Immobilized Plant-Based Presumptive Probiotics as Functional Ingredients for Breakfast Cereals
Abstract
1. Introduction
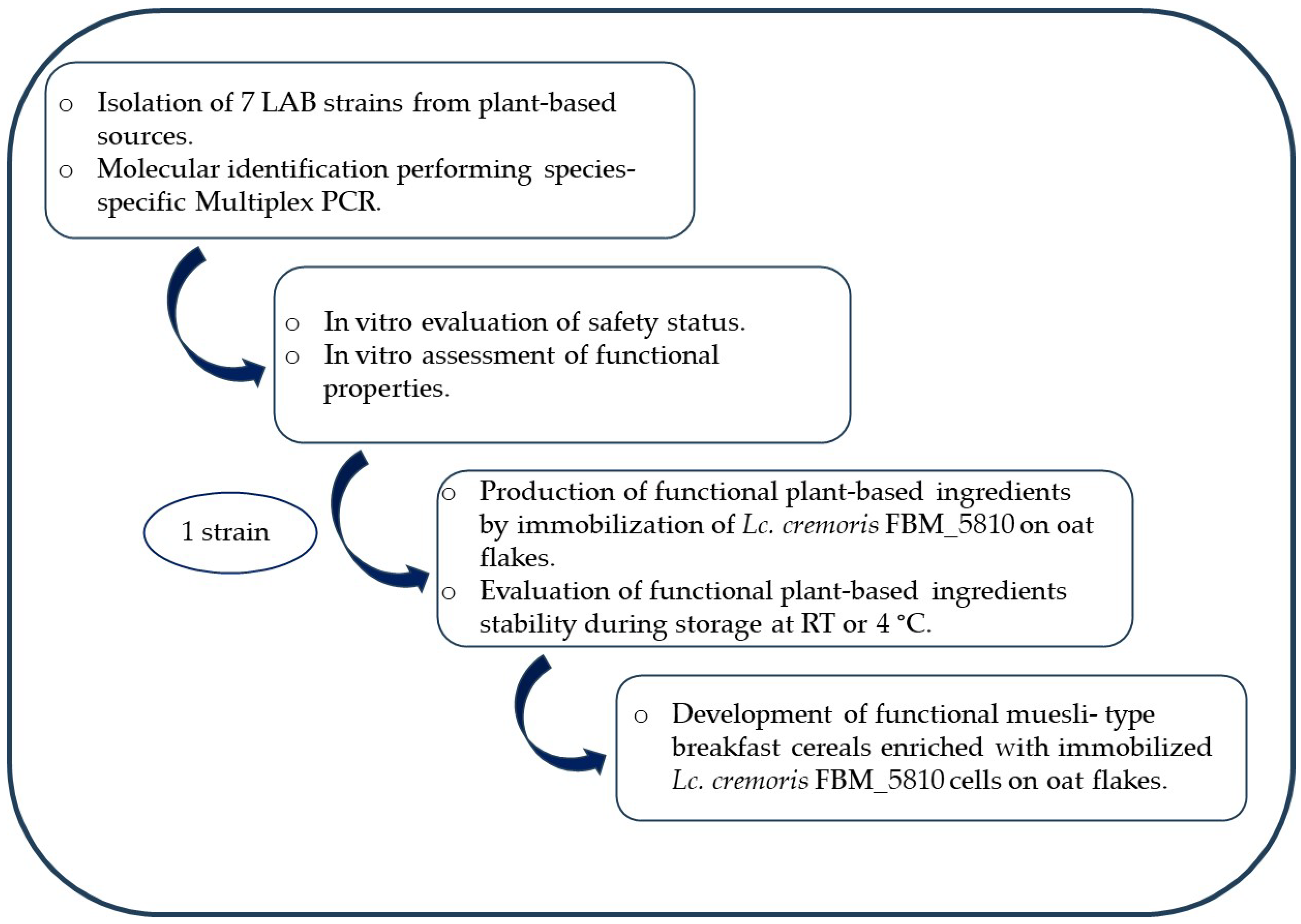
2. Materials and Methods
2.1. Isolation of Wild-Type LAB Strains
2.2. Molecular Identification of Isolated Strains
2.3. Bacterial Growth Conditions
2.4. In Vitro Safety Evaluation
2.4.1. Hemolytic Activity
2.4.2. Antibiotic Susceptibility Test
2.5. In Vitro Assessment of Functional Properties Linked with Probiotic Potential
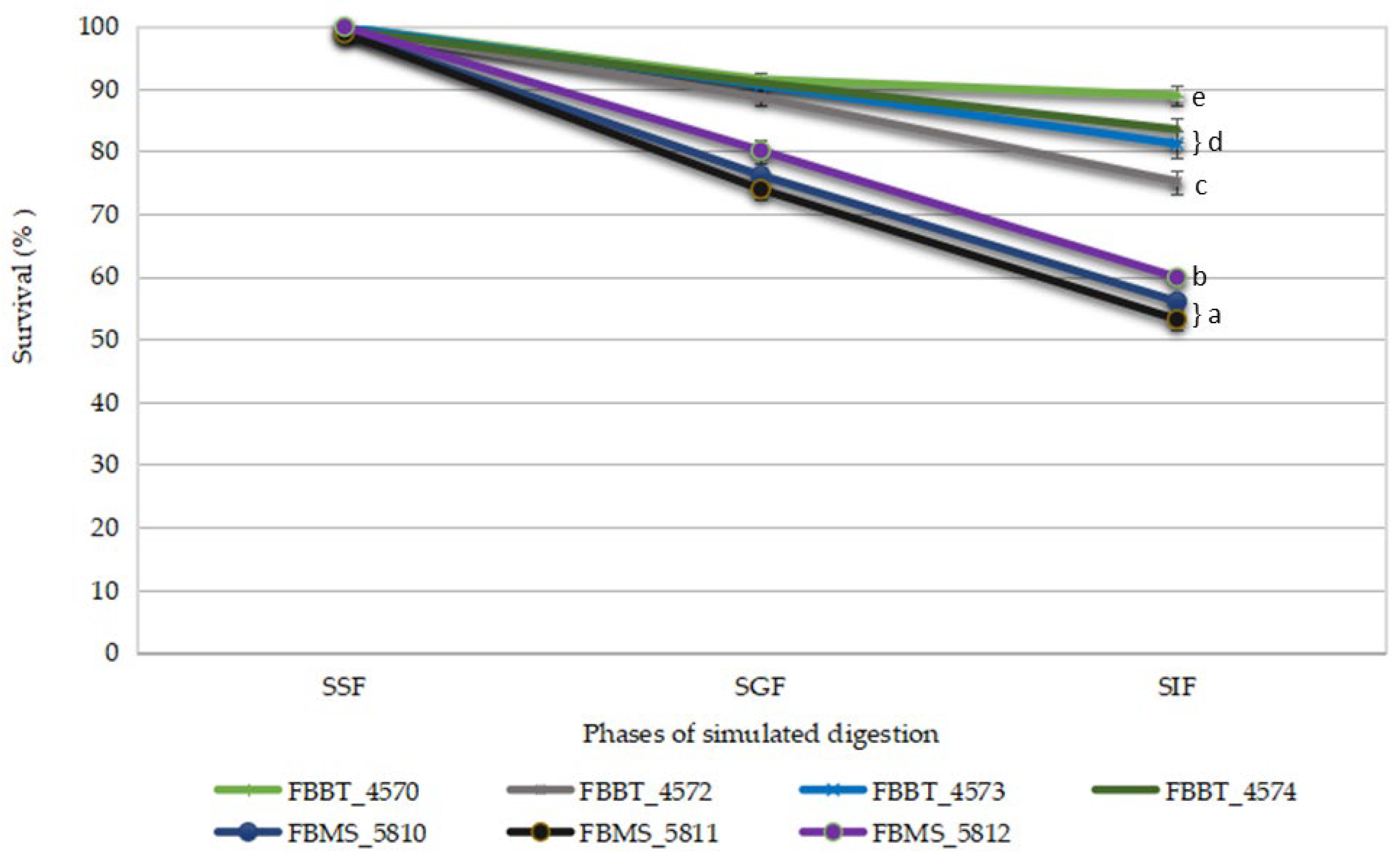
2.5.1. Ability to Survive GI Tract Conditions
2.5.2. Adhesion Properties of Isolates
Hydrophobicity
Auto-Aggregation and Co-Aggregation with Common Foodborne Pathogens
Adhesion to Differentiated Caco-2 Cells
2.5.3. Bile Salt Hydrolase (BSH) Activity
2.5.4. In Vitro Cholesterol Assimilation
2.5.5. Inhibitory Potential of Plant-Origin Isolates Against Foodborne Pathogens
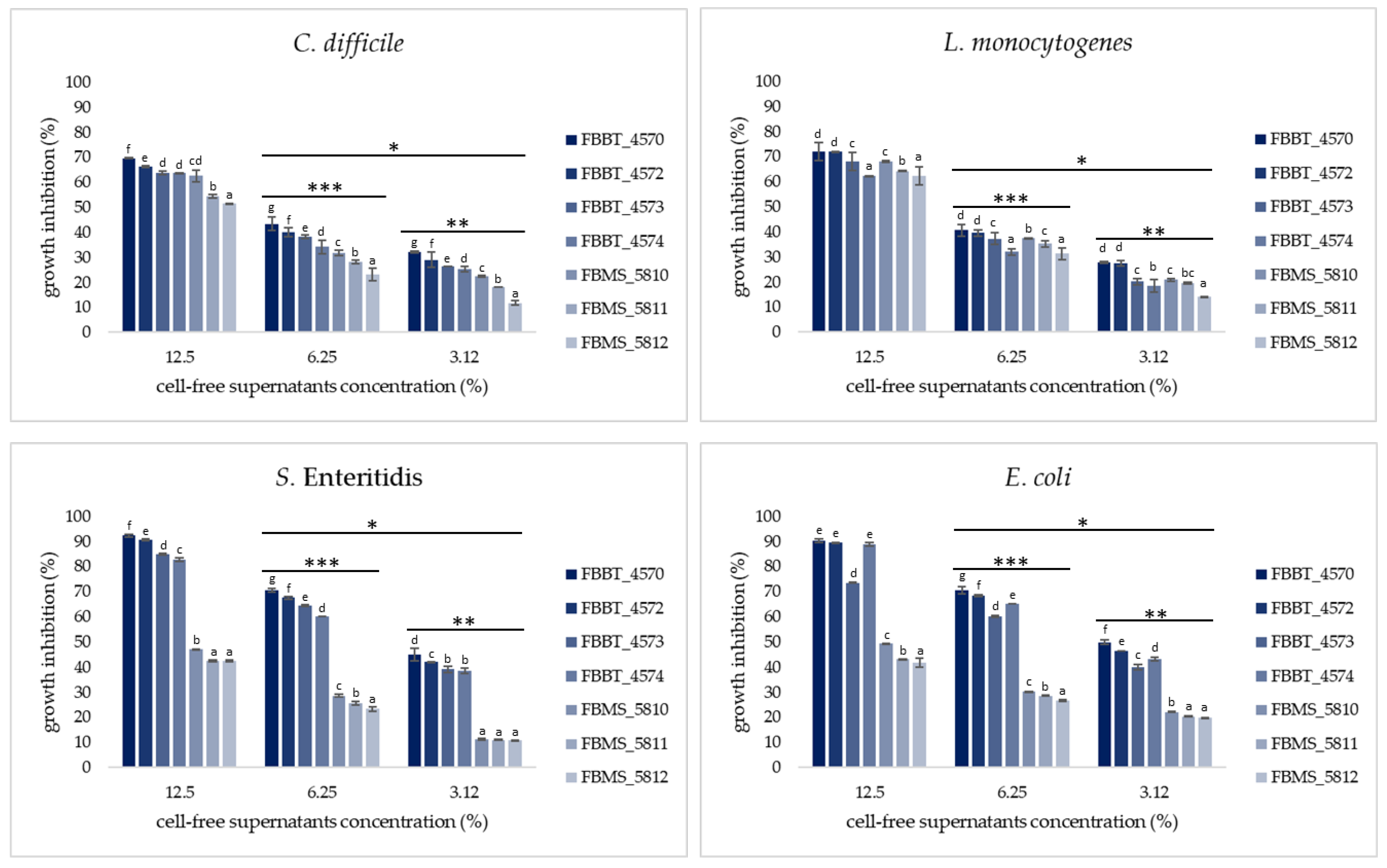
Evaluation of Antagonistic Activity of Isolates Against Foodborne Pathogens
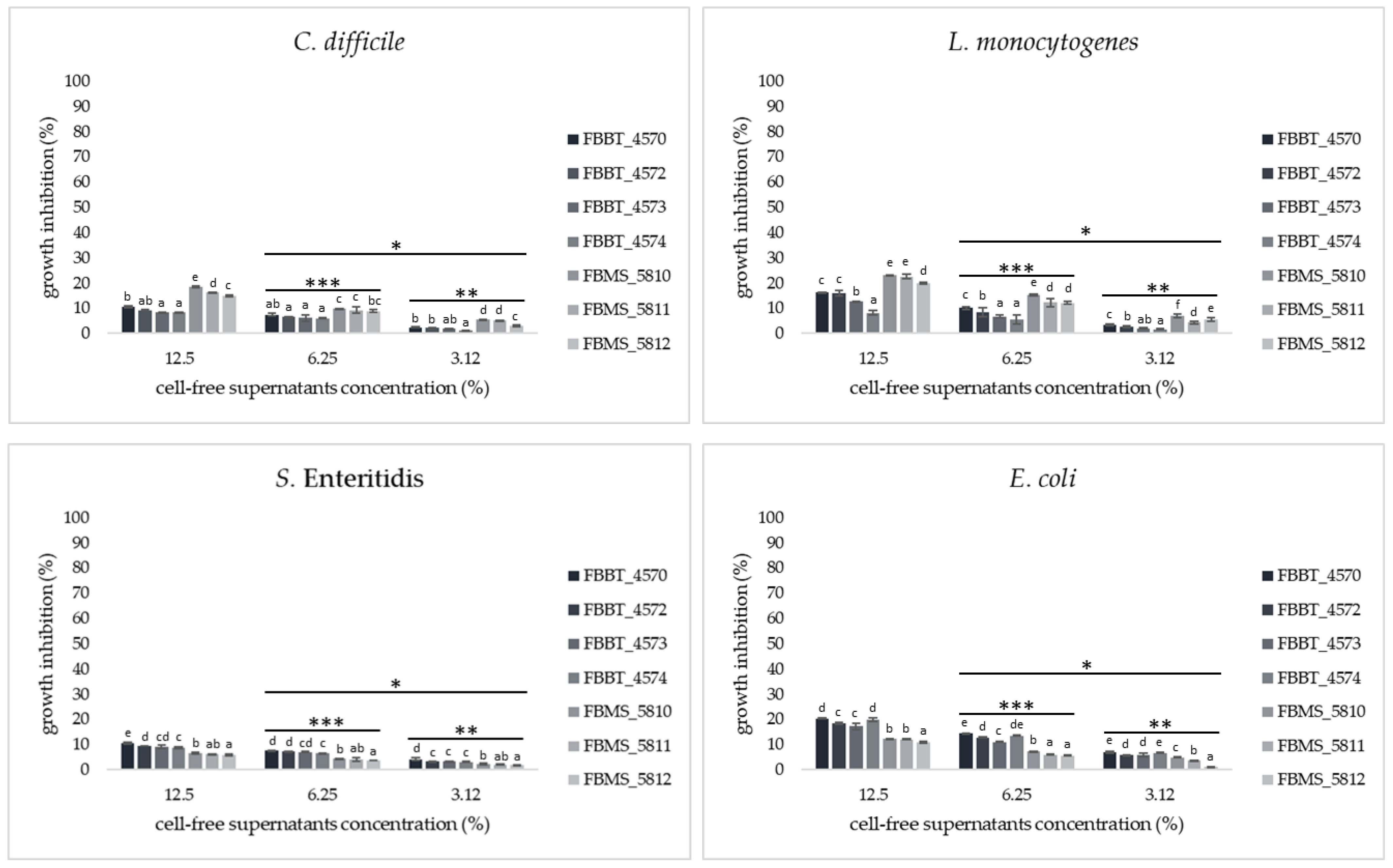
Antimicrobial Activity of CFSs of Isolates Against Common Foodborne Pathogens Using a Broth Microdilution Assay
2.6. Preparation of Freeze-Dried Immobilized Lc. cremoris FBMS_5810 Cells on Oat Flakes as Functional Plant-Based Ingredients
2.7. Production of Functional Muesli-Type Breakfast Cereals Fortified with Immobilized Lc. cremoris FBMS_5810 Cells on Oat Flakes
2.8. Analyses
2.8.1. Scanning Electron Microscopy
2.8.2. Physicochemical Analyses
2.8.3. Microbiological Analyses
2.9. Sensory Evaluation
2.10. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Molecular Identification of LAB Isolates
3.2. Safety Assessment
3.2.1. Hemolytic Activity
3.2.2. Antibiotic Susceptibility Test
3.3. In Vitro Assessment of Functional Properties Linked with Probiotic Potential
3.3.1. Ability to Survive GI Tract Conditions
3.3.2. Adhesion Properties of Isolates
Hydrophobicity and Auto-Aggregation
Co-Aggregation with Common Foodborne Pathogens
Adhesion to Differentiated Caco-2 Cell Lines
