Solid-State Fermentation as a Biotechnological Tool to Reduce Antinutrients and Increase Nutritional Content in Legumes and Cereals for Animal Feed
Abstract
1. Introduction
2. Relevant Sections
2.1. Antinutrients in Legumes and Cereals
2.1.1. Glycosides
2.1.2. Protease Inhibitors
2.1.3. Phenols
2.2. Reduction of ANFs
2.3. Increase in Nutritional Quality
2.4. Improving Sensory Attributes
2.5. Bioproducts Obtained from SSF Applied in Animal Feed
2.6. Applications and Future Perspectives
3. Conclusions
4. Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SST | Solid-State Fermentation |
| ANFs | Antinutritional Factors |
| LAB | Lactic Acid Bacteria |
References
- Halmemies-Beauchet, A.F.; Rinne, M.; Lamminen, M.; Mapato, C.; Ampapon, T.; Wanapat, M.; Vanhatalo, A. Review: Alternative and novel feeds for ruminants: Nutritive value, product quality and environmental aspects. Animal 2018, 12 (Suppl. S2), s295–s309. [Google Scholar] [CrossRef] [PubMed]
- Fritz, M.; Schiefer, G. Food chain management for sustainable food system development: A European research agenda. Agribusiness 2008, 24, 440–452. [Google Scholar] [CrossRef]
- Gonçalves, M.L.M.B.B.; Máximo, G.J. Circular economy in the food chain: Production, processing and waste management. Circ. Econ. Sustain. 2023, 3, 1405–1423. [Google Scholar] [CrossRef]
- Available online: https://www.fao.org/food-safety/background/en/ (accessed on 12 February 2025).
- Available online: www.wfp.org (accessed on 23 January 2025).
- Goenaga, I.; García-Rodríguez, A.; Goiri, I.; León-Ecay, S.; De Las Heras, J.; Aldai, N.; Insausti, K. Vegetable by-products as alternative and sustainable raw materials for ruminant feeding: Nutritive evaluation and their inclusion in a novel ration for calf fattening. Animals 2023, 13, 1391. [Google Scholar] [CrossRef]
- Neethirajan, S.; Kemp, B. Digital Livestock Farming. Sens. Biosens. Res. 2021, 32, 100408. [Google Scholar] [CrossRef]
- Oyerinde, A.M.; Oyun, M.B.; Adeoye, B.O.; Olasupo, O.A.; Ogundipe, G.; Jimoh, B.A.; Oladimeji, O.B.; Ayankanmi, M.O.; Adeagbo, D.O.; Ajiboye, S.D. Determination of nutrients, antinutrients and antioxidants concentrations in some edible forest vegetables in Ondo and Oyo State, south western Nigeria. Niger. J. Nutr. Sci. 2023, 44, 212–222. [Google Scholar]
- Rajat, P.S.; Pradeep, K.S.; Rasna, G.; Ram, L.S. Chapter 2: Biotechnological Tools to Enhance Sustainable Production. In Biotechnology for Sustainable Agriculture Emerging Approaches and Strategies; Singh, R.L., Mondal, S., Eds.; Elsevier Inc. Woodhead Publishing: Amsterdam, The Netherlands, 2018; pp. 19–66. [Google Scholar] [CrossRef]
- Tyczewska, A.; Twardowski, T.; Woźniak-Gientka, E. Agricultural biotechnology for sustainable food security. Trends Biotechnol. 2023, 41, 331–341. [Google Scholar] [CrossRef]
- Doblado, R.; Frias, J.; Muñoz, R.; Vidal-Valverde, C. Fermentation of Vigna sinensis var. carilla flours by natural microflora and Lactobacillus species. J. Food Prot. 2003, 66, 2313–2320. [Google Scholar] [CrossRef]
- Bartkiene, E.; Krungleviciute, V.; Juodeikiene, G.; Vidmantiene, D.; Maknickiene, Z. Solid state fermentation with lactic acid bacteria to improve the nutritional quality of lupin and soya bean. J. Sci. Food Agric. 2015, 95, 1336–1342. [Google Scholar] [CrossRef]
- Rui, X.; Wang, M.; Zhang, Y.; Chen, X.; Li, L.; Liu, Y.; Dong, M. Optimization of soy solid-state fermentation with selected lactic acid bacteria and the effect on the anti-nutritional components. J. Food Process. Preserv. 2017, 41, e13290. [Google Scholar] [CrossRef]
- Olaleye, H.T.; Oresanya, T.O.; Ogundipe, O.O. Comparative study on proximate and antinutritional factors of dehulled and undehulled fermented Lyon bean (Mucuna cochinchinensis). Food Res. 2020, 4, 1611–1615. [Google Scholar] [CrossRef] [PubMed]
- Olagunju, O.F.; Ezekiel, O.O.; Ogunshe, A.O.; Oyeyinka, S.A.; Ijabadeniyi, O.A. Effects of fermentation on proximate composition, mineral profile and antinutrients of tamarind (Tamarindus indica L.) seed in the production of daddawa-type condiment. LWT 2018, 90, 455–459. [Google Scholar] [CrossRef]
- Curiel, J.A.; Coda, R.; Centomani, I.; Summo, C.; Gobbetti, M.; Rizzello, C.G. Exploitation of the nutritional and functional characteristics of traditional Italian legumes: The potential of sourdough fermentation. Int. J. Food Microbiol. 2015, 196, 51–61. [Google Scholar] [CrossRef]
- Álvarez, A.; Rache, L.Y.; Chaparro, S.; Brijaldo, M.H.; Borras, L.M.; Martínez, J.J. Solid-state fermentation of Mucuna deeringiana seed flour using Lacticaseibacillus rhamnosus. Fermentation 2024, 10, 396. [Google Scholar] [CrossRef]
