Characterization of a Vaginal Limosilactobacillus Strain Producing Anti-Virulence Postbiotics: A Potential Probiotic Candidate
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganisms, Culture Conditions, and Antimicrobial Activity Tests
2.2. Fermentation of L. fermentum Lf53, Postbiotics/Metabiotics Production, and Transit Tolerance
2.3. Biofilm Formation Assays
2.4. Screening for Quorum Sensing Inhibition by CFS
2.4.1. Anti-Quorum Sensing Assay—Qualitative Activity by Agar Well Diffusion Method
2.4.2. Anti-Quorum Sensing Assay—Quantitative Inhibition of Violacein Production
2.4.3. Anti-Quorum Sensing Assay—Quantitative Inhibition of Pyocyanin Production
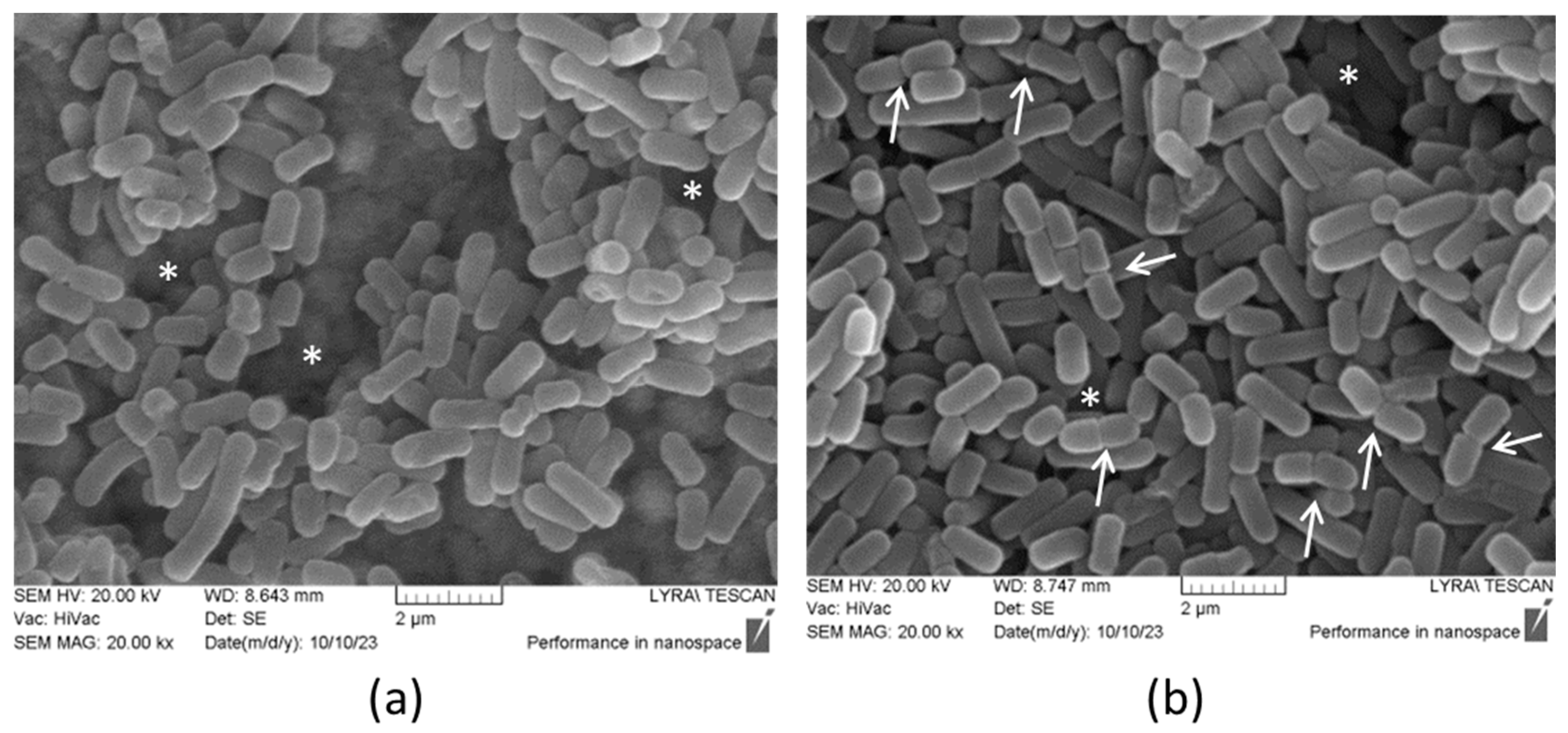
2.5. Scanning Electron Microscopy (SEM)
2.6. Statistical Analysis
3. Results
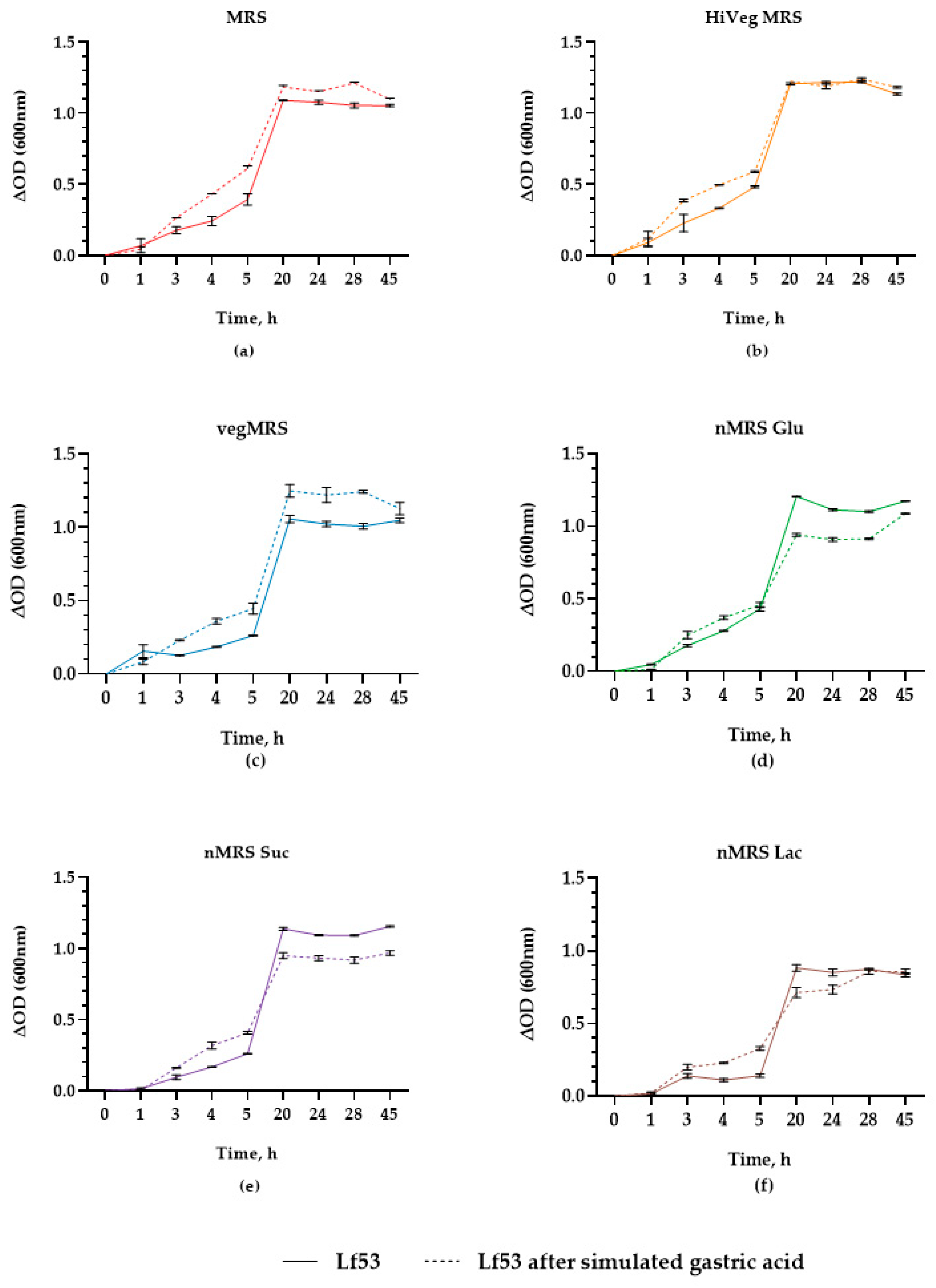
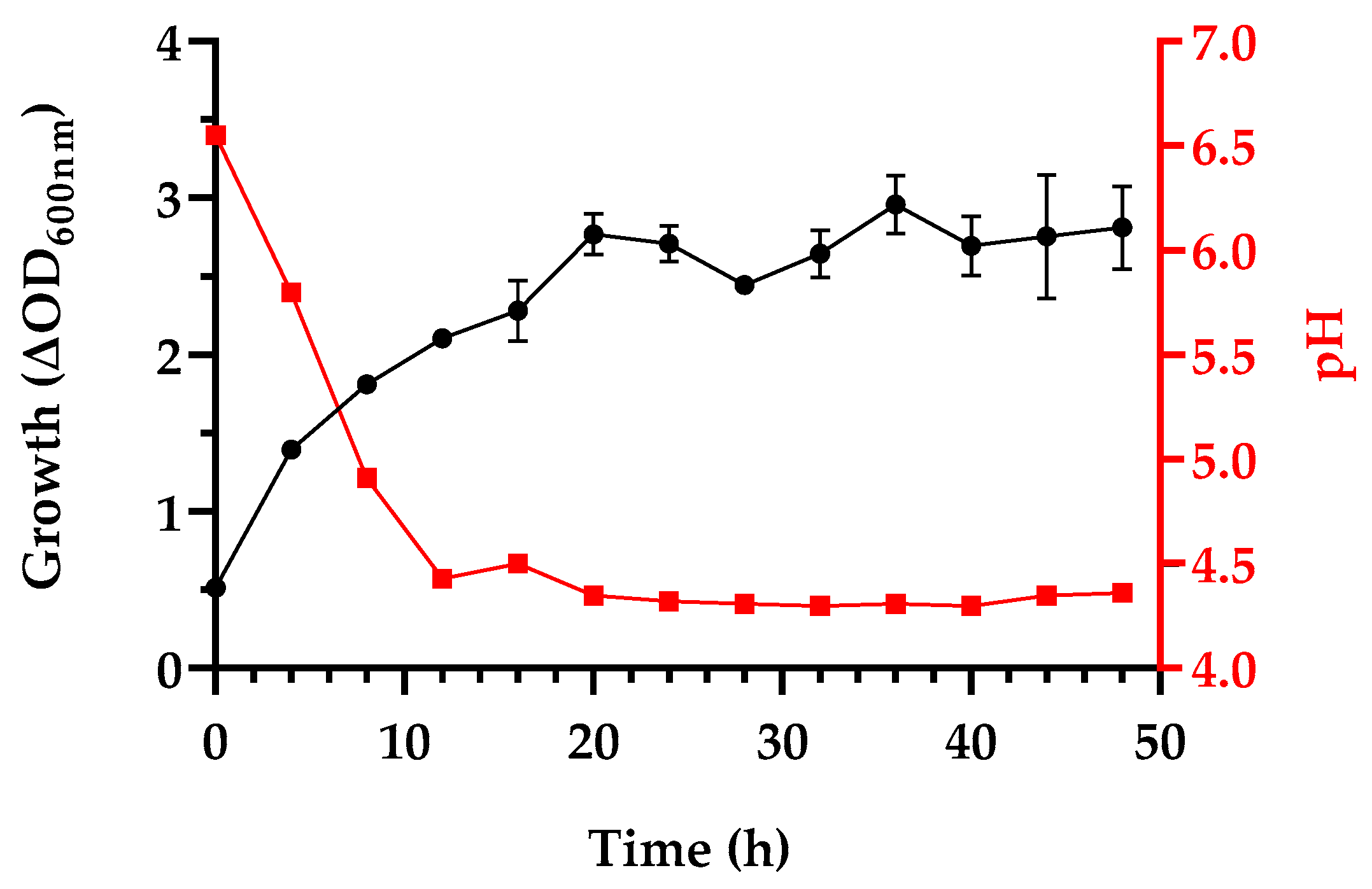
3.1. Growth and Postbiotics Production
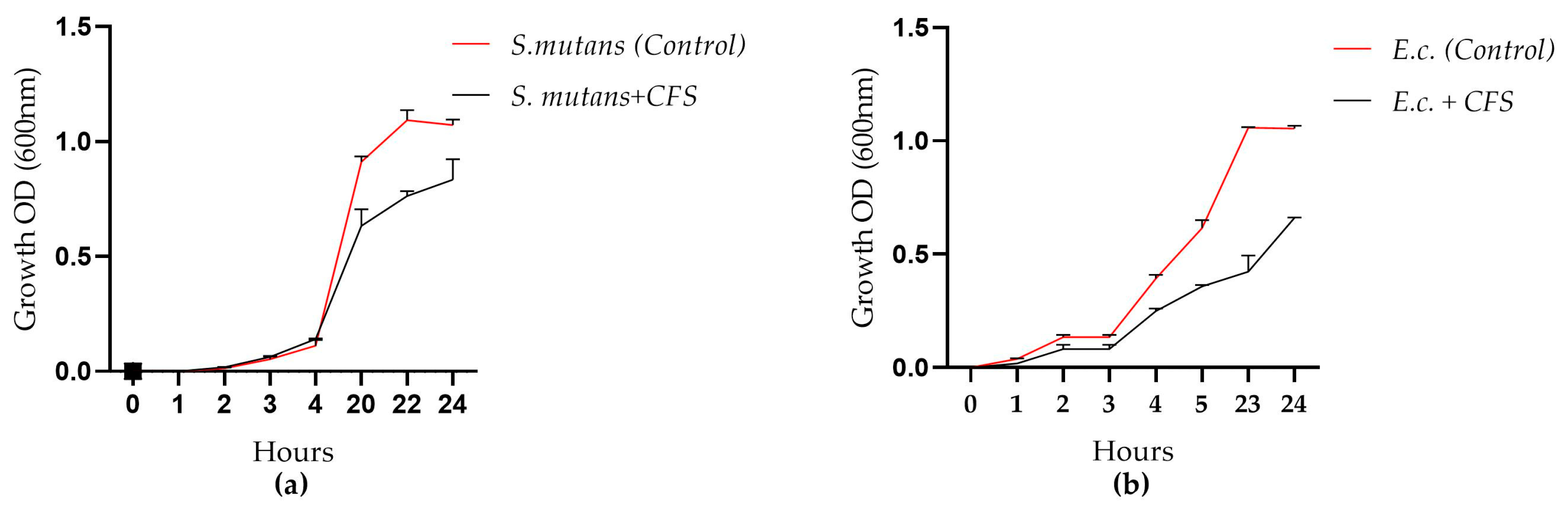
3.2. Antimicrobial Activity Assessment Against Gram (+) and Gram (−) Microorganisms
3.3. In Vitro Assessment Transit Tolerance in Simulated Gastric Conditions
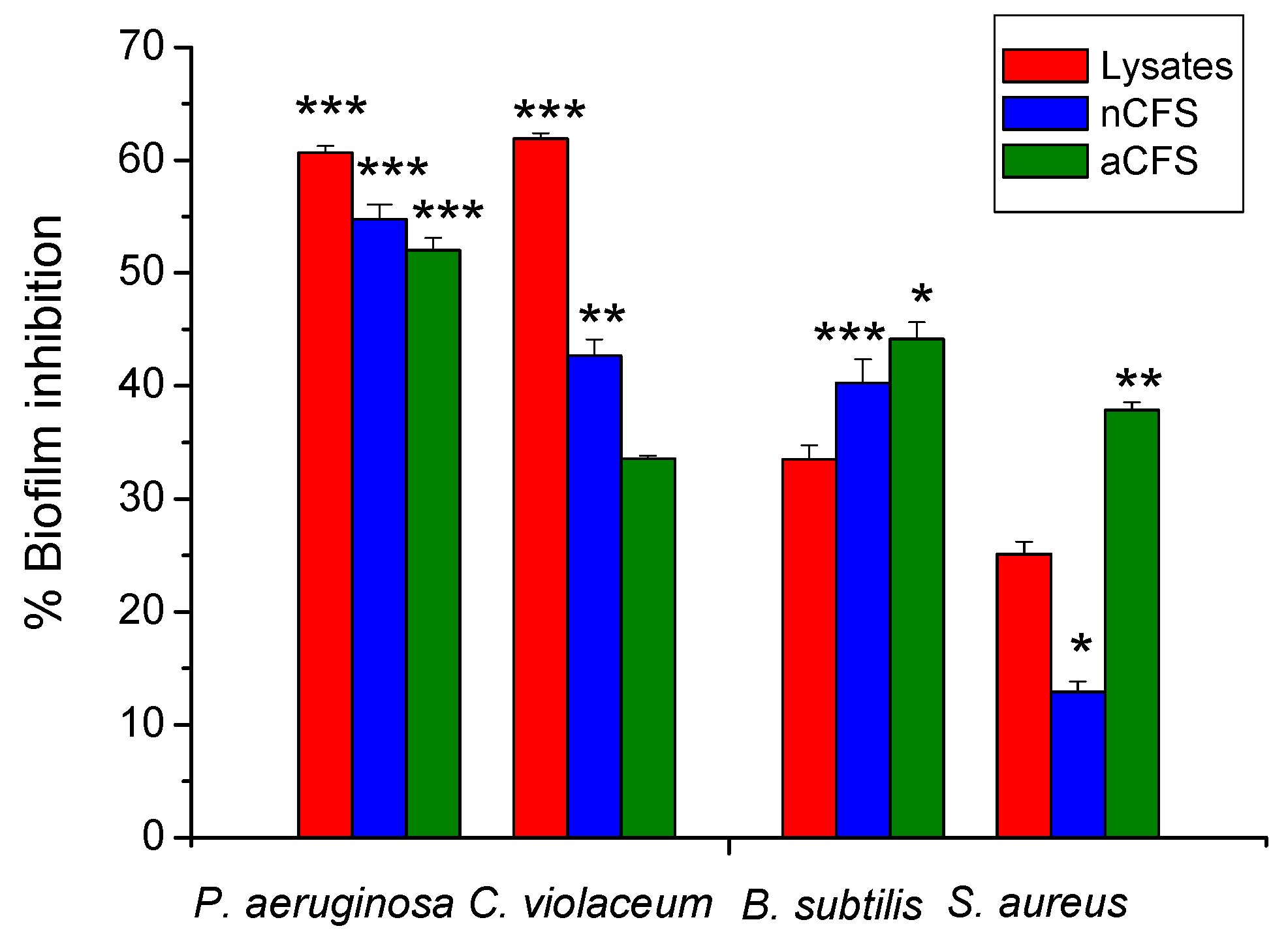
3.4. Inhibitory Activity of Produced Postbiotics on Biofilm Formation
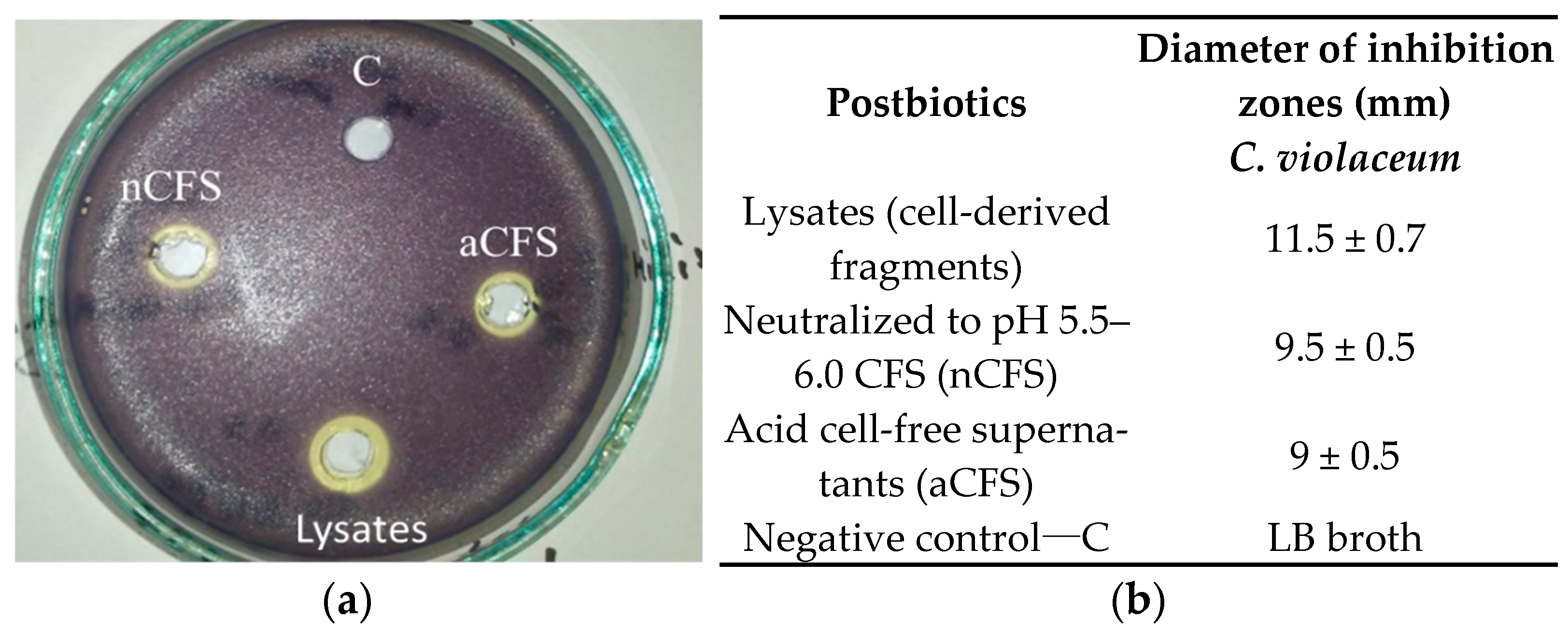
3.5. Effects of Postbiotics on Violacein Production—Qualitative Assay
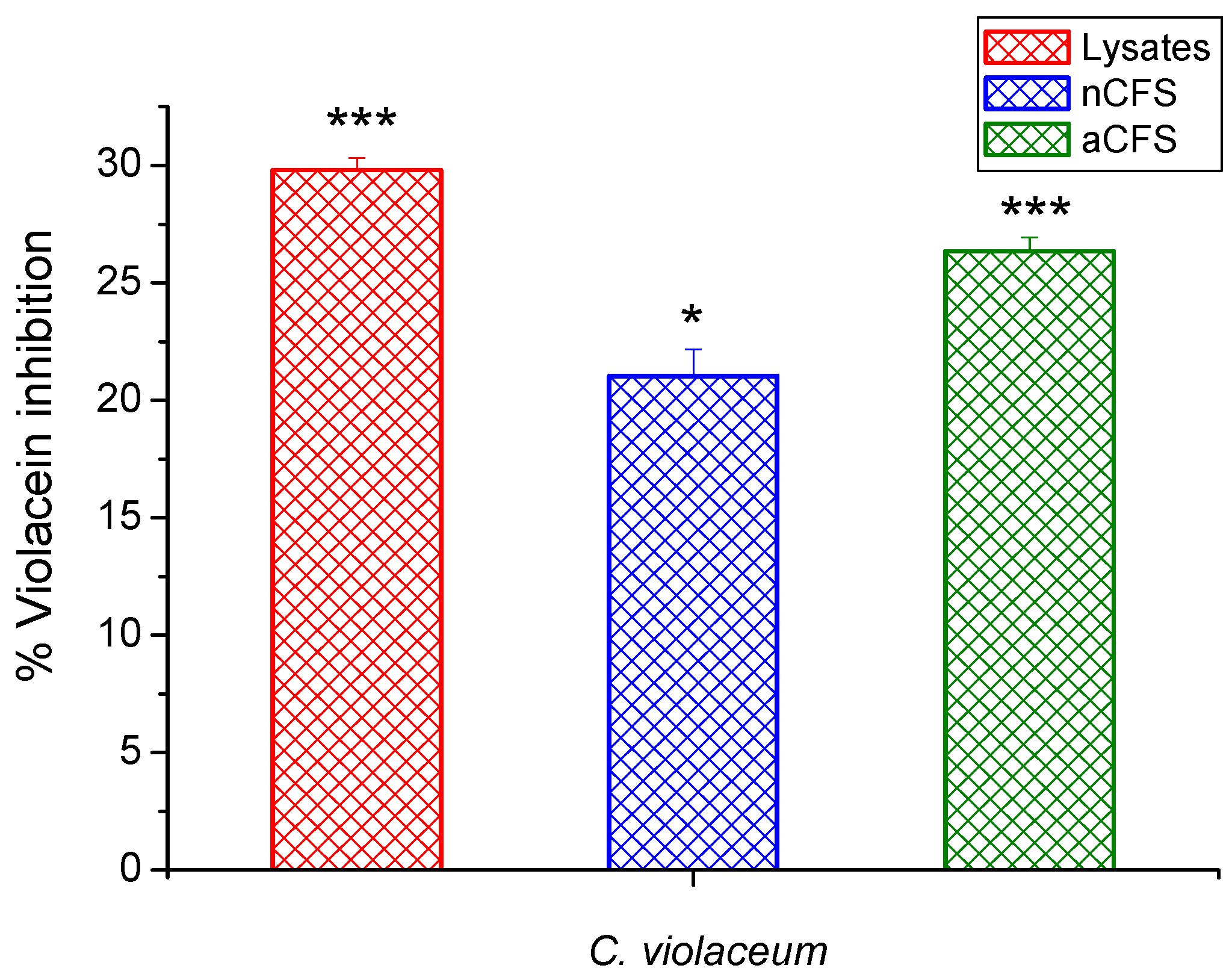
3.6. Effects of Postbiotics on Violacein Production—Quantitative Assay
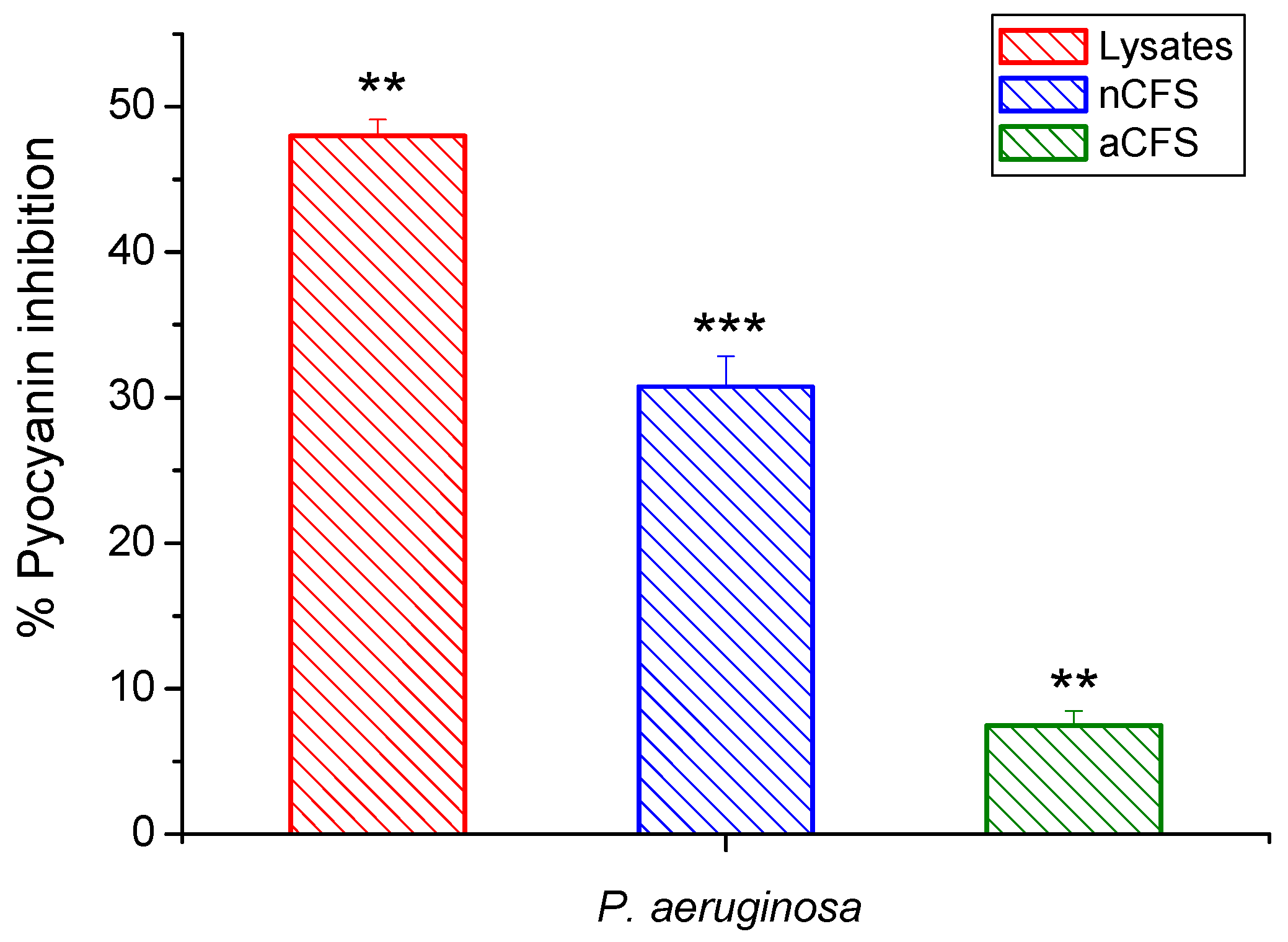
3.7. Effects of Postbiotics on Pyocyanin Production—Quantitative Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Maldonado Galdeano, C.; Cazorla, S.I.; Lemme Dumit, J.M.; Vélez, E.; Perdigón, G. Beneficial Effects of Probiotic Consumption on the Immune System. Ann. Nutr. Metab. 2019, 74, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Safarchi, A.; Al-Qadami, G.; Tran, C.D.; Conlon, M. Understanding dysbiosis and resilience in the human gut microbiome: Biomarkers, interventions, and challenges. Front. Microbiol. 2025, 16, 1559521. [Google Scholar] [CrossRef]
- Mokoena, M.P.; Omatola, C.A.; Olaniran, A.O. Applications of Lactic Acid Bacteria and Their Bacteriocins against Food Spoilage Microorganisms and Foodborne Pathogens. Molecules 2021, 26, 7055. [Google Scholar] [CrossRef]
- Ayivi, R.D.; Gyawali, R.; Krastanov, A.; Aljaloud, S.O.; Worku, M.; Tahergorabi, R.; Silva, R.C.D.; Ibrahim, S.A. Lactic Acid Bacteria: Food Safety and Human Health Applications. Dairy 2020, 1, 202–232. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Resta-Lenert, S. Live probiotics protect intestinal epithelial cells from the effects of infection with enteroinvasive Escherichia coli (EIEC). Gut 2003, 52, 988–997. [Google Scholar] [CrossRef]