3.3.3. Bile Salt Hydrolase Activity
3.3.4. In Vitro Cholesterol Assimilation
3.3.5. Inhibitory Potential of Plant-Origin Isolates Against Common Foodborne Pathogens
Evaluation of Antagonistic Activity of Plant-Origin Isolates Against Common Foodborne Pathogens Using a Co-Culture Assay
Antimicrobial Activity of CFSs of Plant-Origin Isolates Against Common Foodborne Pathogens Using a Broth Microdilution Assay
3.4. Production of Functional Plant-Based Ingredients by Immobilization of Lc. cremoris FBMS_5810 Cells on Oat Flakes
3.4.1. Effect of Storage on Cell Viability of Freeze-Dried Immobilized Lc. cremoris FBMS_5810 Cells on Oat Flakes
Storage at Room Temperature
Storage at Refrigerated Temperature
3.4.2. Water Activity and Moisture Content Values of Freeze-Dried Immobilized Lc. cremoris FBMS_5810 Cells on Oat Flakes During Storage
3.5. Production of Functional Muesli-Type Breakfast Cereals Enriched with Freeze-Dried Immobilized Lc. cremoris FBMS_5810 Cells on Oat Flakes
3.5.1. Cell Viability of Lc. cremoris FBMS_5810 Cells in Functional Muesli-Type Breakfast Cereals During Storage
3.5.2. Effect of Storage on Water Activity (aw) and Moisture Content in Functional Muesli-Type Breakfast Cereals
3.5.3. Sensory Evaluation of Functional Muesli-Type Breakfast Cereals Enriched with Freeze-Dried Immobilized Lc. cremoris FBMS_5810 Cells on Oat Flakes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laparra, J.M.; Sanz, Y. Interactions of gut microbiota with functional food components and nutraceuticals. Pharmacol. Res. 2010, 61, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Danik, M.; Jaishree, S. A New Definition of Functional Food by FFC: What Makes a New Definition Unique? Funct. Foods Health Dis. 2015, 5, 209–223. [Google Scholar] [CrossRef]
- FAO; WHO. Guidelines for the Evaluation of Probiotics in Food; Food and Agriculture Organization of the United Nations/World Health Organization: London, UK, 2002; Available online: https://isappscience.org/wp-content/uploads/2019/04/probiotic_guidelines.pdf (accessed on 2 October 2024).
