1. Introduction
Fungi are one of the most common and diverse organisms on Earth. Multicellular fungi play a central role in bioconversion, as they can produce extracellular enzymes that degrade organic matter into assimilable nutrients [
1]. These organisms grow from filaments (hyphae) that form the mycelium source of mycoproteins. Due to its natural adaptation to growing on solid organic substances with low water content and ability to secrete a variety of extracellular enzymes, fungi are highly suitable for application in fermentation processes [
2]. Fungal fermentation is widely applied in the production of enzymes, organic acids, biofuels, and protein-rich food ingredients and is increasingly explored in solid-state systems for sustainable solutions [
3].
In solid-state fermentation (SSF), fungi grow on moist solid substrates, like those used for mushroom cultivation, without the need for large volumes of free-flowing liquid. This technique offers a sustainable way to utilize waste materials and can enhance the nutritional profile of the final product through fungal activity. This opens the possibilities for the further exploration of using food waste streams for mushroom cultivation via SSF.
Fungal fermentation could be applied to different substrates and enhance a substrate’s nutritional profile. The traditional biomass for fungi cultivation includes agricultural residues, forestry waste, and food processing by-products [
4]. However, these substrates can be limited to availability and can also be expensive due to other possibilities of use [
5,
6].
There are several factors to consider when choosing a substrate for the fungal fermentation process. These include the availability of the substrate, the nutrient and moisture content, and also pH values [
7]. Fruit and vegetable by-products vary in chemical composition and moisture content, so it is important to choose the raw material that is appropriate for the type of fungi being grown. Moreover, these organisms have different pH requirements, and it is crucial to adjust the pH to meet the needs of the fungi’ growth conditions [
8].
Fungi (particularly
Basidiomycetes and some
Ascomycetes) have a long history of medicinal use. Their fruit bodies, cultured mycelia, and broths contain various bioactive compounds (polysaccharides, lectins, etc.) with demonstrated health benefits including antioxidant, antiviral, and antitumor properties [
9,
10]. SSF offers a promising approach for cultivating these medicinal mushrooms and their bioactive products from organic waste. However, scaling up SSF processes presents challenges, particularly in maintaining a consistent temperature throughout the substrate. Beyond medicinal mushrooms, SSF holds potential for producing a diverse range of bioproducts, like biopesticides, biosurfactants, and bioplastics, thereby utilizing waste materials in a sustainable manner.
This review focuses on the emerging applications of SSF, substrate selection, and process optimization aspects. Special attention is given to the diversity of substrates derived from fruit and vegetable by-products as well as crop residues or food industries’ waste. This review also examines critical process parameters–such as inoculum type, moisture content, aeration, temperature, pH, and particle size–that influence fungal growth and product yield in SSF systems. In addition, it explores current challenges in scaling up SSF processes from the laboratory to the industrial scale. By integrating these aspects, this review aims to highlight the potential of SSF as a sustainable platform for producing high-value products in the context of the circular bioeconomy.
2. SSF
While SSF has ancient roots due to tempeh or miso production, modern advancements have led to diverse SSF systems [
11]. There are two main types of SSF systems: natural substrate-based and inert support-based [
12].
SSF has gained attention for its potential to produce enzymes and secondary metabolites in higher quantities than submerged fermentation [
13]. Filamentous fungi secrete significantly more enzymes in SSF, and certain products like antibiotics and spores can only be produced using this method [
14]. Further research into this physiology will be crucial for optimizing SSF processes, improving strains, and developing new technologies.
Filamentous fungi are the primary microorganisms employed in SSF. Their hyphal growth pattern, tolerance to low water activity [
15], and resistance to osmotic pressure confer significant advantages over unicellular organisms in colonizing solid substrates and utilizing nutrients. Filamentous fungi are prolific enzyme producers [
16] capable of synthesizing aromas [
17] and health-promoting compounds [
18] that are valuable to the food industry. Additionally, they serve as natural antagonists to agricultural pests.
Strain selection is a critical aspect of SSF, particularly when aiming for commercially competitive enzyme yields, i.e., for animal feed [
19]. Careful screening of strains for their ability to produce the desired end-product is essential. While fungal strains are commonly used for producing hydrolytic enzymes, bacteria also play a role in natural fermentation applications like composting and food preservation. Several bacterial strains, such as
Staphylococcus sp. and
Brevibacterium sp., have been successfully utilized for enzyme production.
The latest advancements in SSF are characterized by a multidisciplinary approach, combining cutting-edge biotechnological tools with innovative engineering solutions to enhance efficiency, scalability, and the range of valuable products that can be obtained from agro-industrial residues and food waste using fungal cultures. Novel physical pre-treatment techniques like microwave [
20] and ultrasound irradiation [
21] are being optimized to disrupt the recalcitrant structure of agro-residues and food waste, thus enhancing the accessibility of carbohydrates for fungal enzymes and improving overall bioconversion rates. While physical pre-treatment techniques were previously used, biological pre-treatment using specific enzymes or microbial consortia is gaining traction as an environmentally friendly approach to delignify and modify substrates, leading to better fungal colonization and enzyme production.
Metagenomics, transcriptomics, and proteomics [
22] are providing deeper insights into the complex microbial communities and metabolic pathways involved in SSF, allowing for more rational process optimization. Machine learning and artificial intelligence algorithms are being employed to analyze large datasets, predict optimal fermentation conditions, and automate process control, leading to increased efficiency and reduced costs.
While enzyme production remains a key application, there is increasing interest in using SSF to produce high-value compounds like nutraceuticals, biopharmaceuticals, biopesticides, and specialty chemicals from agro-residues or food industries’ waste.
2.1. Parameters Which Influence the SSF Process
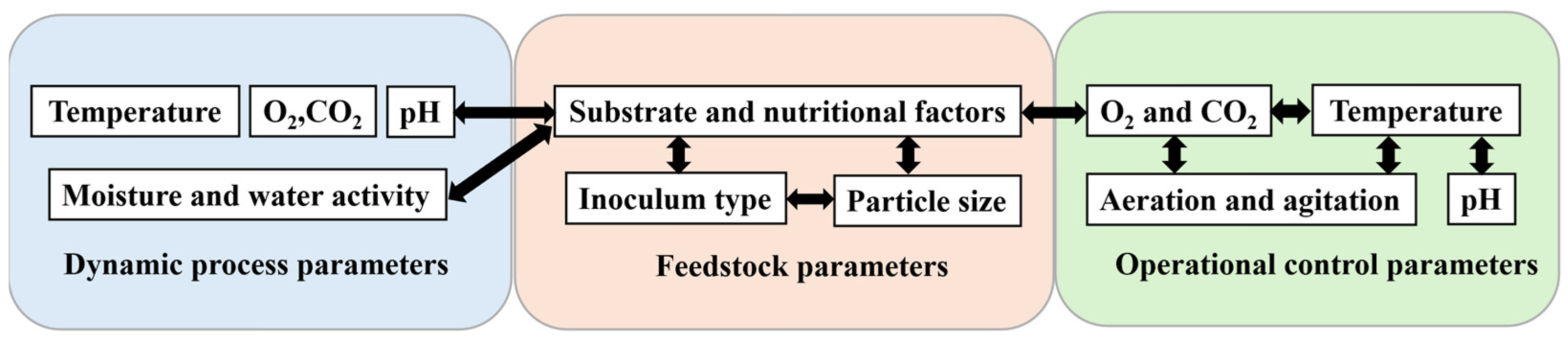
The efficiency and yield of the SSF process are profoundly influenced by a complex interplay of various parameters, encompassing biological, physico-chemical, and environmental factors, as delineated in
Figure 1. These critical parameters include the physico-chemical characteristics of the substrate and its nutritional composition (e.g., the C:N ratio and the availability of micronutrients), thermodynamic conditions such as temperature (influencing enzymatic kinetics and metabolic rates), gaseous phase dynamics encompassing oxygen (O
2) supply and carbon dioxide (CO
2) removal, mass transfer mechanisms like aeration and agitation, the physiological state and concentration of the inoculum, the morphological attributes of the solid matrix (e.g., particle size and porosity), and hydrodynamic properties such as pH and moisture content (influencing water activity and nutrient diffusion). The intricate interactions among these factors mean that their individual significance and synergistic effects can vary considerably depending on the specific substrate employed, the selected filamentous fungal strain, and the operational scale and technical parameters of the bioprocess.
Given that the majority of research in this field has historically concentrated on fungal SSF, the subsequent discussion will primarily address fungal processes unless otherwise noted, with a particular focus on how the optimized control of these parameters contributes to the effective valorization of food industry waste, as highlighted by recent advancements in fungal biomass fermentation.
2.1.1. Inoculum Type
The use of spores as an inoculum in SSF offers several advantages over vegetative cells. Spores can serve as biocatalysts, facilitating bioconversion reactions similar to those performed by the mycelium. Additionally, spores are convenient, adaptable, and have a longer shelf life, making them more resistant to handling errors. However, spores also have drawbacks, including longer lag times, different optimal conditions for germination and growth, and a larger inoculum requirement.
Spores are metabolically dormant, requiring activation and enzyme synthesis before substrate utilization and growth. While some organisms necessitate a vegetative inoculant, the mycelial inoculum has been shown to yield higher protein content in certain fermentation systems, such as wheat straw with
Chaetomium cellulolyticum [
23], due to the immediate availability of enzymes. In the case of
Aspergillus niger, phytase yield and biomass formation have been demonstrated to be strongly correlated with inoculum age, suggesting growth-associated phytase production [
24].
Inoculum density is another crucial factor in SSF, influencing the overall fermentation process [
25]. Inoculum density refers to the concentration or amount of microorganisms (fungal spores or mycelial fragments) introduced into a solid substrate for SSF. This parameter significantly influences the fermentation process, affecting factors such as the growth rate and mycelial development [
26]. Contamination risk: A higher inoculum density can outcompete potential contaminants, reducing the risk of contamination [
25]. Product yield and quality: The optimal inoculum density can lead to higher product yields and improved product quality, whereas excessive inoculum density may result in increased metabolic activity and competition for resources, potentially affecting product formation [
27]. Substrate utilization: A higher inoculum density can more efficiently utilize the available nutrients in the substrate.