- Georgana, L.E.; Ramos, J.A.; Salgado, S.; Aranda, E.M. Elaboración de un alimento basado en caña de azúcar a partir de la fermentación en estado sólido y con diferentes niveles de zeolitas. Rev. Cuba. Cienc. Agrí. 2012, 46, 159–163. [Google Scholar]
- Cubillos Orjuela, D.I.; Rodríguez Montaña, A.; Rache, L.Y.; Borrás Sandoval, L.M. Cereales como suplemento alimenticio procesado por fermentación en estado sólido. Cienc. Desarro. 2024, 15, 55–63. [Google Scholar] [CrossRef]
- Das, D.N.; Paul, D.; Mondal, S. Chapter thirteen—Role of biotechnology on animal breeding and genetic improvement. In Emerging Issues in Climate Smart Livestock Production: Biological Tolos and Techniques; Mondal, S., Singh, R.L., Eds.; Academic Press: Amsterdam, The Netherlands, 2022; pp. 317–337. [Google Scholar] [CrossRef]
- Markowiak, P.; Ṡliżewska, K. The role of probiotics, prebiotcis and synbiotics in animal nutrition. Gut. Pathog. 2018, 6, 21. [Google Scholar] [CrossRef]
- Soussi, A.; Zero, E.; Sacile, R.; Trinchero, D.; Fossa, M. Smart sensors and smart data for precision agriculture: A review. Sensors 2024, 24, 2647. [Google Scholar] [CrossRef]
- Zou, X.; Liu, M.; Li, X.; Pan, F.; Wu, X.; Fang, X.; Zhou, F.; Peng, W.; Tian, W. Applications of insect nutrition resources in animal production. J. Agric. Food Res. 2024, 15, 100966. [Google Scholar] [CrossRef]
- Jhansi Rani, S.; Usha, R. Transgenic plants: Types, benefits, public concerns and future. J. Pharm. Res. 2013, 6, 879–883. [Google Scholar] [CrossRef]
- Hussain, A.; Iqbal, K.; Aziem, S.; Mahato, P.; Negi, A.K. A review on the sciencie of growing crops without soil (Soilless culture)—A novel alternative for growing crops. Int. J. Agric. Crop Sci. 2014, 7, 833–842. [Google Scholar]
- Rubio, L.A.; Molina, E. Las leguminosas en alimentación animal. Arbor Cienc. Pensam. Cult. 2016, 192, a315. [Google Scholar] [CrossRef]
- Mohan, V.R.; Tresina, P.S.; Daffodil, E.D. Antinutritional factors in legume seeds: Characteristics and determination. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic press: Amsterdam, The Netherlands, 2016; pp. 211–220. [Google Scholar] [CrossRef]
- Arsov, A.; Tsigoriyna, L.; Batovska, N.; Mu, W.; Zhang, W.; Petrov, K.; Petrova, P. Bacterial degradation of antinutrients in foods: The genomic insight. Foods 2024, 13, 2408. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Odeh, F.; Jaber, A.M.; Al Sulaibi, M.A.M.; Alshaer, W.; Al Bawab, A.; Mubarak, M.S. Chapter 12—Phytosomes: A modernistic approach to the delivery of herbal drugs. In Advanced and Modern Approaches for Drug Delivery; Nayak, A.K., Md Hasnain, S., Laha, B., Maiti, S., Eds.; Academic Press: Amsterdam, The Netherlands, 2023; pp. 301–355. [Google Scholar] [CrossRef]
- Sandeep, S.G. Chapter 12—Triterpenoids: Structural diversity, biosynthetic pathway, and bioactivity. In Studies in Natural Products Chemistry; Rahman, A., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 411–461. [Google Scholar] [CrossRef]
- Pathaw, N.; Sarda, D.K.; Sapam, R.; Sanasam, J.; Monteshori, S.; Phurailatpam, S.; Chandrajini, D.H.; Tampakleima, C.W.; Wangkhem, B.; Loya, M.N. A comparative review on the anti-nutritional factors of herbal tea concoctions and their reduction strategies. Front. Nutr. 2022, 9, 988964. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Kaur, R.; Kumar, S.; Kumar, S.R.; Sharma, S.; Pawde, V.S.; Kumar, V. Saponins: A concise review on food related aspects, applications and health implications. Food Chem. Adv. 2023, 2, 100191. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T. Plant food anti-nutritional factors and their reduction strategies: An overview. Food Prod. Process. Nutr. 2020, 2, 6. [Google Scholar] [CrossRef]
- Dórea, J.G. Cassava cyanogens and fish mercury are high but safely consumed in the diet of native Amazonians. Ecotoxicol. Environ. Saf. 2004, 57, 248–256. [Google Scholar] [CrossRef]
- Das, G.; Sharma, A.; Sarkar, P.K. Conventional and emerging processing techniques for the post-harvest reduction of antinutrients in edible legumes. Appl. Food Res. 2022, 2, 100112. [Google Scholar] [CrossRef]
- Wezynfeld, N.E.; Frączyk, T.; Bal, W. Metal assisted peptide bond hydrolysis: Chemistry, biotechnology and toxicological implications. Coord. Chem. Rev. 2016, 327–328, 166–187. [Google Scholar] [CrossRef]
- Marathe, K.R.; Patil, R.H.; Vishwakarma, K.S.; Chaudhari, A.B.; Maheshwari, V.L. Protease inhibitors and their applications: An overview. Stud. Nat. Prod. Chem. 2019, 62, 211–242. [Google Scholar] [CrossRef]
- Mandala, D.; Thompson, W.A.; Watts, P. Synthesis routes to anti-HIV drugs. Tetrahedron 2016, 72, 3389–3420. [Google Scholar] [CrossRef]