- Feng, Y.; Qiao, L.; Liu, R.; Yao, H.; Gao, C. Potential Probiotic Properties of Lactic Acid Bacteria Isolated from the Intestinal Mucosa of Healthy Piglets. Ann. Microbiol. 2017, 67, 239–253. [Google Scholar] [CrossRef]
- Miremadi, F.; Ayyash, M.; Sherkat, F.; Stojanovska, L. Cholesterol reduction mechanisms and fatty acid composition of cellular membranes of probiotic Lactobacilli and Bifidobacteria. J. Funct. Foods 2014, 9, 295–305. [Google Scholar] [CrossRef]
- Tsai, C.C.; Lin, P.P.; Hsieh, Y.M.; Zhang, Z.Y.; Wu, H.C.; Huang, C.C. Cholesterol-lowering potentials of lactic acid bacteria based on bile-salt hydrolase activity and effect of potent strains on cholesterol metabolism in vitro and in vivo. Sci. World J. 2014, 2014, 690752. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Albano, C.; Morandi, S.; Silvetti, T.; Casiraghi, M.C.; Manini, F.; Brasca, M. Lactic acid bacteria with cholesterol-lowering properties for dairy applications: In vitro and in situ activity. J. Dairy Sci. 2018, 101, 10807–10818. [Google Scholar] [CrossRef] [PubMed]
- Hor, Y.Y.; Liong, M.T. Use of Extracellular Extracts of Lactic Acid Bacteria and Bifidobacteria for the Inhibition of Dermatological Pathogen Staphylococcus aureus. Dermatol. Sin. 2014, 32, 141–147. [Google Scholar] [CrossRef]
- Arena, M.P.; Silvain, A.; Normanno, G.; Grieco, F.; Drider, D.; Spano, G.; Fiocco, D. Use of Lactobacillus plantarum Strains as a Bio-Control Strategy against Food-Borne Pathogenic Microorganisms. Front. Microbiol. 2016, 7, 464. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, C.C.; Lai, C.C.; Huang, H.L.; Huang, W.Y.; Toh, H.S.; Weng, T.C.; Chuang, Y.C.; Lu, Y.C.; Tang, H.J. Antimicrobial Activity of Lactobacillus Species Against Carbapenem-Resistant Enterobacteriaceae. Front. Microbiol. 2019, 10, 789. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nguyen, T.H.; Kim, Y.; Kim, J.S.; Kim, J.-E.; Kim, H.; Paek, N.-S.; Kang, C.-H. Evaluating the Cryoprotective Encapsulation of the Lactic Acid Bacteria in Simulated Gastrointestinal Conditions. Biotechnol. Bioprocess Eng. 2020, 25, 287–292. [Google Scholar] [CrossRef]
- Mitropoulou, G.; Nedovic, V.; Goyal, A.; Kourkoutas, Y. Immobilization Technologies in Probiotic Food Production. J. Nutr. Metab. 2013, 2013, 716861. [Google Scholar] [CrossRef] [PubMed]
- Prapa, I.; Nikolaou, A.; Panas, P.; Tassou, C.; Kourkoutas, Y. Developing Stable Freeze-Dried Functional Ingredients Containing Wild-Type Presumptive Probiotic Strains for Food Systems. Appl. Sci. 2023, 13, 630. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Salminen, S. In vitro adhesion Assays for Probiotics and Their in vivo relevance: A Review. Microb. Ecol. Health Dis. 2003, 15, 175–184. [Google Scholar]
- Deepika, G.; Charalampopoulos, D. Surface and Adhesion Properties of Lactobacilli. Adv. Appl. Microbiol. 2010, 70, 127–152. [Google Scholar]
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M.; et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701. [Google Scholar] [CrossRef]
- Nikolaou, A.; Sgouros, G.; Mitropoulou, G.; Santarmaki, V.; Kourkoutas, Y. Freeze-Dried Immobilized Kefir Culture in Low Alcohol Winemaking. Foods 2020, 9, 115. [Google Scholar] [CrossRef]
- Mcclements, D.J.; Grossmann, L. Next-Generation Plant-Based Foods Design, Production, and Properties; Springer: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Torriani, S.; Felis, G.E.; Dellaglio, F. Differentiation of Lactobacillus plantarum, L. pentosus, and L. paraplantarum by recA gene sequence analysis and multiplex PCR assay with recA gene-derived primers. Appl. Environ. Microbiol. 2001, 67, 3450–3454. [Google Scholar] [CrossRef]
- Odamaki, T.; Yonezawa, S.; Kitahara, M.; Sugahara, Y.; Xiao, J.Z.; Yaeshima, T.; Iwatsuki, K.; Ohkuma, M. Novel multiplex polymerase chain reaction primer set for identification of Lactococcus species. Lett. Appl. Microbiol. 2011, 52, 491–496. [Google Scholar] [CrossRef]
- ISO 10932/IDF 223:2010; Milk and Milk Products—Determination of the Minimal Inhibitory Concentration (MIC) of Antibiotics Applicable to Bifidobacteria and Non-Enterococcal Lactic Acid Bacteria (LAB). ISO: Geneva, Switzerland, 2010.
- Prapa, I.; Pavlatou, C.; Kompoura, V.; Nikolaou, A.; Stylianopoulou, E.; Skavdis, G.; Grigoriou, M.E.; Kourkoutas, Y. A Novel Wild- Type Lacticaseibacillus paracasei Strain Suitable for the Production of Functional Yoghurt and Ayran Products. Fermentation 2025, 11, 37. [Google Scholar] [CrossRef]
- Nelios, G.; Santarmaki, V.; Pavlatou, C.; Dimitrellou, D.; Kourkoutas, Y. New Wild-Type Lacticaseibacillus rhamnosus Strains as Candidates to Manage Type 1 Diabetes. Microorganisms 2022, 10, 272. [Google Scholar] [CrossRef]
- Li, Q.; Liu, X.; Dong, M.; Zhou, J.; Wang, Y. Aggregation and adhesion abilities of 18 lactic acid bacteria strains isolated from traditional fermented food. Int. J. Agric. Policy Res. 2014, 3, 84–92. [Google Scholar]
- Lappa, I.K.; Natsia, A.; Alimpoumpa, D.; Stylianopoulou, E.; Prapa, I.; Tegopoulos, K.; Pavlatou, C.; Skavdis, G.; Papadaki, A.; Kopsahelis, N. Novel Probiotic Candidates in Artisanal Feta-Type Kefalonian Cheese: Unveiling a Still-Undisclosed Biodiversity. Probiotics Antimicrob. Proteins 2024, 16. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Argyri, A.A.; Zoumpopoulou, G.; Karatzas, K.-A.G.; Tsakalidou, E.; Nychas, G.-J.E.; Panagou, E.Z.; Tassou, C.C. Selection of Potential Probiotic Lactic Acid Bacteria from Fermented Olives by in vitro Tests. Food Microbiol. 2013, 33, 282–291. [Google Scholar] [CrossRef]
- Somalou, P.; Ieronymaki, E.; Feidaki, K.; Prapa, I.; Stylianopoulou, E.; Spyridopoulou, K.; Skavdis, G.; Grigoriou, M.E.; Panas, P.; Argiriou, A.; et al. Novel Wild-Type Pediococcus and Lactiplantibacillus Strains as Probiotic Candidates to Manage Obesity-Associated Insulin Resistance. Microorganisms 2024, 12, 231. [Google Scholar] [CrossRef]
- ISO 5534:2004; Cheese and Processed Cheese. Determination of the Total Solids Content (Reference Method). International Organization for Standardization: Geneva, Switzerland, 2004.
