Sustainable Production of Medium-Chain Fatty Acids from Fresh Leachates in the District of Abidjan: Study of the Feasibility of the Process and Environmental Benefits
Abstract
1. Introduction
2. Materials and Methods
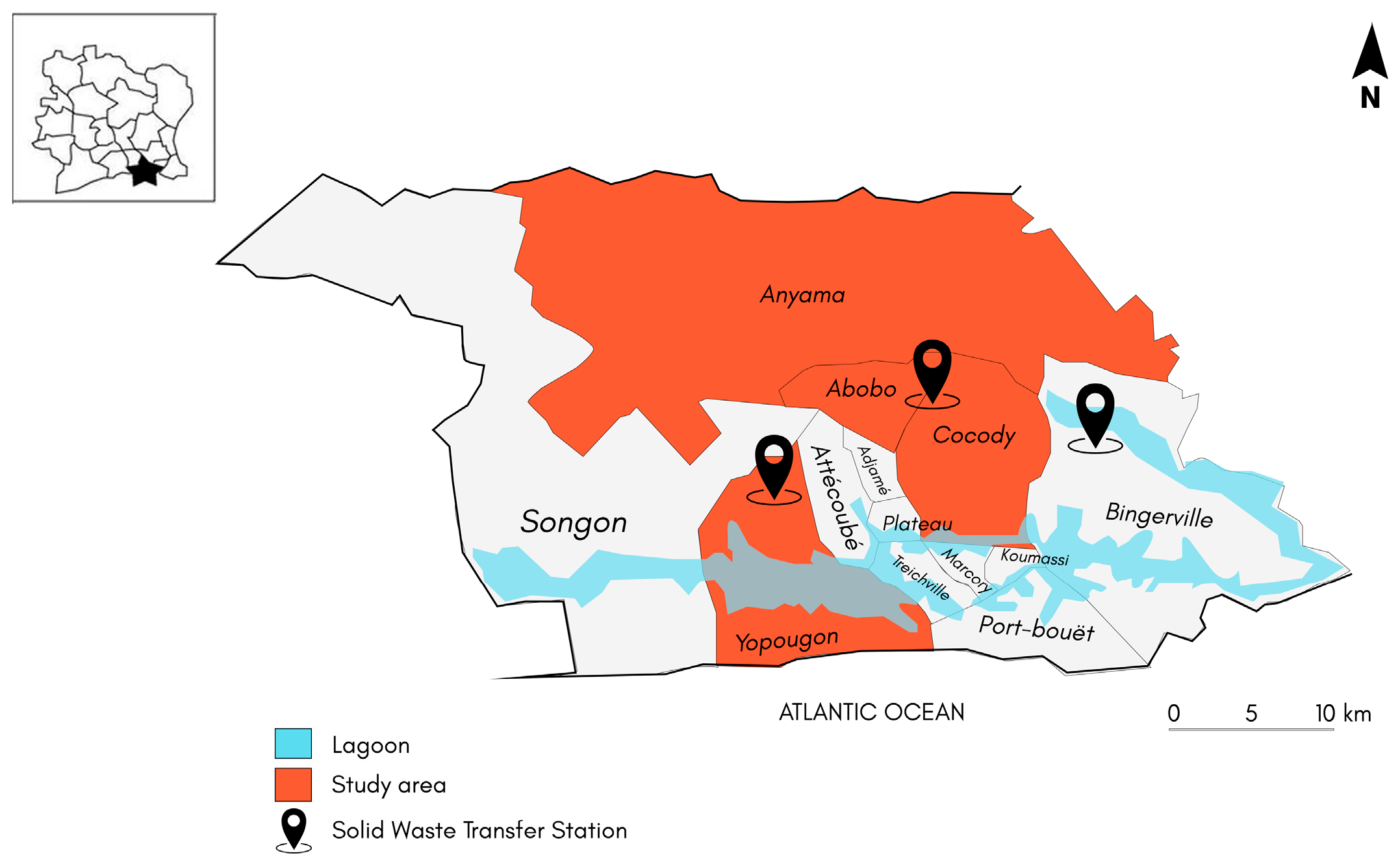
2.1. Description of the Sampling Areas
- -
- The SWTS of Abobo-Dokui: located between the municipalities of Abobo and Cocody. It collects waste that comes largely from Abobo-Dokui and partially from Cocody;
- -
- The SWTS of Bingerville: from the municipality of Bingerville, it nevertheless manages waste from the municipality of Cocody;
- -
- The SWTS of Yopougon: located between the municipalities of Abobo and Yopougon, receives waste from the municipalities of Abobo and Anyama.