- Ribeiro, M.M.S.; Viganó, J.; Meireles, M.A.A.; Veggi, P.C. Recent advances in the recovery of tannins from natural matter. Stud. Nat. Prod. Chem. 2022, 75, 289–328. [Google Scholar] [CrossRef]
- De Deurwaerdère, P.; Di Giovanni, G.; Millan, M.J. Expanding the repertoire of L-DOPA’s actions: A comprehensive review of its functional neurochemistry. Prog. Neurobiol. 2017, 151, 57–100. [Google Scholar] [CrossRef]
- Clark, W.G.; Vivonia, C.A.; Menon, M.K.; Kurtz, S.M.; Mattis, P.A.; Suzuki, Y.; Page, J.G.; Cotzias, G.C. The acute toxicity of L-dopa. Toxicol. Appl. Pharmacol. 1974, 28, 1–7. [Google Scholar] [CrossRef]
- Gupta, P.K. Chapter 15—Veterinary toxicology. In Illustrated Toxicology; Gupta, P.K., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 427–517. [Google Scholar] [CrossRef]
- Silberhorn, E.M. Oxalates. In Encyclopedia of Toxicology, 2nd ed.; Wexlwe, P., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2005; pp. 320–322. [Google Scholar] [CrossRef]
- Salgado, N.; Silva, M.A.; Figueira, M.E.; Costa, H.S.; Albuquerque, T.G. Oxalate in foods: Extraction conditions, analytical methods, occurrence, and health implications. Foods 2023, 12, 3201. [Google Scholar] [CrossRef]
- Sapna; Singh, B. Phytase production by Aspergillus oryzae in solid-state fermentation and its applicability in dephytinization of wheat Ban. Appl. Biochem. Biotechnol. 2014, 173, 1885–1895. [Google Scholar] [CrossRef]
- Joudaki, H.; Aria, N.; Moravej, R.; Rezaei Yazdi, M.; Emami-Karvani, Z.; Hamblin, M.R. Microbial phytases: Properties and applications in the food industry. Curr. Microbiol. 2023, 80, 374. [Google Scholar] [CrossRef]
- López-Moreno, M.; Garcés-Rimón, M.; Miguel, M. Antinutrients: Lectins, goitrogens, phytates and oxalates, friends or foe? J. Funct. Foods 2022, 89, 104938. [Google Scholar] [CrossRef]
- Muleta, C.E. The major potential of non-conventional feed resources in poultry nutrition in Ethiopia: A review. Vet. Anim. Sci. 2024, 12, 68–77. [Google Scholar] [CrossRef]
- Biswas, D.; Ranjan, P.S. Nutritional profile, antinutritional factors, and digestibility of proteins in Non-conventional leaf protein sources. ACS Food Sci. Technol. 2024, 4, 1017–1029. [Google Scholar] [CrossRef]
- Wanjekeche, E.; Wakasa, V.; Mureithi, J.G. Effect of germination, alkaline and acid soaking and boiling on the nutritional value of mature and immature Mucuna (Mucuna pruriens) beans. Trop. Subtrop. Agroecosyst. 2003, 1, 183–192. [Google Scholar]
- Olukomaiya, O.O.; Adiamo, O.Q.; Fernando, W.C.; Mereddy, R.; Li, X.; Sultanbawa, Y. Effect of solid-state fermentation on proximate composition, anti-nutritional factor, microbiological and functional properties of lupin flour. Food Chem. 2020, 315, 126238. [Google Scholar] [CrossRef]
- Acuña, S.P.C.; Torres, I.D.A.; González, J.H.G. Reducción de factores antinutricionales de la semilla de vitabosa (Mucuna deeringiana) mediante procesos físico-químicos. Rev. Fac. Nac. Agron. Medellin. 2009, 62, 5157–5164. [Google Scholar]
- Das, K.; Choudhary, R.; Thompson-Witrick, K.A. Effects of new technology on the current manufacturing process of yogurt-to increase the overall marketability of yogurt. Food Sci. Technol. 2019, 108, 69–80. [Google Scholar] [CrossRef]
- Endalew, H.W.; Atlabachew, M.; Karavoltsos, S.; Sakellari, A.; Farshard, A.M.; Allen, L.; Griffiths, H.; Zoumpoullakis, P.; Kanellou, A.; Fenta, Y.T.; et al. Effect of fermentation on nutrient composition, antinutrients, and mineral bioaccessibility of finger millet based Injera: A traditional Ethiopian food. Food Res. Int. 2024, 190, 114635. [Google Scholar] [CrossRef]
- Behera, S.S.; Ray, R.C. Chapter 12—Forest bioresources for bioethanol and biodiesel production with emphasis on Mohua (Madhuca latifolia L.) flowers and seeds. In Bioethanol Production from Food Crops; Ray, R.C., Ramachandran, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 233–247. [Google Scholar] [CrossRef]
- Lende, S.V.; Karemore, H.; Umekar, M.J. Review on production of citric acid by fermentation technology. GSC Biol. Pharm. Sci. 2021, 17, 085–093. [Google Scholar] [CrossRef]
- Yafetto, L. Application of solid-state fermentation by microbial biotechnology for bioprocessing of agro-industrial wastes from 1970 to 2020: A review and bibliometric análisis. Heliyon 2022, 8, e09173. [Google Scholar] [CrossRef]
- Thomas, L.; Larroche, C.; Pandey, A. Current developments in solid-state fermentation. Biochem. Eng. J. 2013, 81, 146–161. [Google Scholar] [CrossRef]
- Vigoroso, L.; Cavallo, E.; Pampuro, N. Recycling of agro-food and urban wastes according to the circular economy and sustainable paradigms. Agronomy 2024, 14, 1465. [Google Scholar] [CrossRef]