- ISO 6658:2017; Sensory Analysis—Methodology—General Guidance. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 8589:2007; Sensory Analysis—General Guidance for the Design of Test Rooms. International Organization for Standardization: Geneva, Switzerland, 2007.
- EFSA Panel on Additives Products or Substances used in Animal Feed (FEEDAP). Guidance on the assessment of bacterial susceptibility to antimicrobials of human veterinary importance. EFSA J. 2012, 10, 2740. [Google Scholar]
- Campedelli, I.; Mathur, H.; Salvetti, E.; Clarke, S.; Rea, M.C.; Torriani, S.; Ross, R.P.; Hill, C.; O’Toole, P.W. Genus-Wide Assessment of Antibiotic Resistance in Lactobacillus spp. Appl. Environ. Microbiol. 2019, 85, e01738-18. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Balance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standarised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Grimoud, J.; Durand, H.; Courtin, C.; Monsan, P.; Ouarné, F.; Theodorou, V.; Roques, C. In vitro Screening of Probiotic Lactic Acid Bacteria and Prebiotic Glucooligosaccharides to Select Effective Synbiotics. Anaerobe 2010, 16, 493–500. [Google Scholar] [CrossRef]
- Guan, C.; Chen, X.; Jiang, X.; Zhao, R.; Yuan, Y.; Chen, D.; Zhang, C.; Lu, M.; Lu, Z.; Gu, R. In vitro Studies of Adhesion Properties of Six Lactic Acid Bacteria Isolated from the Longevous Population of China. RSC Adv. 2020, 10, 24234–24240. [Google Scholar] [CrossRef]
- Pessoa, W.F.B.; Melgaço, A.C.C.; Almeida, M.E.; Ramos, L.P.; Rezende, R.P.; Romano, C.C. In vitro activity of lactobacilli with probiotic potential isolated from cocoa fermentation against Gardnerella vaginalis. J. Appl. Microbiol. 2018, 125, 1074–1084. [Google Scholar] [CrossRef]
- Krausova, G.; Hyrslova, I.; Hynstova, I. In vitro Evaluation of Adhesion Capacity, Hydrophobicity, and Auto-Aggregation of Newly Isolated Potential Probiotic Strains. Fermentation 2019, 5, 100. [Google Scholar] [CrossRef]
- Collado, M.C.; Meriluoto, J.; Salminen, S. Adhesion and aggregation properties of probiotic and pathogen strains. Eur. Food Res. Technol. 2008, 226, 1065–1073. [Google Scholar] [CrossRef]
- Darmastuti, A.; Hasan, P.N.; Wikandari, R.; Utami, T.; Rahayu, E.S.; Suroto, D.A. Adhesion Properties of Lactobacillus plantarum Dad-13 and Lactobacillus plantarum Mut-7 on Sprague Dawley Rat Intestine. J. Pure Appl. Microbiol. 2020, 14, 173–179. [Google Scholar] [CrossRef]
- Handa, S.; Sharma, N. In vitro study of probiotic properties of Lactobacillus plantarum F22 isolated from chang—A traditional fermented beverage of Himachal Pradesh, India. J. Funct. Foods 2018, 45, 98–106. [Google Scholar]
- Tarazanova, M.; Huppertz, T.; Beerthuyzen, M.; van Schalkwijk, S.; Janssen, P.; Wels, M.; Kok, J.; Bachmann, H. Cell surface properties of Lactococcus lactis reveal milk protein binding specifically evolved in dairy isolates. Front. Microbiol. 2016, 7, 1418. [Google Scholar] [CrossRef]
- Zakaria Gomaa, E. Antimicrobial and anti-adhesive properties of biosurfactant produced by lactobacilli isolates, biofilm formation and aggregation ability. J. Gen. Appl. Microbiol. 2013, 59, 425–436. [Google Scholar] [CrossRef]
- García-Cayuela, T.; Korany, A.M.; Bustos, I.; Gómez de Cadiñanos, L.P.; Requena, T.; Peláez, C.; Martínez-Cuesta, M.C. Adhesion abilities of dairy Lactobacillus plantarum strains showing an aggregation phenotype. Food Res. Int. 2014, 64, 772–778. [Google Scholar] [CrossRef]
- Kaewnopparat, S.; Dangmanee, N.; Kaewnopparat, N.; Srichana, T.; Chulasiri, M.; Settharaksa, S. In vitro probiotic properties of Lactobacillus fermentum SK5 isolated from vagina of a healthy woman. Anaerobe 2013, 22, 6–13. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, K.; Sharma, R. Identification and Evaluation of In vitro Probiotic Attributes of Novel and Potential Strains of Lactic Acid Bacteria Isolated from Traditional Dairy Products of North-West Himalayas. J. Clin. Microbiol. Biochem. Technol. 2016, 2, 018–025. [Google Scholar] [CrossRef]
- Tuo, Y.; Yu, H.; Ai, L.; Wu, Z.; Guo, B.; Chen, W. Aggregation and adhesion properties of 22 Lactobacillus strains. J. Dairy Sci. 2013, 96, 4252–4257. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, Y.; Cui, H.; Li, Y.; Sun, Y.; Qiu, H.J. Characterization of Lactic Acid Bacteria Isolated From the Gastrointestinal Tract of a Wild Boar as Potential Probiotics. Front. Vet. Sci. 2020, 7, 49. [Google Scholar] [CrossRef] [PubMed]
- Alp, D.; KuleaŞan, H. Determination of competition and adhesion abilities of lactic acid bacteria against gut pathogens in a whole-tissue model. Biosci. Microbiota Food Health 2020, 39, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Bhat, B.; Bajaj, B.K. Multifarious cholesterol lowering potential of lactic acid bacteria equipped with desired probiotic functional attributes. 3 Biotech. 2020, 10, 200. [Google Scholar] [CrossRef] [PubMed]
- Shivani, T.M.; Sathiavelu, M. Probiotic evaluation, adherence capability and safety assessment of Lactococcus lactis strain isolated from an important herb “Murraya koenigii”. Sci. Rep. 2024, 14, 15565. [Google Scholar] [CrossRef]
- Jeong, H.; Kim, S.; Hwang, U.-S.; Choi, H.; Park, Y.-S. Immunostimulatory Activity of Lactococcus lactis subsp. lactis CAB701 Isolated from Jeju Cabbage. Microorganisms 2023, 11, 1718. [Google Scholar]
- Cimminiello, C.; Zambon, A.; Polo Friz, H. Hypercholesterolemia and cardiovascular risk: Advantages and limitations of current treatment options. G Ital Cardiol. 2016, 17 (Suppl. 1), 6S–13S. [Google Scholar] [CrossRef]
- Ramkumar, S.; Raghunath, A.; Raghunath, S. Statin Therapy: Review of Safety and Potential Side Effects. Acta Cardiol. Sin. 2016, 32, 631–639. [Google Scholar] [CrossRef]