2.2. Leachates Collection
2.3. Assessment of the Leachate Quality
2.4. Microbial Characterization of Fresh Leachate
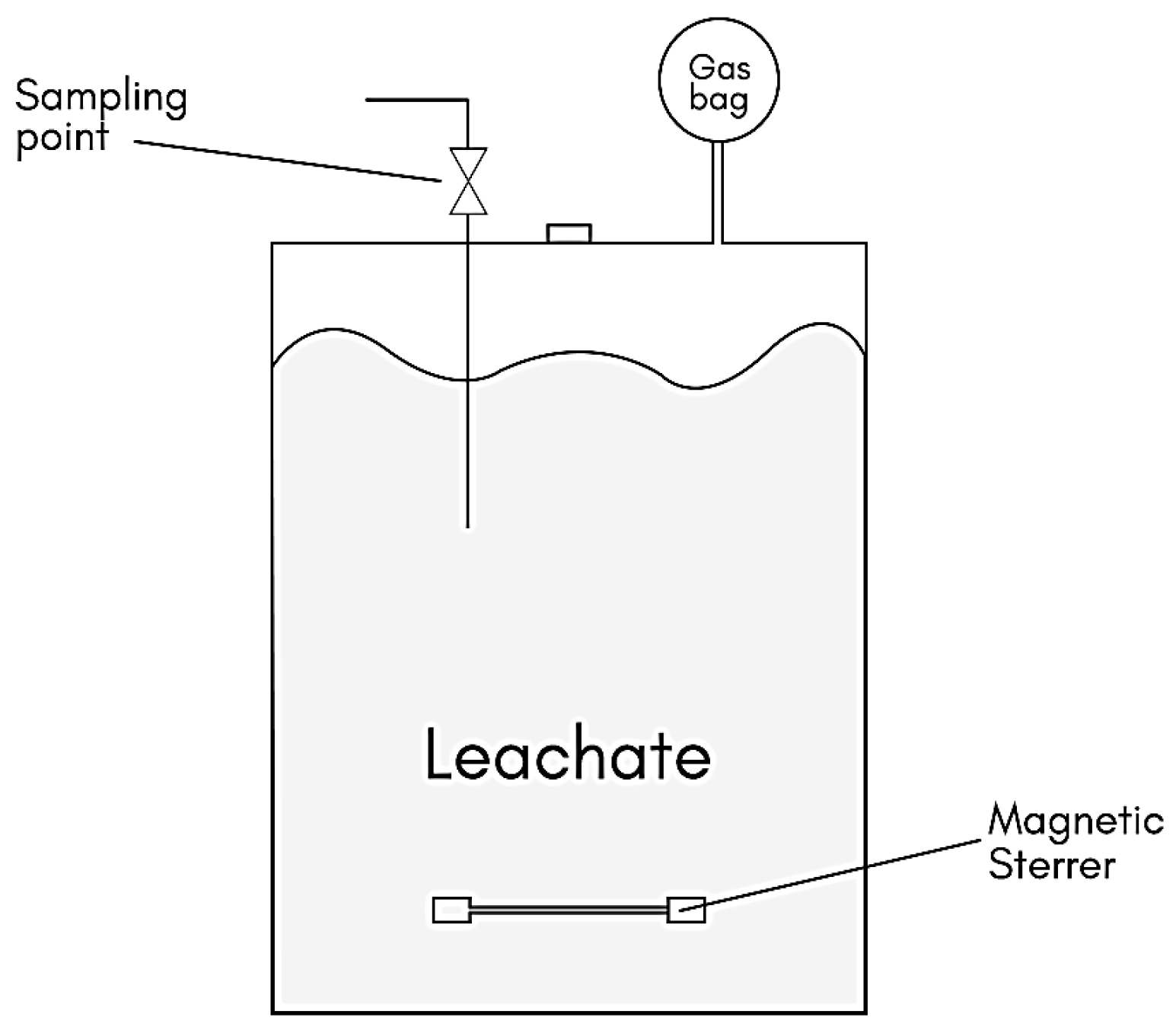
2.5. Setup of Bioreactor and Operation of Chain Elongation
2.6. MCFA Measurement
3. Results and Discussion
3.1. Physicochemical Characteristics of the Fresh Leachates from the SWTS in the District of Abidjan
3.2. Microbial Quality of the Fresh Leachates
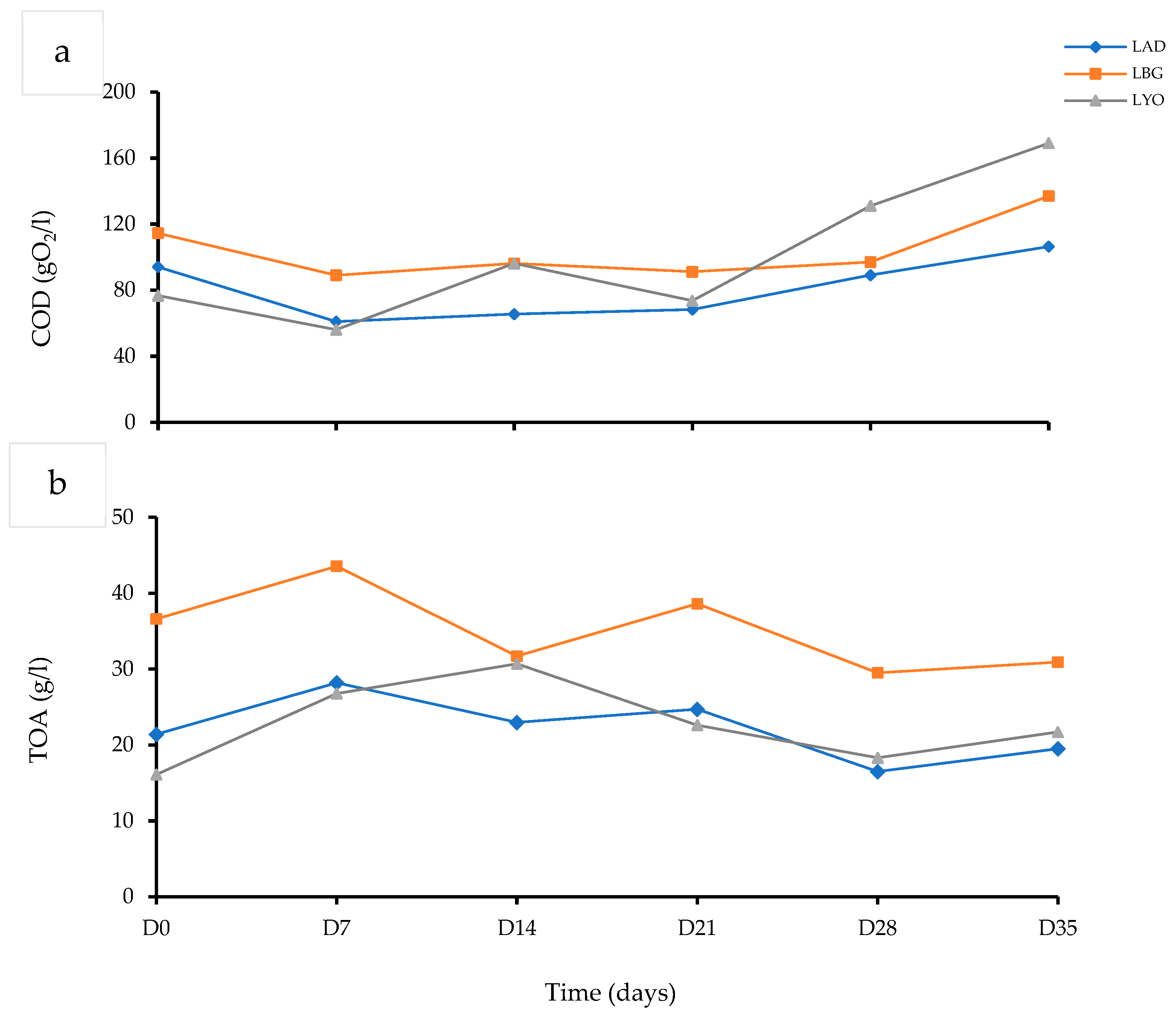
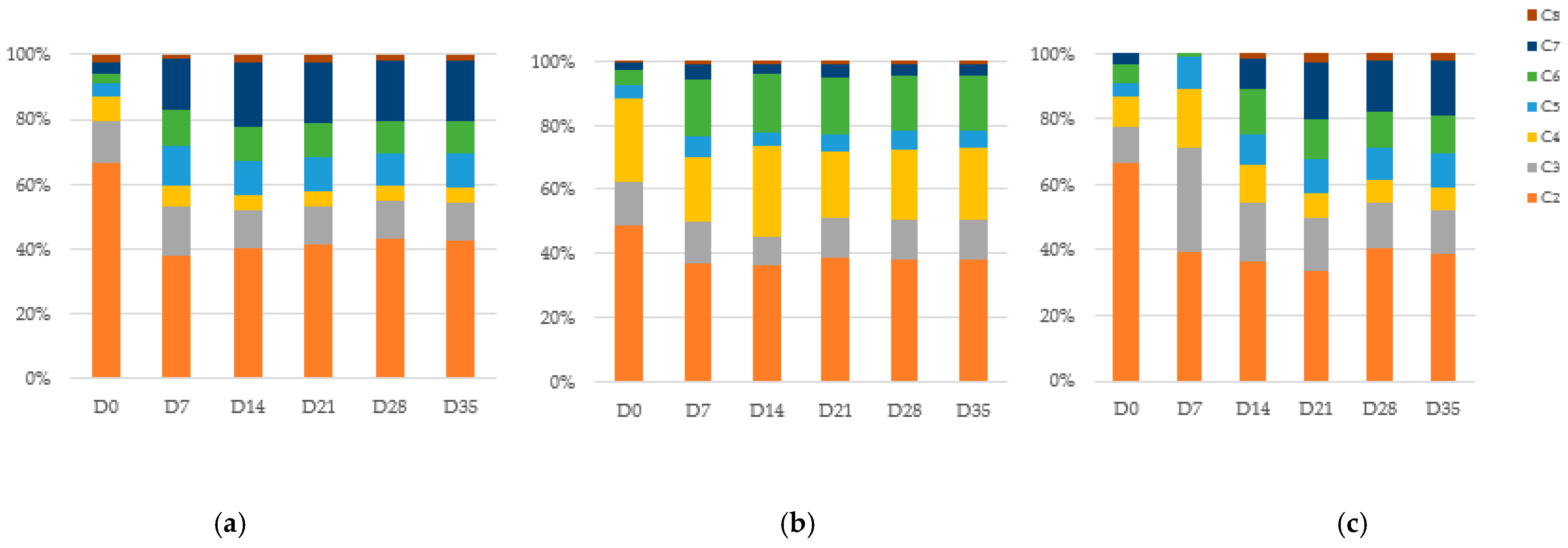
3.3. Changes in COD, TOA During Chain Elongation
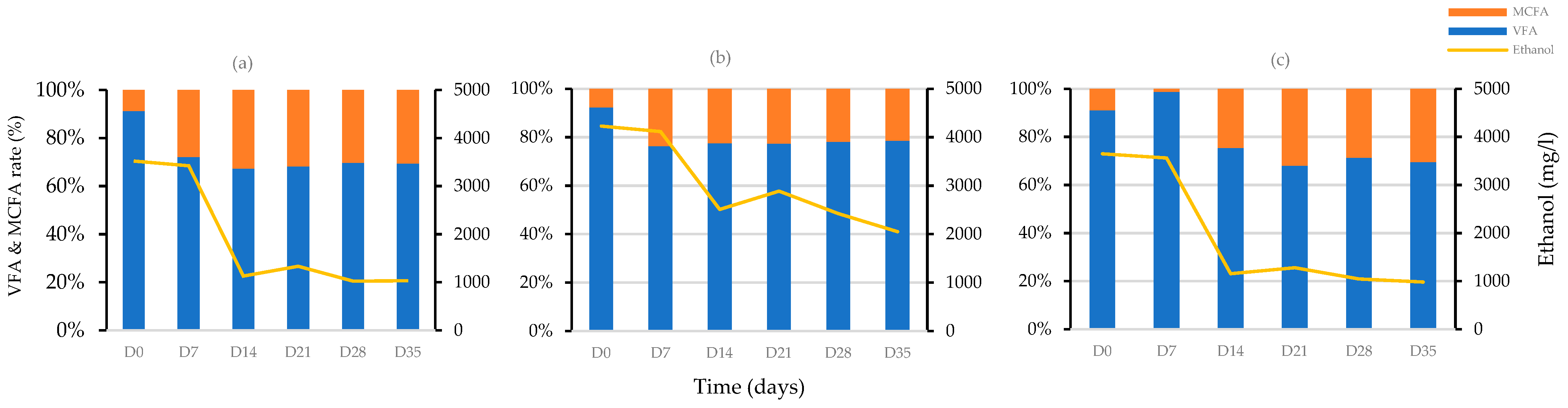
3.4. Effect of Ethanol on the Chain Elongation
3.5. Potential of Fresh Leachates from SWTS in Abidjan for MCFA Production
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| COD | Chemical Oxygen Demand |
| LAD | Leachate from the SWTS of Abobo-Dokui |
| LBG | Leachate from the SWTS of Bingerville |
| LYO | Leachate from the SWTS of Yopougon |
| MCFAs | Medium-chain fatty acids |
| SWTS | Solid waste transfer stations |
| TOA | Total Organic Acids |
| VFAs | Volatile fatty acids |
References
- GBN Partnership Ready Côte d’Ivoire: Gestion et Recyclage des Déchets Organiques—Platforme d’Innovation Cacao 2020. Available online: https://www.giz.de/en/downloads/GBN_Sector%20Brief_CIV_Bioabfall_FRWeb.pdf (accessed on 24 January 2025).
- Xiang, H.; Cheng, L.; Liu, W.; Wang, S.; Zhang, Y.; Su, L.; Tan, C.; Li, Y. Characteristics of leachate from refuse transfer stations in rural China. Environ. Sci. Pollut. Res. 2023, 30, 3056–3069. [Google Scholar] [CrossRef]
