Abstract
This study evaluated the viability of using the solid residues (bagasse) of the mezcal industry produced with Agave durangensis as a substrate for biogas production, using two chemical pretreatments, acid (HCl) and alkaline (KOH + Ca(OH)2), to enhance its biodegradability and improve the anaerobic digestion (AD) process. The chemical composition of bagasse was analyzed before and after the chemical pretreatments and then AD experiments were conducted in anaerobic sequential batch reactors (A-SBR) to analyze the effect of pretreatments on biogas production performance. The results showed that acid pretreatment increased cellulose content to 0.606 g, which represented an increase of 34%, and significantly reduced hemicellulose. In contrast, alkaline pretreatment did not show significant changes in cellulose composition, although it caused a swelling of the Agave durangensis mezcal bagasse (Ad-MB) fibers. In terms of biogas production, Ad-MB pretreated with acid (Ad-MB-acid) increased cumulative production by 76% compared to the Agave durangensis mezcal bagasse that was not pretreated (Ad-MB-not pretreated) and by 135% compared to Agave durangensis mezcal bagasse pretreated with an alkaline solution (Ad-MB-alkaline). These results confirmed that Agave durangensis solid waste from the mezcal industry that receives acidic chemical pretreatment has the potential to generate biogas as a sustainable biofuel that can be used to reduce the ecological footprint of this industry.
1. Introduction
Bioenergy production from renewable sources is becoming essential to meet growing energy demand and the need to reduce greenhouse gas emissions due to the depletion of fossil fuel reserves and the environmental effects of global warming [1,2]. Among renewable sources, lignocellulosic biomass represents a promising alternative due to its abundance and its generation as a residue from various agro-industrial processes, such as mezcal bagasse (MB), one of the main by-products of the mezcal industry [3].
Mezcal is a traditional Mexican alcoholic beverage produced through artisanal processes, giving it unique organoleptic characteristics as well as significant cultural, social, and economic value. In recent years, its production has shown significant growth, reaching more than 12 million liters in 2023. For each liter of mezcal produced, approximately 15 kg of bagasse is generated on a wet basis, which represents more than 180,000 tons of this waste per year, according to data from Mezcal Quality Regulator Council of Mexico [4].
Although a small fraction of bagasse is traditionally used for the manufacture of bricks, furniture, and packaging materials [5], most of it is disposed of on farmland, where its slow decomposition generates environmental problems such as soil contamination, unpleasant odors, harmful pests, and diseases [5,6].
Therefore, to address these environmental challenges and take advantage of the composition of this type of residue, bagasse from Agave tequilana Weber has gained attention as a feedstock in the production of biofuels [7]. However, the main limitation to its utilization is its low biodegradability, due to the recalcitrant structure formed by the stable bonding of its main components (cellulose, hemicellulose, and lignin), which hinders the access of microorganisms during enzymatic hydrolysis. To overcome this barrier, different pretreatment strategies, including physical, physicochemical, chemical, biological, or combinations of these, have been proven to improve the degradation of different lignocellulosic biomasses [8]. Among these strategies, several innovative and highly efficient technological processes stand out, some of which are presented in Table 1. However, despite their effectiveness, these methods usually require high installation and/or operating costs and are complicated to operate. In this sense, and considering that many agro-industries worldwide, such as the mezcal industry, operate in an artisanal manner and with limited technical resources, the present study focused as a first option on evaluating inexpensive and easy to apply pretreatment technologies, with greater potential for adoption by this type of artisanal agro-industries. The novelty of this work lies then in the evaluation of applying two physicochemical pretreatments for the first time to the residues (bagasse) of a plant native to arid and semi-arid zones, the Agave durangensis, which is used in an artisanal way to produce the second most important traditional alcoholic beverage in Mexico, mezcal, which has an exponential growth in consumption worldwide. The study’s objective is to improve the production of biogas and revalorize and reuse the waste of this industry for the generation of biofuels. This waste has been scarcely studied worldwide and, to date, only the use of physical pretreatment (grinding) has been reported for Agave durangensis mezcal bagasse fermentation.

Table 1.
Conventional and emerging pretreatment methods applied to lignocellulosic biomasses.
Among the chemical pretreatments, acid, and alkaline processes stand out for their effectiveness and low cost. Acid pretreatment allows for the hydrolysis of hemicellulose into its main sugars: hexoses (glucose, mannose, and galactose) and pentoses (xylose and arabinose). However, depending on the hydrolysis conditions, such as temperature, reaction time, and acid concentration, it can lead to the formation of toxic compounds, such as furfural, 5-hydroxymethyl furfural, phenolic acids, and aldehydes, which can affect the subsequent fermentation of the released sugars [7,16].
Previous studies have evaluated the effectiveness of acid pretreatment on diverse types of biomasses such as corn stover and bagasse from Agave tequilana Weber (residue from tequila production) using hydrochloric acid (HCl) at different concentrations, temperatures, and reaction times. These works have shown that pretreatment with HCl is effective for the recovery of fermentable sugars necessary for methane (CH4) [3,7,13,17] and hydrogen (H2) generation [18].
On the other hand, alkaline pretreatment is effective in modifying the structure and solubilizing lignin, breaking the bonds between lignin and carbohydrates, thus increasing the accessibility of carbohydrates for enzymatic hydrolysis. Liu et al. [19] studied potassium hydroxide (KOH) to pretreat wheat straw, while further research used KOH, calcium hydroxide (Ca(OH)2), and KOH + Ca(OH)2 to pretreat corn straw, obtaining favorable results in terms of improved biodegradability.
Recent studies have focused on the valorization of agro-industrial waste, mainly from the tequila industry, which is financially and technologically stronger than the mezcal industry. The same agave species (Agave tequilana Weber) is always used to produce tequila, which is internationally recognized, and its residues have been extensively studied as potential sources of biofuels. On the other hand, the mezcal industry, although it uses traditional production methods and operates in small facilities with little technology [20], has significant potential as a source of raw materials for biofuel production, due to the diversity of agave species used in its production.