In general, higher inoculum densities are typically used for faster growth and to reduce contamination, while lower densities may be suitable for specific applications where slower growth or controlled product formation is desired.
2.1.2. Substrate and Nutritional Factors
Solid substrates for SSF are primarily composed of polysaccharides like starch, cellulose, and lignocellulose. These agricultural residues or byproducts serve as both structural matrices and nutrient sources. Substrate preparation involves size reduction, hydrolysis, nutrient supplementation, and pH and moisture adjustment to enhance microbial utilization [
4].
Substrate selection is influenced by factors like the cost, availability, and heterogeneous nature. Two primary SSF systems exist: natural substrate-based and inert support-based. Natural substrates provide both structure and nutrients, while inert supports primarily serve as anchors. Agro-industrial residues are often the preferred substrates due to their nutrient content. Examples include sugarcane bagasse, wheat bran, rice straw, coconut coir, banana waste, tea and coffee waste, cassava waste, and various agricultural by-products [
28].
SSF processes using these substrates have diverse applications, including the production of food, feed, enzymes, chemicals, ethanol, single-cell proteins, mushrooms, organic acids, and secondary metabolites and dye degradation [
29,
30,
31].
Sporulation in microorganisms is influenced by a variety of nutritional factors, including carbon sources, nitrogen sources, minerals, and vitamins. Carbon provides the energy necessary for growth and can be supplied as simple sugars (e.g., glucose) or complex molecules (e.g., cellulose and starch). Nitrogen sources such as ammonium salts, nitrates, urea, peptones, and amino acids stimulate fungal conidiation. Minerals like sodium, calcium, nickel, copper, iron, manganese, potassium, zinc, magnesium, molybdenum, and boron are also essential for sporulation. Additionally, certain organic compounds, including vitamins and steroids, can enhance fungal sporulation when added to the growth medium [
32].
Biomass composition must be considered when formulating media. Cellular biomass typically consists of 40–50% carbon, 30–50% oxygen, 6–8% hydrogen, and 3–12% nitrogen. While phosphorus, sulfur, and metals are also necessary, they are required in smaller quantities. The carbon-to-nitrogen (C:N) ratio is a critical parameter for obtaining specific products in fermentation processes. A C:N ratio of 16 is often considered suitable, but variations can occur depending on the relationship between product formation and growth [
33].
2.1.3. Particle Size
Substrate particle size is a critical factor in SSF, influencing system capacity, microbial growth, and heat and mass transfer. A smaller particle size increases the surface area-to-volume ratio, enhancing the accessibility of the substrate to microorganisms and improving packing density. However, excessively small particles can lead to agglomeration, hindering microbial respiration and aeration [
34].
Particle size also affects oxygen transfer and heat exchange. Smaller particles facilitate these processes, while larger particles may provide better respiration but limit the available surface area for microbial activity. It is important to note that substrate particle size can decrease during SSF due to degradation and erosion.
Optimizing particle size involves balancing the need for an adequate surface area for microbial interaction with considerations for agglomeration, mass transfer, and heat exchange. Selecting the appropriate particle size range is essential for efficient SSF processes.
2.1.4. Moisture and Water Activity
Moisture content is a critical factor in SSF, influencing microbial growth, enzyme stability, and substrate swelling. Optimal moisture levels must be maintained within a specific range to prevent nutrient diffusion limitations, particle agglomeration, and competition from bacteria. Maintaining appropriate water activity (Aw) is essential for microbial growth and product formation.
A
w is a thermodynamic parameter that reflects the availability of water for reactions within the solid substrate. It is influenced by the water-binding properties of the substrate and can change during SSF due to dehydration and solute accumulation. Reduced Aw can negatively impact microbial growth, extending lag phases, decreasing growth rates, and limiting biomass production. Bacteria generally require higher A
w values for growth compared to fungi, giving fungi a competitive advantage in SSF environments [
35].
Measuring water activity is crucial for monitoring and controlling SSF processes. Various methods and equipment can be used to assess the pressure difference between the substrate and the gaseous phase in equilibrium. Maintaining a water activity value close to one is often desirable for optimal spore production.
Water content and water activity are interconnected, with changes in one affecting the other. While fermentation can increase water activity due to water co-production, evaporative losses may counteract this effect. The precise control of moisture content and water activity is essential for successful SSF, requiring careful consideration during substrate preparation and process management [
15].
2.1.5. pH
pH is a critical parameter in fermentation processes and is influenced by metabolic activities. The secretion of organic acids can lead to pH reduction, while the assimilation of organic acids or urea hydrolysis can cause pH elevation. Each microorganism has a specific pH range for optimal growth and activity. Filamentous fungi generally exhibit a wider pH tolerance (2–9) compared to yeasts (4–5). This pH versatility can be advantageous in preventing bacterial contamination by maintaining a lower pH [
36].
pH control is challenging in SSF due to the heterogeneous nature of the system and limitations in equipment and electrodes for measuring pH in solid materials. pH changes can be attributed to the nitrogen source and the growth characteristics of the microorganism.
To address pH variability in SSF, substrate formulation and buffering can be employed. Using urea as a nitrogen source instead of ammonium salts can help control the pH [
37]. Incorporating buffering agents that do not interfere with biological activity can also mitigate pH fluctuations.
2.1.6. Temperature
Temperature is a crucial factor in SSF, influencing growth, enzyme production, and metabolite formation. Fungi exhibit a wide temperature tolerance, but the optimal temperature for growth may differ from that for product formation. Mesophilic strains are commonly used in SSF, but temperature control can be challenging due to the low thermal conductivity of solid substrates and the static nature of many SSF processes [
38]. Heat generation, influenced by metabolic activity and moisture content, can create thermal gradients within the substrate.
Heat removal is another critical aspect of SSF. Forced aeration is a common method for dissipating heat, but the proper control of airflow and humidity is essential to prevent adverse effects. Evaporative cooling can be effective in removing heat but may lead to moisture loss, requiring water addition to maintain optimal moisture levels [
37].
Temperature significantly impacts biological processes, including protein denaturation, enzyme inhibition, metabolite production, and cell death. Understanding and controlling temperature is essential for optimizing SSF processes and ensuring desired outcomes.
2.1.7. Aeration and Agitation
Aeration and agitation are critical parameters in SSF, influencing oxygen transfer, heat exchange, and mass transport within the heterogeneous system. Aeration provides oxygen, removes carbon dioxide and other volatile metabolites, and dissipates heat. The aeration rate is determined by factors like microbial growth requirements, metabolite production, and heat evolution [
25].
Oxygen transfer rates are influenced by various factors, including air pressure, flow rate, substrate porosity, bed depth, vessel design, moisture content, and reactor geometry. To express aeration intensity independently of scale, intensive values are used. Air quality at the fermenter inlet is also essential. Airflow is a primary method for heat removal, with water evaporation and environmental heat transfer also contributing to temperature control.
Agitation is crucial for ensuring homogeneity, providing a gas–liquid interface, and enhancing mass and heat transfer. It allows for homogeneous water addition to compensate for evaporative losses [
39]. However, agitation can affect substrate properties, such as agglomeration and structural integrity. The suitability of agitation depends on the microorganism, substrate, and reactor design. While agitation can improve product yields, it may also negatively impact growth, substrate porosity, and mycelial attachment.
Intermittent agitation may be more appropriate for certain SSF processes to prevent damage to the mycelia and maintain the substrate structure. The choice between agitated and static reactors depends on the specific process, product, and desired outcomes.
2.1.8. O2 and CO2
Aeration plays a multifaceted role in SSF, influencing aerobic conditions, carbon dioxide desorption, temperature regulation, and moisture control. The gas environment significantly impacts biomass and enzyme production. SSF’s open structure facilitates oxygen diffusion into the substrate, minimizing oxygen limitations. The high contact surface between the gas phase, substrate, and aerial mycelium further enhances oxygen transfer [
40,
41].
Controlling the gas phase and airflow is a straightforward method for regulating gas transfer. The theoretical respiratory quotient (RQ) of aerobic microorganisms is 1.0. An RQ below 1.0 indicates insufficient oxygen transfer, leading to impaired growth.
The oxygen uptake rate (OUR) and the carbon dioxide production rate (CDPR) are widely used for estimating growth in SSF. These measurements offer rapid response times and direct insights into microbial metabolism. By evaluating yield coefficients, microbial biomass can be estimated from OUR and CDPR data, although these coefficients may vary with the growth rate [
42].
2.2. Applications of SSF
SSF offers a wide range of applications with significant economic potential, particularly in low-technology settings. Numerous studies have explored the diverse applications of SSF, which include traditional fermented foods [
43] (e.g., koji, tempeh, and ragi) and high-value compounds (enzymes, organic acids, biopesticides, biofuel, and flavors).
In recent years, SSF has emerged as a valuable tool for environmental remediation, addressing issues such as the bioremediation of hazardous compounds and the detoxification of agro-industrial residues.
Table 1 provides a comprehensive overview of SSF applications across various economic sectors, including fermentation, agriculture, and environmental control.
