Integrated Metabolome and Microbiome Analysis Reveals the Regulatory Effects of Fermented Soybean Meal on the Gut Microbiota of Late Gestation
Abstract
1. Introduction
2. Materials and Methods
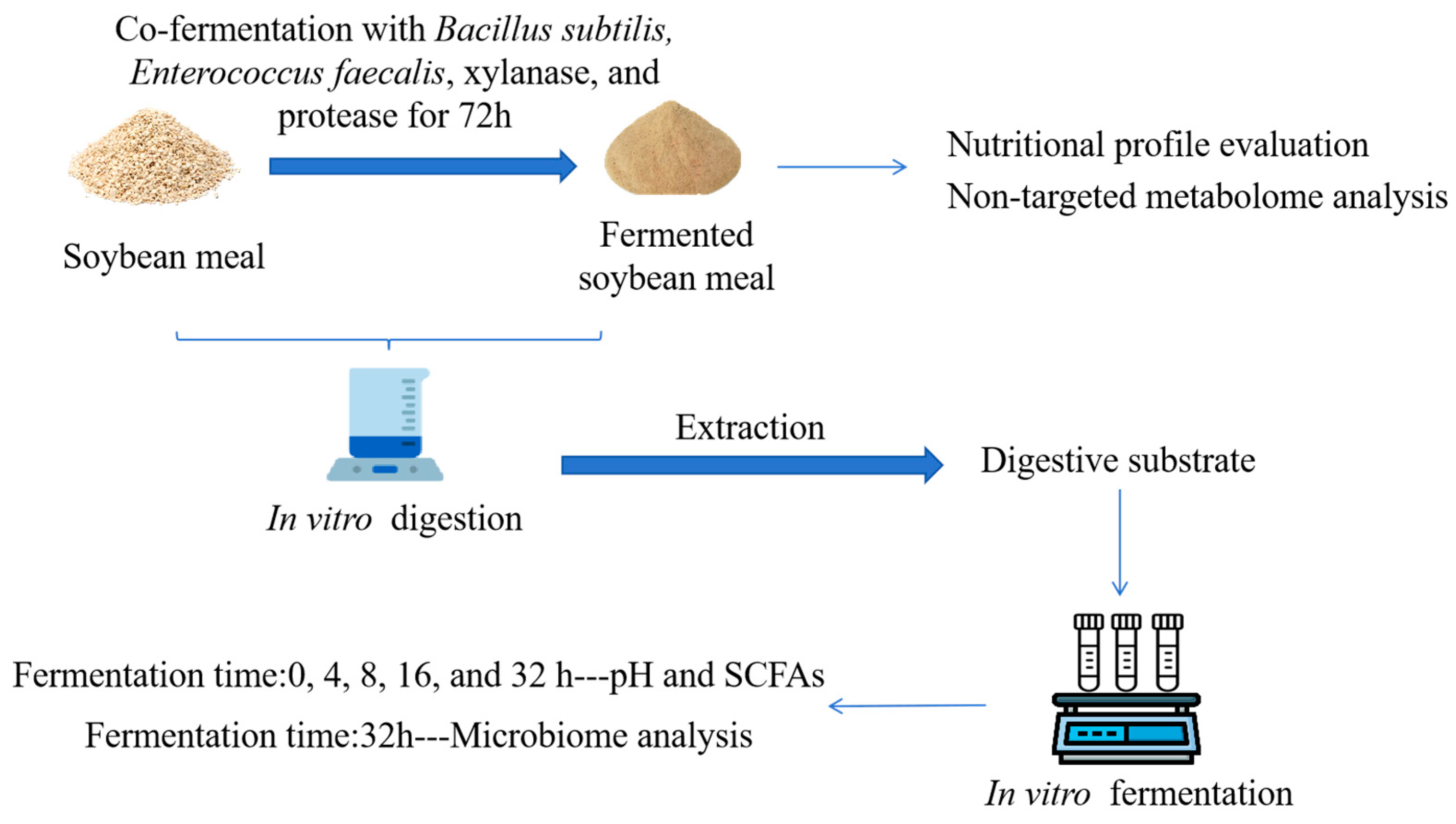
2.1. Preparation and Nutritional Profile Evaluation of FSBM
2.2. Non-Targeted Metabolome Analysis
2.3. In Vitro Digestion
2.4. In Vitro Fermentation
2.5. pH and Short-Chain Fatty Acid Assay
