Multi-Biofuel Production Under Controlled and Noncontrolled pH Conditions by a Glucose-Adapted Enterobacter cloacae
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms and Culture Media
2.2. Batch Fermentations
2.3. Analytical Methods
2.4. Statistical Analysis
3. Results and Discussion
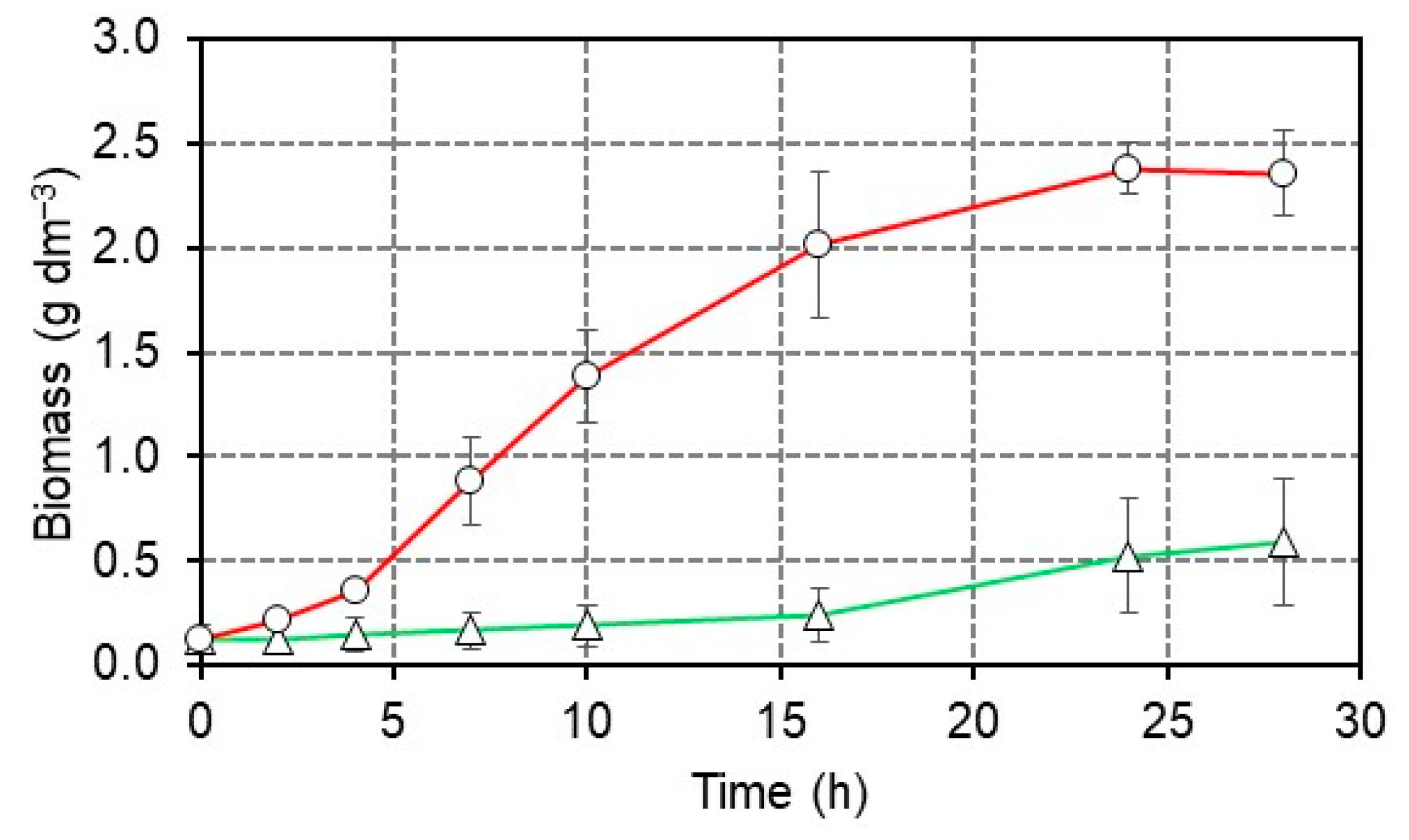
3.1. High-Glucose Concentration Adaptation of E. cloacae K1
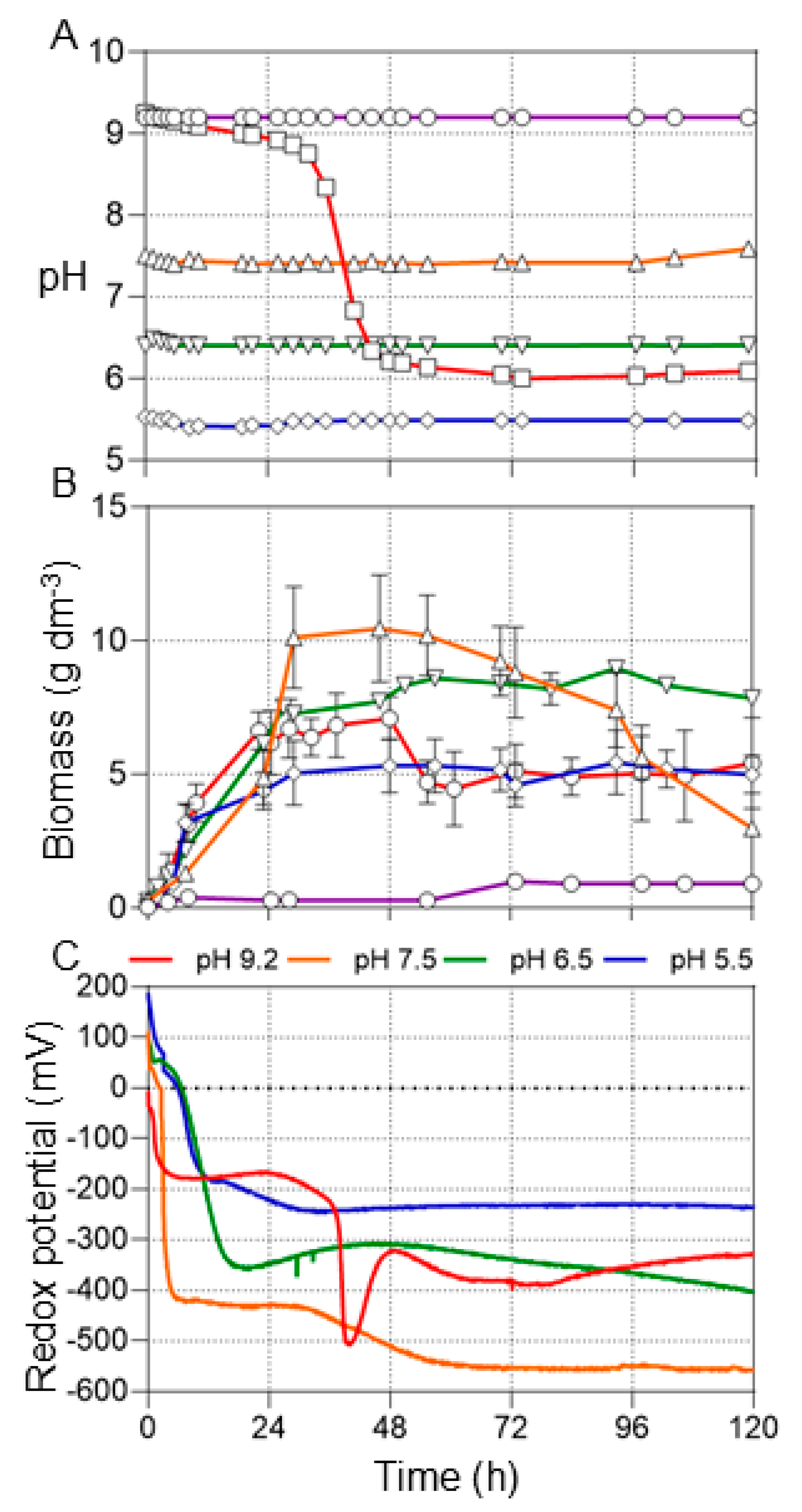
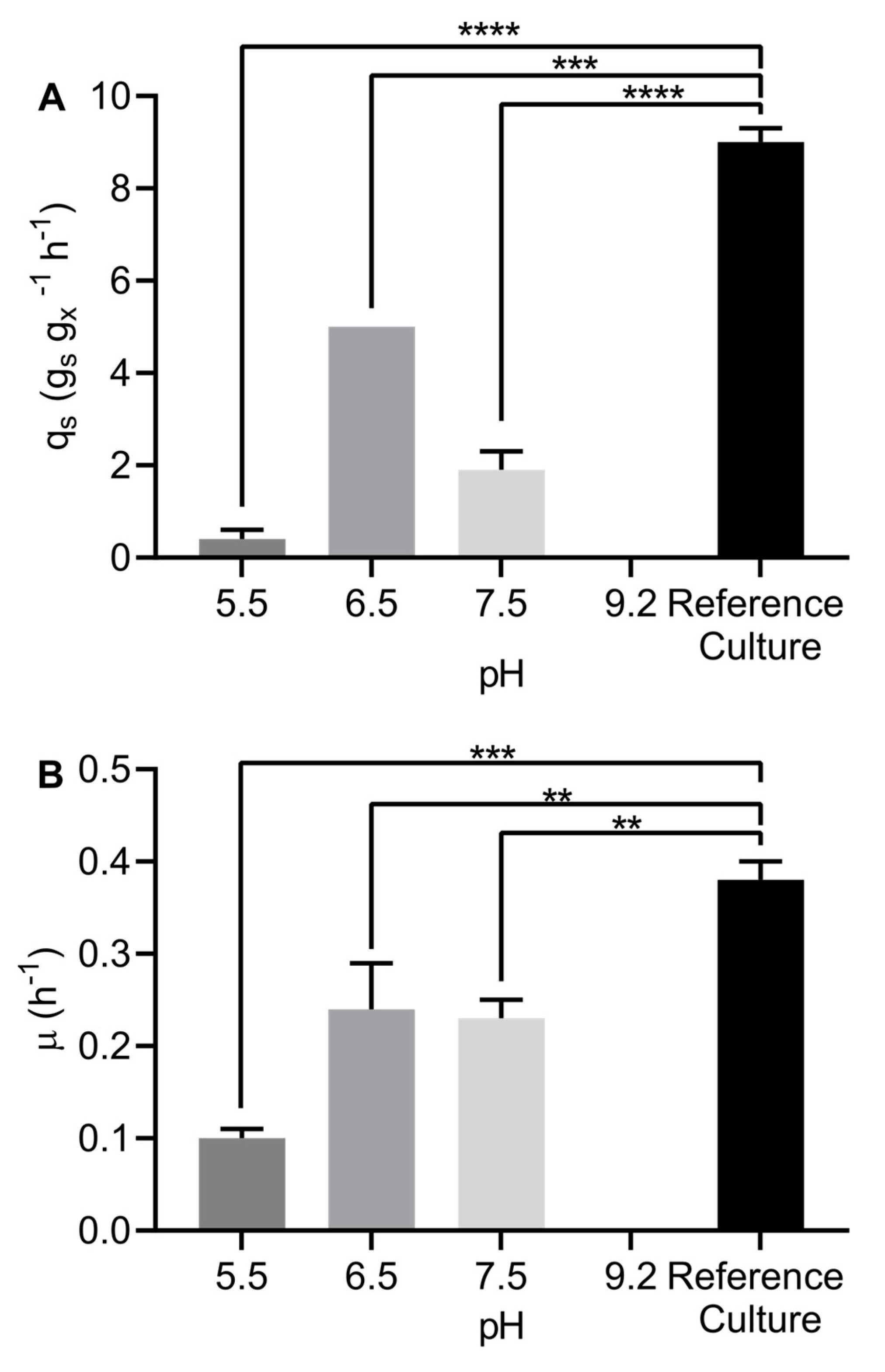
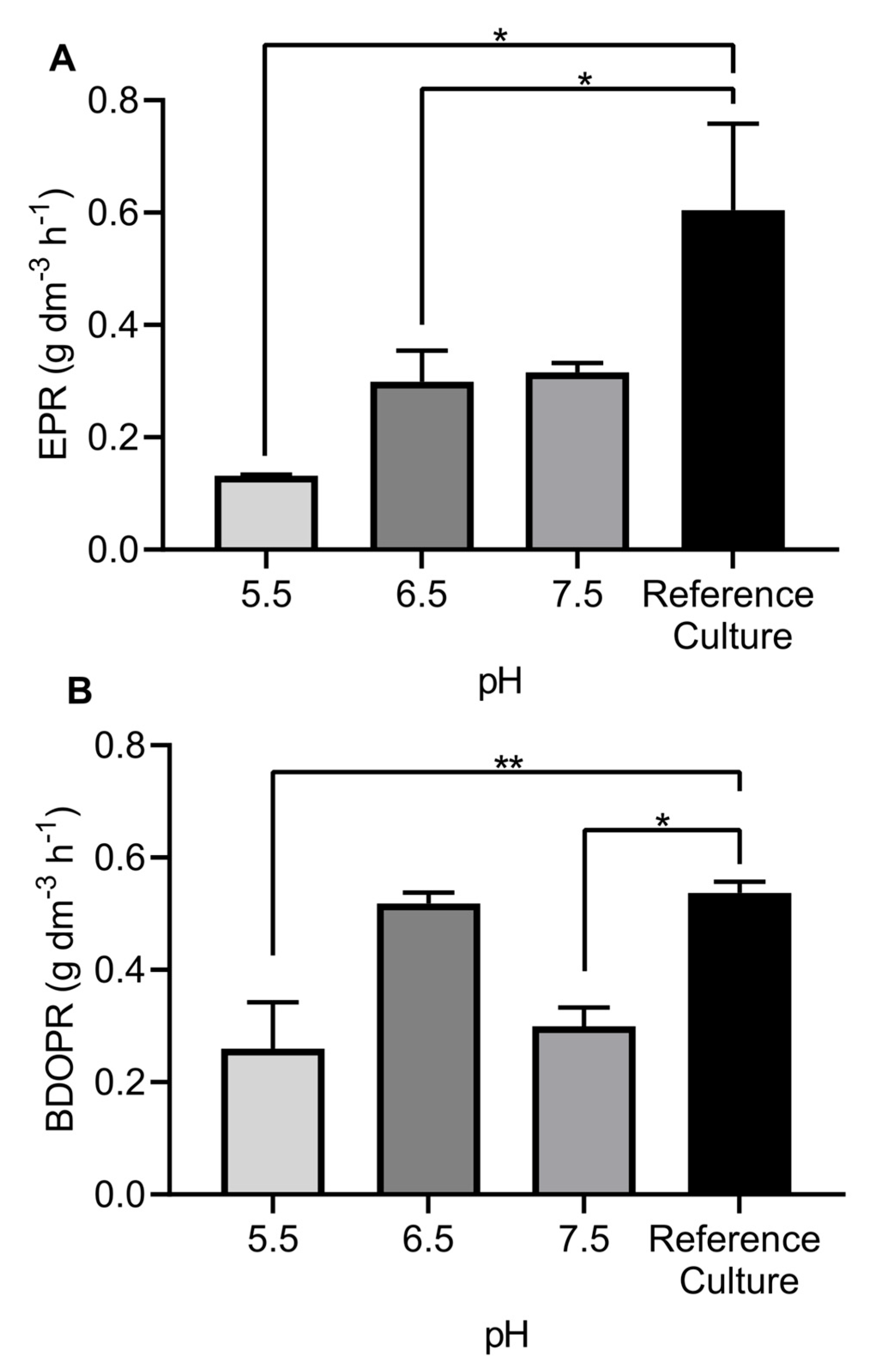
3.2. Effects of pH Control on Production of Biofuels
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Muhammed, N.S.; Gbadamosi, A.O.; Epelle, E.I.; Abdulrasheed, A.A.; Haq, B.; Patil, S.; Al-Shehri, D.; Kamal, M.S. Hydrogen production, transportation, utilization, and storage: Recent advances towards sustainable energy. J. Energy Storage 2023, 73, 109207. [Google Scholar] [CrossRef]
- Phillips, S.D. Technoeconomic Analysis of a Lignocellulosic Biomass Indirect Gasification Process to Make Ethanol via Mixed Alcohols Synthesis. Ind. Eng. Chem. Res. 2007, 46, 8887–8897. [Google Scholar] [CrossRef]
- Toledo-Alarcón, J.; Capson-Tojo, G.; Marone, A.; Paillet, F.; Ferraz Júnior, A.D.N.; Chatellard, L.; Bernet, N.; Trably, E. Basics of bio-hydrogen production by dark fermentation. Green Energy Technol. 2018, 199–220. [Google Scholar] [CrossRef]