- Shibahara-Sone, H.; Gomi, A.; Iino, T.; Kano, M.; Nonaka, C.; Watanabe, O.; Miyazaki, K.; Ohkusa, T. Living cells of probiotic Bifidobacterium bifidum YIT 10347 detected on gastric mucosa in humans. Benef. Microbes 2016, 7, 319–326. [Google Scholar] [CrossRef]
- Nemati, M.; Omrani, G.R.; Ebrahimi, B.; Montazeri-Najafabady, N. The Beneficial Effects of Probiotics via Autophagy: A Systematic Review. BioMed Res. Int. 2021, 2021, 2931580. [Google Scholar] [CrossRef]
- Damyanova, T.; Dimitrova, P.D.; Borisova, D.; Topouzova-Hristova, T.; Haladjova, E.; Paunova-Krasteva, T. An Overview of Biofilm-Associated Infections and the Role of Phytochemicals and Nanomaterials in Their Control and Prevention. Pharmaceutics 2024, 16, 162. [Google Scholar] [CrossRef]
- Damyanova, T.; Paunova-Krasteva, T. What We Still Don’t Know About Biofilms—Current Overview and Key Research Information. Microbiol. Res. 2025, 16, 46. [Google Scholar] [CrossRef]
- Johani, K.; Malone, M.; Jensen, S.; Gosbell, I.; Dickson, H.; Hu, H.; Vickery, K. Microscopy visualisation confirms multi-species biofilms are ubiquitous in diabetic foot ulcers. Int. Wound J. 2017, 14, 1160–1169. [Google Scholar] [CrossRef]
- Garg, S.S.; Dubey, R.; Sharma, S.; Vyas, A.; Gupta, J. Biological macromolecules-based nanoformulation in improving wound healing and bacterial biofilm-associated infection: A review. Int. J. Biol. Macromol. 2023, 247, 125636. [Google Scholar] [CrossRef]
- Borisova, D.; Strateva, T.; Dimov, S.G.; Atanassova, B.; Paunova-Krasteva, T.; Topouzova-Hristova, T.; Danova, S.T.; Tropcheva, R.; Stoitsova, S. Diversification of Pseudomonas aeruginosa After Inhaled Tobramycin Therapy of Cystic Fibrosis Patients: Genotypic and Phenotypic Characteristics of Paired Pre- and Post-Treatment Isolates. Microorganisms 2025, 13, 730. [Google Scholar] [CrossRef]
- Fatima, H.; Gupta, S.; Khare, S.K. Meropenem incorporated nanoflowers as nano antibiotics: A promising strategy for combating antimicrobial resistance and biofilm-associated infections. Inorg. Chem. Commun. 2025, 177, 114362. [Google Scholar] [CrossRef]
- Assefa, M.; Amare, A. Biofilm-Associated Multi-Drug Resistance in Hospital-Acquired Infections: A Review. Infect. Drug Resist. 2022, 15, 5061–5068. [Google Scholar] [CrossRef]
- Ciofu, O.; Moser, C.; Jensen, P.Ø.; Høiby, N. Tolerance and resistance of microbial biofilms. Nat. Rev. Microbiol. 2022, 20, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Zahra, A.; Kamthan, M.; Husain, F.M.; Albalawi, T.; Zubair, M.; Alatawy, R.; Abid, M.; Noorani, M.S. Microbial Biofilms: Applications, Clinical Consequences, and Alternative Therapies. Microorganisms 2023, 11, 1934. [Google Scholar] [CrossRef]
- Shaikh, S.; Lapin, N.A.; Prasad, B.; Sturge, C.R.; Pybus, C.; Pifer, R.; Wang, Q.; Evers, B.M.; Chopra, R.; Greenberg, D.E. Intermittent alternating magnetic fields diminish metal-associated biofilm in vivo. Sci. Rep. 2023, 13, 22456. [Google Scholar] [CrossRef]