More than 150 species of agave can be used in the production of mezcal, depending on their availability in each region. The main species include Agave angustifolia, Agave potatorum, Agave karwinskii, and Agave durangensis. In the state of Durango, the fourth largest mezcal producer in Mexico, Agave durangensis is widely used for mezcal production [4]. However, to date, no studies have been reported on pretreatment methods or on the utilization of bagasse generated from Agave durangensis as a raw material for biofuel production. Therefore, the present study aims to establish the effect of applying two chemical pretreatments (acid and alkaline) on biogas production from the anaerobic digestion (AD) of Agave durangensis mezcal bagasse (Ad-MB).
2. Materials and Methods
2.1. Inoculum and Bagasse
The inoculum for the AD treatments in this work consisted of anaerobic sludge obtained from the nitrification/denitrification process of the wastewater treatment plant (WWTP) south of Durango City, Mexico (23°97′55″ N, 104°63′67″ W). The sludge was thermally treated at 105 °C for 24 h to dry it and inhibit the activity of H2-consuming microorganisms and favor H2-generating and spore-forming anaerobic microorganisms. Subsequently, this inoculum was ground in a porcelain mortar until a powdered sample was obtained [21].
Bagasse was obtained from the facilities of an artisanal mezcal factory located in Nombre de Dios, Durango, Mexico (23°86′85″ N, 104°26′05″ W), a region where mezcal is made from Agave durangensis. The bagasse collected was washed with tap water, sun dried, ground, and sieved to an average particle size of 0.3 mm using a grain mill (Molino Nixtamatic NG-02, NIXTAMATIC, Ciudad de México, México) and stored at room temperature. The physicochemical characterization of Ad-MB was determined on a dry weight basis, per gram of Ad-MB, in terms of moisture content, ash, total organic carbon (TOC), total Kjeldahl nitrogen (TKN), carbon/nitrogen ratio (C/N), and quantity of cellulose, hemicellulose and lignin.
2.2. Chemical Pretreatment of Bagasse
In this study, two different chemical pretreatments were applied to Ad-MB: one with hydrochloric acid (HCl, analytical grade, 37%, Fermont, PRODUCTOS QUÍMICOS MONTERREY, Monterrey, México) according to the methodology of Arreola-Vargas et al. [7] and another with a mixture of potassium hydroxide (KOH, analytical grade, 85%, Fermont) and calcium hydroxide (Ca(OH)2, analytical grade, 96%, Fermont) according to the methodology of Li et al. [22]. In the HCl pretreatment, the ground and dried Ad-MB fibers were kept at constant mixing for 2 h with 2% w/w HCl at a controlled temperature of 90 °C. In the pretreatment with KOH + Ca(OH)2, the ground and dried Ad-MB was mixed with a solution of 0.5% w/w KOH + 2% w/w Ca(OH)2. The pretreatment was conducted at room temperature for 24 h, stirring manually at 4 h intervals. At the end of each pretreatment, the liquid fraction was separated by filtration, and the Ad-MB fibers were washed with tap water until the liquid fraction reached a pH between 6 and 6.5.
2.3. Anaerobic Reactors and Experimental System
To evaluate the biodegradability of Ad-MB without chemical pretreatment, with HCl pretreatment, and with KOH + Ca(OH)2 pretreatment, anaerobic sequential batch reactors (A-SBRs) were used. The A-SBRs consisted of 1000 mL glass flasks with a working volume of 700 mL and an exchange ratio for each new cycle of 50% of the total working volume. A water bath maintained the A-SBRs at a temperature of 35 ± 2 °C. The pH was adjusted daily to a value of 5.5 ± 0.2 using Ca(OH)2, and H2SO4 as needed. Homogeneous conditions of the A-SBRs were maintained by continuous mixing at 250 rpm. Biogas production was measured daily using the water displacement technique by placing an inverted 250 mL test tube on a base containing water and preventing air from entering the tube.
2.4. Inoculum Activation
The spore-forming and H2-generating anaerobic microorganisms present in the inoculum were activated according to the methodology described by Buitrón and Carvajal [21], which consisted of loading the A-SBR with 20 g/L of heat-treated powdered inoculum and 2 g glucose/L as a source of readily assimilable carbon. The pH was stabilized at 5.5 ± 0.2 using calcium hydroxide (Ca(OH)2) or sulfuric acid (H2SO4) as needed, and a retention time (RT) of 1 day per operation cycle was applied. The inoculum was considered activated when a constant removal of organic matter, measured as chemical oxygen demand (COD), and a stable production of volatile fatty acids (VFAs) were observed.
2.5. Acclimatization of Inoculum
Once the inoculum was activated, the microorganisms were acclimated to use Ad-MB as a carbon source. Three A-SBRs were used for acclimatization: one A-SBR was fed with Agave durangensis mezcal bagasse pretreated with acid (Ad-MB-acid), one was fed with Agave durangensis mezcal bagasse pretreated with alkalis (Ad-MB-alkaline), and finally, one A-SBR was fed with Agave durangensis mezcal bagasse not pretreated (Ad-MB-not pretreated). The A-SBRs were loaded with the previously activated inoculum and a 10 g/L solution of Ad-MB with a particle size of 0.3 mm. At this stage, glucose was completely removed to stimulate microorganisms to use the carbohydrates present in the Ad-MB as a carbon source. The A-SBRs operated for 5 cycles, each cycle lasting 21 days, equivalent to 105 days of operation. The other operating conditions of each of the A-SBRs were kept constant.
2.6. Biodegradability of Agave durangensis Mezcal Bagasse
Once the acclimatization of the microorganisms to the Ad-MB was completed, the operation of the A-SBRs continued for 11 more cycles of 21 days each, corresponding to 231 days of operation. The other operating conditions of each of the A-SBRs were kept constant. The performance of each A-SBR was monitored by following the behavior of each operating cycle, taking samples every 4 days to determine VFA and reducing sugars (Rsu); biogas production was measured daily.