- Vuyst, L.D.; Leroy, F. Bacteriocins from lactic acid bacteria: Production, purification, and food applications. Microb. Physiol. 2007, 13, 194–199. [Google Scholar] [CrossRef]
- Beshkova, D.; Frengova, G.I. Bacteriocins from lactic acid bacteria: Microorganisms of potential biotechnological importance for the dairy industry. Eng. Life Sci. 2012, 12, 419–432. [Google Scholar] [CrossRef]
- Borrás-Sandoval, L.M.; Valiño-Cabrera, E.C.; Rodríguez-Molano, C.E. Preparado microbiano con actividad ácido láctica como acelerante biológico en los procesos de fermentación para alimento animal. Cienc. Agric. 2017, 14, 7–13. [Google Scholar] [CrossRef]
- Ramachandra, S.G.; Motiram, H.P.; Venkateswaran, G. Identification and characterization of a calcium dependent bacillopeptidase from Bacillus subtilis CFR5 with novel kunitz trypsin inhibitor degradation activity. Food Res. Int. 2017, 103, 263–272. [Google Scholar] [CrossRef]
- Caminero, A.; McCarville, J.L.; Zevallos, V.F.; Pigrau, M.; Yu, X.B.; Jury, J.; Galipeau, H.J.; Clarizio, A.V.; Casqueiro, J.; Murray, J.A.; et al. Lactobacilli degrade wheat amylase trypsin inhibitors to reduce intestinal dysfunction induced by immunogenic wheat proteins. Gastroenterology 2019, 156, 2266–2280. [Google Scholar] [CrossRef]
- Gao, Y.L.; Wang, C.S.; Zhu, Q.H.; Qian, G.Y. Optimization of solid-state fermentation with Lactobacillus brevis and Aspergillus oryzae for trypsin inhibitor degradation in soybean meal. J. Integr. Agric. 2013, 12, 869–876. [Google Scholar] [CrossRef]
- Hoffmann, E.M.; Muetzel, S.; Becker, K. The fermentation of soybean meal by rumen microbes in vitro reveals different kinetic features for the inactivation and the degradation of trypsin inhibitor protein. Anim. Feed Sci. Technol. 2003, 106, 189–197. [Google Scholar] [CrossRef]
- Priyadarshini, A.; Madhusmita, M.; Ranjan, P.; Mahanty, B.; Kumar, N. Kinetic modelling and process engineering of phenolics microbial and enzymatic biodegradation: A current outlook and challenges. J. Water Process. Eng. 2021, 44, 102421. [Google Scholar] [CrossRef]
- Sharma, K.P. Tannin degradation by phytopathogen’s tannase: A Plant’s defense perspective. Biocatal. Agric. Biotechnol. 2019, 21, 101342. [Google Scholar] [CrossRef]
- Sigona, C.; Bardi, A.; Modeo, L.; Mori, G.; Potekhin, A.; Verni, F.; Munz, G.; Petroni, G. Role of bacterivorous organisms on fungal-based systems for natural tannin degradation. Heliyon 2020, 6, e03604. [Google Scholar] [CrossRef]
- Polanowska, K.; Szwengiel, A.; Kuligowski, M.; Nowak, J. Degradation of pyrimidine glycosides and L-DOPA in the Faba bean by Rhizopus oligosporus. LWT 2020, 127, 109353. [Google Scholar] [CrossRef]
- Espinosa-Páez, E.; Alanis-Guzmán, M.G.; Hernández-Luna, C.; Báez-González, J.; Amaya-Guerra, C.; Andrés-Grau, A. Increasing antioxidant activity and protein digestibility in Phaseolus vulgaris and Avena sativa by fermentation with the Pleurotus ostreatus fungus. Molecules 2017, 22, 2275. [Google Scholar] [CrossRef]
- Raghuwanshi, S.; Misra, S.; Saxena, R.K. Treatment of wheat straw using tannase and white-rot fungus to improve feed utilization by ruminants. J. Anim. Sci. Biotechnol. 2014, 5, 13. [Google Scholar] [CrossRef]
- Kupski, L.; Cipolatti, E.; da Rocha, M.; Oliveira, M.d.S.; Souza-Soares, L.d.A.; Badiale-Furlong, E. Solid-state fermentation for the enrichment and extraction of proteins and antioxidant compounds in rice bran by Rhizopus oryzae. Braz. Arch. Biol. Technol. 2012, 55, 937–942. [Google Scholar] [CrossRef]
- Xu, L.N.; Guo, S.; Zhang, S. Effects of solid-state fermentation with three higher fungi on the total phenol contents and antioxidant properties of diverse cereal grains. FEMS Microbiol. Lett. 2018, 365, fny163. [Google Scholar] [CrossRef]
- Adeyinka, A.J.; Berka, N.P.; Gbashi, S.; Bamikole, O.A.; Mayowa, O.O.; Adeoye, O.S.; Ayodeji, A.O. Fermentation of cereals and legumes: Impact on nutritional constituents and nutrient bioavailability. Fermentation 2022, 8, 63. [Google Scholar] [CrossRef]
- Parra, R.A. Review. Bacterias ácido-lácticas: Papel funcional en los alimentos. Rev. Bio. Agro. 2010, 8, 93–105. [Google Scholar]
- Anumudu, C.K.; Miri, T.; Onyeaka, H. Multifunctional applications of lactic acid bacteria: Enhancing safety, quality, and nutritional value in foods and fermented beverages. Foods 2024, 13, 3714. [Google Scholar] [CrossRef]
- Abedi, E.; Bagher, S.M. Lactic acid production—Producing microorganisms and substrates sources-state of art. Heliyon 2020, 6, e04974. [Google Scholar] [CrossRef]