- Kjeldsen, P.; Barlaz, M.A.; Rooker, A.P.; Baun, A.; Ledin, A.; Christensen, T.H. Present and Long-Term Composition of MSW Landfill Leachate: A Review. Crit. Rev. Environ. Sci. Technol. 2002, 32, 297–336. [Google Scholar] [CrossRef]
- Foo, K.Y.; Hameed, B.H. An overview of landfill leachate treatment via activated carbon adsorption process. J. Hazard. Mater. 2009, 171, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Thong, S.; Zhu, X.; Angelidaki, I.; Zhang, S.; Luo, G. Medium chain fatty acids production by microbial chain elongation: Recent advances. In Advances in Bioenergy; Elsevier: Amsterdam, The Netherlands, 2020; Volume 5, pp. 63–99. ISBN 978-0-12-820744-4. [Google Scholar]
- Kouadio, M.C.; Ekra, E.; Akichi, A.; Trokourey, A. Characterization of the Parameters and Estimation of Potential Biogas of A Landfill in Tropical Area: Case Study of the Principal Landfill of Abidjan Akouedo Landfill. Res. Rev. J. Ecol. Environ. Sci. 2018, 6, 1–7. [Google Scholar]
- Yan, W.; Wang, Y.; Li, Y.; Rong, C.; Wang, D.; Wang, C.; Wang, Y.; Yuen, Y.; Wong, F.F.; Chui, H.-K.; et al. Treatment of fresh leachate by anaerobic membrane bioreactor: On-site investigation, long-term performance and response of microbial community. Bioresour. Technol. 2023, 383, 129243. [Google Scholar] [CrossRef]