2.7. Analytical Methods
Moisture content, pH, ash, TKN, VS, and COD were measured following the procedures of standard methods [23]. TOC was calculated using the following equation described by Gouluke [24] (Equation (1)):
% TOC = (100 − ash)/1.8
The carbon/nitrogen ratio was determined based on TOC and TKN analysis. Rsu was determined by the 3,5-dinitrosalicylic acid (DNS) colorimetric method proposed by Miller Gail Lorenz [25]. VFA content was measured by Hach method 8196 [26]. Cellulose, hemicellulose, and lignin content were determined by neutral detergent fiber (NDF), acid detergent fiber (ADF), and acid detergent lignin (ADL) analyses according to the technique described by Van Soest et al. [27]. The hemicellulose content was calculated by the difference in weight between NDF and ADF. Cellulose content was determined by the difference in weight between ADF and ADL. All analyses were performed in triplicate and results were expressed as average ± standard deviation (SD).
2.8. Kinetic Analysis of Biogas Production
The cumulative biogas production data for each cycle were adjusted to the modified Gompertz equation (Equation (2)). The modified Gompertz model described the cumulative biogas production in the batch experiments, assuming that biogas production is a function of bacterial growth.
In this equation, B represents the cumulative biogas production (NmL biogas/Lreactor) and t the cycle time (d). In addition, it estimates the maximum cumulative biogas production B0 (NmL biogas/Lreactor), the Euler coefficient e (2.71828), the maximum rate of biogas production Rm (NmL biogas/Lreactor⋅d), and the latency phase time λ (d).
The adjustment was performed using the Excel solver tool, minimizing the error between the experimental values and the estimated values. The degree of fit was determined by means of the squared correlation factor (R2).
2.9. Statistical Analysis
The statistical analysis of data was conducted using ANOVA to determine the presence of significant differences (p < 0.05) in terms of biogas production in each treatment. For this purpose, Tukey tests were performed for comparison between treatments. Statistical analyses were conducted using GraphPad 8 software.
3. Results and Discussion
3.1. Characteristics of Mezcal Bagasse
The results of the physicochemical characterization of Agave durangensis mezcal bagasse (Ad-MB) from the present study showed an average moisture content of 82.3%. The properties related to ash content, TOC, and TKN presented similar values to those reported by Martínez-Gutiérrez et al. [28] for Agave angustifolia Haw mezcal bagasse (AaH-MB). On the other hand, the VS content was 89.9 ± 0.64%, a value comparable to those reported by Martínez-Gutiérrez et al. [28] and Gómez-Guerrero et al. [6] for Agave angustifolia Haw bagasse, and equal to the value reported by Valdez-Vázquez et al. [13] for Agave tequilana Weber bagasse. In terms of structural components, the cellulose, hemicellulose, and lignin content per gram on a dry basis of Ad-MB were 0.451, 0.032, and 0.222 g, respectively. Although cellulose and hemicellulose values are below previously reported values, lignin content is higher compared to data reported by Gómez-Guerrero et al. [6] and Valdez-Vázquez et al. [13] for (AaH-MB) and Agave tequilana Weber tequila bagasse (AtW-TB) (Table 2).

Table 2.
Physicochemical composition of bagasse of different origins.
According to these results, structural carbohydrates (cellulose and hemicellulose) represent more than 48% of the dry weight of Ad-MB, which makes it a suitable substrate to produce fermentable sugars, essential in anaerobic digestion processes to produce biofuels. However, Ad-MB also presented a high lignin content (0.222 g), with lignin being the second most abundant component of its composition. Lignin is an overly complex molecule that represents a barrier to accessing carbohydrates, limiting the release of sugars for fermentation. To extract the sugars, the carbohydrates must first be separated from the lignin and then the newly released carbohydrates must be hydrolyzed to break them down into simple monosaccharides [29].
These results highlight that the composition of MB can be different depending on the agave species, the area of cultivation, and the influence of agricultural practices. In addition, as a by-product of an agro-industrial process, its composition may also vary due to factors related to production methods. However, we consider that the results obtained in this study can be a reference for similar biomasses from other regions, especially those species with similar lignocellulosic characteristics. The application of these results in other areas is possible if a prior characterization of the available residue is carried out, in terms of its cellulose, hemicellulose, and lignin content.
3.2. Effect of Chemical Pretreatments on Cellulose, Hemicellulose, and Lignin Content
The composition of Ad-MB after chemical pretreatment is presented in Table 3. In the case of Ad-MB-acid, a significant decrease in the amount of hemicellulose was observed, while the cellulose content increased from 0.451 to 0.606 g, representing an increase of 34%. On the other hand, in the Ad-MB-alkaline, the values of cellulose, hemicellulose, and lignin remained like those observed in the Ad-MB-not pretreated.

Table 3.
Composition of chemically pretreated Agave durangensis mezcal bagasse (Ad-MB).
The decrease in hemicellulose content observed in Ad-BM-acid may be attributed to the branched and amorphous structure of hemicellulose, making it less resistant to hydrolysis and, consequently, more easily hydrolyzed by acid [30].
On the other hand, the chemical pretreatments applied failed to dissolve the lignin present in the Ad-MB, maintaining the same proportion as that of the Ad-BM-not pretreated. Lignin is a complex molecule composed of phenylpropane units linked in a three-dimensional structure, which makes it resistant to acid hydrolysis [31]. Although alkaline pretreatment is the most widely used method to eliminate lignin in different biomasses, its efficiency depends on factors such as temperature (room temperature for long periods of time over 24 h or high temperatures for less pretreatment time) and lignin content, which is most efficient for lignocelluloses with low lignin content [29,32].
Previous studies have successfully reported the use of sodium hydroxide (Na(OH)) at different concentrations, temperatures, and pretreatment times for the removal of lignin from wheat straw, rice straw, and sugarcane bagasse [33,34,35,36]. Liu et al. [19] reported a reduction in lignin in wheat straw by increasing KOH concentration from 2% to 50% at 21 °C, while Li et al. [22] reported a 39% reduction in lignin in corn straw by using 0.5% KOH + 2% Ca(OH)2 at 21 °C.