3. Fruit and Vegetable By-Products as a Substrate for SSF
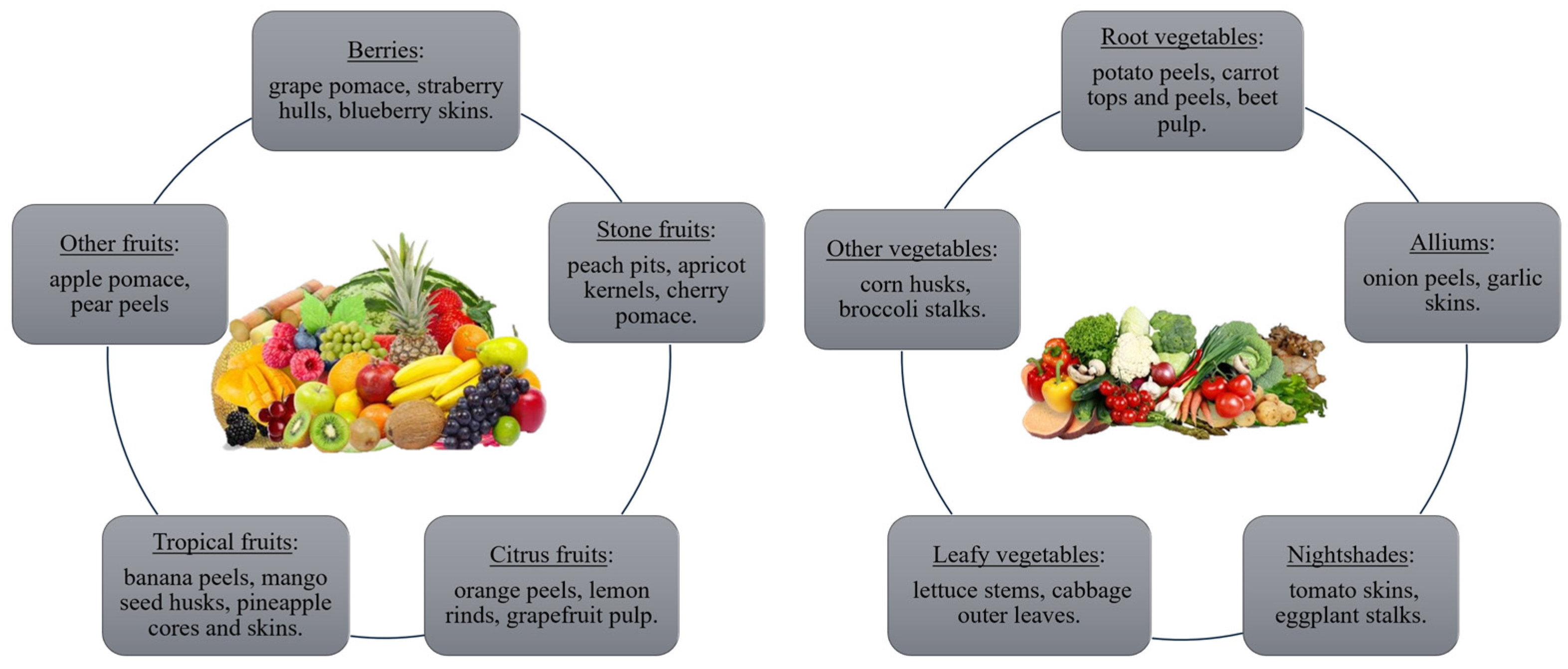
Fruits and vegetables constitute a significant portion of global food consumption but contribute disproportionately to food waste. The increasing global population and technological advancements have exacerbated the imbalance between supply and demand, leading to a rise in food wastage. While source reduction and recycling are promising strategies, a further evaluation of their economic and environmental impacts is warranted. Residues of primary processing of fruits and vegetables are valued materials due to their chemical compounds, structure, and composition for SSF [
80]; they are used as precursors for the formation of new metabolites [
81]. The list of fruit and vegetable by-products can be found in
Figure 2.
Fruits and vegetables are a rich source of dietary components essential for human health. They contain carbohydrates, acids, minerals, polyphenols, water-soluble vitamins (C and B-complex), provitamin A, amino acids, aromatic compounds, carotenoids, fibers, phytosterols, and other bioactive substances. Their water content typically ranges from 70 to 90%, with lipids concentrated primarily in seeds, and their protein content is generally low [
82].
Fruit and vegetable consumption is associated with a reduced risk of chronic diseases like cancer, heart disease, and stroke [
83]. This is likely due to the presence of bioactive compounds. Additionally, fruit juices are low in sodium and potassium, contributing to healthy blood pressure, and their lack of fat benefits the cardiovascular system. Some studies suggest potential benefits for Alzheimer’s disease and cancer prevention [
84]. While consumption has increased in recent decades, it remains below recommended levels.
Fruits, vegetables, and their by-products are valuable sources for extracting bioactive compounds used in nutraceuticals and functional foods. Functional foods are processed or natural foods with demonstrated health benefits beyond basic nutrition. They may have increased levels of beneficial compounds, reduce undesirable components, or added functional ingredients [
85]. Fruits and vegetables themselves can be considered functional foods due to their content of health-promoting bioactive compounds [
86].
Fruit and vegetable residues (FVRs) represent a significant source of waste generated by the food processing industry. The high carbohydrate content of these residues, ranging from 20–30% dry weight in citrus pulps to 60–70% dry weight in potato peels, makes them promising substrates for fermentation processes.
FVRs can be valorized through various approaches, including biofuel production and the extraction of bioactive compounds. Bioethanol, biobutanol, biomethane, biohydrogen, and organic acids can be obtained from FVRs via fermentation [
47]. Additionally, extraction techniques can be employed to isolate bioactive compounds such as phenolic compounds from olive, orange, tomato, and raspberry [
87] waste.
A literature search revealed a growing interest in utilizing FVRs for biofuel and bioactive compound production. Cassava, potato, orange, and coffee waste was frequently studied for biofuel production [
88], while olive, orange, and tomato waste was commonly used for extracting phenolic compounds [
89].
Recommendations and future perspectives are provided to guide the development of techno-economically efficient waste management techniques for fruit and vegetable valorization. An example of FVR fermentation processes is shown in
Figure 3.
3.1. Variety of Bioactive Compounds in Fruit and Vegetable By-Products
Bioactive compounds from fruit and vegetable by-products, with their potential to mitigate the risk of various diseases, have garnered significant research attention. Studies have demonstrated the association between bioactive compound intake and reduced risk of cardiovascular disease, cancer, and degenerative diseases. The antioxidant activity of these compounds, which neutralize free radicals, plays a crucial role in their health-promoting effects [
90].
Fruit and vegetable waste (FVW) contains a diverse array of bioactive fractions, including carbohydrates, proteins, lipids, and secondary metabolites. Polysaccharides in FVW have been identified as anticancer and anti-inflammatory agents [
91]. Moreover, the protein content of fruit and vegetable seeds, when hydrolyzed, releases bioactive peptides with pharmacological properties. Additionally, FVW contains bioactive lipids such as carotenoids, sterols, and fatty acids. Groups of chemicals and compounds from a particular FVW are listed in
Figure 4.
The potential of FVW as a source of bioactive compounds, particularly polysaccharides, has gained increasing interest [
92]. Researchers have explored the use of FVW as substrates for obtaining secondary metabolites, including polyphenols [
93], alkaloids, and volatile acids [
94].
3.2. Fruit and Vegetable By-Product Use in SSF
Fungi are among the earliest microorganisms exploited for fermentation processes. Their ability to synthesize a diverse range of extracellular enzymes, antibiotics, and pigments has made them ideal for utilizing vegetable waste [
95]. Examples of fermentation products include lactic acid [
96], functional carbohydrates, organic acids, carotenoids, and enzymes like glycosidases and lipases. Additionally, fungal fermentation has been employed to enhance the nutritional properties of substrates, such as increasing the fiber and protein content [
97].
Aspergillus species have been extensively used for producing enzymes with technological applications. Xylanases, phytases, α-amylases, proteases, and lipases are examples of enzymes obtained through fungal fermentation of various agricultural residues including fruit and vegetable by-products such as tomatoes pomace [
98].
Fungal fermentation is also employed for producing natural pigments from corn cobs, such as those generated by
Monascus purpureus, which also yields an anti-hypercholesterolemic agent [
99].
The fermentation of agri-food waste including fruit and vegetable by-products using fungal species can lead to the simultaneous production of multiple value-added ingredients. For example, olive-mill wastewater fermentation with
Yarrowia lipolytica produces citric acid and oleic acid [
100], while a combination of
Aspergillus,
Pleurotus, and
Hericium spp. can generate fractions rich in polyphenols, antioxidants, and fiber from cooked maize residue [
101]. The diverse metabolic capabilities of fungi offer opportunities for designing fermentation strategies that maximize the sustainable production of value-added ingredients from vegetable waste.
4. Brewer’s Spent Grain (BSG) as a Substrate for SSF
One of the biggest waste producers are breweries where brewing generates significant quantities of BSG, a by-product primarily composed of barley husks. While traditionally disposed of as waste, BSG presents opportunities for valorization due to its high carbohydrate content. BSG production is substantial, with an estimated 4.5 × 10
10 kg generated annually worldwide. Its disposal through landfills or animal feed has environmental drawbacks, including methane emissions from ruminant livestock and uncontrolled anaerobic fermentation in landfills [
102].
The application of BSG as a primary substrate in SSF represents a compelling and increasingly explored avenue for biowaste valorization. While BSG’s potential as an animal feed component is well-established, its direct utilization as a structured matrix for microbial cultivation in SSF offers a novel approach to harness its inherent lignocellulosic and proteinaceous composition [
103]. This strategy moves beyond simple disposal or low-value applications, enabling the production of higher-value bioproducts such as enzymes, organic acids [
104], biofuels, and protein-enriched food [
105] and feed. The inherent solid nature and nutrient richness of BSG [
106] provide a cost-effective and environmentally sound platform for microbial growth and biotransformation, positioning it as a promising substrate for sustainable biotechnology and circular economy initiatives within the brewing and related industries.