- Kannengiesser, J.; Kuhn, C.; Mrukwia, T.; Stanojkovski, D.; Jager, J.; Schebek, L. Generation of Bio-Based Products from Omsw by Using a Solid-Liquid Separation Technique and an Anaerobic Treatment. Detritus 2018, 4, 78. [Google Scholar] [CrossRef]
- Grootscholten, T.I.M.; Kinsky dal Borgo, F.; Hamelers, H.V.M.; Buisman, C.J.N. Promoting chain elongation in mixed culture acidification reactors by addition of ethanol. Biomass Bioenergy 2013, 48, 10–16. [Google Scholar] [CrossRef]
- Agler, M.T.; Wrenn, B.A.; Zinder, S.H.; Angenent, L.T. Waste to bioproduct conversion with undefined mixed cultures: The carboxylate platform. Trends Biotechnol. 2011, 29, 70–78. [Google Scholar] [CrossRef]
- Steinbusch, K.J.J.; Hamelers, H.V.M.; Plugge, C.M.; Buisman, C.J.N. Biological formation of caproate and caprylate from acetate: Fuel and chemical production from low grade biomass. Energy Environ. Sci. 2010, 4, 216–224. [Google Scholar] [CrossRef]
- Spirito, C.M.; Richter, H.; Rabaey, K.; Stams, A.J.; Angenent, L.T. Chain elongation in anaerobic reactor microbiomes to recover resources from waste. Curr. Opin. Biotechnol. 2014, 27, 115–122. [Google Scholar] [CrossRef]
- Saadoun, L.; Campitelli, A.; Kannengiesser, J.; Stanojkovski, D.; El Alaoui El Fels, A.; Mandi, L.; Ouazzani, N. Potential of medium chain fatty acids production from municipal solid waste leachate: Effect of age and external electron donors. Waste Manag. 2021, 120, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Menon, A.; Lyng, J.G. Circular bioeconomy solutions: Driving anaerobic digestion of waste streams towards production of high value medium chain fatty acids. Rev. Environ. Sci. Biotechnol. 2021, 20, 189–208. [Google Scholar] [CrossRef]