In the present study, the alkaline pretreatment applied to Ad-BM did not show a reduction in lignin content. This result may be attributed to varied factors, including the high initial lignin content in Ad-BM, as well as the concentration of the alkaline solution, temperature, and reaction time employed. These factors, taken together, appear not to have been sufficient to induce an effective depolymerization of the complex and recalcitrant lignin structure present in Ad-BM. However, a swelling of the Ad-BM-fibers was observed, due to the initial alkaline pretreatment reactions taking place, such as solvation and saponification. These reactions cause the biomass to expand, leading to an increase in internal surface area, which, in principle, would favor its subsequent biodegradation [29,37].
3.3. Biodegradability of Agave durangensis Mezcal Bagasse
3.3.1. Inoculum Activation
Figure 1 shows that during operation cycles 2 and 3 of the activation process (each cycle of one day), there was a decrease in COD output with respect to COD input of 183 mg COD/L, equivalent to a non-significant COD removal of 4 ± 1%; a constant VFA generation of 157 to 401 mg VFA/L was observed as well. However, from operation cycle 4 forward, a constant COD removal of 446 ± 129 mg COD/L was observed, which is equivalent to a removal efficiency of 21 ± 7%. In addition, a constant VFA production of 198 to 338 mg VFA/L was obtained, equivalent to a production efficiency of 61 ± 18%. Therefore, by obtaining an organic matter (COD) removal efficiency of 21% and a constant VFA production (61%), the inoculum was considered successfully activated for a fermentation process.
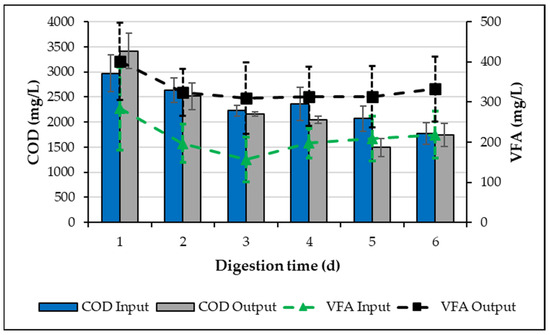
Figure 1.
VFA and COD from inoculum activation.
3.3.2. Acclimatization of Inoculum to Agave durangensis Mezcal Bagasse
Figure 2 presents the average values of Rsu and VFA concentrations, as well as the kinetics of cumulative biogas production obtained during the five treatment cycles that comprised the acclimatization process of the microorganisms to the Agave durangensis mezcal bagasse not pretreated (Ad-MB-not pretreated) throughout the 21 days of operation of each cycle. A cycle length of 21 days was selected based on the cumulative biogas production results observed in the A-SBR with acid pretreatment during the acclimatization period (Figure S1, Supplementary Materials) where it was observed that there was no more biogas generation after day 19 of operation. As mentioned above, the acclimatization process was conducted in five cycles, corresponding to 105 days of operation. The average Rsu concentration was 180 ± 90 mg Rsu/L, remaining at elevated levels with moderate variability. The high Rsu concentration can be attributed to insufficient hydrolysis and fermentation, possibly because the microorganisms were still in the adaptation phase to this new carbon source, which is both complex and difficult to biodegrade. Additionally, a steady increase in VFA accumulation was observed during the entire duration of the cycles (21 days), from 538 ± 250 to 1107 ± 263 mg VFA/L, with a high variability. This VFA accumulation could be explained by the fact that the inoculum is composed of H2-producing microorganisms and not methanogenic archaea, which consume VFA for methane production. Meanwhile, the average accumulated biogas production at the end of the cycle duration was 119 ± 100 NmL/Lreactor, also presenting high variability. This behavior may be related to the slow adaptation of methanogenic archaea, whose activity depends on a stable balance in the production and consumption of VFA.
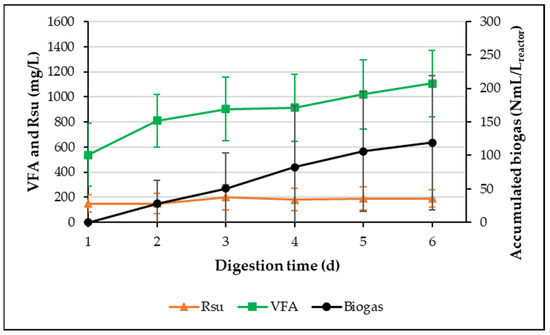
Figure 2.
The production of Rsu, VFA, and normalized biogas during the acclimatization process of the microorganisms to the Ad-MB-not pretreated (cycles 1 to 5).
In general, the high variability observed in Rsu, VFA, and biogas values during the initial cycles reflects the stage of adaptation of the microbial community to the Ad-MB-not pretreated composition.
Figure 3 shows the average values of Rsu and VFA concentrations and the accumulated biogas production kinetics for the treatment cycle duration (21 days), obtained from 11 treatment cycles once the microorganisms were acclimatized to the Ad-MB. From cycles 6 to 16 of operation, it was observed that the average concentration of Rsu and VFA dropped notably to values of 80 ± 20 mg Rsu/L and 172 ± 119 mg VFA/L, respectively. This result suggests the development of a population of fermentation bacteria and methanogenic microorganisms, which are better acclimated to use Ad-MB carbohydrates for anaerobic digestion. In addition, it was observed that the variability of the concentration values of Rsu and VFA decreased notably compared to the acclimatization period (Figure 2), with the variability for VFA being moderate and that of Rsu almost disappearing. Meanwhile, the average maximum accumulated biogas production reached at the end of the cycle was 113 ± 47 NmL/Lreactor, which is very similar to the one obtained during the acclimatization process but with notably less variability. This volume, generated from 3.1465 gVS of Ad-MB, corresponds to a normalized biogas yield of 35.91 ± 10.00 NmL/gVS. Therefore, the differences observed between cycles 1 to 5 and cycles 6 to 16 evidence a period of acclimatization, where the microorganisms present in the inoculum needed 5 cycles of operation (cycles 1 to 5) for their acclimatization to the Ad-MB.