- Matthews, A.; Grimaldi, A.; Walker, M.; Bartowsky, E.; Grbin, P.; Jiranek, V. Lactic acid bacteria as a potential source of enzymes for use in vinification. Appl. Environ. Microbiol. 2004, 70, 5715–5731. [Google Scholar] [CrossRef]
- Ng’ong’ola-Manani, T.A.; Marit, H.O.; Mbachi, A.M.; Wicklund, T. Metabolite changes during natural and lactic acid bacteria fermentations in pastes of soybeans and soybean–maize blends. Food Sci. Nutr. 2014, 2, 768–785. [Google Scholar] [CrossRef]
- Zhang, S.; Shi, Y.; Zhang, S.; Shang, W.; Gao, X.; Wang, H. Whole soybean as probiotic lactic acid bacteria carrier food in solid-state fermentation. Food Control. 2014, 41, 1–6. [Google Scholar] [CrossRef]
- Dai, C.; Hou, Y.; Xu, H.; Huang, L.; Dabbour, M.; Mintah, B.K.; He, R.; Ma, H. Effect of solid-state fermentation by three different Bacillus species on composition and protein structure of soybean meal. J. Sci. Food Agric. 2022, 102, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Terefe, Z.; Omwamba, M.; Nduko, J. Effect of solid state fermentation on proximate composition, antinutritional factors and in vitro protein digestibility of maize flour. Food Sci. Nutr. 2021, 9, 6343–6352. [Google Scholar] [CrossRef]
- Xu, R.; Han, J.; Tian, T.; Zhu, N.; Chen, Y.; Wangkahart, E.; Zhang, Z.; Liu, J.; Yu, D.; Wei, J.; et al. Comparative study of solid-state fermentation with Lactobacillus plantarum versus Saccharomycopsis fibuligera on anti-nutritional factors, nutritional values and antioxidant activity of faba bean (Vicia faba L.) meal. Int. J. Food Sci. Technol. 2023, 59, 288–298. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, Z.; Yu, W.; Zheng, L.; Li, L.; Gu, W.; Xu, H.; Wei, B.; Yan, X. Nutritional quality improvement of soybean meal by Bacillus velezensis and Lactobacillus plantarum during two-stage solid-state fermentation. AMB Express 2021, 11, 23. [Google Scholar] [CrossRef]
- Yao, Y.; Hong-ya, L.; Li, J.; Zhu, B.; Gao, T. Anaerobic solid-state fermentation of soybean meal with Bacillus sp. to improve nutritional quality. Front. Nutr. 2021, 8, 706977. [Google Scholar] [CrossRef]
- Qi, N.; Zhan, X.; Milmine, J.; Chang, K.-H.; Li, J. A novel thermophilic strain of Bacillus subtilis with antimicrobial activity and its potential application in solid-state fermentation of soybean meal. Microbiol. Spectr. 2024, 12, e0278423. [Google Scholar] [CrossRef]
- Adegbehingbe, K.T.; Daramola, O.B. Fermentation of single and mixed substrates of lima bean seeds (Phaseolus lunatus) and African locust bean seeds (Parkia biglobosa). Ife J. Sci. 2019, 21, 333–343. [Google Scholar] [CrossRef]
- Martín-Cabrejas, M.A. Chapter 1. Legumes: An Overview. In Nutritional Quality, Processing and Potential Health Benefits; Martín-Cabrejas, M.A., Ed.; The Royal Society of Chemistry: London, UK, 2019; pp. 1–18. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Z.; Jiang, Q.; Roubík, H.; Xu, Q.; Cai, M.; Yang, K.; Sun, P. Fungal solid-state fermentation of crops and their by-products to obtain protein resources: The next frontier of food industry. Trends Food Sci. Technol. 2023, 138, 628–644. [Google Scholar] [CrossRef]
- Starzyńska-Janiszewska, A.; Stodolak, B.; Wikiera, A. Proteolysis in tempeh-type products obtained with rhizopus and Aspergillus strains from grass pea (Lathyrus sativus) seeds. Acta Sci. Pol. Technol. Aliment. 2015, 14, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-García, J.; Muñoz-Pina, S.; García-Hernández, J.; Heredia, A.; Andrés, A. Impact of air-drying temperature on antioxidant properties and ACE-inhibiting activity of fungal fermented lentil flour. Foods 2023, 12, 999. [Google Scholar] [CrossRef] [PubMed]
- Starzyńska-Janiszewska, A.; Stodolak, B.; Mickowska, B. Effect of controlled lactic acid fermentation on selected bioactive and nutritional parameters of tempeh obtained from unhulled common bean (Phaseolus vulgaris) seeds. J. Sci. Food Agric. 2014, 94, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Tie, Y.; Li, L.; Liu, J.; Liu, C.; Fu, J.; Xiao, X.; Wan, G.; Wang, J. Two-step biological approach for treatment of rapeseed meal. J. Food Sci. 2020, 85, 340–348. [Google Scholar] [CrossRef]
- Gbenle, J.; Mert, M.; Phasha, N.; Madibana, M.; Manyeula, F.; Bamidele, O.; Toefy, R.; Dibakoane, S.; Mlambo, V. Fungal-mediated solid-state fermentation ameliorates antinutritional factors but does not improve in vitro digestibility of marama (Tylosema esculentum) beans. Future Foods. 2025, 11, 100664–100672. [Google Scholar] [CrossRef]
- Pascual, M.; Painefilú, J.; Herbert, L.; Campos, M.; Jurski, V.; Luquet, C. Nutritional improvement of wheat grains and soybeans by solid-state fermentation with Pleurotus ostreatus mycelium. Innov. Food Sci. Emerg. Technol. 2025, 102, 104021. [Google Scholar] [CrossRef]