- Yebouet, O.F.; Campitelli, A.; Amoikon, S.L.T.; Kannengiesser, J.; Stanojkovski, D.; Mrukwia, T.; Dje Koffi, M.; Djeni, T.N. Assessment of the diversity and abundance of bacterial population and its correlation with medium chain fatty acids production from fermentation of two leachate qualities. Bioresour. Technol. Rep. 2021, 16, 100840. [Google Scholar] [CrossRef]
- Deza, A.D. Cartographie de la Pauvreté non Financière Dans le District D’ABIDJAN à Partir du Recensement Général de la Population et de L’habitat 2014 de la Côte D’ivoire; Observatoire Démographique et Statistique de L’espace Francophone/Université Laval: Québec, QC, Canada, 2017; p. 32. [Google Scholar]
- Sabki, M.H.; Lee, C.T.; Bong, C.P.C.; Klemes, J.J. A Review on the Economic Feasibility of Composting for Organic Waste Management in Asian Countries. Chem. Eng. Trans. 2018, 70, 49–54. [Google Scholar] [CrossRef]
- Sané, Y. Une Ville Face à ses déchets; une Problématique géographique de la Pollution à Abidjan (Côte d’Ivoire). Ph.D. Thesis, Université Laval, Quebec, QC, Canada, 1999. [Google Scholar]
- RGPH Recensement Général de la Population et de L’habitat 2021; 2021; p. 68. Available online: https://rp2021.anstat.ci/ (accessed on 7 February 2025).