- Sánchez-Pascuala, A.; De Lorenzo, V.; Nikel, P.I. Refactoring the Embden—Meyerhof-Parnas Pathway as a Whole of Portable GlucoBricks for Implantation of Glycolytic Modules in Gram-Negative Bacteria. ACS Synth. Biol. 2017, 6, 793–805. [Google Scholar] [CrossRef]
- Kumar, G.; Shobana, S.; Nagarajan, D.; Lee, D.J.; Lee, K.S.; Lin, C.Y.; Chen, C.Y.; Chang, J.S. Biomass Based Hydrogen Production by Dark Fermentation—Recent Trends and Opportunities for Greener Processes. Curr. Opin. Biotechnol. 2018, 50, 136–145. [Google Scholar] [CrossRef]
- Ju, J.H.; Jo, M.H.; Heo, S.Y.; Kim, M.S.; Kim, C.H.; Paul, N.C.; Sang, H.; Oh, B.R. Production of Highly Pure R,R-2,3-Butanediol for Biological Plant Growth Promoting Agent Using Carbon Feeding Control of Paenibacillus Polymyxa MDBDO. Microb. Cell Factories 2023, 22, 121. [Google Scholar] [CrossRef]
- Łukajtis, R.; Hołowacz, I.; Kucharska, K.; Glinka, M.; Rybarczyk, P.; Przyjazny, A.; Kamiński, M. Hydrogen Production from Biomass Using Dark Fermentation. Renew. Sustain. Energy Rev. 2018, 91, 665–694. [Google Scholar] [CrossRef]