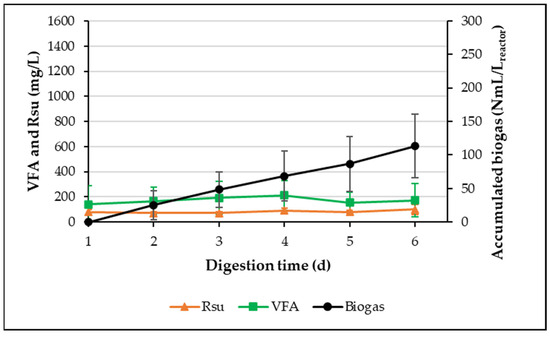
Figure 3.
The production of Rsu, VFA, and normalized biogas after the acclimatization process of Ad-MB-not pretreated (cycles 6 to 16).
3.4. Effect of Chemical Pretreatments on Biodegradability of Agave durangensis Mezcal Bagasse
Figure 4 shows the average values of Rsu and VFA obtained at the end of the treatment cycles and the average value of the maximum volume that the accumulated biogas reached at the end of each cycle during 11 operation cycles (cycles 6 to 16) for Ad-MB-not pretreated, Ad-MB-acid, and Ad-MB-alkaline once the microorganisms were acclimatized to the Ad-MB. The highest cumulative biogas production was 200 ± 73.07 NmL/Lreactor, equivalent to a specific yield of 63.56 ± 15.40 NmL/gVS, which represents a statistically significant increase (p > 0.05) of 76%, compared to the Ad-MB-not pretreated (113 ± 47.46 NmL/Lreactor).
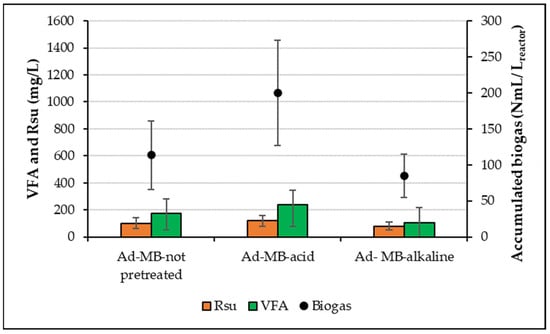
Figure 4.
Rsu, VFA, and normalized biogas production from AD process of Ad-MB-not pretreated, Ad-MB-acid, and Ad-MB-alkaline.
These results agree with previous studies that have reported significant improvements in biogas production when using HCl as a pretreatment in diverse types of lignocellulosic biomass. For example, Song et al. [17] reported a 62% increase in methane production by pretreatment of corn stover with 2% HCl. Similarly, Breton-Deval et al. [3] and Valdez-Vázquez et al. [13] reported 1.3–1.9 fold increases in methane production using 1.9% HCl and a temperature of 130 °C to pretreat Agave tequilana Weber tequila bagasse (AtW-TB). Additionally, acid pretreatment promotes the release of Rsu (120 mg/L) and favors a higher production of VFA (237 mg/L), which accelerates the rate of biogas production, since Rsu are a main source of carbon for microorganisms, while VFA are key intermediates for methane production.
In contrast, Ad-MB-alkaline presented the lowest cumulative biogas production, with an average of 85 ± 29.86 NmL/Lreactor, which represents a decrease of 25% compared to Ad-MB-not pretreated. This result differs from that reported in the literature, where significant increases in biogas production have been observed with the use of alkaline pretreatments in other lignocellulosic biomasses. For example, Li et al. [22] reported increases of 77% and 79.9% in biogas production from corn straw pretreated with 0.5% KOH + 2% Ca(OH)2. Likewise, Liu et al. [19] reported 77.5% and 95.6% increases in methane production when using KOH to pretreat wheat and corn straw, respectively, while with Ca(OH)2 in corn straw, they observed a 39.7% increase.
The difference in results can be attributed in part to the chemical composition of Ad-MB, which contains 22% lignin, representing 16% more than corn stover. Lignin is the second most abundant component of Ad-MB and is a significant structural barrier to degradation. Lignocellulosic biomasses with high lignin content are more difficult to effectively pretreat by alkaline processes, as lignin is highly recalcitrant and requires specific high-intensity conditions for depolymerization. In addition, it is likely that some components of the mezcal bagasse reacted with the alkaline compounds used, which would have caused the release of phenolic compounds with an inhibitory effect, or favored the formation of more resistant crystalline structures, hindering microbial activity during AD as has be reported by Jönsson & Martín [38].
Additionally, Ad-MB-acid showed a significant increase (p > 0.05) of 135% in biogas production compared to Ad-MB-alkaline. This behavior indicates that acid pretreatment is more effective in improving the digestibility of Ad-MB by facilitating the release of fermentable carbohydrates that favor the development of acidogenic bacteria and the production of VFA, which are fundamental precursors for biogas generation in anaerobic digestion processes.
3.5. Kinetics of Biogas Production
Figure 5 presents the cumulative biogas production curves for three experimental cycles representative of the anaerobic digestion processes conducted in the A-SBRs, working with Ad-MB-not pretreated (a), Ad-MB-acid (b), and Ad-MB-alkaline (c). Additionally, the fit of the experimental data to the modified Gompertz model, used to evaluate and compare biogas production under the different pretreatment conditions, is included. The estimated kinetic parameters are summarized in Table 4.