- Wang, S.C.; Chang, C.K.; Chan, S.C.; Shieh, J.S.; Chiu, C.K.; Duh, P.-D. Effects of Lactic Acid Bacteria Isolated from Fermented Mustard on Lowering Cholesterol. Asian Pac. J. Trop. Biomed. 2014, 4, 523–528. [Google Scholar] [CrossRef]
- Hassanein, W.A.; Awny, N.M.; Ibraheim, S.M. Reduction of cholesterol by Lactococcus lactis KF147. Sch. J. Biol. Sci. 2013, 2, 30–38. [Google Scholar]
- Shehata, M.G.; El Sohaimy, S.A.; El-Sahn, M.A.; Youssef, M.M. Screening of Isolated Potential Probiotic Lactic Acid Bacteria for Cholesterol Lowering Property and Bile Salt Hydrolase Activity. Ann. Agric. Sci. 2016, 61, 65–75. [Google Scholar] [CrossRef]
- Bandyopadhyay, B.; Das, S.; Mitra, P.K.; Kundu, A.; Mandal, V.; Adhikary, R.; Mandal, N.C. Characterization of two new strains of Lactococcus lactis for their probiotic efficacy over commercial synbiotics consortia. Braz. J. Microbiol. 2022, 53, 903–920. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Xue, W.-J.; Ding, H.; An, C.; Ma, S.-J.; Liu, Y. Probiotic potential of Lactobacillus strains isolated from fermented vegetables in Shaanxi, China. Front. Microbiol. 2022, 12, 774903. [Google Scholar] [CrossRef]
- Patangia, D.V.; Anthony Ryan, C.; Dempsey, E.; Paul Ross, R.; Stanton, C. Impact of antibiotics on the human microbiome and consequences for host health. Microbiologyopen 2022, 11, e1260. [Google Scholar] [CrossRef] [PubMed]
- Kesavelu , D.; Jog, P. Current understanding of antibiotic-associated dysbiosis and approaches for its management. Ther. Adv. Infect. Dis. 2023, 10, 20499361231154443. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lathakumari, R.H.; Vajravelu, L.K.; Satheesan, A.; Ravi, S.; Thulukanam, J. Antibiotics and the gut microbiome: Understanding the impact on human health. Med. Microecol. 2024, 20, 100106. [Google Scholar] [CrossRef]
- Wadhwa, A.; AlNahhas, M.F.; Dierkhising, R.; Patel, R.; Kashyap, P.; Pardi, D.S.; Khanna, S.; Grover, M. High risk of post-infectious irritable bowel syndrome in patientswith Clostridium difficile infection. Aliment. Pharmacol. Ther. 2016, 44, 576–582. [Google Scholar] [CrossRef]
- Alkalbani, N.S.; Turner, M.S.; Ayyash, M.M. Isolation, Identification, and Potential Probiotic Characterization of Isolated Lactic Acid Bacteria and in vitro Investigation of the Cytotoxicity, Antioxidant, and Antidiabetic Activities in Fermented Sausage. Microb. Cell Factories 2019, 18, 188. [Google Scholar] [CrossRef]
- Choi, A.R.; Patra, J.K.; Kim, W.J.; Kang, S.S. Antagonistic activities and probiotic potential of lactic acid bacteria derived from a plant-based fermented food. Front. Microbiol. 2018, 9, 1963. [Google Scholar] [CrossRef]
- Ratsep, M.; Naaber, P.; Kõljalg, S.; Smidt, I.; Shkut, E.; Sepp, E. Effect of Lactobacillus plantarum strains on clinical isolates of Clostridium difficile in vitro. J. Probiotics Health 2014, 2, 1000119. [Google Scholar] [CrossRef]
- Reuben, R.C.; Roy, P.C.; Sarkar, S.L.; Alam, R.-U.; Jahid, I.K. Isolation, characterization, and assessment of lactic acid bacteria toward their selection as poultry probiotics. BMC Microbiol. 2019, 19, 253. [Google Scholar] [CrossRef]
- Maalaoui, A.; Trimeche, A.; Marnet, P.; Demarigny, Y. Use of Lactococcus lactis subsp. lactis strains to inhibit the development of pathogens. Food Nutr. Sci. 2020, 11, 98–112. [Google Scholar] [CrossRef]
- Tejero-Sariñena, S.; Barlow, J.; Costabile, A.; Gibson, G.R.; Rowland, I. In vitro evaluation of the antimicrobial activity of a range of probiotics against pathogens: Evidence for the effects of organic acids. Anaerobe 2012, 18, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Enan, G.; Abdel-Shafi, S.; Ouda, S.; Negm, S. Novel antibacterial activity of Lactococcus lactis subspecies lactis z11 isolated from zabady. Int. J. Biomed. Sci. 2013, 9, 174–180. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suzuki, A.; Suzuki, M. Antimicrobial Activity of Lactococcus lactis subsp. lactis Isolated from a Stranded Cuvier’s Beaked Whale (Ziphius cavirostris) against Gram-Positive and -Negative Bacteria. Microorganisms 2021, 9, 243. [Google Scholar] [CrossRef]
- Sanca, F.M.M.; Blanco, I.R.; Dias, M.; Moreno, A.M.; Martins, S.M.M.K.; Stephano, M.A.; Mendes, M.A.; Mendonça, C.M.N.; Pereira, W.A.; Azevedo, P.O.S.; et al. Antimicrobial Activity of Peptides Produced by Lactococcus lactis subsp. lactis on Swine Pathogens. Animals 2023, 13, 2442. [Google Scholar] [CrossRef]
- Alakomi, H.L.; Skyttä, E.; Saarela, M.; Mattila-Sandholm, T.; Latva-Kala, K.; Helander, I.M. Lactic acid permeabilizes gram-negative bacteria by disrupting the outer membrane. Appl. Environ. Microbiol. 2000, 66, 2001–2005. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sukrita, P.; Phumkhachorn, P.; Rattanachaikunsopon, P. Nisin: Production and mechanism of antimicrobial action. Int. J. Curr. Res. Rev. 2015, 7, 47. [Google Scholar]
- Muñoz-Quezada, S.; Bermudez-Brito, M.; Chenoll, E.; Genov’cs, S.; Gomez-Llorente, C.; Plaza-Diaz, J.; Matencio, E.; Gil, A.; Omero, F.; Ramon, D.; et al. Competitive inhibition of three novel bacteria isolated from faeces of breast milk-fed infants against selected enteropathogens. Br. J. Nutr. 2013, 109, S63–S69. [Google Scholar] [CrossRef]
- Hladíková, Z.; Smetanková, J.; Greif, G.; Greifová, M. Antimicrobial activity of selected lactic acid cocci and production of organic acids. Acta Chim. Slovaca 2012, 5, 80–85. [Google Scholar] [CrossRef]
- Ribeiro, L.L.S.M.; Araújo, G.P.; de Oliveira Ribeiro, K.; Torres, I.M.S.; De Martinis, E.C.P.; Marreto, R.N.; Alves, V.F. Use of Encapsulated Lactic Acid Bacteria as Bioprotective Cultures in Fresh Brazilian Cheese. Braz. J. Microbiol. 2021, 52, 2247–2256. [Google Scholar] [CrossRef]
- Tripathi, M.K.; Giri, S.K. Probiotic Functional Foods: Survival of Probiotics during Processing and Storage. J. Funct. Foods 2014, 9, 225–241. [Google Scholar] [CrossRef]