4.1. Variety of Bioactive Compounds in BSG
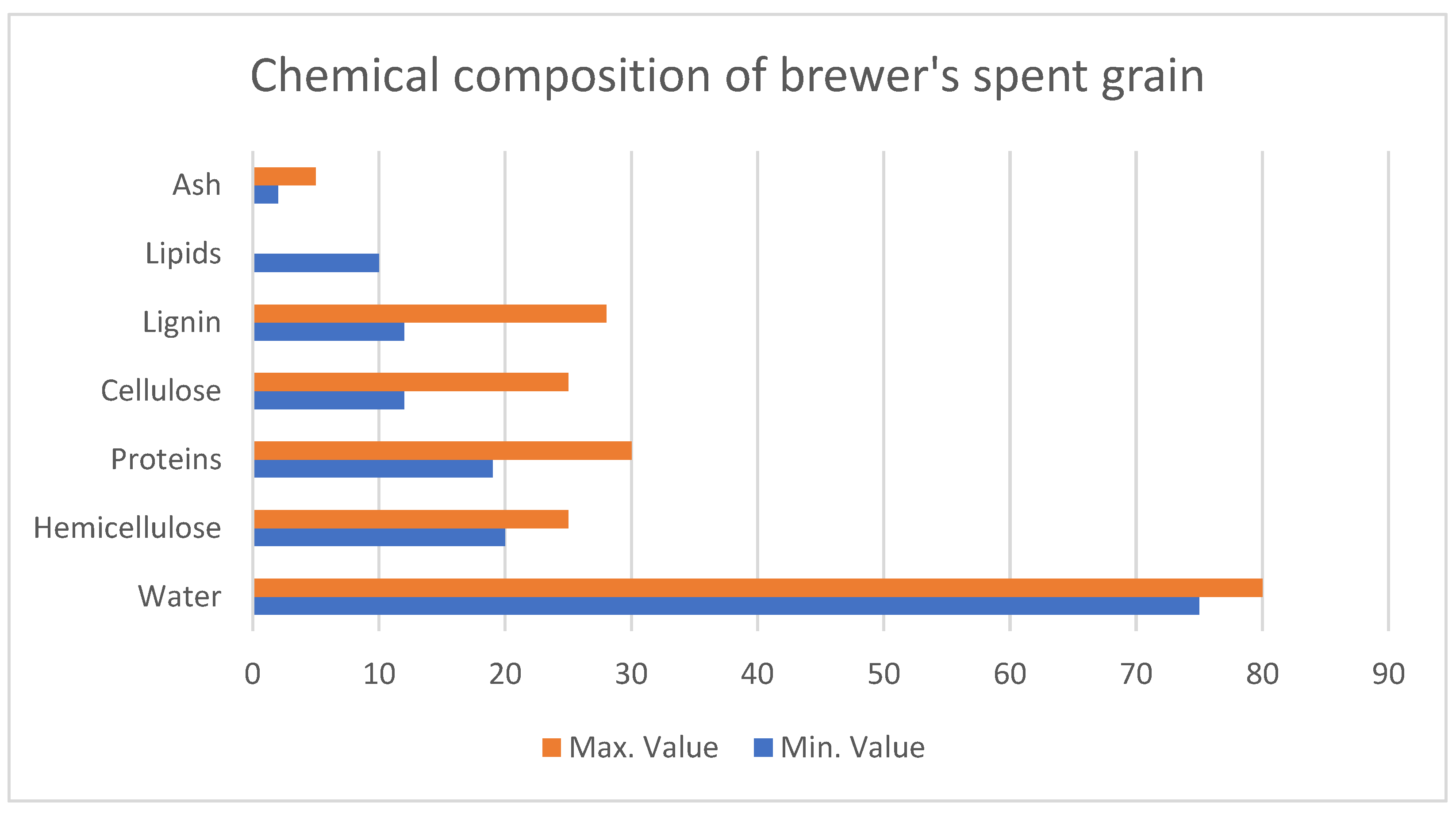
According to the different sources, BSG’s chemical composition, which is presented in
Figure 5, can vary significantly due to factors like barley variety, harvest conditions, and processing methods [
107]. While primarily consisting of recalcitrant barley husks, BSG also contains portions of the endosperm, including starch, proteins, and lipids.
Non-starch polysaccharides (NSPs) dominate BSG composition, accounting for over 60% of the dry weight. Arabinoxylan is the most abundant NSP, followed by lignin. Protein content is also substantial, ranging from 10 to 26% [
108].
Lignocellulosic compounds, including cellulose, hemicellulose, and lignin, form the structural components of plant cell walls. Cellulose is a linear polymer of glucose units, while hemicellulose is a branched heteropolymer primarily composed of arabinoxylan. Lignin is a complex phenolic polymer that provides structural rigidity and resistance to microbial degradation [
109].
The heterogeneous nature of lignocellulosic compounds, including their branching and varying degrees of polymerization, makes them challenging to degrade enzymatically. This resistance is further compounded by the presence of lignin, which binds and inhibits hydrolytic enzymes. BSG’s lignocellulosic composition is similar to other agricultural and industrial waste streams, suggesting that similar biovalorization techniques could be applied to these materials [
102]. Wet corn distillers’ grain and barley straws are examples of other lignocellulosic-rich byproducts.
BSG also consists of several types of phenols including
Hydroxycinnamic acids which include ferulic acid, p-coumaric acid, and caffeic acid, which have antioxidant and anti-inflammatory properties [
110];
Flavonoids like catechins, quercetin, and rutin, which are known for their antioxidants, anti-inflammatory, and anticancer activities [
111];
Resveratrol is a prominent stilbene found in BSG, as it is known for its antioxidant and cardioprotective effects [
112].
Other bioactive compounds present in BSG include
Tocopherols and vitamin E compounds with antioxidant properties [
113];
Carotenoids, including beta-carotene, have antioxidant and pro-vitamin A activities [
114];
Phytosterols which are plant-derived sterols which have cholesterol-lowering effects [
115].
The specific composition of phenols and other bioactive compounds in BSG can vary depending on factors such as barley variety, malting conditions, and brewing processes. These compounds contribute to the nutritional value and functional properties of BSG, making it a promising substrate to produce nutraceuticals, functional foods, and other value-added products [
116].
4.2. BSG Use in Fungal SSF
Lignocellulosic polymers, such as cellulose, hemicellulose, and lignin, are recalcitrant to human digestion but serve as nutrient sources for microorganisms, particularly fungi. Fungi are well-adapted to degrade these compounds, as they encounter similar materials in their natural environments. The complex structure of hemicellulose and lignin requires a suite of enzymes for complete hydrolysis. Arabinoxylan, a major component of BSG, is particularly challenging to degrade due to its structural complexity. Filamentous fungi produce a variety of enzymes capable of penetrating the lignocellulosic matrix and degrading its components. BSG’s moisture content falls within the optimal range for SSF processes, making it a suitable substrate without requiring additional drying or water addition [
117].
Fungal species can work synergistically to degrade lignocellulosic compounds. Xylanase enzymes, including endo-β-1,4-xylanase and β-D-xylosidase, are crucial for arabinoxylan degradation. Accessory enzymes like α-L-arabinofuranosidase and β-glucuronidase can also contribute to the process [
48].
SSF is a well-suited bioreactor model for the fungal degradation of BSG. Its solid-state nature and high density provide an optimal environment for filamentous fungi. This approach offers a more cost-effective alternative to traditional methods involving heat, chemicals, and purchased enzymes [
103].
5. Challenges, Economics, and Future Prospects
Fruit and vegetable by-products are promising alternative substrates for fungi and fungal culture cultivation. They are a renewable resource that is available in large quantities. They are also a good source of nutrients for fungi, including carbon, nitrogen, calcium, phosphorus, potassium, and other minerals [
118]. The use of fruit and vegetable by-products in mushroom cultivation can help to reduce major food waste and environmental problems while enhancing circular economy approaches. Reusing fruit and vegetable by-products as raw materials for fungal fermentation could divert these substances from landfills and incinerators to create value-added biobased products.
Challenges to BSG utilization as food industries’ waste include its high moisture content, which makes it unsuitable for combustion-based energy generation, and the dispersed nature of breweries, which increases transportation costs. Microbial spoilage can also occur during storage and transfer if BSG is not properly cooled [
107].
From an economic perspective, the choice between SSF and submerged fermentation for fungal biomass valorization, particularly using food industry waste, presents a complex trade-off between capital expenditure (CAPEX) and operational expenditure (OPEX). SSF typically demonstrates lower initial capital investment due to simpler bioreactor designs, reduced requirements for extensive agitation and aeration systems, and minimized downstream processing complexity when the entire fermented solid can be utilized [
119]. Furthermore, the utilization of readily available, low-cost agro-industrial residues as substrates significantly reduces raw material costs, a major contributor to overall production expenses (often exceeding 40% in bioprocesses) [
120]. This advantage is particularly pronounced in waste valorization schemes, where SSF can concurrently address waste disposal challenges. Conversely, submerged fermentation, while offering superior control over process parameters and generally achieving higher microbial growth rates and productivity due to homogenous nutrient distribution, often necessitates more sophisticated bioreactors, stringent sterilization protocols, and elaborate product recovery and purification stages, contributing to a higher CAPEX and energy consumption [
121]. However, the inherent challenges in large-scale SSF operations, such as difficulties in heat removal and ensuring uniform conditions, can lead to lower overall yields in scaled-up systems for certain products compared to well-established submerged fermentation processes, potentially impacting economic feasibility if high volumetric productivity is paramount. Future research endeavors are focused on optimizing SSF bioreactor designs and implementing advanced process control strategies to mitigate these scalability limitations, thereby enhancing the economic competitiveness and broader industrial applicability of SSF for the sustainable valorization of food industry by-products [
122,
123].
There are advantages and disadvantages of SSF listed in
Table 2, along with its’ future prospects.
While SSF offers compelling advantages rooted in its ecological and economic sustainability, particularly its inherent capacity to valorize diverse, low-cost agro-industrial by-products as substrates, thereby mitigating waste disposal challenges and reducing production expenses, its industrial application is often constrained by specific intrinsic limitations. The inherent low water activity characteristic of SSF environments, while providing a physical barrier to contamination by undesirable microorganisms as a key advantage over submerged fermentation [
125], concurrently impedes the kinetics of microbial growth and mass transfer, often resulting in prolonged fermentation cycles and potentially constrained volumetric productivities [
128]. This reduced moisture content also exacerbates the challenge of precise, real-time monitoring and the dynamic control of critical process parameters such as oxygen tension, carbon dioxide accumulation, and moisture gradients within the heterogeneous solid matrix, leading to spatial and temporal heterogeneities that can compromise optimal bioprocess performance and product consistency [
129].
The most significant impediment to the widespread industrial adoption of SSF remains the formidable challenge of process scalability. Translating successful laboratory-scale SSF operations to industrial production is complicated by the difficulty in maintaining uniform temperature profiles, gas exchange, and moisture distribution throughout large-volume solid beds, often leading to issues such as localized overheating, anoxia, and desiccation [
130].