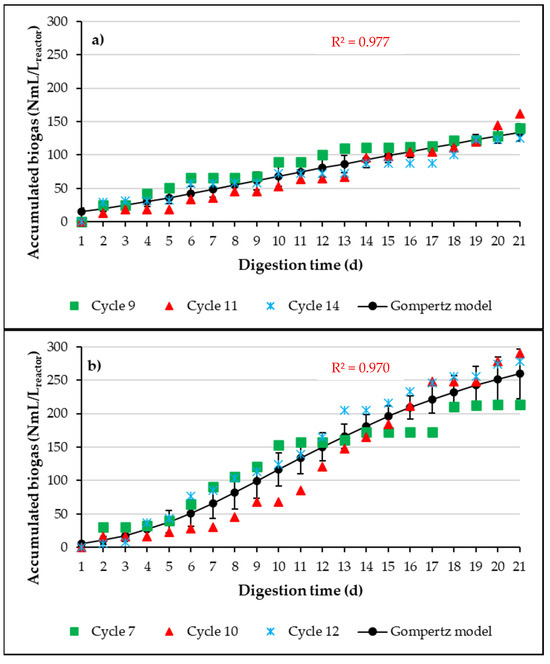
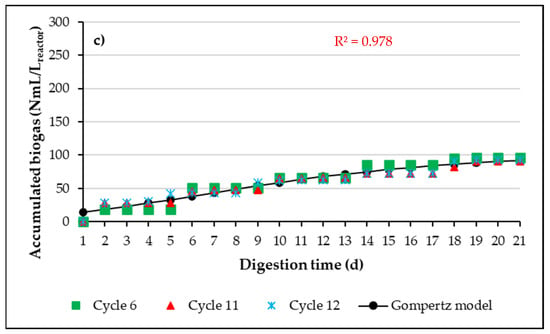
Figure 5.
Experimental results and average adjustment curve to Gompertz model of cumulative biogas production. (a) Ad-MB-not pretreated, (b) Ad-MB-acid, (c) Ad-MB-alkaline.

Table 4.
Kinetic parameters of modified Gompertz model.
The fit of the experimental data to the modified Gompertz model showed a coefficient of determination (R2 > 0.970), indicating that the model explains more than 97% of the variability observed in the data. This result suggests that the modified Gompertz model is a suitable and accurate tool for describing the kinetics of biogas production in the anaerobic digestion processes evaluated.
The estimated kinetic parameters highlight the positive effect of HCl pretreatment on Ad-MB. Specifically, the latency time (λ) was significantly shorter for Ad-MB-acid (5.9 days) and Ad-MB-alkaline (6.0 days) compared to Ad-MB-not pretreated (10.9 days). However, although the latency time was similar between the acid and alkaline pretreatments, only the Ad-MB-acid showed a significant improvement in cumulative biogas production, reaching much higher values than those observed for Ad-MB-alkaline.
The higher biogas production associated with Ad-MB-acid can be explained by the effects of HCl pretreatment, which modified the structure of Ad-MB favorably, increasing the proportion of soluble and easily digestible compounds. In contrast, the alkaline pretreatment was not severe enough to significantly alter the Ad-MB structure, resulting in lower cumulative biogas production.
In terms of maximum biogas production rate (Rm), Ad-MB-acid showed the highest value, 9.70 mL/Lreactor·d, which represents an increase of 98.96% in the biogas velocity production compared to Ad-MB-not pretreated and 98.55% compared to Ad-MB-alkaline (Table 4). These results highlight the effectiveness of acid pretreatment in improving the digestibility of Ad-MB and optimizing biogas production in anaerobic digestion processes.
In comparison, Breton-Deval et al. [3] reported a methane production rate of 4.02 ± 0.12 mL CH4/h by pretreatment of Agave tequilana Weber tequila bagasse (AtW-TB) with 1.9% HCl at a temperature of 130 °C. When converting this rate to a daily period, production of 96.48 mL CH4/d is estimated, significantly higher than the rate reported for Ad-MB-acid. These differences can be attributed to factors such as the intrinsic characteristics of each biomass, specific operational pretreatment conditions, and differences in bagasse composition. While Agave tequilana Weber tequila bagasse (AtW-TB) comes from standardized industrial processes, Ad-MB has a more heterogeneous composition due to its artisanal origin, which could limit its efficiency in biogas production. In addition, the more severe conditions of the acid pretreatment employed by Breton-Deval et al. [3] (e.g., high temperature) may have contributed to a higher solubilization of easily digestible organic compounds, which explains the difference in the reported production rates.
4. Conclusions
The results obtained in the present study demonstrate the efficacy of acid pretreatment in improving the biodegradability of the solid residue of the mezcal industry (Agave durangensis mezcal bagasse) in an anaerobic digestion (AD) process. This is an organic residue constituted by bagasse of a plant from arid zones, Agave durangensis, which had not been previously studied about the effect of chemical pretreatments. This treatment favored hemicellulose decomposition and increased the proportion of cellulose, favoring the release of fermentable sugars, which boosted the AD process and, consequently, increased biogas production by 76% compared with the anaerobic digestion of the Agave durangensis mezcal bagasse that was not pretreated. Acid pretreatment is presented as an efficient strategy to enhance the use of this agro-industrial waste. Unexpected results showed that alkaline pretreatment, as was performed, did not transform the chemical composition of mezcal bagasse enough to enhance its biodegradation and did not improve biogas production during the AD process. In addition, the anaerobic digestion of mezcal bagasse offers a sustainable alternative for waste management, promoting the valorization of by-products. These findings highlight the potential of Agave durangensis mezcal bagasse as a renewable energy source, opening opportunities for its implementation in large-scale industrial processes.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation11070399/s1, Figure S1: Biogas production from the Ad-MB-acid acclimatization process (cycles 1 to 5).