- Savedboworn, W.; Teawsomboonkit, K.; Surichay, S.; Riansa-Ngawong, W.; Rittisak, S.; Charoen, R.; Phattayakorn, K. Impact of protectants on the storage stability of freeze-dried probiotic Lactobacillus plantarum. Food Sci. Biotechnol. 2018, 28, 795–805. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mercier, S.; Villeneuve, S.; Mondor, M.; Uysal, I. Time-Temperature Management along the Food Cold Chain: A Review of Recent Developments: Food Preservation along the Cold Chain: A Review of Recent Developments. Compr. Rev. Food Sci. Food Saf. 2017, 16, 647–667. [Google Scholar] [CrossRef]
- Dimitrellou, D.; Kandylis, P.; Kourkoutas, Y. Effect of Cooling Rate, Freeze-Drying, and Storage on Survival of Free and Immobilized Lactobacillus casei ATCC 393. Lebenson. Wiss. Technol. 2016, 69, 468–473. [Google Scholar] [CrossRef]
- Pavlatou, C.; Nikolaou, A.; Prapa, I.; Tegopoulos, K.; Plesssas, S.; Grigoriou, M.E.; Bezirtzoglou, E.; Kourkoutas, Y. Effect of Immobilized Pediococcus acidilactici ORE5 Cells on Pistachio Nuts on the Functional Regulation of the Novel Katiki Domokou-Type Cheese Microbiome. Appl. Sci. 2023, 13, 8047. [Google Scholar] [CrossRef]
- Mani-López, E.; Palou, E.; López-Malo, A. Probiotic Viability and Storage Stability of Yogurts and Fermented Milks Prepared with Several Mixtures of Lactic Acid Bacteria. J. Dairy Sci. 2014, 97, 2578–2590. [Google Scholar] [CrossRef]
- Hoobin, P.; Burgar, I.; Zhu, S.; Ying, D.; Sanguansri, L.; Augustin, M.A. Water Sorption Properties, Molecular Mobility and Probiotic Survival in Freeze Dried Protein-Carbohydrate Matrices. Food Funct. 2013, 4, 1376–1386. [Google Scholar] [CrossRef]
- Jofré, A.; Aymerich, T.; Garriga, M. Impact of different cryoprotectants on the survival of freeze-dried Lactobacillus rhamnosus and Lactobacillus casei/paracasei during long-term storage. Benef. Microbes. 2015, 6, 381–386. [Google Scholar] [CrossRef]
- Dianawati, D.; Mishra, V.; Shah, N.P. Survival of Microencapsulated Probiotic Bacteria after Processing and during Storage: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 1685–1716. [Google Scholar] [CrossRef]
- Poddar, D.; Das, S.; Jones, G.; Palmer, J.; Jameson, G.B.; Haverkamp, R.G.; Singh, H. Stability of probiotic Lactobacillus paracasei during storage as affected by the drying method. Int. Dairy J. 2014, 39, 1–7. [Google Scholar] [CrossRef]
- Aspri, M.; Papademas, P.; Tsaltas, D. Review on Non-Dairy Probiotics and Their Use in Non-Dairy Based Products. Fermentation 2020, 6, 30. [Google Scholar] [CrossRef]
- Monfared, K.E.; Gharachorloo, M.; Jafarpour, A.; Varvani, J. Production feasibility of functional probiotic muesli containing matcha and investigation of its physicochemical, microbial, and sensory properties. J. Food Meas. Charact. 2022, 16, 975–986. [Google Scholar] [CrossRef]
- Saarela, M.; Virkajärvi, I.; Nohynek, L.; Vaari, A.; Mättö, J. Fibres as carriers for Lactobacillus rhamnosus during freeze-drying and storage in apple juice and chocolate-coated breakfast cereals. Int. J. Food Microbiol. 2006, 112, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Burca-Busaga, C.G.; Betoret, N.; Seguí, L.; Betoret, E.; Barrera, C. Survival of Lactobacillus salivarius CECT 4063 and Stability of Antioxidant Compounds in Dried Apple Snacks as Affected by the Water Activity, the Addition of Trehalose and High Pressure Homogenization. Microorganisms 2020, 8, 1095. [Google Scholar] [CrossRef]
- Albadran, H.A.; Chatzifragkou, A.; Khutoryanskiy, V.V.; Charalampopoulos, D. Stability of Probiotic Lactobacillus plantarum in Dry Microcapsules under Accelerated Storage Conditions. Food Res. Int. 2015, 74, 208–216. [Google Scholar] [CrossRef]

| Ingredients | g/100 g of Product |
|---|---|
| Oat flakes | 40 |
| Sunflower seeds | 20 |
| Almonds | 10 |
| Dark chocolate (60% cocoa content) | 10 |
| Corinthian currants | 5 |
| Goji berries | 5 |
| Coconut flakes | 5 |
| Chia seeds | 5 |
| Source of Isolation | Microbial Species | Strain Code |
|---|---|---|
| Beetroot | Lactiplantibacillus plantarum | FBBT_4570 |
| Beetroot | Lactiplantibacillus plantarum | FBBT_4572 |
| Beetroot | Lactiplantibacillus plantarum | FBBT_4573 |
| Beetroot | Lactiplantibacillus plantarum | FBBT_4574 |
| White Mushroom | Lactococcus cremoris | FBMS_5810 |
| White Mushroom | Lactococcus cremoris | FBMS_5811 |
| White Mushroom | Lactococcus cremoris | FBMS_5812 |
| Isolated Strain | AM | C | Ch | E | G | S | T | V | K |
|---|---|---|---|---|---|---|---|---|---|
| Cut-off values for L. plantarum (mg/L) (EFSA, 2012) | 2 | 2 | 8 | 1 | 16 | n. r. 1 | 32 | n. r. 1 | 64 |
| L. plantarum FBBT_4570 | 0.25 | 2 | 8 | 2 R | 64 R | n. r. 1 | 16 | n. r. 1 | 512 R |
| L. plantarum FBBT_4572 | 0.5 | 0.5 | 4 | 2 R | 64 R | n. r. 1 | 16 | n. r. 1 | 512 R |
| L. plantarum FBBT_4573 | 0.25 | 4 R | 4 | 1 | 32 R | n. r. 1 | 16 | n. r. 1 | 128 R |
| L. plantarum FBBT_4574 | 0.125 | 4 R | 8 | 2 R | 32 R | n. r. 1 | 16 | n. r. 1 | 128 R |
| Cut-off values for Lactococcus spp. (mg/L) (EFSA, 2012) | 2 | 1 | 8 | 1 | 32 | 32 | 4 | 4 | 64 |
| Lc. cremoris FBMS_5810 | 0.5 | 0.25 | 4 | 0.5 | 8 | 16 | 0.5 | 1 | 32 |
| Lc. cremoris FBMS_5811 | 0.5 | 0.5 | 4 | 0.25 | 32 | 32 | 0.5 | 1 | 16 |
| Lc. cremoris FBMS_5812 | 0.5 | 0.25 | 4 | 0.25 | 16 | 16 | 0.25 | 1 | 32 |
| Isolates | Hydrophobicity (%) | Auto-Aggregation (%) | Adhesion to Caco-2 Cell Lines (%) | Co-Aggregation (%) | |||
|---|---|---|---|---|---|---|---|
| C. difficile | L. monocytogenes | S. Enteritidis | E. coli | ||||
| L. plantarum FBBT_4570 | 23.30 ± 0.09 d | 47.70 ± 0.20 e | 3.92 ± 0.12 b | 68.89 ± 0.76 e | 67.87 ± 0.51 d | 69.62 ± 0.36 g | 68.08 ± 0.47 d |
| L. plantarum FBBT_4572 | 14.80 ± 0.10 b | 26.70 ± 0.21 b | 5.80 ± 0.17 a | 68.72 ± 0.74 d | 67.76 ± 0.28 c | 69.14 ± 0.86 f | 69.20 ± 0.07 f |
