed Packages; CRAN: Windhoek, Namibia, 2007. [Google Scholar]
- Miura, T.; Sánchez, R.; Castañeda, L.E.; Godoy, K.; Barbosa, O. Is Microbial Terroir Related to Geographic Distance between Vineyards? Environ. Microbiol. Rep. 2017, 9, 742–749. [Google Scholar] [CrossRef] [PubMed]
- Fatemi, S.; Haelewaters, D.; Urbina, H.; Brown, S.; Houston, M.L.; Aime, M.C. Sporobolomyces lactucae sp. nov. (Pucciniomycotina, Microbotryomycetes, Sporidiobolales): An Abundant Component of Romaine Lettuce Phylloplanes. J. Fungi 2022, 8, 302. [Google Scholar] [CrossRef] [PubMed]
- Libkind, D.; Gadanho, M.; van Broock, M.; Sampaio, J.P. Sporidiobolus longiusculus sp. nov. and Sporobolomyces patagonicus sp. nov., Novel Yeasts of the Sporidiobolales Isolated from Aquatic Environments in Patagonia, Argentina. Int. J. Syst. Evol. Microbiol. 2005, 55, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Fazio, N.A.; Albertin, W.; Masneuf-Pomarede, I.; Randazzo, C.L.; Caggia, C. Structure of Culturable Indigenous Yeast Population and Genetic Diversity of Saccharomyces cerevisiae and Non-Saccharomyces Yeasts during Spontaneous Fermentation of Etna Vineyards Grapes. Int. J. Food Microbiol. 2025, 440, 111282. [Google Scholar] [CrossRef]
- Liu, Q.; Hao, N.; Mi, L.; Peng, S.; Marie-Colette, A.K.; Zhao, X.; Wang, J. From Microbial Communities to Aroma Profiles: A Comparative Study of Spontaneous Fermentation in Merlot and Cabernet Sauvignon Wines. Food Chem. X 2025, 26, 102317. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perron, G.G.; Miller, L.C.; Lau, P.; Davila-Santiago, L.; Jain, S.S. Orchards and Varietals Shape Apple and Cider Local Microbial Terroirs in the Hudson Valley of New York. Fermentation 2025, 11, 369. https://doi.org/10.3390/fermentation11070369
Perron GG, Miller LC, Lau P, Davila-Santiago L, Jain SS. Orchards and Varietals Shape Apple and Cider Local Microbial Terroirs in the Hudson Valley of New York. Fermentation. 2025; 11(7):369. https://doi.org/10.3390/fermentation11070369
Chicago/Turabian StylePerron, Gabriel G., Lee C. Miller, Pearson Lau, Lizbeth Davila-Santiago, and Swapan S. Jain. 2025. "Orchards and Varietals Shape Apple and Cider Local Microbial Terroirs in the Hudson Valley of New York" Fermentation 11, no. 7: 369. https://doi.org/10.3390/fermentation11070369
APA StylePerron, G. G., Miller, L. C., Lau, P., Davila-Santiago, L., & Jain, S. S. (2025). Orchards and Varietals Shape Apple and Cider Local Microbial Terroirs in the Hudson Valley of New York. Fermentation, 11(7), 369. https://doi.org/10.3390/fermentation11070369