Author Contributions
Conceptualization, M.A.G.-Z. and R.H.-L.; methodology, M.A.G.-Z., I.M.-A., B.E.B.-H., E.B.E.-A. and R.H.-L.; validation, M.A.G.-Z., I.M.-A., B.E.B.-H. and E.B.E.-A.; formal analysis, M.A.G.-Z. and R.H.-L.; investigation, M.A.G.-Z., I.M.-A., B.E.B.-H., E.B.E.-A. and R.H.-L.; resources, M.A.G.-Z.; data curation, R.H.-L.; writing—original draft preparation, M.A.G.-Z. and R.H.-L.; writing—review and editing, M.A.G.-Z., I.M.-A., B.E.B.-H., E.B.E.-A. and R.H.-L.; visualization, M.A.G.-Z.; supervision M.A.G.-Z.; project administration, M.A.G.-Z.; funding acquisition, M.A.G.-Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by SECRETARÍA DE INVESTIGACIÓN Y POSGRADO, INSTITUTO POLITÉCNICO NACIONAL (Mexico), grant number SIP-20231195; and Ma. del Refugio Hernández-López received a scholarship from SECRETARIA DE CIENCIA, HUMANIDADES, TECNOLOGÍA E INNOVACIÓN (Mexico).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- Alemán-Nava, G.S.; Casiano-Flores, V.H.; Cárdenas-Chávez, D.L.; Díaz-Chavez, R.; Scarlat, N.; Mahlknecht, J.; Dallemand, J.F.; Parra, R. Renewable energy research progress in Mexico: A review. Renew. Sustain. Energy Rev. 2014, 32, 140–153. [Google Scholar] [CrossRef]
- Morales Ramos, A.C.; Pérez Figueroa, M.; Pérez Gallardo, J.R.; Almaraz, S.D.L. Energías renovables y el hidrógeno: Un par prometedor en la transición energética de México. Investig. Cienc. Univ. Autónoma Aguascalientes 2017, 70, 92–101. [Google Scholar] [CrossRef]
- Breton-Deval, L.; Méndez-Acosta, H.O.; González-Álvarez, V.; Snell-Castro, R.; Gutiérrez-Sánchez, D.; Arreola-Vargas, J. Agave tequilana bagasse for methane production in batch and sequencing batch reactors: Acid catalyst effect, batch optimization and stability of the semi-continuous process. J. Environ. Manag. 2018, 224, 156–163. [Google Scholar] [CrossRef]
- Comercam. Informe Estaditico 2024; Consejo Mexicano Regulador de la Calidad del Mezcal, A.C.: Oaxaca, Mexico, 2024. [Google Scholar]
- Palomo-Briones, R.; López-Gutiérrez, I.; Islas-Lugo, F.; Galindo-Hernández, K.L.; Munguía-Aguilar, D.; Rincón-Pérez, J.A.; Cortés-Carmona, M.Á.; Alatriste-Mondragón, F.; Razo-Flores, E. Agave bagasse biorefinery: Processing and perspectives. Clean. Technol. Environ. Policy 2018, 20, 1423–1441. [Google Scholar] [CrossRef]
- Gómez-Guerrero, A.V.; Valdez-Vazquez, I.; Caballero-Caballero, M.; Chiñas-Castillo, F.; Alavéz-Ramírez, R.; Montes-Bernabéde, J.L. Co-digestion of Agave angustifolia haw bagasse and vinasses for biogas production from mezcal industry. Rev. Mex. Ing. Quim. 2019, 18, 1073–1083. [Google Scholar] [CrossRef]
- Arreola-Vargas, J.; Ojeda-Castillo, V.; Snell-Castro, R.; Corona-González, R.I.; Alatriste-Mondragón, F.; Méndez-Acosta, H.O. Methane production from acid hydrolysates of Agave tequilana bagasse: Evaluation of hydrolysis conditions and methane yield. Bioresour. Technol. 2015, 181, 191–199. [Google Scholar] [CrossRef]
- Pérez-pimienta, J.A.; Vargas-tah, A.; López-ortega, K.M.; Medina-lópez, Y.N.; Mendoza-pérez, J.A.; Avila, S.; Singh, S.; Simmons, B.A.; Loaces, I.; Martinez, A. Bioresource Technology Sequential enzymatic saccharification and fermentation of ionic liquid and organosolv pretreated agave bagasse for ethanol production. Bioresour. Technol. 2017, 225, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Olugbemide, A.D.; Oberlintner, A.; Novak, U.; Likozar, B. Lignocellulosic corn stover biomass pre-treatment by deep eutectic solvents (Des) for biomethane production process by bioresource anaerobic digestion. Sustainability 2021, 13, 10504. [Google Scholar] [CrossRef]
- Olatunji, K.O.; Madyira, D.M. Enhancing the biomethane yield of groundnut shells using deep eutectic solvents for sustainable energy production. Front. Energy Res. 2024, 12, 1346764. [Google Scholar] [CrossRef]
- Li, J.; Liu, W.; Meng, J.; Zhao, L.; Li, J.; Zheng, M. Mesothermal pretreatment using FeCl3 enhances methane production from rice straw. Renew. Energy 2022, 188, 670–677. [Google Scholar] [CrossRef]
- Al Afif, R.; Wendland, M.; Amon, T.; Pfeifer, C. Supercritical carbon dioxide enhanced pre-treatment of cotton stalks for methane production. Energy 2020, 194, 116903. [Google Scholar] [CrossRef]
- Valdez-Vazquez, I.; Alatriste-Mondragón, F.; Arreola-Vargas, J.; Buitrón, G.; Carrillo-Reyes, J.; León-Becerril, E.; Mendez-Acosta, H.O.; Ortíz, I.; Weber, B. A comparison of biological, enzymatic, chemical and hydrothermal pretreatments for producing biomethane from Agave bagasse. Ind. Crops Prod. 2020, 145, 112160. [Google Scholar] [CrossRef]
- Heiske, S.; Schultz-Jensen, N.; Leipold, F.; Schmidt, J.E. Improving Anaerobic Digestion of Wheat Straw by Plasma-Assisted Pretreatment. J. At. Mol. Phys. 2013, 2013, 791353. [Google Scholar] [CrossRef]
- Wang, C.; Shao, Z.; Qiu, L.; Hao, W.; Qu, Q.; Sun, G. The solid-state physicochemical properties and biogas production of the anaerobic digestion of corn straw pretreated by microwave irradiation. RSC Adv. 2021, 11, 3575–3584. [Google Scholar] [CrossRef]