- Andersen, S.J.; De Groof, V.; Khor, W.C.; Roume, H.; Props, R.; Coma, M.; Rabaey, K. A Clostridium Group IV Species Dominates and Suppresses a Mixed Culture Fermentation by Tolerance to Medium Chain Fatty Acids Products. Front. Bioeng. Biotechnol. 2017, 5, 8. [Google Scholar] [CrossRef]
- Scarborough, M.J.; Lynch, G.; Dickson, M.; McGee, M.; Donohue, T.J.; Noguera, D.R. Increasing the economic value of lignocellulosic stillage through medium-chain fatty acid production. Biotechnol. Biofuels 2018, 11, 200. [Google Scholar] [CrossRef]
- Naminata, S.; Kwa-Koffi, K.E.; Marcel, K.A.; Marcellin, Y.K. Assessment and Impact of Leachate Generated by the Landfill City in Abidjan on the Quality of Ground Water and Surface Water (M’Badon Bay, Côte d’Ivoire). J. Water Resour. Prot. 2018, 10, 145–165. [Google Scholar] [CrossRef]
- Sackey, L.N.A.; Meizah, K. Assesement of the quality of leachate at Sarbah landfill site at Weija in Accra. J. Environ. Chem. Ecotoxicol. 2015, 7, 56–61. [Google Scholar] [CrossRef]
- Ye, J.; Mu, Y.; Cheng, X.; Sun, D. Treatment of fresh leachate with high-strength organics and calcium from municipal solid waste incineration plant using UASB reactor. Bioresour. Technol. 2011, 102, 5498–5503. [Google Scholar] [CrossRef]
- Grisey, E. Impact de l’évolution des déchets d’une installation de stockage de déchets non dangereux sur l’environnement—Site d’étude: l’ISDND d’Etueffont (Territoire de Belfort—France). In Ecologie, Environnement; Université de Franche-Comté: Besançon, France, 2013; Available online: https://theses.hal.science/tel-01136468 (accessed on 16 February 2025).