| L. plantarum FBBT_4573 | 31.30 ± 0.03 f | 73.50 ± 0.07 g | 5.83 ± 0.15 a | 69.34 ± 0.18 f | 69.03 ± 0.72 f | 68.55 ± 0.27 d | 69.09 ± 0.20 e |
| L. plantarum FBBT_4574 | 27.90 ± 0.05 e | 35.20 ± 0.04 c | 8.95 ± 0.28 c | 67.91 ± 0.46 c | 67.96 ± 0.54 e | 68.93 ± 0.25 e | 69.39 ± 0.22 g |
| Lc. cremoris FBMS_5810 | 55.41 ± 0.05 g | 55.40 ± 0.05 f | 32.14 ± 3.64 g | 70.85 ± 1.38 g | 58.45 ± 0.33 a | 41.90 ± 1.96 c | 65.43 ± 2.86 c |
| Lc. cremoris FBMS_5811 | 17.96 ± 0.02 c | 16.40 ± 0.10 a | 10.35 ± 0.21 d | 61.67 ± 0.62 b | 62.22 ± 2.74 b | 41.76 ± 1.71 b | 60.42 ± 5.83 a |
| Lc. cremoris FBMS_5812 | 7.43 ± 0.05 a | 38.40 ± 0.20 d | 15.21 ± 0.29 e | 58.13 ± 0.33 a | 58.48 ± 0.52 a | 34.20 ± 1.98 a | 60.61 ± 0.74 b |
| Isolates | CFS pH | BSH Activity | Cholesterol Assimilation (%) |
|---|---|---|---|
| L. plantarum FBBT_4570 | 3.75 ± 0.02 | + | 36.00 ± 1.41 b |
| L. plantarum FBBT_4572 | 3.76 ±0.01 | + | 10.22 ± 0.58 c |
| L. plantarum FBBT_4573 | 3.78 ± 0.01 | + | 29.05 ± 0.98 d |
| L. plantarum FBBT_4574 | 3.89 ± 0.01 | + | 26.92 ± 0.62 a |
| Lc. cremoris FBMS_5810 | 5.17 ± 0.02 | + | 51.89 ± 1.00 e |
| Lc. cremoris FBMS_5811 | 5.12 ± 0.01 | + | 37.16 ± 1.05 b |
| Lc. cremoris FBMS_5812 | 5.21 ± 0.04 | + | 26.08 ± 1.12 a |
| Isolates | C. difficile | L. monocytogenes | S. Enteritidis | E. coli |
|---|---|---|---|---|
| L. plantarum FBBT_4570 | 3.71 ± 0.08 b* | 5.69 ± 0.11 b* | 6.20 ± 0.04 c* | 5.72 ± 0.19 b* |
| L. plantarum FBBT_4572 | 5.57 ± 0.14 d* | 5.79 ± 0.18 b* | 6.60 ± 0.08 c* | 6.39 ± 0.20 a* |
| L. plantarum FBBT_4573 | 4.46 ± 0.04 c* | 4.74 ± 0.11 a* | 5.42 ± 0.68 b* | 6.66 ± 0.10 a* |
| L. plantarum FBBT_4574 | 3.08 ± 0.17 a* | 4.83 ± 0.10 a* | 5.23 ± 0.96 b* | 6.38 ± 0.09 a* |
| Lc. cremoris FBMS_5810 | 3.94 ± 0.35 b* | 6.49 ± 0.66 c* | 8.82 ± 0.07 a | 9.09 ± 0.25 c |
| Lc. cremoris FBMS_5811 | 7.53 ± 0.04 e* | 8.58 ± 0.12 d | 9.10 ± 0.01 a | 9.14 ± 0.05 c |
| Lc. cremoris FBMS_5812 | 8.03 ± 0.09 e* | 8.62 ± 0.37 d | 8.72 ± 0.03 a | 9.22 ± 0.02 c |
| Growth control | 9.09 ± 0.01 | 9.08 ± 0.01 | 8.79 ± 0.04 | 9.05 ± 0.11 |
| Logcfu/g | % Survival | |||||||
|---|---|---|---|---|---|---|---|---|
| d0 | d30 | d90 | d180 | d0 | d30 | d90 | d180 | |
| Oat flakes W | 9.01 ± 0.03 c | 0 * | 0 * | 0 * | 100 | ND | ND | ND |
| Oat flakes FD | 8.59 ± 0.03 a,c | 7.52 ± 0.01 c,d,f,g | 6.35 ± 0.01 c,d,e,g | 2.22 ± 0.02 c,d,e,f | 100 | 87.54 ± 1.01 c,d,f,g | 73.92 ± 1.15 c,d,e,g | 25.84 ± 0.31 c,d,e,f |
| Oat flakes FD (DW) | 9.00 ± 0.06 a,c | 8.23 ± 0.01 c,d,f,g | 7.15 ± 0.01 c,d,e,g | 4.55 ± 0.06 c,d,e,f | 100 | 91.44 ± 1.18 c,d,f,g | 79.44 ± 1.85 c,d,e,g | 50.56 ± 0.87 c,d,e,f |
| Oat flakes TR W | 9.15 ± 0.05 b,c | ND * | ND * | ND * | 100 | ND | ND | ND |
| Oat flakes TR FD | 9.10 ± 0.01 a,b,c | 8.25 ± 0.03 b,c,d,f,g | 7.82 ± 0.05 b,c,d,e,g | 6.55 ± 0.07 b,c,d,e,f | 100 | 90.66 ± 1.46 b,c,d,f,g | 85.93 ± 1.68 b,c,d,e,g | 71.98 ± 0.96 b,c,d,e,f |
| Oat flakes TR FD (DW) | 9.79 ± 0.08 a,b,c | 9.01 ± 0.01 b,c,d,f,g | 8.55 ± 0.03 b,c,d,e,g | 7.42 ± 0.05 b,c,d,e,f | 100 | 92.03 ± 1.24 b,c,d,f,g | 87.33 ± 1.97 b,c,d,e,g | 75.59 ± 1.28 b,c,d,e,f |
| Free cells W | 9.42 ± 0.06 | 3.12 ± 0.01 d | <1 | <1 | 100 | 33.12 ± 1.45 d | ND | ND |
| Free cells FD | 8.94 ± 0.05 a | 5.22 ± 0.03 a,d | <1 | <1 | 100 | 58.39 ± 0.77 a,d | ND | ND |
| Free cells FD (DW) | 10.47 ± 0.08 a | 6.54 ± 0.07 a,d | <1 | <1 | 100 | 62.46 ± 1.75 a,d | ND | ND |
| Free cells TR W | 9.52 ± 0.06 b | 3.45 ± 0.01 b,d | <1 | <1 | 100 | 36.24 ± 2.22 b,d | ND | ND |
| Free cells TR FD | 9.21 ± 0.02 a,b | 7.11 ± 0.05 a,b,d,f | 3.24 ± 0.02 b,d,e | <1 | 100 | 77.20 ± 1.72 a,b,d,f | 35.18 ± 1.92 b,d,e | ND |
| Free cells TR FD (DW) | 11.56 ± 0.05 a,b | 8.34 ± 0.01 a,b,d,f | 5.35 ± 0.04 b,d,e | <1 | 100 | 72.15 ± 1.28 a,b,d,f | 46.28 ± 1.48 b,d,e | ND |
| Logcfu | % Survival | |||||||
|---|---|---|---|---|---|---|---|---|
| d0 | d30 | d90 | d180 | d0 | d30 | d90 | d180 | |
| Oat flakes W | 9.01 ± 0.03 c | 8.22 ± 0.02 c,d | 0 * | 0 * | 100 | 91.23 ± 1.11 c,d | ND | ND |
| Oat flakes FD | 8.59 ± 0.03 a,c | 8.11 ± 0.01 a,c,d,f,g | 7.74 ± 0.05 c,d,e,g | 6.67 ± 0.08 d,e,f | 100 | 94.41 ± 0.67 a,c,d,f,g | 90.10 ± 1.58 c,d,e,g | 77.65 ± 2.55 d,e,f |
| Oat flakes FD (DW) | 9.00 ± 0.06 a,c | 8.65 ± 0.01 a,c,d,f,g | 8.31 ± 0.05 c,d,e,g | 7.32 ± 0.09 d,e,f | 100 | 96.11 ± 0.88 a,c,d,f,g | 92.33 ± 1.74 c,d,e,g | 81.33 ± 2.02 d,e,f |
| Oat flakes TR W | 9.15 ± 0.05 b,c | 8.74 ± 0.04 b,c,d | ND * | ND * | 100 | 95.52 ± 1.12 b,c,d | ND | ND |
| Oat flakes TR FD | 9.10 ± 0.01 a,b,c | 9.05 ± 0.03 a,b,c,d,f,g | 8.75 ± 0.05 b,c,d,e,g | 8.19 ± 0.07 b,c,d,e,f | 100 | 99.45 ± 1.58 a,b,c,d,f,g | 96.15 ± 0.75 b,c,d,e,g | 90.00 ± 1.88 b,c,d,e,f |
| Oat flakes TR FD (DW) | 9.79 ± 0.08 a,b,c | 9.70 ± 0.01 a,b,c,d,f,g | 9.31 ± 0.03 b,c,d,e,g | 8.75 ± 0.05 b,c,d,e,f | 100 | 99.08 ± 1.77 a,b,c,d,f,g | 95.10 ± 0.88 b,c,d,e,g | 89.38 ± 1.44 b,c,d,e,f |
| Free cells W | 9.42 ± 0.06 | 5.45 ± 0.01 d | <1 | <1 | 100 | 57.86 ± 0.82 d | ND | ND |
| Free cells FD | 8.94 ± 0.05 a | 7.52 ± 0.03 a,d,f | 5.55 ± 0.05 d,e | <1 | 100 | 84.12 ± 1.15 a,d,f | 62.08 ± 1.74 d,e | ND |
| Free cells FD (DW) | 10.47 ± 0.08 a | 9.45 ± 0.07 a,d,f | 7.69 ± 0.08 d,e | <1 | 100 | 90.26 ± 2.17 a,d,f | 73.45 ± 2.38 d,e | ND |
| Free cells TR W | 9.52 ± 0.06 b | 7.22 ± 0.01 b,d,f | 3.51 ± 0.07 d,e | <1 | 100 | 75.84 ± 1.23 b,d,f | 36.87 ± 0.88 d,e | ND |
| Free cells TR FD | 9.21 ± 0.02 a,b | 8.75 ± 0.07 a,b,d,f,g | 7.12 ± 0.02 a,b,d,e,g | 3.08 ± 0.0 d,e,f | 100 | 95.01 ± 1.66 a,b,d,f,g | 77.31 ± 2.05 a,b,d,e,g | 33.44 ± 1.08 d,e,f |
| Free cells TR FD (DW) | 11.56 ± 0.05 a,b | 10.47 ± 0.01 a,b,d,f,g | 9.21 ± 0.04 a,b,d,e,g | 5.33 ± 0.05 d,e,f | 100 | 90.57 ± 1.48 a,b,d,f,g | 79.67 ± 1.18 a,b,d,e,g | 46.11 ± 1.85 d,e,f |