- Werneburg, G.T.; Hettel, D.; Goldman, H.B.; Vasavada, S.P.; Miller, A.W. Indwelling Urological Device Biofilm Composition and Characteristics in the Presence and Absence of Infection. Urology 2025, 196, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Adnan, M.; Siddiqui, A.J.; Noumi, E.; Ashraf, S.A.; Awadelkareem, A.M.; Hadi, S.; Snoussi, M.; Badraoui, R.; Bardakci, F.; Sachidanandan, M.; et al. Biosurfactant derived from probiotic Lactobacillus acidophilus exhibits broad-spectrum antibiofilm activity and inhibits the quorum sensing-regulated virulence. Biomol. Biomed. 2023, 23, 1051–1068. [Google Scholar] [CrossRef] [PubMed]
- Poon, Y.; Hui, M. Inhibitory effect of lactobacilli supernatants on biofilm and filamentation of Candida albicans, Candida tropicalis, and Candida parapsilosis. Front. Microbiol. 2023, 14, 1105949. [Google Scholar] [CrossRef]
- Parappilly, S.J.; Radhakrishnan, E.K.; George, S.M. Antibacterial and antibiofilm activity of human gut lactic acid bacteria. Braz. J. Microbiol. 2024, 55, 3529–3539. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Bahador, A. In vitro Anti-biofilm and Anti-adhesion Effects of Lactic Acid Bacteria- derived Biosurfactants against Streptococcus mutans. Infect. Disord.-Drug Targets 2024, 25, 4. [Google Scholar] [CrossRef]
- Dimitrova, P.D.; Damyanova, T.; Paunova-Krasteva, T. Chromobacterium Violaceum: A Model for Evaluating the Anti-Quorum Sensing Activities of Plant Substances. Sci. Pharm. 2023, 91, 33. [Google Scholar] [CrossRef]
- Zhong, S.; He, S. Quorum Sensing Inhibition or Quenching in Acinetobacter baumannii: The Novel Therapeutic Strategies for New Drug Development. Front. Microbiol. 2021, 12, 558003. [Google Scholar] [CrossRef] [PubMed]
- Aman, M.; Aneeqha, N.; Bristi, K.; Deeksha, J.; Afza, N.; Sindhuja, V.; Shastry, R.P. Lactic acid bacteria inhibits quorum sensing and biofilm formation of Pseudomonas aeruginosa strain JUPG01 isolated from rancid butter. Biocatal. Agric. Biotechnol. 2021, 36, 102115. [Google Scholar] [CrossRef]
- Díaz, M.A.; González, S.N.; Alberto, M.R.; Arena, M.E. Human probiotic bacteria attenuate Pseudomonas aeruginosa biofilm and virulence by quorum-sensing inhibition. Biofouling 2020, 36, 597–609. [Google Scholar] [CrossRef]
- FAO/WHO. Joint FAO/WHO Working Group Guidelines for the Evaluation of Probiotics in Food: Report of a Joint FAO/WHO Working Group on Drafting Guidelines for the Evaluation of Probiotics in Food; World Health Organization and Food and Agriculture Organization of the United Nations: Rome, Italy, 2002. [Google Scholar]
- Lin, T.-L.; Shu, C.-C.; Lai, W.-F.; Tzeng, C.-M.; Lai, H.-C.; Lu, C.-C. Investiture of next generation probiotics on amelioration of diseases—Strains do matter. Med. Microecol. 2019, 1, 100002. [Google Scholar] [CrossRef]