- Reddy, M.V.; Mohan, S.V.; Chang, Y.-C. Medium-Chain Fatty Acids (MCFA) Production Through Anaerobic Fermentation Using Clostridium kluyveri: Effect of Ethanol and Acetate. Appl. Biochem. Biotechnol. 2018, 185, 594–605. [Google Scholar] [CrossRef]
- Gupta, R.; Gigras, P.; Mohapatra, H.; Goswami, V.K.; Chauhan, B. Microbial α-amylases: A biotechnological perspective. Process Biochem. 2003, 38, 1599–1616. [Google Scholar] [CrossRef]
- Pandey, A.; Nigam, P.; Soccol, C.; Thomaz-Soccol, V.; Singh, D.; Mohan, R. Advances in Microbial Amylases. Biotechnol. Appl. Biochem. 2000, 31 Pt 2, 135–152. [Google Scholar] [CrossRef]
- Cruz Ramos, H.; Hoffmann, T.; Marino, M.; Nedjari, H.; Presecan-Siedel, E.; Dreesen, O.; Glaser, P.; Jahn, D. Fermentative Metabolism of Bacillus subtilis: Physiology and Regulation of Gene Expression. J. Bacteriol. 2000, 182, 3072–3080. [Google Scholar] [CrossRef]
- Hwang, H.; Lee, J.-H. Evaluation of Metabolites Derived from Lactic Acid Bacteria Isolated from Kimchi. In Chemistry of Korean Foods and Beverages; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2019; Volume 1303, pp. 3–10. ISBN 978-0-8412-3369-0. [Google Scholar]
- Roghair, M.; Liu, Y.; Strik, D.P.B.T.B.; Weusthuis, R.A.; Bruins, M.E.; Buisman, C.J.N. Development of an Effective Chain Elongation Process From Acidified Food Waste and Ethanol Into n-Caproate. Front. Bioeng. Biotechnol. 2018, 6, 50. [Google Scholar] [CrossRef]
- Sambusiti, C. Physical, Chemical and Biological Pretreatments to Enhance Biogas Production from Lignocellulosic Substrates. 2013. Available online: https://www.politesi.polimi.it/handle/10589/74843 (accessed on 16 February 2025).
- Hoornweg, D.; Bhada-Tata, P. What a waste: A global review of solid waste management. Urban Dev. Ser. Knowl. Pap. 2012, 15, 87–88. [Google Scholar]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Grootscholten, T.I.M.; Strik, D.P.B.T.B.; Steinbusch, K.J.J.; Buisman, C.J.N.; Hamelers, H.V.M. Two-stage medium chain fatty acid (MCFA) production from municipal solid waste and ethanol. Appl. Energy 2014, 116, 223–229. [Google Scholar] [CrossRef]
- Xu, J.; Hao, J.; Guzman, J.J.L.; Spirito, C.M.; Harroff, L.A.; Angenent, L.T. Temperature-Phased Conversion of Acid Whey Waste Into Medium-Chain Carboxylic Acids via Lactic Acid: No External e-Donor. Joule 2018, 2, 280–295. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, N.; Cai, Y.; Zhang, R.; Wu, Y.; Ma, W.; Fu, C.; Zhang, P.; Zhang, G. Advances in understanding entire process of medium chain carboxylic acid production from organic wastes via chain elongation. Chemosphere 2023, 339, 139723. [Google Scholar] [CrossRef]
| Group | Municipalities | Poverty Level (%) |
|---|---|---|
| Group 1 | Plateau | 15% |
| Group 2 | Cocody | 25% |
| Group 3 | Yopougon Treichville Marcory | 30 to 35% |
| Group 4 | Koumassi Attécoubé Adjamé Abobo | 40 to 45% |