However, cutting-edge research and engineering innovations are actively addressing these trade-offs. Advances in bioreactor design are crucial, with the development of novel configurations such as optimized packed-bed bioreactors, intermittently mixed rotating drums, and fluidized bed systems aimed at enhancing heat and mass transfer efficiency and promoting homogeneity across larger scales [
131]. The integration of advanced sensor technologies and computational fluid dynamics (CFD) modeling is enabling more accurate, in situ monitoring of key parameters like oxygen consumption and water activity, facilitating the implementation of sophisticated feedback control strategies that mitigate heterogeneity issues and optimize process conditions [
130]. Moreover, ongoing efforts in microbial strain engineering are developing robust fungal strains with enhanced tolerance to a broader range of water activities and improved metabolic efficiencies under SSF conditions. These concerted advancements in bioreactor technology, process control, and microbial biotechnology are progressively overcoming historical limitations, positioning SSF as a more competitive and industrially viable platform for sustainable bioproduction from complex organic residues.
6. Conclusions
SSF stands as a highly sustainable and ecologically advantageous bioprocess for valorizing a diverse array of low-cost, readily available substrates, including various fruit and vegetable waste streams, agro-industrial residues like brewers’ spent grain, and other food industry or agricultural by-products. By strategically cultivating fungal strains on these matrices, SSF not only facilitates the production of a wide range of value-added compounds—from enzymes and bioactive molecules to enhanced nutritional profiles in fermented biomass—but also critically contributes to waste reduction and circular economy initiatives. The efficiency of this bioconversion hinges on the precise optimization of key parameters, such as substrate selection, nutritional supplementation, inoculum type, physiological state, moisture content, water activity, pH, temperature, and the specific aeration and agitation strategies employed. Achieving optimal conditions across these interconnected factors is paramount for maximizing product yield, ensuring product quality, and mitigating contamination risks.
Despite its inherent advantages and broad applications, from traditional fermented foods to high-value industrial products, the widespread industrial adoption of SSF faces identifiable scaling challenges. The inherent heterogeneity of solid substrates complicates uniform heat and mass transfer, leading to issues like localized temperature gradients, oxygen depletion, and uneven moisture distribution in large-scale bioreactors. Addressing these engineering bottlenecks is crucial for transitioning SSF from laboratory success to industrial viability.
Future research and development could focus on improving process efficiency by optimizing SSF parameters, developing advanced bioreactors, and enhancing scale-up capabilities, as well as expanding the substrate range by exploring new and unconventional substrates for SSF, enhancing product yield and quality by developing strategies to increase the production of desired compounds and improve their quality, and integrating SSF with other technologies by combining SSF with downstream processing techniques to create novel products or improve production efficiency. By addressing these areas, SSF can continue to play a vital role in sustainable production and resource utilization.
Author Contributions
Conceptualization, S.B.; methodology, S.B.; software, S.B.; validation, S.B., D.U. and P.S.; formal analysis, S.B.; investigation, S.B.; resources, S.B.; data curation, S.B.; writing—original draft preparation, S.B., D.U. and U.G.; writing—review and editing, S.B., D.U., P.S. and U.G.; visualization, U.G. and S.B.; supervision, J.V. and P.V.; project administration, J.V.; funding acquisition, P.V. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed by the Lithuania Research Centre for Agriculture and Forestry and attributed the long-term research program (2022–2026), “Horticulture: agrobiological foundations and technologies”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated during this study are including in this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Bahram, M.; Netherway, T. Fungi as mediators linking organisms and ecosystems. FEMS Microbiol. Rev. 2022, 46, fuab058. [Google Scholar] [CrossRef] [PubMed]
- Hölker, U.; Höfer, M.; Lenz, J. Biotechnological advantages of laboratory-scale solid-state fermentation with fungi. Appl. Microbiol. Biotechnol. 2004, 64, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Mienda, B.S.; Idi, A.; Umar, A. Microbiological Features of Solid State Fermentation and its Applications—An overview. Res. Biotechnol. 2011, 2, 21–26. [Google Scholar]
- Oiza, N.; Moral-Vico, J.; Sánchez, A.; Oviedo, E.R.; Gea, T. Solid-State Fermentation from Organic Wastes: A New Generation of Bioproducts. Processes 2022, 10, 2675. [Google Scholar] [CrossRef]
- Roudneshin, M.; Sosa, A. Optimising Agricultural Waste Supply Chains for Sustainable Bioenergy Production: A Comprehensive Literature Review. Energies 2024, 17, 2542. [Google Scholar] [CrossRef]
- Liang, K. Energy Utilization of Agricultural Waste: From Waste Management to Energy Production. J. Energy Biosci. 2024, 15, 147–159. [Google Scholar] [CrossRef]
- Bellettini, M.B.; Fiorda, F.A.; Maieves, H.A.; Teixeira, G.L.; Avila, S.; Hornung, P.S.; Junior, A.M.; Ribani, R.H. Factors affecting mushroom Pleurotus spp. Saudi J. Biol. Sci. 2019, 26, 633–646. [Google Scholar] [CrossRef]
- Siwulski, M.; Rzymski, P.; Budka, A.; Kalač, P.; Budzyńska, S.; Dawidowicz, L.; Hajduk, E.; Kozak, L.; Budzulak, J.; Sobieralski, K.; et al. The effect of different substrates on the growth of six cultivated mushroom species and composition of macro and trace elements in their fruiting bodies. Eur. Food Res. Technol. 2019, 245, 419–431. [Google Scholar] [CrossRef]
- Lindequist, U.; Niedermeyer, T.H.J.; Julich, W. The pharmacological potential of mushrooms. Evid. Based Complement. Altern. Med. 2005, 2, 285–299. [Google Scholar] [CrossRef]
- Vallavan, V.; Krishnasamy, G.; Zin, N.M.; Abdul Latif, M. A Review on Antistaphylococcal Secondary Metabolites from Basidiomycetes. Molecules 2020, 25, 5848. [Google Scholar] [CrossRef]
- Jin, G.; Zhao, Y.; Xin, S.; Li, T.; Xu, Y. Solid-State Fermentation Engineering of Traditional Chinese Fermented Food. Foods 2024, 13, 3003. [Google Scholar] [CrossRef] [PubMed]
- Barrios-González, J.; Mejýa, A. Production of Antibiotics and other Commercially Valuable Secondary Metabolites. In Current Developments in Solid-State Fermentation; Springer: New York, NY, USA, 2008; pp. 302–336. [Google Scholar] [CrossRef]
- Tsuchiya, K.; Nagashima, T.; Yamamoto, Y.; Gomi, K.; Kitamoto, K.; Kumagai, C.; Tamura, G. High level secretion of calf chymosin using a glucoamylase-prochymosin fusion gene in Aspergillus oryzae. Biosci. Biotechnol. Biochem. 1994, 58, 895–899. [Google Scholar] [CrossRef] [PubMed]
- Javier, B. Solid-state fermentation: Physiology of solid medium, its molecular basis and applications. Process Biochem. 2012, 47, 175–185. [Google Scholar] [CrossRef]
- Pandey, A.; Ashakumary, L.; Selvakumar, P.; Vijayalakshmi, K.S. Influence of water activity on growth and activity of Aspergillus niger for glycoamylase production in solid-state fermentation. World J. Microbiol. Biotechnol. 1994, 10, 485–486. [Google Scholar] [CrossRef]
- Copetti, M.V. Fungi as industrial producers of food ingredients. Curr. Opin. Food Sci. 2019, 25, 52–56. [Google Scholar] [CrossRef]
- Lindsay, M.A.; Granucci, N.; Greenwood, D.R.; Villas-Boas, S.G. Identification of New Natural Sources of Flavour and Aroma Metabolites from Solid-State Fermentation of Agro-Industrial By-Products. Metabolites 2022, 12, 157. [Google Scholar] [CrossRef]
- Zervakis, G.I.; Koutrotsios, G. Solid-State Fermentation of Plant Residues and Agro-industrial Wastes for the Production of Medicinal Mushrooms. In Medicinal Plants and Fungi: Recent Advances in Research and Development; Springer: Singapore, 2017; pp. 365–396. [Google Scholar] [CrossRef]
- Graminha, E.B.N.; Gonçalves, A.Z.L.; Pirota, R.D.P.B.; Balsalobre, M.A.A.; Da Silva, R.; Gomes, E. Enzyme production by solid-state fermentation: Application to animal nutrition. Anim. Feed Sci. Technol. 2008, 144, 1–22. [Google Scholar] [CrossRef]
- Soni, A.; Smith, J.; Thompson, A.; Brightwell, G. Microwave-induced thermal sterilization—A review on history, technical progress, advantages and challenges as compared to the conventional methods. Trends Food Sci. Technol. 2020, 97, 433–442. [Google Scholar] [CrossRef]