- Liao, J.C.; Mi, L.; Pontrelli, S.; Luo, S. Fuelling the Future: Microbial Engineering for the Production of Sustainable Biofuels. Nat. Rev. Microbiol. 2016, 14, 288–304. [Google Scholar] [CrossRef]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudie, R.; Lens, P.N.L.; Esposito, G. A Review on Dark Fermentative Biohydrogen Production from Organic Biomass: Process Parameters and Use of by-Products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- Isikgor, F.H.; Becer, C.R. Lignocellulosic biomass: A sustainable platform for production of bio-based chemicals and polymers. Polym. Chem. 2015, 6, 4497–4559. [Google Scholar] [CrossRef]
- Jang, Y.S.; Kim, B.; Shin, J.H.; Choi, Y.J.; Choi, S.; Song, C.W.; Lee, J.; Park, H.G.; Lee, S.Y. Bio-Based Production of C2-C6 Platform Chemicals. Biotechnol. Bioeng. 2012, 109, 2437–2459. [Google Scholar] [CrossRef]
- Song, C.W.; Park, J.M.; Chung, S.C.; Lee, S.Y.; Song, H. Microbial Production of 2,3-Butanediol for Industrial Applications. J. Ind. Microbiol. Biotechnol. 2019, 46, 1583–1601. [Google Scholar] [CrossRef]
- Chiariotti, A.; Crisà, A. Bio-hydrogen production from buffalo waste with rumen inoculum and metagenomic characterization of bacterial and archaeal community. Front. Sustain. Food Syst. 2018, 2, 354674. [Google Scholar] [CrossRef]
- Singh, S.; Thavamani, P.; Megharaj, M.; Naidu, R. Multifarious Activities of Cellulose Degrading Bacteria from Koala (Phascolarctos cinereus) Faeces. J. Anim. Sci. Technol. 2015, 57, 23. [Google Scholar] [CrossRef]