- Asensio-Grau, A.; Calvo-Lerma, J.; Heredia, A.; Andrés, A. Enhancing the nutritional profile and digestibility of lentil flour by solid state fermentation with Pleurotus ostreatus. Food Funct. 2020, 11, 7905–7912. [Google Scholar] [CrossRef]
- Pérez-Velasco, R.; Gómez-Gil, B.; Martínez-Montaño, E.; González-Córdova, A.F.; Hernández, C. Nutritional attributes and microbial metagenomic profile during solid-state fermentation of soybean meal inoculated with Lactobacillus acidophilus under non-sterile conditions. J. Sci. Food Agric. 2024, 104, 8219–8229. [Google Scholar] [CrossRef]
- Gautheron, O.; Nyhan, L.; Torreiro, M.G.; Tlais, A.Z.A.; Cappello, C.; Gobbetti, M.; Hammer, A.K.; Zannini, E.; Arendt, E.K.; Sahin, A.W. Exploring the impact of solid-state fermentation on fava bean flour: A comparative study of Aspergillus oryzae and Rhizopus oligosporus. Foods 2024, 13, 2922. [Google Scholar] [CrossRef]
- Patrick, N.K.; Martial-Didier, A.K.; Florent, N.K.; Constant, A.K.; Kablan, T. Effect of spontaneous fermentation time on physicochemical, nutrient, anti-nutrient and microbiological composition of lima bean (Phaseolus lunatus) flour. J. Appl. Biosci. 2021, 162, 16707–16725. [Google Scholar] [CrossRef]
- Nadhifa, D.G.; Mahendradatta, M.; Bastian, F. Effect of spontaneous fermentation and germination on antinutrients, total ash, and iron contents of several types of legumes. Acta Aliment. 2025, 54, 109–119. [Google Scholar] [CrossRef]
- Chen, W.; Xie, C.; He, Q.; Sun, J.; Bai, W. Improvement in color expression and antioxidant activity of strawberry juice fermented with lactic acid bacteria: A phenolic-based research. Food Chem. 2023, 17, 100535. [Google Scholar] [CrossRef] [PubMed]
- Vanbelle, M.; Teller, E.; Focant, M. Probiotics in animal nutrition: A review. Archiv. Tierernaehrung 1989, 40, 543–567. [Google Scholar] [CrossRef]
- Chaucheyras-Durand, F.; Durand, H. Probiotics in animal nutrition and health. Benef. Microbes 2010, 1, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Bernardeau, M.; Guguen, M.; Vernoux, J.P. Beneficial lactobacilli in food and feed: Long-term use, biodiversity and proposals for specific and realistic safety assessments. FEMS Microbiol. Rev. 2006, 30, 487–513. [Google Scholar] [CrossRef]
- Gaggìa, F.; Mattarelli, P.; Biavati, B. Probiotics and prebiotics in animal feeding for safe food production. Int. J. Food Microbiol. 2010, 141, S15–S28. [Google Scholar] [CrossRef] [PubMed]
- Lutgendorff, F.; Akkermans, L.; Söderholm, J.D. The role of microbiota and probiotics in stress-induced gastro-intestinal damage. Curr. Mol. Med. 2008, 8, 282–298. [Google Scholar] [CrossRef]
- Fijan, S. Microorganisms with claimed probiotic properties: An overview of recent literatura. Int. J. Environ. Res. Public Health 2014, 11, 4745–4767. [Google Scholar] [CrossRef]
- Anadón, A.; Martínez-Larrañaga, M.R.; Ares, I.; Aránzazu, M.M. Chapter 55—Probiotics: Safety and toxicity considerations. In Nutraceuticals, Efficacy, Safety And Toxicity; Gupta, R.C., Ed.; Academic press: Cambridge, MA, USA, 2016; pp. 777–798. [Google Scholar] [CrossRef]
- Dupont, M.S.; Muzquiz, M.; Estrella, I.; Fenwick, G.R.; Price, K.R. Relationship between the sensory properties of lupin seed with alkaloid and tannin content. J. Sci. Food Agric. 1994, 65, 95–100. [Google Scholar] [CrossRef]
- Pfister, J.A.; Manners, G.D.; Gardner, D.R.; Price, K.W.; Ralphs, M.H. Influence of alkaloid concentration on acceptability of tall larkspur (Delphinium spp.) to cattle and sheep. J. Chem. Ecol. 1996, 22, 1147–1168. [Google Scholar] [CrossRef]
- Elsanhoty, R.M.; Al-Soqeer, A.A.; Ramadan, M.F. Effect of detoxification methods on the quality and safety of jojoba (Simmondsia chinensis) meal. J. Food Biochem. 2017, 41, e12400. [Google Scholar] [CrossRef]
- Zhang, D.; Ye, Y.; Wang, L.; Tan, B. Nutrition and sensory evaluation of solid-state fermented brown rice based on cluster and principal component analysis. Foods 2022, 11, 1560. [Google Scholar] [CrossRef] [PubMed]
- Vandenberghe, L.P.S.; Pandey, A.; Carvalho, J.C.; Letti, L.A.J.; Woiciechowski, A.L.; Karp, S.G.; Thomaz-Soccol, V.; MartínezBurgos, W.J.; Penha, R.O.; Herrmann, L.W.; et al. Solid-state fermentation technology and innovation for the production of agricultural and animal feed bioproducts. Syst. Microbiol. Biomanuf. 2021, 1, 142–165. [Google Scholar] [CrossRef]
- Hassaan, M.S.; Soltan, M.A.; Abdel-Moez, A.M. Nutritive value of soybean meal after solid state fermentation with Saccharomyces cerevisiae for Nile tilapia, Oreochromis niloticus. Anim. Feed Sci. Technol. 2015, 201, 89–98. [Google Scholar] [CrossRef]