- Gavahian, M.; Manyatsi, T.S.; Morata, A.; Tiwari, B.K. Ultrasound-assisted production of alcoholic beverages: From fermentation and sterilization to extraction and aging. Compr. Rev. Food Sci. Food Saf. 2022, 21, 5243–5271. [Google Scholar] [CrossRef]
- Abrahams, A.M. Chapter 23—Proteomics and transcriptomics and their application in fermented foods. In Indigenous Fermented Foods for the Tropics; Academic Press: Cambridge, MA, USA, 2023; pp. 377–391. [Google Scholar] [CrossRef]
- Abdullah, A.L.; Tengerdy, R.P.; Murphy, V.G. Optimization of solid substrate fermentation of wheat straw. Biotechnol. Bioeng. 1985, 27, 20–27. [Google Scholar] [CrossRef]
- Neira-Vielma, A.A.; Aguilar, C.N.; Ilyina, A.; Contreras-Esquivel, J.C.; Carneiro-da-Cunha, M.D.G.; Michelena-Alvarez, G.; Martinez-Hernandez, J.L. Purification and biochemical characterization of an Aspergillus niger phytase produced by solid-state fermentation using triticale residues as substrate. Biotechnol. Rep. 2017, 17, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Singhania, R.R.; Patel, A.K.; Thomas, L.; Pandey, A. Solid-State Fermentation. In Industrial Biotechnology; Wiley: Hoboken, NJ, USA, 2017; pp. 187–204. [Google Scholar] [CrossRef]
- Steinkraus, K.H. Solid-state (solid-substrate) food/beverage fermentations involving fungi. Acta Biotechnol. 1984, 4, 83–88. [Google Scholar] [CrossRef]
- Ikasari, L.; Mitchell, D.A. Protease production by Rhizopus oligosporus in solid-state fermentation. World J. Microbiol. Biotechnol. 1994, 10, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, C.; Liao, J.C. Industrial Biotechnology; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2017; Volume 4. [Google Scholar] [CrossRef]
- Farag El, A.; Ray, R.C. Bioprocessing of Horticultural Wastes by Solid-State Fermentation into Value-Added/Innovative Bioproducts: A Review. Food Rev. Int. 2022, 39, 3009–3065. [Google Scholar] [CrossRef]
- Taneja, A.; Sharma, R.; Khetrapal, S.; Sharma, A.; Nagraik, R.; Venkidasamy, B.; Ghate, M.N.; Azizov, S.; Sharma, S.; Kumar, D. Value Addition Employing Waste Bio-Materials in Environmental Remedies and Food Sector. Metabolites 2023, 13, 624. [Google Scholar] [CrossRef]
- Singhania, R.R.; Patel, A.K.; Soccol, C.R.; Pandey, A. Recent advances in solid-state fermentation. Biochem. Eng. J. 2009, 44, 13–18. [Google Scholar] [CrossRef]
- Bernabé-González, T.; Cayetano-Catarino, M. Cultivation of Pleurotus pulmonarius on substrates treated by immersion in alkaline water in Guerrero, Mexico. Micol. Apl. Int. 2009, 21, 19–23. [Google Scholar]
- Desgranges, C.; Vergoignan, C.; Georges, M.; Durand, A. Biomass estimation in solid state fermentation I. Manual biochemical methods. Biochem. Eng. 1991, 35, 200–205. [Google Scholar] [CrossRef]
- Tosuner, Z.V.; Taylan, G.G.; Özmıhçı, S. Effects of rice husk particle size on biohydrogen production under solid state fermentation. Int. J. Hydrogen Energy 2019, 44, 18785–18791. [Google Scholar] [CrossRef]
- Chabite, I.T.; Lei, Z.; Ningning, Y.; Qiang, F.; Haiye, Y. Mode of Managing Nutrient Solution Based on N Use Efficiency for Lettuce (Lactuca sativa L.). J. Food Sci. Eng. 2017, 7, 29–37. [Google Scholar] [CrossRef][Green Version]
- Robledo-Narvaez, P.N.; Munoz-Paez, K.M.; Poggi-Varaldo, H.M.; Rios-Leal, E.; Calva-Calva, G.; Ortega-Clemente, L.A.; Rinderknecht-Seijas, N.; Estrada-Vazquez, C.; Ponce-Noyola, M.T.; Salazar-Montoya, J.A. The influence of total solids content and initial pH on batch biohydrogen production by solid substrate fermentation of agroindustrial wastes. J. Environ. Manag. 2013, 128, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Smits, J.P.; Rinzema, A.; Tramper, J.; Sonsbeek, H.M.; Hage, J.C.; Kaynak, A.; Knol, W. The influence of temperature on kinetics in solid-state fermentation. Enzym. Microb. Technol. 1998, 22, 50–57. [Google Scholar] [CrossRef]
- Soccol, C.R.; Vandenberghe, L.P.S.; Rodrigues, C.; Medeiros, A.B.P.; Larroche, C.; Pandey, A. Production of Organic Acids by Solid-state Fermentation. In Current Developments in Solid-State Fermentation; Springer: New York, NY, USA, 2008; pp. 205–229. [Google Scholar] [CrossRef]
- Gassara, F.; Ajila, C.M.; Brar, S.K.; Tyagi, R.D.; Verma, M.; Valero, J. Influence of aeration and agitation modes on solid-state fermentation of apple pomace waste by Phanerochaete chrysosporium to produce ligninolytic enzymes and co-extract polyphenols. Int. J. Food Sci. Technol. 2013, 48, 2119–2126. [Google Scholar] [CrossRef]
- Han, O.; Mudgett, R.E. Effects of oxygen and carbon dioxide partial pressures on Monascus growth and pigment production in solid-state fermentations. Biotechnol. Prog. 1992, 8, 5–10. [Google Scholar] [CrossRef]
- Kamra, D.N.; Zadrazil, F. Influence of oxygen and carbon dioxide on lignin degradation in solid state fermentation of wheat straw with Stropharia rugosoannulata. Biotechnol. Lett. 1985, 7, 335–340. [Google Scholar] [CrossRef]
- Arora, S.; Singh, P.; Rani, R.; Ghosh, S. Oxygen uptake rate as a tool for on-line estimation of cell biomass and bed temperature in a novel solid-state fermentation bioreactor. Bioprocess. Biosyst. Eng. 2018, 41, 917–929. [Google Scholar] [CrossRef]
- Reyes-Moreno, C.; Cuevas-Rodriguez, E.O.; Milan-Carrillo, J.; Cardenas-Valenzuela, O.G.; Barron-Hoyos, J. Solid state fermentation process for producing chickpea (Cicer arietinum L) tempeh flour. Physicochemical and nutritional characteristics of the product. J. Sci. Food Agric. 2004, 84, 271–278. [Google Scholar] [CrossRef]
- Pérez-Guerra, N.; Torrado-Agrasar, A.; López-Macias, C.; Pastrana, L. Main characteristics and applications of solid substrate fermentation. Electron. J. Environ. Agric. Food Chem. 2003, 2, 1. [Google Scholar]
- Iram, A.; Özcan, A.; Yatmaz, E.; Turhan, İ.; Demirci, A. Effect of Microparticles on Fungal Fermentation for Fermentation-Based Product Productions. Processes 2022, 10, 2681. [Google Scholar] [CrossRef]
- Melikoglu, M.; Lin, C.S.K.; Webb, C. Stepwise optimisation of enzyme production in solid state fermentation of waste bread pieces. Food Bioprod. Process. 2013, 91, 638–646. [Google Scholar] [CrossRef]
- Mansour, A.A.; Arnaud, T.; Lu-Chau, T.A.; Fdz-Polanco, M.; Moreira, M.T.; Rivero, J.A.C. Review of solid state fermentation for lignocellulolytic enzyme production: Challenges for environmental applications. Rev. Environ. Sci. Biotechnol. 2016, 15, 31–46. [Google Scholar] [CrossRef]
- Moran-Aguilar, M.G.; Costa-Trigo, I.; Calderón-Santoyo, M.; Domínguez, J.M.; Aguilar-Uscanga, M.G. Production of cellulases and xylanases in solid-state fermentation by different strains of Aspergillus niger using sugarcane bagasse and brewery spent grain. Biochem. Eng. J. 2021, 172, 108060. [Google Scholar] [CrossRef]
- Vasquez-Bonilla, J.N.; Barranco-Florido, J.E.; Ponce-Alquicira, E.; Rincon-Guevara, M.A.; Loera, O. Improvement of beauvericin production by Fusarium oxysporum AB2 under solid-state fermentation using an optimised liquid medium and co-cultures. Mycotoxin Res. 2022, 38, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Molelekoa, T.B.J.; Regnier, T.; da Silva, L.S.; Augustyn, W. Production of Pigments by Filamentous Fungi Cultured on Agro-Industrial by-Products Using Submerged and Solid-State Fermentation Methods. Fermentation 2021, 7, 295. [Google Scholar] [CrossRef]
- De Villa, R.; Roasa, J.; Mine, Y.; Tsao, R. Impact of solid-state fermentation on factors and mechanisms influencing the bioactive compounds of grains and processing by-products. Crit. Rev. Food Sci. Nutr. 2023, 63, 5388–5413. [Google Scholar] [CrossRef]
- Vandenberghe, L.; Karp, S.; de Carvalho, J.; Soccol, C.; Rodrigues, C.; de Oliveira, P. Solid-State Fermentation for the Production of Organic Acids. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 415–434. [Google Scholar] [CrossRef]
- El-Naggar, N.E.-A.; El-Hersh, M.S. Organic acids associated with saccharification of cellulosic wastes during solid-state fermentation. J. Microbiol. 2011, 49, 58–65. [Google Scholar] [CrossRef]
- Groff, M.C.; Noriega, S.E.; Díaz Meglioli, M.E.; Rodríguez, L.; Kuchen, B.; Scaglia, G. Determination of Variable Humidity Profile for Lactic Acid Maximization in Fungal Solid-State Fermentation. Fermentation 2024, 10, 406. [Google Scholar] [CrossRef]
- West, T.P. Citric Acid Production by Aspergillus niger Using Solid-State Fermentation of Agricultural Processing Coproducts. Appl. Biosci. 2023, 2, 1–13. [Google Scholar] [CrossRef]
- Li, S.; Li, G.; Zhang, L.; Zhou, Z.; Han, B.; Hou, W.; Wang, J.; Li, T. A demonstration study of ethanol production from sweet sorghum stems with advanced solid state fermentation technology. Appl. Energy 2013, 102, 260–265. [Google Scholar] [CrossRef]
- Karimi, F.; Mazaheri, D.; Saei Moghaddam, M.; Mataei Moghaddam, A.; Sanati, A.L.; Orooji, Y. Solid-state fermentation as an alternative technology for cost-effective production of bioethanol as useful renewable energy: A review. Biomass Convers. Biorefinery 2021, 1–17. [Google Scholar] [CrossRef]