- Osawa, R.; Blanshard, W.H.; O’callaghan, P.G. Microbiological Studies of the Intestinal Microflora of the Koala, Phascolarctos Cinereus. II. Pap, a Special Maternal Faeces Consumed by Juvenile Koalas. Aust. J. Zool. 1993, 41, 527–536. [Google Scholar] [CrossRef]
- Cork, S.J.; Hume, I.D.; Dawson, T.J. Digestion and Metabolism of a Natural Foliar Diet (Eucalyptus punctata) by an Arboreal Marsupial, the Koala (Phascolarctos cinereus). J. Comp. Physiol. B 1983, 153, 181–190. [Google Scholar] [CrossRef]
- Shiffman, M.E.; Soo, R.M.; Dennis, P.G.; Morrison, M.; Tyson, G.W.; Hugenholtz, P. Gene and genome-centric analyses of koala and wombat fecal microbiomes point to metabolic specialization for eucalyptus digestion. PeerJ 2017, 5, e4075. [Google Scholar] [CrossRef]
- Palaiogeorgou, A.M.; Papanikolaou, S.; De Castro, A.M.H.; Freire, D.M.G.; Kookos, I.K.; Koutinas, A.A. A newly isolated Enterobacter sp. strain produces 2,3-butanediol during its cultivation on low-cost carbohydrate-based substrates. FEMS Microbiol. Lett. 2019, 366, fny280. [Google Scholar] [CrossRef]
- Shi, A.; Fan, F.; Broach, J.R. Microbial adaptive evolution. J. Ind. Microbiol. Biotechnol. 2022, 49, kuab076. [Google Scholar] [CrossRef]