- Petrova, M.; Georgieva, R.; Dojchinovska, L.; Kirilov, N.; Iliev, I.; Antonova, S.; Hadjieva, N.; Ivanova, I.; Danova, S. Lactic acid bacteria against pathogenic microbes. Trakia J. Sci. 2009, 7, 33–39. [Google Scholar]
- Moskova-Doumanova, V.; Vaseva, A.; Veleva, R.; Mladenova, K.; Melniska, D.; Doumanov, J.; Videv, P.; Topouzova-Hristova, T.; Dobreva, L.; Atanasova, N.; et al. In Vitro Effects of Postmetabolites from Limosilactobacillus fermentum 53 on the Survival and Proliferation of HT-29 Cells. Microorganisms 2024, 12, 1365. [Google Scholar] [CrossRef] [PubMed]
- Danova, S. Biodiversity and Probiotic Potential of Lactic Acid Bacteria from Various Ecological Niches. Ph.D. Thesis, Stefan Angeloff Institute of Microbiology, Bulgarian Academy of Sciences, Sofia, Bulgaria, 2015. [Google Scholar]
- Dobreva, L.; Atanasova, N.; Donchev, P.; Krumova, E.; Abrashev, R.; Karakirova, Y.; Mladenova, R.; Tolchkov, V.; Ralchev, N.; Dishliyska, V.; et al. Candidate-Probiotic Lactobacilli and Their Postbiotics as Health-Benefit Promoters. Microorganisms 2024, 12, 1910. [Google Scholar] [CrossRef]
- De Soyza, A.; Hall, A.J.; Mahenthiralingam, E.; Drevinek, P.; Kaca, W.; Drulis-Kawa, Z.; Stoitsova, S.R.; Toth, V.; Coenye, T.; Zlosnik, J.E.A.; et al. Developing an international Pseudomonas aeruginosa reference panel. MicrobiologyOpen 2013, 2, 1010–1023. [Google Scholar] [CrossRef] [PubMed]
- Dimitrova, P.D.; Ivanova, V.; Trendafilova, A.; Paunova-Krasteva, T. Anti-Biofilm and Anti-Quorum-Sensing Activity of Inula Extracts: A Strategy for Modulating Chromobacterium violaceum Virulence Factors. Pharmaceuticals 2024, 17, 573. [Google Scholar] [CrossRef]
- Dobreva, L. Functional Characteristic of Lactic Acid Bacteria from Different Habitats. Ph.D. Thesis, The Stephan Angeloff Institute of Microbiology, Bulgarian Academy of Sciences, Sofia, Bulgaria, 2023. [Google Scholar]
- Lou, Z.; Wang, H.; Tang, Y.; Chen, X. The effect of burdock leaf fraction on adhesion, biofilm formation, quorum sensing and virulence factors of Pseudomonas aeruginosa. J. Appl. Microbiol. 2017, 122, 615–624. [Google Scholar] [CrossRef]
- Nemska, V.; Danova, S.; Georgieva, N. In Vitro Assessment of Lactobacillus spp. to Grow in the Presence of Different Carbon Sources in the Medium. J. Chem. Technol. Met. 2022, 57, 487–495. [Google Scholar]
- Garrote Achou, C.; Cantalejo Díez, M.J.; Diaz Cano, J.; Molinos Equiza, X. Evaluation of Different Nutritional Sources in Lactic Acid Bacteria Fermentation for Sustainable Postbiotic Production. Foods 2025, 14, 649. [Google Scholar] [CrossRef]
- Singh, V.; Haque, S.; Niwas, R.; Srivastava, A.; Pasupuleti, M.; Tripathi, C.K.M. Strategies for Fermentation Medium Optimization: An In-Depth Review. Front. Microbiol. 2017, 7, 2087. [Google Scholar] [CrossRef] [PubMed]
- Dimitonova, S. Biological Properties of Vaginal Lactobacilli. Ph.D. Thesis, SU, St Kl. Ohridski, Sofia, Bulgaria, 2007. [Google Scholar]