- Ruslan, N.F.; Ahmad, N.; Abas, A.; Sanfilippo, A.; Mahmoud, K.; Munaim, M.S.A.; Nour, A.H. Sustainable bioethanol production by solid-state fermentation: A systematic review. Environ. Sci. Pollut. Res. Int. 2024, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Ghoshal, G.; Jain, S. Bio-utilization of fruits and vegetables waste to produce β-carotene in solid-state fermentation: Characterization and antioxidant activity. Process Biochem. 2019, 76, 155–164. [Google Scholar] [CrossRef]
- de Carvalho, J.C.; Pandey, A.; Oishi, B.O.; Brand, D.; Rodriguez-Léon, J.A.; Soccol, C.R. Relation between growth, respirometric analysis and biopigments production from Monascus by solid-state fermentation. Biochem. Eng. J. 2006, 29, 262–269. [Google Scholar] [CrossRef]
- Brahma, D.; Dutta, D.; Mukherjee, S. Mass Production of Valuable Pro-Vitamin a Pigment from a Microbe, Cost Analysis and Targeting It for Health Benefiting Purpose. In Industrial Microbiology Based Entrepreneurship; Springer: Singapore, 2022; pp. 147–178. [Google Scholar] [CrossRef]
- Keivani, H.; Jahadi, M. Solid-state fermentation for the production of Monascus pigments from soybean meals. Biocatal. Agric. Biotechnol. 2022, 46, 102531. [Google Scholar] [CrossRef]
- Feng, X.; Ng, K.; Ajlouni, S.; Zhang, P.; Fang, Z. Effect of Solid-State Fermentation on Plant-Sourced Proteins: A Review. Food Rev. Int. 2024, 40, 2580–2617. [Google Scholar] [CrossRef]
- Allwood, J.G.; Wakeling, L.T.; Bean, D.C. Fermentation and the microbial community of Japanese koji and miso: A review. J. Food Sci. 2021, 86, 2194–2207. [Google Scholar] [CrossRef]
- He, M.; Peng, Q.; Xu, X.; Shi, B.; Qiao, Y. Antioxidant capacities and non-volatile metabolites changes after solid-state fermentation of soybean using oyster mushroom (Pleurotus ostreatus) mycelium. Front. Nutr. 2024, 11, 1509341. [Google Scholar] [CrossRef]
- Karp, S.; da Costa, E.; Bissoqui, L.; Medeiros, A.; Destéfanis Vítola, F.; de Melo Pereira, G.; Letti, L.; Soccol, C. Solid-State Fermentation for the Production of Mushrooms. In Current Developments in Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2018; pp. 285–318. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Z.; Jiang, Q.; Roubík, H.; Xu, Q.; Gharsallaoui, A.; Cai, M.; Yang, K.; Sun, P. Fungal solid-state fermentation of crops and their by-products to obtain protein resources: The next frontier of food industry. Trends Food Sci. Technol. 2023, 138, 628–644. [Google Scholar] [CrossRef]
- Streimikyte, P.; Balciunaitiene, A.; Liapman, T.D.; Streimikyte-Mockeliune, Z.; Puzeryte, V.; Borkertas, S.; Viskelis, P.; Viskelis, J. Enzymatically Hydrolysed Common Buckwheat (Fagopyrum esculentum M.) as a Fermentable Source of Oligosaccharides and Sugars. Appl. Sci. 2022, 12, 8210. [Google Scholar] [CrossRef]
- Stodolak, B.; Starzyńska-Janiszewska, A.; Wywrocka-Gurgul, A.; Wikiera, A. Solid-State Fermented Flaxseed Oil Cake of Improved Antioxidant Capacity as Potential Food Additive. J. Food Process. Preserv. 2016, 41, e12855. [Google Scholar] [CrossRef]
- Jiang, K.; Tang, B.; Wang, Q.; Xu, Z.; Sun, L.; Ma, J.; Li, S.; Xu, H.; Lei, P. The bio-processing of soybean dregs by solid state fermentation using a poly γ-glutamic acid producing strain and its effect as feed additive. Bioresour. Technol. 2019, 291, 121841. [Google Scholar] [CrossRef] [PubMed]
- Couto, S.R.; Sanromán, M.Á. Application of solid-state fermentation to food industry—A review. J. Food Eng. 2006, 76, 291–302. [Google Scholar] [CrossRef]
- Bai, Y.; Liang, H.; Wang, L.; Tang, T.; Li, Y.; Cheng, L.; Gao, D. Bioremediation of Diesel-Contaminated Soil by Fungal Solid-State Fermentation. Bull. Environ. Contam. Toxicol. 2024, 112, 13. [Google Scholar] [CrossRef] [PubMed]
- Kaewlaoyoong, A.; Cheng, C.; Lin, C.; Chen, J.; Huang, W.; Sriprom, P. White rot fungus Pleurotus pulmonarius enhanced bioremediation of highly PCDD/F-contaminated field soil via solid state fermentation. Sci. Total Environ. 2020, 738, 139670. [Google Scholar] [CrossRef]
- Martins, V.G.; Kalil, S.J.; Costa, J.A.V. In situ bioremediation using biosurfactant produced by solid state fermentation. World J. Microbiol. Biotechnol. 2009, 25, 843–851. [Google Scholar] [CrossRef]
- Penaloza, W.; Molina, M.R.; Brenes, R.G.; Bressani, R. Solid-state fermentation: An alternative to improve the nutritive value of coffee pulp. Appl. Environ. Microbiol. 1985, 49, 388–393. [Google Scholar] [CrossRef]
- Alhomodi, A.F.; Zavadil, A.; Berhow, M.; Gibbons, W.R.; Karki, B. Application of Cocultures of Fungal Mycelium during Solid-State Fermentation of Canola Meal for Potential Feed Application. J. Am. Oil Chem. Soc. 2021, 98, 509–517. [Google Scholar] [CrossRef]
- Heidari, F.; Øverland, M.; Hansen, J.Ø.; Mydland, L.T.; Urriola, P.E.; Chen, C.; Shurson, G.C.; Hu, B. Solid-state fermentation of Pleurotus ostreatus to improve the nutritional profile of mechanically-fractionated canola meal. Biochem. Eng. J. 2022, 187, 108591. [Google Scholar] [CrossRef]
- Machado, C.M.M.; Soccol, C.R.; de Oliveira, B.H.; Pandey, A. Gibberellic acid production by solid-state fermentation in coffee husk. Appl. Biochem. Biotechnol. 2002, 102, 179–191. [Google Scholar] [CrossRef]
- Londoño-Hernandez, L.; Ruiz, H.A.; Cristina Ramírez, T.; Ascacio, J.A.; Rodríguez-Herrera, R.; Aguilar, C.N. Fungal detoxification of coffee pulp by solid-state fermentation. Biocatal. Agric. Biotechnol. 2020, 23, 101467. [Google Scholar] [CrossRef]
- Salas-Millán, J.Á.; Aguayo, E. Fermentation for Revalorisation of Fruit and Vegetable By-Products: A Sustainable Approach Towards Minimising Food Loss and Waste. Foods 2024, 13, 3680. [Google Scholar] [CrossRef]
- Ibarruri, J.; Goiri, I.; Cebrián, M.; García-Rodríguez, A. Solid State Fermentation as a Tool to Stabilize and Improve Nutritive Value of Fruit and Vegetable Discards: Effect on Nutritional Composition, In Vitro Ruminal Fermentation and Organic Matter Digestibility. Animals 2021, 11, 1653. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Rodriguez, L.G.; Zamora Gasga, V.M.; Pescuma, M.; Van Nieuwenhove, C.; Mozzi, F.; Sanchez Burgos, J.A. Fruits and fruit by-products as sources of bioactive compounds. Benefits and trends of lactic acid fermentation in the development of novel fruit-based functional beverages. Food Res. Int. 2021, 140, 109854. [Google Scholar] [CrossRef] [PubMed]
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Muller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef] [PubMed]
- Roman, G.C.; Jackson, R.E.; Gadhia, R.; Roman, A.N.; Reis, J. Mediterranean diet: The role of long-chain omega-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev. Neurol. 2019, 175, 724–741. [Google Scholar] [CrossRef]
- Serafini, M.; Peluso, I. Functional Foods for Health: The Interrelated Antioxidant and Anti-Inflammatory Role of Fruits, Vegetables, Herbs, Spices and Cocoa in Humans. Curr. Pharm. Des. 2016, 22, 6701–6715. [Google Scholar] [CrossRef]
- Del Rio-Celestino, M.; Font, R. The Health Benefits of Fruits and Vegetables. Foods 2020, 9, 369. [Google Scholar] [CrossRef]
- Ispiryan, A.; Viškelis, J.; Viškelis, P.; Urbonavičienė, D.; Raudonė, L. Biochemical and Antioxidant Profiling of Raspberry Plant Parts for Sustainable Processing. Plants 2023, 12, 2424. [Google Scholar] [CrossRef]
- Shehu, I.; Akanbi, T.O.; Wyatt, V.; Aryee, A.N.A. Fruit, Nut, Cereal, and Vegetable Waste Valorization to Produce Biofuel. In Byproducts from Agriculture and Fisheries; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2019; pp. 665–684. [Google Scholar] [CrossRef]
- Garcia-Salas, P.; Morales-Soto, A.; Segura-Carretero, A.; Fernandez-Gutierrez, A. Phenolic-compound-extraction systems for fruit and vegetable samples. Molecules 2010, 15, 8813–8826. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhou, W.; Yu, J.; Zhao, L.; Wang, K.; Hu, Z.; Liu, X. By-Products of Fruit and Vegetables: Antioxidant Properties of Extractable and Non-Extractable Phenolic Compounds. Antioxidants 2023, 12, 418. [Google Scholar] [CrossRef]
- Ozcan, B.E.; Tetik, N.; Aloglu, H.S. Polysaccharides from fruit and vegetable wastes and their food applications: A review. Int. J. Biol. Macromol. 2024, 276, 134007. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Tan, J.; Sang, Y.; Tang, J.; Cai, X.; Xue, H. Preparation, structure, and biological activities of the polysaccharides from fruits and vegetables: A review. Food Biosci. 2023, 54, 102909. [Google Scholar] [CrossRef]
- Bhanja Dey, T.; Chakraborty, S.; Jain, K.K.; Sharma, A.; Kuhad, R.C. Antioxidant phenolics and their microbial production by submerged and solid state fermentation process: A review. Trends Food Sci. Technol. 2016, 53, 60–74. [Google Scholar] [CrossRef]