- Maeda, T.; Furusawa, C. Laboratory evolution of antimicrobial resistance in bacteria to develop rational treatment strategies. Antibiotics 2024, 13, 94. [Google Scholar] [CrossRef]
- Hirasawa, T.; Maeda, T. Adaptive laboratory evolution of microorganisms: Methodology and application for bioproduction. Microorganisms 2023, 11, 92. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Tremaine, M.; Grass, J.A.; Purdy, H.M.; Landick, R.; Kiley, P.J.; Reed, J.L. Systems metabolic engineering of Escherichia coli improves coconversion of lignocellulose-derived Sugars. Biotechnol. J. 2019, 14, e1800441. [Google Scholar] [CrossRef]
- Flores-Montiel, F.; Balderas-Hernández, V.E.; Rosales-Colunga, L.M.; De León-Rodríguez, A. Simultaneous production of biohydrogen, bioethanol, and bio-2,3-butanediol by enterobacter cloacae K1 isolated from koala using glucose as substrate. Int. J. Hydrogen Energy 2025, 101, 826–836. [Google Scholar] [CrossRef]
- Davila-Vazquez, G.; de León-Rodríguez, A.; Alatriste-Mondragón, F.; Razo-Flores, E. The buffer composition impacts the hydrogen production and the microbial community composition in non-axenic cultures. Biomass Bioenergy 2011, 35, 3174–3181. [Google Scholar] [CrossRef]
- Lu, P.; Bai, R.; Gao, T.; Chen, J.; Jiang, K.; Zhu, Y.; Lu, Y.; Zhang, S.; Xu, F.; Zhao, H. Systemic metabolic engineering of Enterobacter aerogenes for efficient 2,3-butanediol production. Appl. Microbiol. Biotechnol. 2024, 108, 146. [Google Scholar] [CrossRef]