| Room Temperature | Refrigerated Temperature | |||||||
|---|---|---|---|---|---|---|---|---|
| d0 | d30 | d90 | d180 | d0 | d30 | d90 | d180 | |
| (a) Water activity (aw) | ||||||||
| Oat flakes W | 0.957 ± 0.01 b | ND | ND | ND | 0.957 ± 0.01 b | 0.931 ± 0.01 a,b,c | ND | ND |
| Oat flakes FD | 0.085 ± 0.02 b | 0.091 ± 0.01 b,c,e | 0.125 ± 0.03 c,d | ND | 0.085 ± 0.02 b | 0.091 ± 0.02 c,e,f | 0.099 ± 0.02 a,b,c,d,f | 0.117 ±0.01 c,d,e |
| Oat flakes TR W | 0.984 ± 0.03 b | ND | ND | ND | 0.984 ± 0.03 b | 0.936 ± 0.01 b,c | ND | ND |
| Oat flakes TR FD | 0.089 ± 0.01 b | 0.095 ± 0.02 c,e,f | 0.131 ± 0.01 b,c,d,f | 0.202 ± 0.02 c,d,e | 0.089 ± 0.01 b | 0.096 ± 0.01 c,e,f | 0.105 ± 0.02 a,b,c,d,f | 0.122 ± 0.01 a,b,c,d,e |
| Free cells W | 0.915 ± 0.02 | 0.897 ± 0.01 c | ND | ND | 0.915 ± 0.02 | 0.899 ± 0.03 c | ND | ND |
| Free cells FD | 0.072 ± 0.01 | 0.098 ± 0.02 c | ND | ND | 0.072 ± 0.01 | 0.091 ± 0.02 a,c,e | 0.107 ± 0.01 c,d | ND |
| Free cells TR W | 0.956 ± 0.01 | 0.931 ± 0.02 c | ND | ND | 0.956 ± 0.01 | 0.901 ± 0.01 a,c,e | 0.895 ± 0.03 c,d | ND |
| Free cells TR FD | 0.084 ± 0.02 | 0.095 ± 0.01 c,e | 0.123 ± 0.02 c,d | ND | 0.084 ± 0.02 | 0.096 ± 0.04 c,e,f | 0.117 ± 0.01 a,c,d,f | 0.151 ± 0.02 c,d,e |
| (b) Moisture content (%) | ||||||||
| Oat flakes W | 62.48 ± 0.06 b | ND | ND | ND | 62.48 ± 0.13 b | 69.15 ± 0.09 b,c | ND | ND |
| Oat flakes FD | 3.61 ± 0.11 b | 4.08 ± 0.05 b,c,e | 6.28 ± 0.08 c,d | ND | 3.61 ± 0.02 b | 3.81 ± 0.07 a,b,c,e,f | 4.79 ± 0.02 a,b,c,d,f | 7.61 ± 0.11 c,d,e |
| Oat flakes TR W | 65.28 ± 0.22 b | ND | ND | ND | 65.28 ± 0.05 b | 72.22 ± 0.15 b,c | ND | ND |
| Oat flakes TR FD | 3.65 ± 0.04 b | 4.11 ± 0.03 b,c,e | 6.24 ± 0.12 b,c,d,f | 9.81 ± 0.02 b,c,d,e | 3.67 ± 0.08 b | 3.92 ± 0.02 a,b,c,e,f | 4.83 ± 0.09 a,b,c,d,f | 7.77 ± 0.05 a,b,c,d,e |
| Free cells W | 52.23 ± 0.09 | 66.74 ± 0.05 c | ND | ND | 52.23 ± 0.11 | 59.24 ± 0.04 a,c | ND | ND |
| Free cells FD | 2.55 ± 0.11 | 3.16 ± 0.01 c | ND | ND | 2.85 ± 0.04 | 3.04 ± 0.02 a,c,e | 4.11 ± 0.22 c,d | ND |
| Free cells TR W | 55.42 ± 0.08 | 64.15 ± 0.09 c | ND | ND | 55.42 ± 0.02 | 60.87 ± 0.11 a,c,e | 71.55 ± 0.08 c,e | ND |
| Free cells TR FD | 2.88 ± 0.03 | 3.21 ± 0.05 c,e,f | 4.51 ± 0.08 c,d,f | 6.11 ± 0.11 c,d,e | 2.88 ± 0.06 | 3.08 ± 0.03 a,c,e,f | 4.15 ± 0.08 a,c,d,f | 5.87 ± 0.04 a,c,d,e |
| Storage Time (Days) | MC | ML | MLT | |||
|---|---|---|---|---|---|---|
| RT | 4 °C | RT | 4 °C | RT | 4 °C | |
| log CFU/g | ||||||
| 0 | NA | ΝA | 9.18 ± 0.10 | 9.18 ± 0.10 | 9.21 ± 0.01 | 9.21 ± 0.01 |
| 30 | NA | ΝA | 8.76 ± 0.12 c,e,f,g | 9.12 ± 0.04 a,c,e,f,g | 9.11 ± 0.11 b,e,f,g | 9.18 ± 0.04 b,e.f.g |
| 60 | NA | ΝA | 7.54 ± 0.02 c,d,f,g | 8.74 ± 0.02 a,c,d,f,g | 8.61 ± 0.06 b,c,d,f,g | 9.14 ± 0.09 a,b,c,f,g |
| 90 | NA | ΝA | 7.18 ± 0.04 c,d,e,g | 8.35 ± 0.05 a,c,d,e,g | 8.19 ± 0.08 b,c,d,e | 9.09 ± 0.11 a,b,c,d,e,g |
| 180 | NA | ΝA | 6.52 ± 0.02 c,d,e,f | 7.52 ± 0.08 a,c,d,e,f | 7.75 ± 0.11 b,c,d,e | 8.65 ± 0.07 a,b,c,d,e,f |
| Water activity (aw) | ||||||
| 0 | 0.222 ± 0.05 | 0.222 ± 0.05 | 0.131 ± 0.07 h | 0.131 ± 0.07 h | 0.134 ± 0.11 h | 0.134 ± 0.11 h |
| 30 | 0.254 ± 0.07 c,e,f,g | 0.242 ± 0.13 a,c,e,f,g | 0.177 ± 0.12 c,e,f,g,h | 0.153 ± 0.03 a,c,e,f,g,h | 0.189 ± 0.05 b,c,e,f,g,h | 0.163 ± 0.08 a,b,c,e,f,g,h |
| 60 | 0.323 ± 0.11 c,d,f,g | 0.287 ± 0.02 a,c,d,f,g | 0.224 ± 0.08 c,d,f,g,h | 0.174 ± 0.05 a,c,d,f,g,h | 0.242 ± 0.03 b,c,d,f,g,h | 0.179 ± 0.09 a,b,c,d,f,g,h |
| 90 | 0.382 ± 0.08 c,d,e,g | 0.311 ± 0.05 a,c,d,e,g | 0.271 ± 0.09 c,d,e,g,h | 0.184 ± 0.11 a,c,d,e,g,h | 0.283 ± 0.10 b,c,d,e,g,h | 0.188 ± 0.02 a,b,c,d,e,g,h |
| 180 | 0.446 ± 0.12 c,d,e,f | 0.413 ± 0.07 a,c,d,e,f | 0.358 ± 0.05 c,d,e,f,h | 0.265 ± 0.02 a,c,d,e,f,h | 0.365 ± 0.09 b,c,d,e,f,h | 0.273 ± 0.06 a,b,c,d,e,f,h |
| Moisture content (%) | ||||||
| 0 | 8.56 ± 0.11 | 8.56 ± 0.11 | 5.09 ± 0.05 h | 5.09 ± 0.05 h | 5.16 ± 0.09 b,h | 5.16 ± 0.09 b,h |
| 30 | 10.05 ± 0.08 c,e,f,g | 8.73 ± 0.04 a,c,e,f,g | 5.77 ± 0.04 c,e,f,g,h | 5.32 ± 0.07 a,c,e,f,g,h | 5.88 ± 0.02 b,c,e,f,g,h | 5.40 ± 0.04 a,b,c,e,f,g,h |
| 60 | 10.84 ± 0.02 c,d,f,g | 9.03 ± 0.07 a,c,d,f,g | 6.10 ± 0.09 c,d,f,g,h | 5.50 ± 0.01 a,c,d,f,g,h | 6.21 ± 0.07 b,c,d,f,g,h | 5.57 ± 0.11 a,b,c,d,f,g,h |
| 90 | 11.32 ± 0.06 c,d,e,g | 10.60 ± 0.12 a,c,d,e,g | 6.96 ± 0.11 c,d,e,g,h | 5.75 ± 0.03 a,c,d,e,g,h | 7.05 ± 0.05 b,c,d,e,g,h | 5.73 ± 0.08 a,c,d,e,g,h |
| 180 | 13.35 ± 0.07 c,d,e,f | 12.54 ± 0.03 a,c,d,e,f | 8.00 ± 0.05 c,d,e,f,h | 7.12 ± 0.07 a,c,d,e,f,h | 8.03 ± 0.08 c,d,e,f,h | 7.15 ± 0.05 a,c,d,e,f,h |
| Overall sensory evaluation | ||||||
| 4.08 ± 0.90 | 4.08 ± 0.88 | 4.75 ± 0.45 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pavlatou, C.; Prapa, I.; Stylianopoulou, E.; Mitropoulou, G.; Skavdis, G.; Kourkoutas, Y. Immobilized Plant-Based Presumptive Probiotics as Functional Ingredients for Breakfast Cereals. Fermentation 2025, 11, 335. https://doi.org/10.3390/fermentation11060335
Pavlatou C, Prapa I, Stylianopoulou E, Mitropoulou G, Skavdis G, Kourkoutas Y. Immobilized Plant-Based Presumptive Probiotics as Functional Ingredients for Breakfast Cereals. Fermentation. 2025; 11(6):335. https://doi.org/10.3390/fermentation11060335
Chicago/Turabian StylePavlatou, Chrysoula, Ioanna Prapa, Electra Stylianopoulou, Gregoria Mitropoulou, George Skavdis, and Yiannis Kourkoutas. 2025. "Immobilized Plant-Based Presumptive Probiotics as Functional Ingredients for Breakfast Cereals" Fermentation 11, no. 6: 335. https://doi.org/10.3390/fermentation11060335
APA StylePavlatou, C., Prapa, I., Stylianopoulou, E., Mitropoulou, G., Skavdis, G., & Kourkoutas, Y. (2025). Immobilized Plant-Based Presumptive Probiotics as Functional Ingredients for Breakfast Cereals. Fermentation, 11(6), 335. https://doi.org/10.3390/fermentation11060335