- Poulsen, H.D.; Blaabjerg, K. Fermentation of rapeseed meal, sunflower meal and faba beans in combination with wheat bran increases solubility of protein and phosphorus. J. Sci. Food Agric. 2017, 97, 244–251. [Google Scholar] [CrossRef]
- Zhai, S.S.; Zhou, T.; Li, M.M.; Zhu, Y.W.; Li, M.C.; Feng, P.S.; Zhang, X.F.; Ye, H.; Wang, W.C.; Yang, L. Fermentation of flaxseed cake increases its nutritional value and utilization in ducklings. Poult. Sci. 2019, 98, 5636–5647. [Google Scholar] [CrossRef]
- Wang, C.C.; Lin, L.J.; Chao, Y.P.; Chiang, C.J.; Lee, M.T.; Chang, S.C.; Yu, B.; Lee, T.T. Antioxidant molecular targets of wheat bran fermented by white rot fungi and its potential modulation of antioxidative status in broiler chickens. Br. Poult. Sci. 2017, 58, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wang, W.; Li, J.; Li, H.; Gao, T.; Zhu, B. Anaerobic fermentation of soybean meal by Bacillus subtilis ED-3-7 and its effect on the intestinal microbial community of chicken. Poult. Sci. 2025, 104, 104564–104572. [Google Scholar] [CrossRef]
- Duodu, C.P.; Adjei-Boateng, D.; Edziyie, R.E.; Agbo, N.W.; Owusu-Boateng, G.; Larsen, B.K.; Skov, P.V. Processing techniques of selected oilseed by-products of potential use in animal feed: Effects on proximate nutrient composition, amino acid profile and antinutrients. Anim. Nutr. 2018, 4, 442–451. [Google Scholar] [CrossRef]
- Chebaibi, S.; Leriche Grandchamp, M.; Burgé, G.; Clément, T.; Allais, F.; Laziri, F. Improvement of protein content and decrease of anti-nutritional factors in olive cake by solid-state fermentation: A way to valorize this industrial by-product in animal feed. J. Biosci. Bioeng. 2019, 128, 384–390. [Google Scholar] [CrossRef]
- Chilakamarry, C.R.; Mimi, A.M.; Zularisam, A.W.; Sirohi, R.; Ahamad, K.I.; Ahmad, N.; Pandey, A. Advances in solid-state fermentation for bioconversion of agricultural wastes to value-added products: Opportunities and challenges. Bioresour. Technol. 2022, 343, 126065. [Google Scholar] [CrossRef]
- Castro, R.; Sato, H. Enzyme production by solid state fermentation: General aspects and an analysis of the physicochemical characteristics of substrates for agro-industrial wastes valorization. Waste Biomass Valori. 2015, 6, 1085–1093. [Google Scholar] [CrossRef]
- Hadush, M. Does it pay to switch from free grazing to stall feeding? impact of stall feeding practice on household welfare in tigrai ethiopia. Agric. Food Econ. 2021, 9, 4. [Google Scholar] [CrossRef]
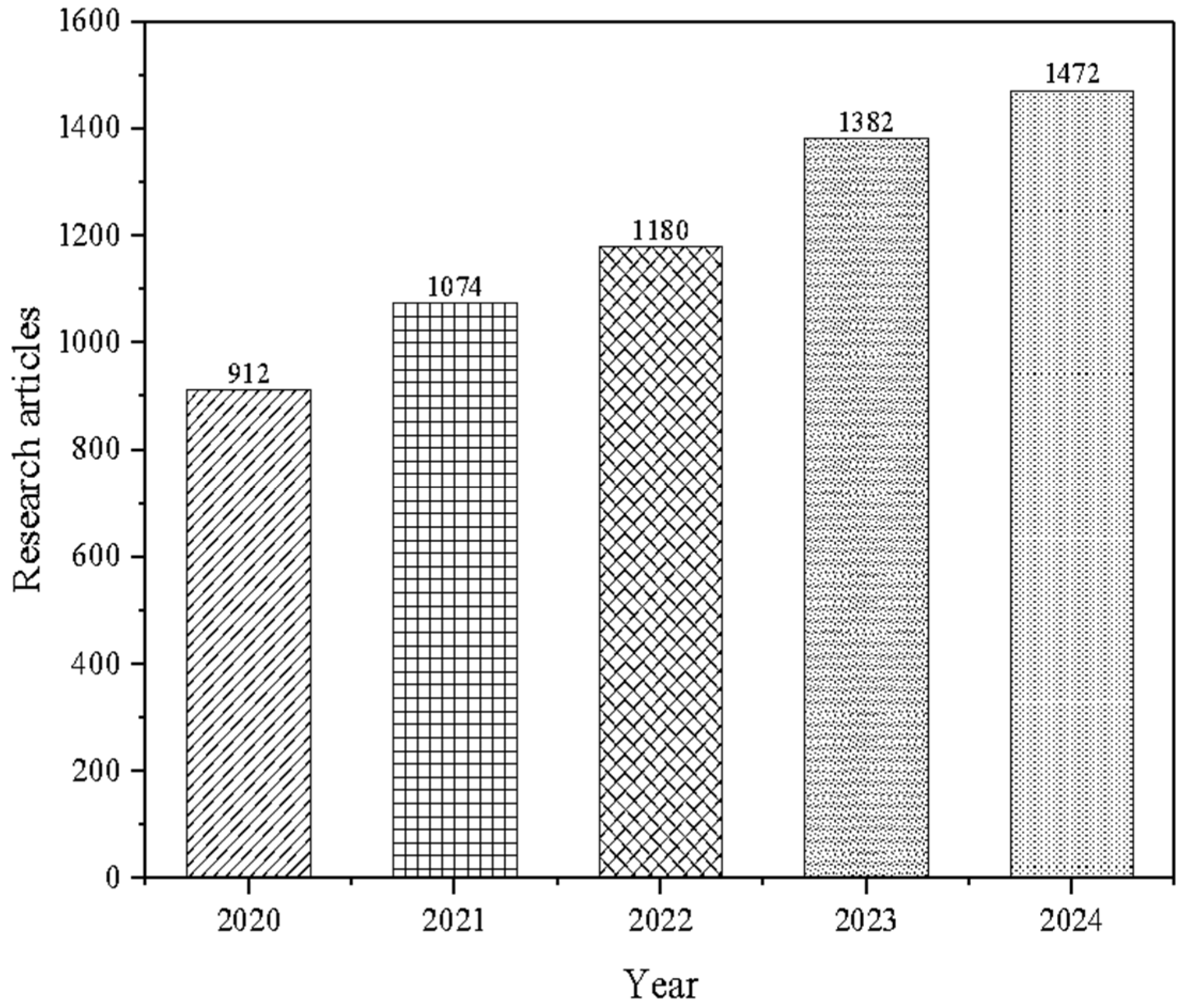
| Antinutrients Classification | Chemical structure | Glycosides | Saponins Cyanogens |
| Proteins | Protease inhibitors: Trypsin Lectins | ||
| Phenols | Tannins L-DOPA | ||
| Functional significance | Anti-minerals | Phytates Oxalates |
| Legume | Microorganism | Main Findings | Reference |
|---|---|---|---|
| Vigna sinnensis var | L. plantarum | ↓ Phytates (85%), ↓ TIs (50%) | [11] |
| Glycine max | L. plantarum | ↑ TPC (21%), ↓ Phytic acid (55%), ↓ Saponins (4%), ↓ TIs (95%) | [13] |
| Mucuna cochinchinensis | L. plantarum | ↓ Phytates (32%), ↓ Tannins (72%) | [14] |
| Tamarindus indica L. | L. licheniformis | ↓ TPC (50%), ↓ TIs (86%), ↓ Tannins (75%) | [15] |
| Phaseolus vulgaris Cicer arietinum Lathyrus satires Lens culinaris Pisum sativum | L. plantarum L. brevis | ↑ TPC (20–70%), ↓ Tannins (18%) | [16] |
| Mucuna deeringiana | L. rhamnosus | ↓ TPC (51%), ↓ L-DOPA (91%), ↓ Tannins (97%) | [17] |
| Glycine max | Bifidobacterium animalis 937, Lactobacillus casei Zhang and Lactobacillus plantarum P-8 mixed with Bacillus subtilis natto | ↑ Free amino nitrogen (100%), ↑ peptide with molecular weight less than 1000 Da increased (30.7–81.3%) | [81] |
| Glycine max | Bacillus licheniformis YYC4, Geobacillus stearothermophilus A75, Bacillus subtilis 10160 | ↑ Protein (12–18%), ↓ TIs (38–74%) | [82] |
| Zea mays | Lactobacillus plantarum and Saccharomyces cerevisiae | ↑ Protein (38–55%), ↓ Phytate (66%), ↓ Tannins (75%), ↓ Trypsin inhibitor (64%) | [83] |
| Vicia faba L. | Lactobacillus plantarum | ↓ Tannin (68–77%), ↓ phytic acid (18–35%), ↑ Protein (10–11%) | [84] |
| Glycine max | Bacillus velezensis Lactobacillus plantarum | ↑ Protein (47–52%), ↑ amino acid profile (5%), ↓ glycinin (79%), ↓ β-conglycinin (73%) | [85] |
| Glycine max | Bacillus sp. | ↑ Protein (11–13%), ↓ glycinin (82%), ↓ β-conglycinin (88%), ↓ ITs (99%), ↓ phytic acid (72%) | [86] |
| Glycine max | Bacillus subtilis | ↓ ITs (99%), ↓ phytic Effect of solid-state fermentation on proximate composition, acid (20–38%), ↑ crude protein (49–51%) | [87] |