- Julkipli, J.; Babel, S.; Bilyaminu, A.M.; Rene, E.R. Hydrogen and biodiesel production from food waste: A review. Environ. Chem. Lett. 2024, 22, 585–607. [Google Scholar] [CrossRef]
- Kim, K.; Choe, D.; Kang, M.; Cho, S.H.; Cho, S.; Jeong, K.J.; Palsson, B.; Cho, B.K. Serial adaptive laboratory evolution enhances mixed carbon metabolic capacity of Escherichia coli. Metab. Eng. 2024, 83, 160–171. [Google Scholar] [CrossRef]
- Corrao, M.; Abrahams, G.; Steel, H. Optimised control of adaptive evolution with competing selective pressures. bioRxiv, 2024; preprint. [Google Scholar] [CrossRef]
- Dongare, P.P.; Pawar, H.S. Biohydrogen Production from Dark Fermentation of Lignocellulosic Biomass; Elsevier Inc.: Amsterdam, The Netherlands, 2023; ISBN 9780128243183. [Google Scholar]
- Sawasdee, V.; Vikromvarasiri, N.; Pisutpaisal, N. Optimization of ethanol production from co-substrate of waste glycerol and acetic acid by enterobacter aerogenes. Biomass Convers. Biorefin. 2023, 13, 10505–10512. [Google Scholar] [CrossRef]
- Ewing, T.A.; Nouse, N.; van Lint, M.; van Haveren, J.; Hugenholtz, J.; van Es, D.S. Fermentation for the production of biobased chemicals in a Circular Economy: A Perspective for the Period 2022–2050. Green Chem. 2022, 24, 6373–6405. [Google Scholar] [CrossRef]