- Hao, H.; Nie, Z.; Wu, Y.; Liu, Z.; Luo, F.; Deng, F.; Zhao, L. Probiotic Characteristics and Anti-Inflammatory Effects of Limosilactobacillus fermentum 664 Isolated from Chinese Fermented Pickles. Antioxidants 2024, 13, 703. [Google Scholar] [CrossRef]
- Barman, I.; Seo, H.; Kim, S.; Rahim, M.A.; Yoon, Y.; Hossain, M.S.; Shuvo, M.S.H.; Song, H.-Y. Isolation of New Strains of Lactic Acid Bacteria from the Vaginal Microbiome of Postmenopausal Women and their Probiotic Characteristics. Curr. Microbiol. 2025, 82, 76. [Google Scholar] [CrossRef]
- Abebe, G.M. The Role of Bacterial Biofilm in Antibiotic Resistance and Food Contamination. Int. J. Microbiol. 2020, 2020, 1705814. [Google Scholar] [CrossRef] [PubMed]
- Davies, O.L.; Simeon, B. WHO Publishes List of Bacteria for Which New Antibiotics are Urgently Needed; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Chatterjee, M.; D’Morris, S.; Paul, V.; Warrier, S.; Vasudevan, A.K.; Vanuopadath, M.; Nair, S.S.; Paul-Prasanth, B.; Mohan, C.G.; Biswas, R. Mechanistic understanding of Phenyllactic acid mediated inhibition of quorum sensing and biofilm development in Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2017, 101, 8223–8236. [Google Scholar] [CrossRef]
- Anokhina, I.V.; Kravtsov, E.G.; Protsenko, A.V.; Yashina, N.V.; Yermolaev, A.V.; Chesnokova, V.L.; Dalin, M.V. Bactericidal activity of culture fluid components of Lactobacillus fermentum strain 90 TS-4 (21) clone 3, and their capacity to modulate adhesion of Candida Albicans yeast-like fungi to vaginal epithelial cells. Bull. Exp. Biol. Med. 2007, 143, 359–362. [Google Scholar] [CrossRef] [PubMed]
- Stanojević-Nikolić, S.; Dimić, G.; Mojović, L.; Pejin, J.; Djukić-Vuković, A.; Kocić-Tanackov, S. Antimicrobial Activity of Lactic Acid Against Pathogen and Spoilage Microorganisms: Antimicrobial Activity of Lactic Acid. J. Food Process. Preserv. 2016, 40, 990–998. [Google Scholar] [CrossRef]
- Girma, A.; Aemiro, A. Antibacterial Activity of Lactic Acid Bacteria Isolated from Fermented Ethiopian Traditional Dairy Products against Food Spoilage and Pathogenic Bacterial Strains. J. Food Qual. 2021, 2021, 9978561. [Google Scholar] [CrossRef]
- Amenu, D.; Bacha, K. Antagonistic Effects of Lactic Acid Bacteria Isolated from Ethiopian Traditional Fermented Foods and Beverages Against Foodborne Pathogens. Probiotics Antimicrob. Proteins 2025, 17, 1674–1687. [Google Scholar] [CrossRef]
- Anwer, A.W.; Ahmed, M.E. Antimicrobial Susceptibility of Fructophilic Lactic Acid Bacteria on PHZM Gene of Pseudomonas Aeruginosa Isolates from Wounds Infected. Acta Medica Bulg. 2024, 51, 52–58. [Google Scholar] [CrossRef]
- Jiang, X.; Yan, X.; Gu, S.; Yang, Y.; Zhao, L.; He, X.; Chen, H.; Ge, J.; Liu, D. Biosurfactants of Lactobacillus helveticus for Biodiversity Inhibit the Biofilm Formation of Staphylococcus aureus and Cell Invasion. Future Microbiol. 2019, 14, 1133–1146. [Google Scholar] [CrossRef]