| Phaseolus lunatus, Parkia biglobosa | Bacillus subtilis, B. polymyxa, Lactobacillus casei, Leuconostoc mesenteroides, Micrococcus rubens and Staphylococcus aureus | ↑ Protein (4–11%), ↓ Phytates (40–47%), ↓ Tannins (48–56%) | [88] |
| Faba bean meal | Lactobacillus plantarum | ↑ Protein (10%), ↑ in vitro protein digestibility (4%), ↓ Tannins (80%), ↓ phytic acid (18%) | [89] |
| Legume | Microorganism | Main Findings | Reference |
|---|---|---|---|
| Lathyrus sativus | R. microsporus var. Chinensis, A. oryzae | ↑ Free amino acids (12%) | [91] |
| Lens culinaris | Pleurotus ostreatus | ↓ Phytic acid (88%), ↓ TPC (33%) | [92] |
| Phaseolus vulgaris | Lactobacillus plantarum | ↓ Tannins (20%), ↑ protein (18%) | [93] |
| Brassica napus | Aspergillus niger | ↑ protein (81.7%), ↓ Phytic acid (37.5%), ↑ TPC (64.5%) | [94] |
| Tylosema esculentum | Aspergillus oryzae, Aspergillus sojae | ↓ Phytic acid (99%), ↓ TIs (68%), ↑ TPC (9%) | [95] |
| Glycine max | Pleurotus ostreatus | ↑ Protein (53%), ↑ Phosphorus (36%), ↓ Phytic acid (29%), ↑ TPC (12%) | [96] |
| Lens culinaris | Pleurotus ostreatus | ↑ Protein (23%), ↑ TPC (52%) | [97] |
| Lupinus spp | Aspergillus sojae, Aspergillus ficuum | ↑ TPC (21.2–37.3%), ↓ Phytic acid (53.3 to 73.2%) | [51] |
| Glycine max | Lactobacillus acidophilus | ↓ TIs (90%) | [98] |
| Phaseolus vulgaris | Pleurotus ostreatus | ↓ Tannins (34–66%) | [71] |
| Glycine max | Aspergillus oryzae | ↓ TIs (89.2%), ↓ Phytic acid (34.8%) | [65] |
| Vicia Faba L. | Aspergillus oryzae and Rhizopus oligosporus | ↑ Protein (8–20%), a reduction in most antinutrients, with the exception of trypsin inhibitors | [99] |
| Application | Substrate | Microorganism | Improvement in Animal Feed | Reference |
|---|---|---|---|---|
| Pig feed | Soybean meal | L. plantarum, B. subtilis and S. cerevisiae | Reduction of trypsin inhibitor levels (97%) and enhanced crude protein levels (13%). | [114] |
| Fish feed | Soybean meal | Saccharomyces cerevisiae | Elevated levels of crude protein (13.7%) and amino acids (16.3%), with a decrease in phytic acid (93%) and trypsin inhibitor concentrations (8.5%). | [115] |
| Poultry feed | Faba beans, wheat bran, potato pulp | Lactic acid bacteria from a commercial product | Improved protein (13–16%) and phosphorus solubility (10–17%). | [116] |
| Ducklings | Flaxseed cake | Aspergillus niger, Candida utilis | Enhanced crude protein content (15,8%) and reduced hydrocyanic acid levels (73%), leading to increased nutrient bioavailability. | [117] |
| Broiler chickens | Wheat bran | Pleurotus eryngii | Boost in lignocellulolytic enzyme activity and antioxidant molecule expression following broiler consumption. | [118] |
| Chickens | Soybean meal | Bacillus subtilis ED-3-7 | Crude protein and acid-soluble protein contents increased by 12% and 343%, and the trypsin inhibitor content was lower than the range specified in the detection kit. | [119] |
| Ovine feed | Groundnut meal | Saccharomyces cerevisiae | Increase in total protein (11–27%) and amino acids content (44%) with a reduction in phytic acid levels (69–72%). | [120] |
| Ruminants feed | Olive cake | Rhizodiscina cf. lignyota, Aspergillus niger | Rise in protein levels (94%) and decrease in phenolic compounds (43%), flavonoids (70%), and condensed tannins (42%). | [121] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Álvarez, A.; Rodríguez, A.; Chaparro, S.; Borrás, L.M.; Rache, L.Y.; Brijaldo, M.H.; Martínez, J.J. Solid-State Fermentation as a Biotechnological Tool to Reduce Antinutrients and Increase Nutritional Content in Legumes and Cereals for Animal Feed. Fermentation 2025, 11, 359. https://doi.org/10.3390/fermentation11070359
Álvarez A, Rodríguez A, Chaparro S, Borrás LM, Rache LY, Brijaldo MH, Martínez JJ. Solid-State Fermentation as a Biotechnological Tool to Reduce Antinutrients and Increase Nutritional Content in Legumes and Cereals for Animal Feed. Fermentation. 2025; 11(7):359. https://doi.org/10.3390/fermentation11070359
Chicago/Turabian StyleÁlvarez, Andrés, Alejandra Rodríguez, Sandra Chaparro, Luis Miguel Borrás, Leidy Y. Rache, Maria H. Brijaldo, and José J. Martínez. 2025. "Solid-State Fermentation as a Biotechnological Tool to Reduce Antinutrients and Increase Nutritional Content in Legumes and Cereals for Animal Feed" Fermentation 11, no. 7: 359. https://doi.org/10.3390/fermentation11070359
APA StyleÁlvarez, A., Rodríguez, A., Chaparro, S., Borrás, L. M., Rache, L. Y., Brijaldo, M. H., & Martínez, J. J. (2025). Solid-State Fermentation as a Biotechnological Tool to Reduce Antinutrients and Increase Nutritional Content in Legumes and Cereals for Animal Feed. Fermentation, 11(7), 359. https://doi.org/10.3390/fermentation11070359