- Cano-Lamadrid, M.; Artés-Hernández, F. By-Products Revalorization with Non-Thermal Treatments to Enhance Phytochemical Compounds of Fruit and Vegetables Derived Products: A Review. Foods 2021, 11, 59. [Google Scholar] [CrossRef]
- Amara, A.A.; El-Baky, N.A. Fungi as a Source of Edible Proteins and Animal Feed. J. Fungi 2023, 9, 73. [Google Scholar] [CrossRef]
- Urbonaviciene, D. The Use of Lactic Acid Bacteria in the Fermentation of Fruits and Vegetables—Technological and Functional Properties. In Biotechnology; IntechOpen: Savini, Slovenia, 2015. [Google Scholar] [CrossRef]
- Bechman, A.; Phillips, R.D.; Chen, J. Changes in selected physical property and enzyme activity of rice and barley koji during fermentation and storage. J. Food Sci. 2012, 77, M318–M322. [Google Scholar] [CrossRef]
- Umsza-Guez, M.A.; Diaz, A.B.; de Ory, I.; Blandino, A.; Gomes, E.; Caro, I. Xylanase production by Aspergillus awamori under solid state fermentation conditions on tomato pomace. Braz. J. Microbiol. 2011, 42, 1585–1597. [Google Scholar] [CrossRef]
- Velmurugan, P.; Hur, H.; Balachandar, V.; Kamala-Kannan, S.; Lee, K.; Lee, S.; Chae, J.; Shea, P.J.; Oh, B. Monascus pigment production by solid-state fermentation with corn cob substrate. J. Biosci. Bioeng. 2011, 112, 590–594. [Google Scholar] [CrossRef]
- Papanikolaou, S.; Galiotou-Panayotou, M.; Fakas, S.; Komaitis, M.; Aggelis, G. Citric acid production by Yarrowia lipolytica cultivated on olive-mill wastewater-based media. Bioresour. Technol. 2008, 99, 2419–2428. [Google Scholar] [CrossRef]
- Acosta-Estrada, B.A.; Villela-Castrejón, J.; Perez-Carrillo, E.; Gómez-Sánchez, C.E.; Gutiérrez-Uribe, J.A. Effects of solid-state fungi fermentation on phenolic content, antioxidant properties and fiber composition of lime cooked maize by-product (nejayote). J. Cereal Sci. 2019, 90, 102837. [Google Scholar] [CrossRef]
- Marcus, A.; Fox, G. Fungal Biovalorization of a Brewing Industry Byproduct, Brewer’s Spent Grain: A Review. Foods 2021, 10, 2159. [Google Scholar] [CrossRef] [PubMed]
- Eliopoulos, C.; Arapoglou, D.; Chorianopoulos, N.; Markou, G.; Haroutounian, S.A. Conversion of brewers’ spent grain into proteinaceous animal feed using solid state fermentation. Environ. Sci. Pollut. Res. 2022, 29, 29562–29569. [Google Scholar] [CrossRef] [PubMed]
- Mollea, C.; Bosco, F. Solid-State Fermentation of Brewery Spent Grains to Enhance Biomolecule Extraction. Separations 2025, 12, 58. [Google Scholar] [CrossRef]
- Zhang, J.; Perez-Gavilan, A.; Cunha Neves, A. Optimization of Solid-State Fermentation Conditions with Mixed Strains Using Box-Behnken Design for the Production of brewers’ Spent Grain Protein. J. Am. Soc. Brew. Chem. 2024, 82, 468–479. [Google Scholar] [CrossRef]
- Ibarruri, J.; Cebrián, M.; Hernández, I. Solid State Fermentation of Brewer’s Spent Grain Using Rhizopus sp. to Enhance Nutritional Value. Waste Biomass Valor. 2019, 10, 3687–3700. [Google Scholar] [CrossRef]
- Zanker, G.; Kepplinger, W.; Pecher, C. Incineration of Solid Food Waste: A Project About Spent Grain. In Utilization of By-Products and Treatment of Waste in the Food Industry; Springer: New York, NY, USA, 2007; pp. 273–281. [Google Scholar] [CrossRef]
- Coelho, E.; Rocha, M.A.M.; Moreira, A.S.P.; Domingues, M.R.M.; Coimbra, M.A. Revisiting the structural features of arabinoxylans from brewers’ spent grain. Carbohydr. Polym. 2016, 139, 167–176. [Google Scholar] [CrossRef]
- Wagner, E.; Pería, M.E.; Ortiz, G.E.; Rojas, N.L.; Ghiringhelli, P.D. Valorization of brewer’s spent grain by different strategies of structural destabilization and enzymatic saccharification. Ind. Crops Prod. 2021, 163, 113329. [Google Scholar] [CrossRef]
- Bartolomé, B.; Santos, M.; Jiménez, J.J.; del Nozal, M.J.; Gómez-Cordovés, C. Pentoses and Hydroxycinnamic Acids in Brewer’s Spent Grain. J. Cereal Sci. 2002, 36, 51–58. [Google Scholar] [CrossRef]
- Socaci, S.A.; Fărcaş, A.C.; Diaconeasa, Z.M.; Vodnar, D.C.; Rusu, B.; Tofană, M. Influence of the extraction solvent on phenolic content, antioxidant, antimicrobial and antimutagenic activities of brewers’ spent grain. J. Cereal Sci. 2018, 80, 180–187. [Google Scholar] [CrossRef]
- da Costa Maia, I.; Thomaz dos Santos D’Almeida, C.; Guimarães Freire, D.M.; d’Avila Costa Cavalcanti, E.; Cameron, L.C.; Furtado Dias, J.; Simões Larraz Ferreira, M. Effect of solid-state fermentation over the release of phenolic compounds from brewer’s spent grain revealed by UPLC-MSE. Food Sci. Technol. 2020, 133, 110136. [Google Scholar] [CrossRef]
- Bohnsack, C.; Ternes, W.; Büsing, A.; Drotleff, A.M. Tocotrienol levels in sieving fraction extracts of brewer’s spent grain. Eur. Food Res. Technol. 2011, 232, 563–573. [Google Scholar] [CrossRef]
- Mollea, C.; Bosco, F. Valorisation Proposal of Brewery Spent Grain: Carotenoids and Protein Films Production. Chem. Eng. Trans. 2024, 110, 331–336. [Google Scholar] [CrossRef]
- del Río, J.C.; Prinsen, P.; Gutiérrez, A. Chemical composition of lipids in brewer’s spent grain: A promising source of valuable phytochemicals. J. Cereal Sci. 2013, 58, 248–254. [Google Scholar] [CrossRef]
- Ikram, S.; Huang, L.; Zhang, H.; Wang, J.; Yin, M. Composition and Nutrient Value Proposition of Brewers Spent Grain. J. Food Sci. 2017, 82, 2232–2242. [Google Scholar] [CrossRef]
- Cooray, S.T.; Chen, W.N. Valorization of brewer’s spent grain using fungi solid-state fermentation to enhance nutritional value. J. Funct. Foods 2018, 42, 85–94. [Google Scholar] [CrossRef]
- Aggelopoulos, T.; Katsieris, K.; Bekatorou, A.; Pandey, A.; Banat, I.M.; Koutinas, A.A. Solid state fermentation of food waste mixtures for single cell protein, aroma volatiles and fat production. Food Chem. 2014, 145, 710–716. [Google Scholar] [CrossRef]
- Dukare, A.; Mhatre, P.; Maheshwari, H.S.; Bagul, S.; Manjunatha, B.S.; Khade, Y.; Kamble, U. Delineation of mechanistic approaches of rhizosphere microorganisms facilitated plant health and resilience under challenging conditions. 3 Biotech 2022, 12, 57. [Google Scholar] [CrossRef]
- Martínez, M.; Rodríguez, A.; Gea, T.; Font, X. A Simplified Techno-Economic Analysis for Sophorolipid Production in a Solid-State Fermentation Process. Energies 2022, 15, 4077. [Google Scholar] [CrossRef]
- Khootama, A.; Putri, D.N.; Hermansyah, H. Techno-economic analysis of lipase enzyme production from Aspergillus niger using agro-industrial waste by solid state fermentation. Energy Procedia 2018, 153, 143–148. [Google Scholar] [CrossRef]
- Mendes, F.B.; Ibraim Pires Atala, D.; Thoméo, J.C. Is cellulase production by solid-state fermentation economically attractive for the second generation ethanol production? Renew. Energy 2017, 114, 525–533. [Google Scholar] [CrossRef]
- Sadh, P.K.; Duhan, S.; Duhan, J.S. Agro-industrial wastes and their utilization using solid state fermentation: A review. Bioresour. Bioprocess. 2018, 5, 1. [Google Scholar] [CrossRef]
- Soccol, C.R.; Costa, E.S.F.d.; Letti, L.A.J.; Karp, S.G.; Woiciechowski, A.L.; Vandenberghe, L.P.d.S. Recent developments and innovations in solid state fermentation. Biotechnol. Res. Innov. 2017, 1, 52–71. [Google Scholar] [CrossRef]
- Hölker, U.; Lenz, J. Solid-state fermentation—Are there any biotechnological advantages? Curr. Opin. Microbiol. 2005, 8, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Chilakamarry, C.R.; Mimi Sakinah, A.M.; Zularisam, A.W.; Sirohi, R.; Khilji, I.A.; Ahmad, N.; Pandey, A. Advances in solid-state fermentation for bioconversion of agricultural wastes to value-added products: Opportunities and challenges. Bioresour. Technol. 2022, 343, 126065. [Google Scholar] [CrossRef]
- Thomas, L.; Larroche, C.; Pandey, A. Current developments in solid-state fermentation. Biochem. Eng. J. 2013, 81, 146–161. [Google Scholar] [CrossRef]
- Dasari, P.R.; Ramteke, P.W.; Kesri, S.; Kongala, P.R. Comparative Study of Cellulase Production Using Submerged and Solid-State Fermentation. In Approaches to Enhance Industrial Production of Fungal Cellulases; Springer International Publishing AG: Cham, Switzerland, 2019; pp. 37–52. [Google Scholar]
- Huang, C.; Liao, Y.; Tsai, G. Solid-State Fermentation of Grain-Derived By-Products by Aspergillus kawachii and Rhizopus oryzae: Preparation and Evaluation of Anti-Allergic Activity. Fermentation 2024, 10, 457. [Google Scholar] [CrossRef]
- Pessoa, D.R.; Finkler, A.T.J.; Machado, A.V.L.; Mitchell, D.A.; de Lima Luz, L.F., Jr. CFD simulation of a packed-bed solid-state fermentation bioreactor. Appl. Math. Model. 2019, 70, 439–458. [Google Scholar] [CrossRef]
- Finkler, A.T.J.; Weber, M.Z.; Fuchs, G.A.; Scholz, L.A.; de Lima Luz, L.F., Jr.; Krieger, N.; Mitchell, D.A.; de Matos Jorge, L.M. Estimation of heat and mass transfer coefficients in a pilot packed-bed solid-state fermentation bioreactor. Chem. Eng. J. 2021, 408, 127246. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).