- Akova, B.; Kıvanç, S.A.; Kıvanç, M. Antibiofilm effect of probiotic lactic acid bacteria against Bacillus spp obtained from the ocular surface. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 7799–7805. [Google Scholar] [CrossRef]
- Hindieh, P.; Yaghi, J.; Assaf, J.C.; Chokr, A.; Atoui, A.; Louka, N.; Khoury, A.E. Unlocking the potential of lactic acid bacteria mature biofilm extracts as antibiofilm agents. AMB Express 2024, 14, 112. [Google Scholar] [CrossRef]
- Göksel, Ş.; Akçelik, N.; Özdemir, C.; Akçelik, M. The Effects of Lactic Acid Bacteria on Salmonella Biofilms. Microbiology 2022, 91, 278–285. [Google Scholar] [CrossRef]
- Gong, H.; Meng, X.; Wang, H. Mode of action of plantaricin MG, a bacteriocin active against Salmonella typhimurium. J. Basic. Microbiol. 2010, 50, S37–S45. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; An, H.; Hao, Y.; Qin, Q.; Huang, Y.; Luo, Y.; Zhang, L. Characterization of an anti-Listeria bacteriocin produced by Lactobacillus plantarum LB-B1 isolated from koumiss, a traditionally fermented dairy product from China. Food Control 2011, 22, 1027–1031. [Google Scholar] [CrossRef]
- Mangalanayaki, R.; Bala, A. Bacteriocin production using lactic acid bacteria. Biomed. Pharmacol. J. 2015, 3, 413–416. [Google Scholar]
- Ahmed, M. Enterococcus faecium Bacteriocin Efflux Pump MexA Gene and promote skin wound healing in mice. ESS Open Arch. Eprints 2023, 11, 01143350. [Google Scholar] [CrossRef]
- Ahmed, S.O.; Zedan, H.H.; Ibrahim, Y.M. Quorum sensing inhibitory effect of bergamot oil and aspidosperma extract against Chromobacterium violaceum and Pseudomonas aeruginosa. Arch. Microbiol. 2021, 203, 4663–4675. [Google Scholar] [CrossRef]
- Juszczuk-Kubiak, E. Molecular Aspects of the Functioning of Pathogenic Bacteria Biofilm Based on Quorum Sensing (QS) Signal-Response System and Innovative Non-Antibiotic Strategies for Their Elimination. Int. J. Mol. Sci. 2024, 25, 2655. [Google Scholar] [CrossRef]
- Mohan, M.S.; Salim, S.A.; Ranganathan, S.; Parasuraman, P.; Anju, V.T.; Ampasala, D.R.; Dyavaiah, M.; Lee, J.-K.; Busi, S. Attenuation of Las/Rhl quorum sensing regulated virulence and biofilm formation in Pseudomonas aeruginosa PAO1 by Artocarpesin. Microb. Pathog. 2024, 189, 106609. [Google Scholar] [CrossRef]
- Das, T.; Young, B.C. Biofilm Formation by Pathogenic Bacteria: The Role of Quorum Sensing and Physical-Chemical Interactions. In Focus on Bacterial Biofilms; Das, T., Ed.; IntechOpen: London, UK, 2022; ISBN 978-1-80355-795-3. [Google Scholar]
- Aarti, C.; Khusro, A.; Varghese, R.; Arasu, M.V.; Agastian, P.; Al-Dhabi, N.A.; Ilavenil, S.; Choi, K.C. In vitro investigation on probiotic, anti-Candida, and antibiofilm properties of Lactobacillus pentosus strain LAP1. Arch. Oral. Biol. 2018, 89, 99–106. [Google Scholar] [CrossRef]