- Lu, P.; Gao, T.; Bai, R.; Yang, J.; Xu, Y.; Chu, W.; Jiang, K.; Zhang, J.; Xu, F.; Zhao, H. Regulation of carbon flux and NADH/NAD+ supply to enhance 2,3-butanediol production in enterobacter aerogenes. J. Biotechnol. 2022, 358, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Celińska, E.; Grajek, W. Biotechnological production of 2,3-butanediol-current state and prospects. Biotechnol. Adv. 2009, 27, 715–725. [Google Scholar] [CrossRef] [PubMed]
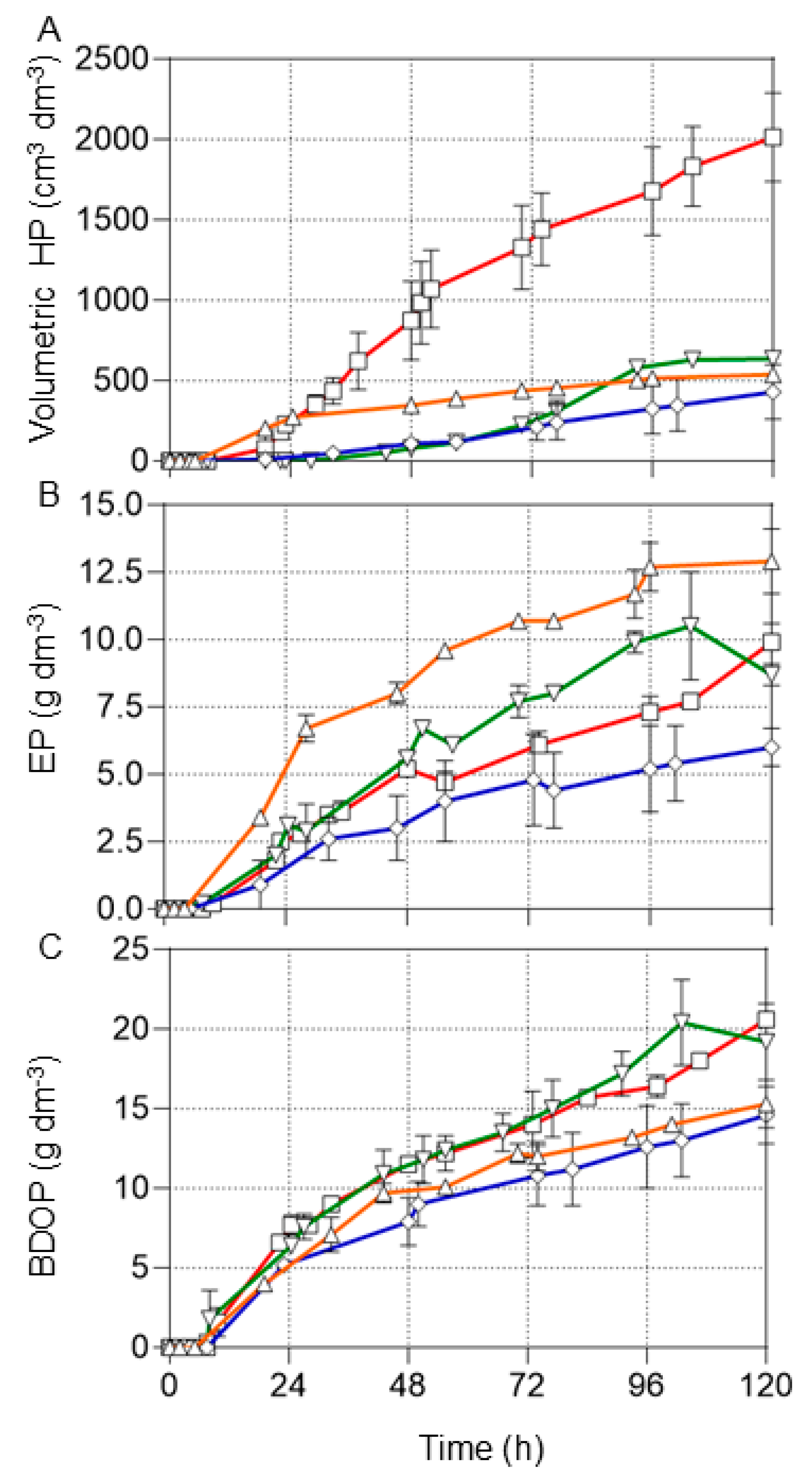

| Parameter | pH 5.5 | pH 6.5 | pH 7.5 | Reference Culture |
|---|---|---|---|---|
| HP (cm3 dm−3) a | 462.4 ± 200 | 637.2 ± 6.9 | 543.4 ± 1.45 | 2013.1 ± 275.7 |
| EP (g dm−3) b | 8.05 ± 0.35 | 11.4 ± 1.1 | 14.0 ± 0.05 | 9.9 ± 0.7 |
| BDOP (g dm−3) c | 18.9 ± 0.55 | 21.7 ± 1.3 | 17.1 ± 0.35 | 22.9 ± 2.1 |
| HPR (cm3 dm−3 h−1) d | 4.0 ± 1.2 | 6.3 ± 0.05 | 10.5 ± 2.5 | 21.1 ± 6.3 |
| EPR (g dm−3 h−1) e | 0.13 ± 0.002 | 0.29 ± 0.05 | 0.31 ± 0.01 | 0.6 ± 0.15 |
| BDOPR (g dm−3 h−1) f | 0.26 ± 0.08 | 0.51 ± 0.02 | 0.29 ± 0.03 | 0.53 ± 0.02 |
| YH2 (mol mol−1) g | 0.06 ± 0.005 | 0.05 ± 0.002 | 0.04 ± 0.00 | 0.17 ± 0.02 |
| YEtOH (g g−1) h | 0.12 ± 0.005 | 0.14 ± 0.014 | 0.18 ± 0.001 | 0.13 ± 0.009 |