- Arreola-Vargas, J.; Flores-Larios, A.; González-Álvarez, V.; Corona-González, R.I.; Méndez-Acosta, H.O. Single and two-stage anaerobic digestion for hydrogen and methane production from acid and enzymatic hydrolysates of Agave tequilana bagasse. Int. J. Hydrogen Energy 2016, 41, 897–904. [Google Scholar] [CrossRef]
- Song, Z.; Yang, G.; Liu, X.; Yan, Z.; Yuan, Y.; Liao, Y. Comparison of seven chemical pretreatments of corn straw for improving methane yield by anaerobic digestion. PLoS ONE 2014, 9, e93801. [Google Scholar] [CrossRef]
- Muñoz-Páez, K.M.; Alvarado-Michi, E.L.; Moreno-Andrade, I.; Buitrón, G.; Valdez-Vazquez, I. Comparison of suspended and granular cell anaerobic bioreactors for hydrogen production from acid agave bagasse hydrolyzates. Int. J. Hydrogen Energy 2020, 45, 275–285. [Google Scholar] [CrossRef]
- Liu, X.; Zicari, S.M.; Liu, G.; Li, Y.; Zhang, R. Pretreatment of wheat straw with potassium hydroxide for increasing enzymatic and microbial degradability. Bioresour. Technol. 2015, 185, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.C.; Dohleman, F.G.; Long, S.P. The global potential for Agave as a biofuel feedstock. GCB Bioenergy 2011, 3, 68–78. [Google Scholar] [CrossRef]
- Buitrón, G.; Carvajal, C. Biohydrogen production from Tequila vinasses in an anaerobic sequencing batch reactor: Effect of initial substrate concentration, temperature and hydraulic retention time. Bioresour. Technol. 2010, 101, 9071–9077. [Google Scholar] [CrossRef]
- Li, L.; Chen, C.; Zhang, R.; He, Y.; Wang, W.; Liu, G. Pretreatment of Corn Stover for Methane Production with the Combination of Potassium Hydroxide and Calcium Hydroxide. Energy Fuels 2015, 29, 5841–5846. [Google Scholar] [CrossRef]
- American Public Health Association; American Water Works Association; Water Environment Federation; Lipps, W.C.; Braun-Howland, E.B.; Baxter, T.E. (Eds.) Standard Methods for the Examination of Water and Wastewater, 24th ed.; APHA Press: Washington, DC, USA, 2023. [Google Scholar]
- Gouluke, C.G. Biological Processing: Composting and Hydrolysis. In Handbook of Solid Waste Management; Wilson, D.G., Ed.; Reinhold van Norstrand: New York, NY, USA, 1977; pp. 195–225. [Google Scholar]
- Miller Gail Lorenz. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- HACH. Method 8196: Volatile acids, total. In DR5000 Spectrophotometer Procedures Manual, 2nd ed.; Hach Company: Duesseldorf, Germany, 2005. [Google Scholar]
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for Dietary Fiber, Neutral Detergent Fiber, and Nonstarch Polysaccharides in Relation to Animal Nutrition. J. Dairy. Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gutiérrez, G.A.; Ortiz-Hernández, Y.D.; Aquino-Bolaños, T.; Bautista-Cruz, A.; López-Cruz, J.Y. Properties of Agave angustifolia Haw. bagasse before and after its composting. Comun. Sci. 2015, 6, 418–429. [Google Scholar] [CrossRef][Green Version]
- Jȩdrzejczyk, M.; Soszka, E.; Czapnik, M.; Ruppert, A.M.; Grams, J. Physical and chemical pretreatment of lignocellulosic biomass. In Second and Third Generation of Feedstocks: The Evolution of Biofuels; Elsevier: Amsterdam, The Netherlands, 2019; pp. 143–196. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of Lignocellulosic Wastes to Improve Ethanol and Biogas Production: A Review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef]
- Momayez, F.; Karimi, K.; Taherzadeh, M.J. Energy recovery from industrial crop wastes by dry anaerobic digestion: A review. Ind. Crops Prod. 2019, 129, 673–687. [Google Scholar] [CrossRef]
- Kumar, B.; Bhardwaj, N.; Agrawal, K.; Chaturvedi, V.; Verma, P. Current perspective on pretreatment technologies using lignocellulosic biomass: An emerging biorefinery concept. Fuel Process. Technol. 2020, 199, 106244. [Google Scholar] [CrossRef]
- González Suárez, A.; Hernández Alfonso, G.; Pereda Reyes, I.; PretrHernández, C.; Ziarelli, F.; Gaime, I.; Marie, A.; García, G.; García-pérez, J.; Gutierrez-rivera, B.; et al. Bagasse to Enhance its Enzymatic Hydrolysis. Cent. Azúcar 2019, 46, 79–88. [Google Scholar]
- Mancini, G.; Papirio, S.; Lens, P.N.L.; Esposito, G. Increased biogas production from wheat straw by chemical pretreatments. Renew. Energy 2018, 119, 608–614. [Google Scholar] [CrossRef]
- Shetty, D.J.; Kshirsagar, P.; Tapadia-Maheshwari, S.; Lanjekar, V.; Singh, S.K.; Dhakephalkar, P.K. Alkali pretreatment at ambient temperature: A promising method to enhance biomethanation of rice straw. Bioresour. Technol. 2017, 226, 80–88. [Google Scholar] [CrossRef]
- Talha, Z.; Ding, W.; Mehryar, E.; Hassan, M.; Bi, J. Pretratamiento Alcalino de Bagazo de Caña de Azúcar y Lodo de Filtrado Codigerido para Mejorar la Producción de Biometano; Universidad Nacional de Cuyo: Mendoza, Argentina, 2016. [Google Scholar]
- Mankar, A.R.; Pandey, A.; Modak, A.; Pant, K. Pretreatment of lignocellulosic biomass: A review on recent advances. Bioresour. Technol. 2021, 334, 125235. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory by-products and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).