- Savijoki, K.; San-Martin-Galindo, P.; Pitkänen, K.; Edelmann, M.; Sillanpää, A.; Van Der Velde, C.; Miettinen, I.; Patel, J.Z.; Yli-Kauhaluoma, J.; Parikka, M.; et al. Food-Grade Bacteria Combat Pathogens by Blocking AHL-Mediated Quorum Sensing and Biofilm Formation. Foods 2022, 12, 90. [Google Scholar] [CrossRef]
- Patel, M.; Siddiqui, A.J.; Ashraf, S.A.; Surti, M.; Awadelkareem, A.M.; Snoussi, M.; Hamadou, W.S.; Bardakci, F.; Jamal, A.; Jahan, S.; et al. Lactiplantibacillus plantarum-Derived Biosurfactant Attenuates Quorum Sensing-Mediated Virulence and Biofilm Formation in Pseudomonas aeruginosa and Chromobacterium violaceum. Microorganisms 2022, 10, 1026. [Google Scholar] [CrossRef] [PubMed]
- Kostoglou, D.; Giaouris, E. Screening of Lactic Acid Bacteria Isolated from Foods for Interference with Bacterial Quorum Sensing Systems. In Proceedings of the 5th International Electronic Conference on Foods, Basel, Switzerland, 28–30 October 2024; MDPI: Basel, Switzerland, 2025; p. 19. [Google Scholar]
- Natesan, S.; Boopathi, S.; Selvakumar, G. Quorum Quenching Potentials of Probiotic Enterococcus Durans Lab38 Against Methicillin Resistant Staphylococcus Aureus. Asian J. Pharm. Clin. Res. 2017, 10, 445. [Google Scholar] [CrossRef]
- Miller, L.C.; O’Loughlin, C.T.; Zhang, Z.; Siryaporn, A.; Silpe, J.E.; Bassler, B.L.; Semmelhack, M.F. Development of Potent Inhibitors of Pyocyanin Production in Pseudomonas aeruginosa. J. Med. Chem. 2015, 58, 1298–1306. [Google Scholar] [CrossRef]
- Rai, D.; Sulthana, F.; Neeksha; Divyashree, M. Production and Antimicrobial Activities of Pyocyanin Extracts from Pseudomonas aeruginosa. J. Pure Appl. Microbiol. 2024, 18, 2371–2379. [Google Scholar] [CrossRef]
- Marey, M.A.; Abozahra, R.; El-Nikhely, N.A.; Kamal, M.F.; Abdelhamid, S.M.; El-Kholy, M.A. Transforming microbial pigment into therapeutic revelation: Extraction and characterization of pyocyanin from Pseudomonas aeruginosa and its therapeutic potential as an antibacterial and anticancer agent. Microb. Cell Factories 2024, 23, 174. [Google Scholar] [CrossRef]
- Algburi, A.; Comito, N.; Kashtanov, D.; Dicks, L.M.T.; Chikindas, M.L. Control of Biofilm Formation: Antibiotics and Beyond. Appl. Environ. Microbiol. 2017, 83, e02508-16. [Google Scholar] [CrossRef]
- Van Zyl, W.F.; Deane, S.M.; Dicks, L.M.T. Molecular insights into probiotic mechanisms of action employed against intestinal pathogenic bacteria. Gut Microbes 2020, 12, 1831339. [Google Scholar] [CrossRef]
- Yao, W.; Yang, L.; Shao, Z.; Xie, L.; Chen, L. Identification of salt tolerance-related genes of Lactobacillus plantarum D31 and T9 strains by genomic analysis. Ann. Microbiol. 2020, 70, 10. [Google Scholar] [CrossRef]