| YBDO (g g−1) i | 0.28 ± 0.008 | 0.27 ± 0.016 | 0.22 ± 0.004 | 0.3 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flores-Montiel, F.; Balderas-Hernández, V.E.; Márquez-Rivera, K.L.; De Leon-Rodriguez, A. Multi-Biofuel Production Under Controlled and Noncontrolled pH Conditions by a Glucose-Adapted Enterobacter cloacae. Fermentation 2025, 11, 357. https://doi.org/10.3390/fermentation11060357
Flores-Montiel F, Balderas-Hernández VE, Márquez-Rivera KL, De Leon-Rodriguez A. Multi-Biofuel Production Under Controlled and Noncontrolled pH Conditions by a Glucose-Adapted Enterobacter cloacae. Fermentation. 2025; 11(6):357. https://doi.org/10.3390/fermentation11060357
Chicago/Turabian StyleFlores-Montiel, Francisco, Victor E. Balderas-Hernández, Karla L. Márquez-Rivera, and Antonio De Leon-Rodriguez. 2025. "Multi-Biofuel Production Under Controlled and Noncontrolled pH Conditions by a Glucose-Adapted Enterobacter cloacae" Fermentation 11, no. 6: 357. https://doi.org/10.3390/fermentation11060357
APA StyleFlores-Montiel, F., Balderas-Hernández, V. E., Márquez-Rivera, K. L., & De Leon-Rodriguez, A. (2025). Multi-Biofuel Production Under Controlled and Noncontrolled pH Conditions by a Glucose-Adapted Enterobacter cloacae. Fermentation, 11(6), 357. https://doi.org/10.3390/fermentation11060357