| Group 5 | Port-Bouët Bingerville | 45 to 50% |
| Group 6 | Songon | 60 to 70% |
| Group 7 | Anyama | Up to 80% |
| Parameter | Unit | LAD | LBG | LYO |
|---|---|---|---|---|
| pH | - | 4.5 ± 0.7 a | 5.1 ± 0.5 a | 4.6 ± 0.3 a |
| Conductivity | mS/cm | 17.0 ± 2.3 a | 41.9 ± 1.3 b | 18.7 ± 1.5 a |
| COD | g·L−1 | 94.1 ± 5.2 a | 114.5 ± 14.2 b | 76.7 ± 2.3 c |
| TOA | g·L−1 | 21.4 ± 4.7 a | 36.6 ± 0.8 b | 16.1 ± 0.2 c |
| TFA | g·L−1 | 13 ± 1.3 b | 33.7 ± 2.4 a | 6.4 ± 0.7 c |
| VFA | g·L−1 | 11.9 ± 0.8 b (91.3% TFA) | 31.1 ± 1.9 a (92% TFA) | 5.8 ± 0.6 c (91% TFA) |
| MCFA | g·L−1 | 1.1 ± 0.6 (8.7% TFA) | 2.6 ± 0.3 (8% TFA) | 0.6 ± 0.2 (9% TFA) |
| K | mg·L−1 | 2 400 ± 480 b | 3 665 ± 325 a | 1 735 ± 176 b |
| P | mg·L−1 | 305 ± 27 a | 477 ± 38 a | 136 ± 45 b |
| Fe | mg·L−1 | 280 ± 19 b | 506 ± 23 a | 422 ± 18 a |
| Zn | mg·L−1 | <1 | <1 | <1 |
| Cd | mg·L−1 | <1 | <1 | <1 |
| Cr | mg·L−1 | <1 | <1 | <1 |
| Cu | mg·L−1 | <1 | <1 | <1 |
| Pb | mg·L−1 | <1 | <1 | <1 |
| Microorganisms | Unit | LAD | LBG | LYO |
|---|---|---|---|---|
| Clostridium | log10 CFU/mL | 6.06 ± 0.36 a | 6.20 ± 0.52 a | 5.86 ± 0.53 a |
| Lactobacillus | log10 CFU/mL | 7.14 ± 1.29 a | 7.36 ± 2.15 a | 7.25 ± 2.11 a |
| Bacillus | log10 CFU/mL | 7.09 ± 0.13 a | 7.11 ± 0.01 a | 7.31 ± 0.09 a |
| Pseudomonas | log10 CFU/mL | 7.64 ± 0.25 a | 7.83 ± 0.48 a | 7.68 ± 0.57 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koffi, A.R.H.; Campitelli, A.; Stanojkovski, D.; Yapo, E.G.-A.S.; N’guessan, A.R.; Yebouet, F.O.; Djeni, N.T. Sustainable Production of Medium-Chain Fatty Acids from Fresh Leachates in the District of Abidjan: Study of the Feasibility of the Process and Environmental Benefits. Fermentation 2025, 11, 330. https://doi.org/10.3390/fermentation11060330
Koffi ARH, Campitelli A, Stanojkovski D, Yapo EG-AS, N’guessan AR, Yebouet FO, Djeni NT. Sustainable Production of Medium-Chain Fatty Acids from Fresh Leachates in the District of Abidjan: Study of the Feasibility of the Process and Environmental Benefits. Fermentation. 2025; 11(6):330. https://doi.org/10.3390/fermentation11060330
Chicago/Turabian StyleKoffi, Akeyt Richmond Hervé, Alessio Campitelli, Daniel Stanojkovski, Edi Guy-Alain Serges Yapo, Alane Romaric N’guessan, Franck Orlando Yebouet, and N’Dédé Théodore Djeni. 2025. "Sustainable Production of Medium-Chain Fatty Acids from Fresh Leachates in the District of Abidjan: Study of the Feasibility of the Process and Environmental Benefits" Fermentation 11, no. 6: 330. https://doi.org/10.3390/fermentation11060330
APA StyleKoffi, A. R. H., Campitelli, A., Stanojkovski, D., Yapo, E. G.-A. S., N’guessan, A. R., Yebouet, F. O., & Djeni, N. T. (2025). Sustainable Production of Medium-Chain Fatty Acids from Fresh Leachates in the District of Abidjan: Study of the Feasibility of the Process and Environmental Benefits. Fermentation, 11(6), 330. https://doi.org/10.3390/fermentation11060330