- Georgieva, R.N.; Iliev, I.N.; Chipeva, V.A.; Dimitonova, S.P.; Samelis, J.; Danova, S.T. Identification and in vitro characterisation of Lactobacillus plantarum strains from artisanal Bulgarian white brined cheeses. J. Basic. Microbiol. 2008, 48, 234–244. [Google Scholar] [CrossRef]
- Dobreva, L.; Borisova, D.; Paunova-Krasteva, T.; Dimitrova, P.D.; Hubenov, V.; Atanasova, N.; Ivanov, I.; Danova, S. From Traditional Dairy Product “Katak” to Beneficial Lactiplantibacillus plantarum Strains. Microorganisms 2023, 11, 2847. [Google Scholar] [CrossRef]
- Albene, D.; Lema, N.K.; Tesfaye, G.; Andeta, A.F.; Ali, K.; Guadie, A. Probiotic potential of lactic acid bacteria isolated from Ethiopian traditional fermented Cheka beverage. Ann. Microbiol. 2024, 74, 25. [Google Scholar] [CrossRef]
- Sana, M.; Ouarda, A.Z.; Nassereddine, Z.M.; Magalie, B.; Mohamed, B.A.; Nathalie, C. Probiotic potential of indigenous Lactobacillus strains isolated from Algerian goatskin bag cheese Bouhezza using physiological and comparative genomic analyses. Int. Dairy J. 2025, 165, 106206. [Google Scholar] [CrossRef]
- Madushanka, D.; Vidanarachchi, J.K.; Kodithuwakku, S.; Nayanajith, G.R.A.; Jayatilake, S.; Priyashantha, H. Isolation and characterization of probiotic lactic acid bacteria from fermented traditional rice for potential applications in food and livestock production. Appl. Food Res. 2025, 5, 100865. [Google Scholar] [CrossRef]
- Wei, G.; Wang, D.; Wang, T.; Wang, G.; Chai, Y.; Li, Y.; Mei, M.; Wang, H.; Huang, A. Probiotic potential and safety properties of Limosilactobacillus fermentum A51 with high exopolysaccharide production. Front. Microbiol. 2025, 16, 1498352. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paunova-Krasteva, T.; Dimitrova, P.D.; Borisova, D.; Dobreva, L.; Atanasova, N.; Danova, S. Characterization of a Vaginal Limosilactobacillus Strain Producing Anti-Virulence Postbiotics: A Potential Probiotic Candidate. Fermentation 2025, 11, 350. https://doi.org/10.3390/fermentation11060350
Paunova-Krasteva T, Dimitrova PD, Borisova D, Dobreva L, Atanasova N, Danova S. Characterization of a Vaginal Limosilactobacillus Strain Producing Anti-Virulence Postbiotics: A Potential Probiotic Candidate. Fermentation. 2025; 11(6):350. https://doi.org/10.3390/fermentation11060350
Chicago/Turabian StylePaunova-Krasteva, Tsvetelina, Petya D. Dimitrova, Dayana Borisova, Lili Dobreva, Nikoleta Atanasova, and Svetla Danova. 2025. "Characterization of a Vaginal Limosilactobacillus Strain Producing Anti-Virulence Postbiotics: A Potential Probiotic Candidate" Fermentation 11, no. 6: 350. https://doi.org/10.3390/fermentation11060350
APA StylePaunova-Krasteva, T., Dimitrova, P. D., Borisova, D., Dobreva, L., Atanasova, N., & Danova, S. (2025). Characterization of a Vaginal Limosilactobacillus Strain Producing Anti-Virulence Postbiotics: A Potential Probiotic Candidate. Fermentation, 11(6), 350. https://doi.org/10.3390/fermentation11060350