2.6. Microbiome Analysis
2.7. Correlation Analysis
2.8. Statistical Analysis
3. Results
3.1. Changes in Nutrient Composition of SBM Before and After Fermentation
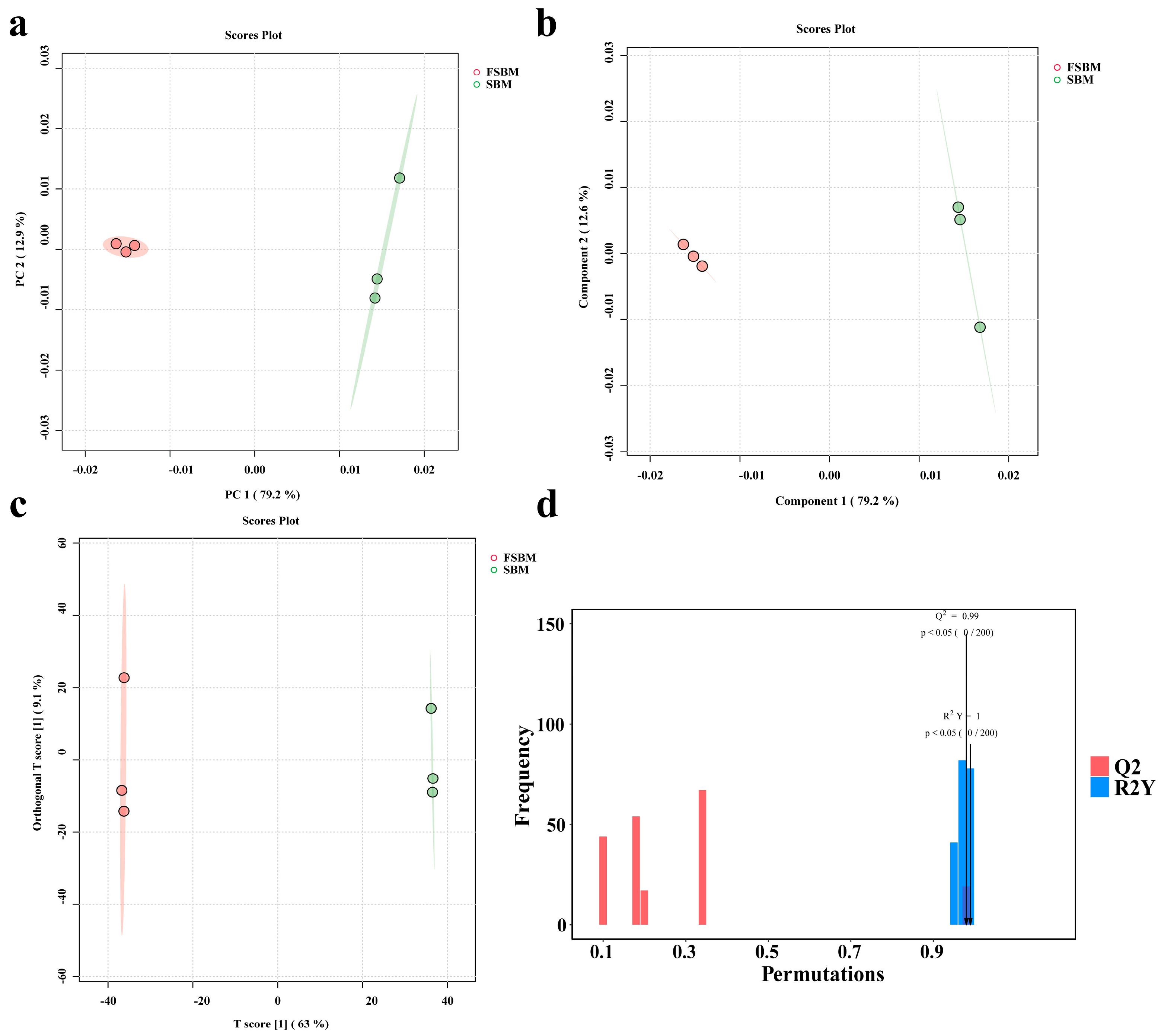
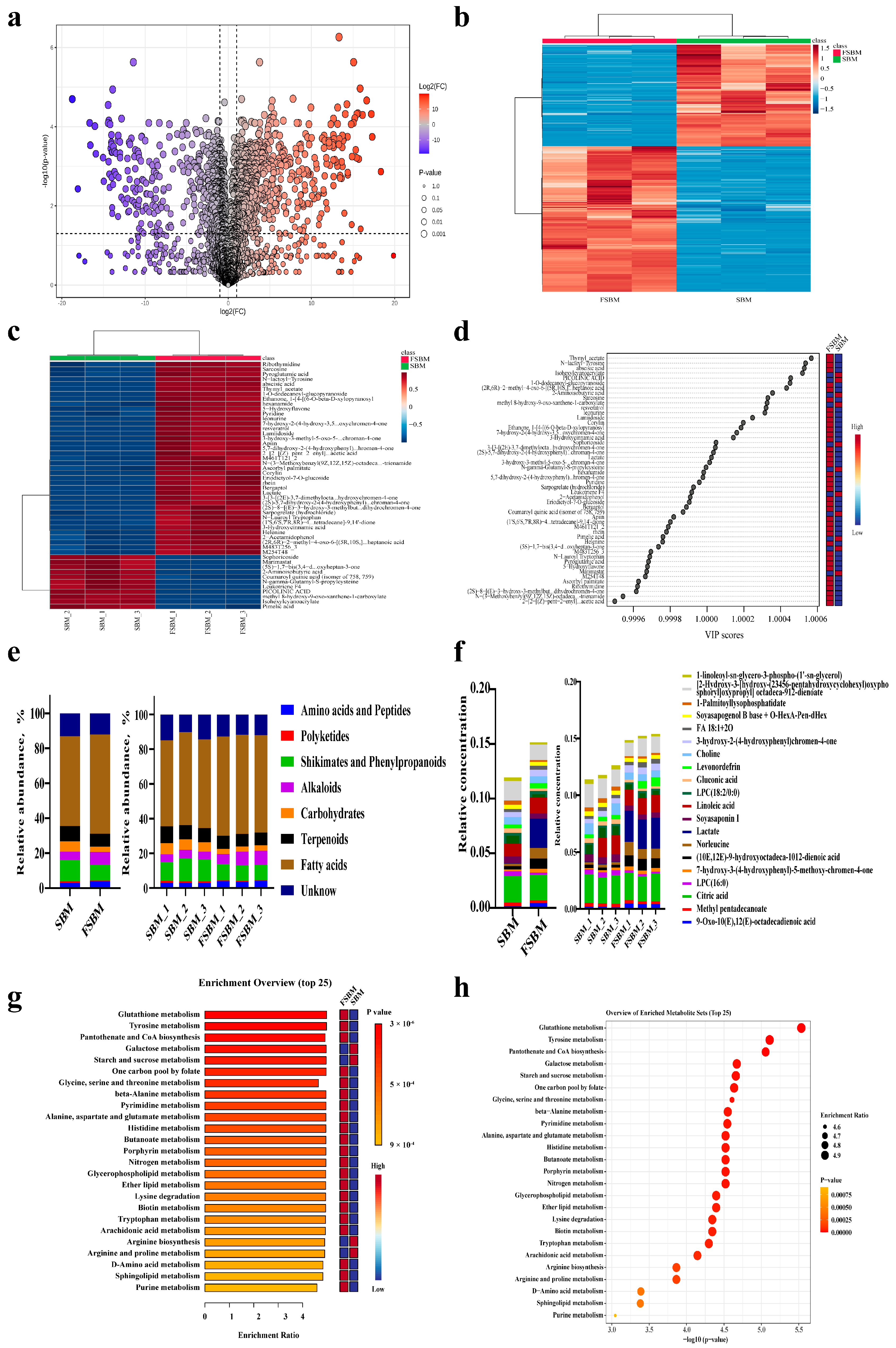
3.2. Untargeted Metabolomics Analysis During Fermentation
3.3. Changes of pH and SCFAs During In Vitro Fermentation
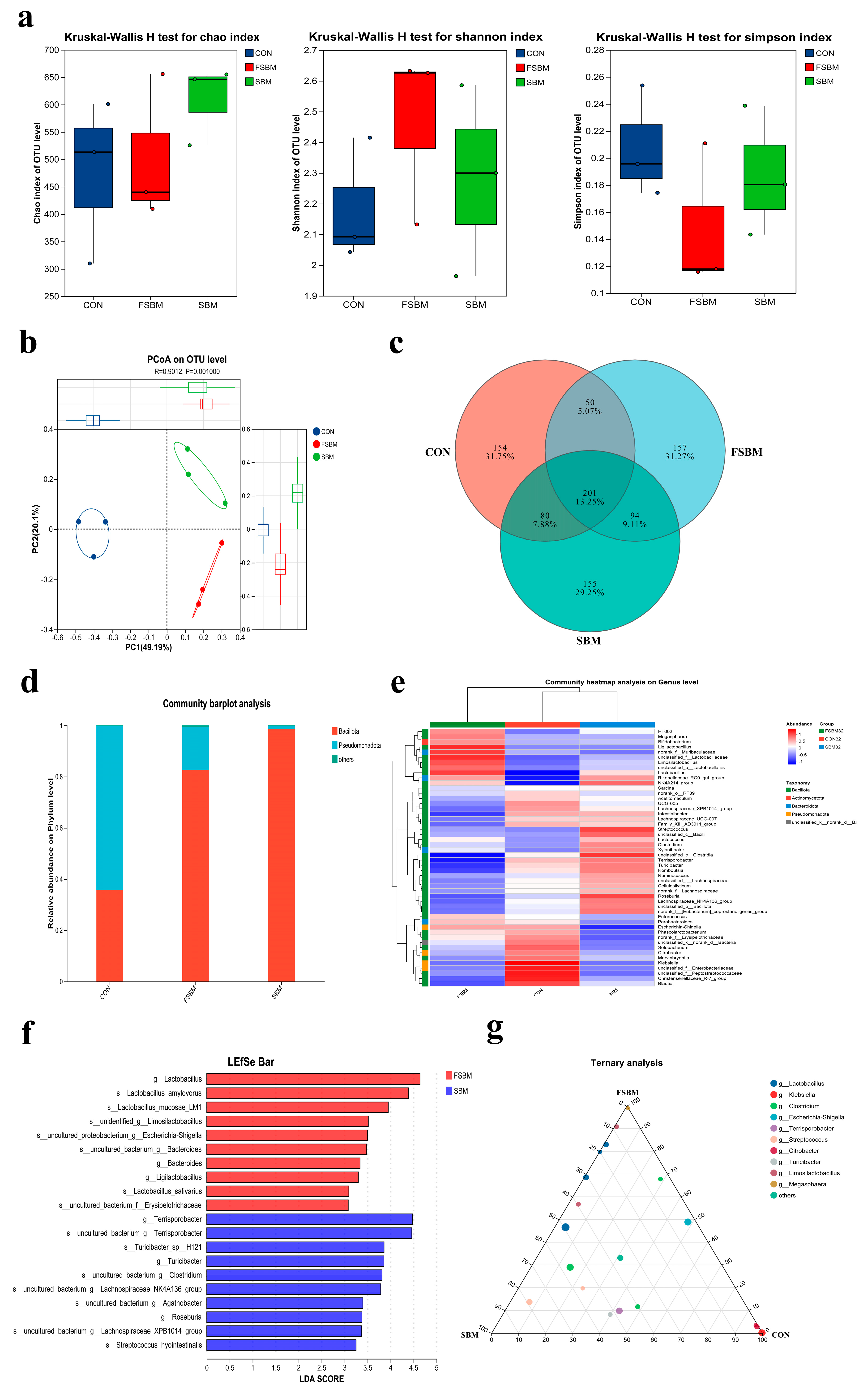
3.4. Modulation of Gut Microbiota in Late Gestation
3.5. Correlation Analysis of Microbiome with the Metabolome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feyera, T.; Pedersen, T.F.; Krogh, U.; Foldager, L.; Theil, P.K. Impact of sow energy status during farrowing on farrowing kinetics, frequency of stillborn piglets, and farrowing assistance. J. Anim. Sci. 2018, 96, 2320–2331. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.; Wei, H.; Yu, H.; Xu, C.; Jiang, S.; Peng, J. Metabolic Syndrome During Perinatal Period in Sows and the Link With Gut Microbiota and Metabolites. Front. Microbiol. 2018, 9, 1989. [Google Scholar] [CrossRef] [PubMed]
- Shang, Q.; Liu, S.; Liu, H.; Mahfuz, S.; Piao, X. Impact of sugar beet pulp and wheat bran on serum biochemical profile, inflammatory responses and gut microbiota in sows during late gestation and lactation. J. Anim. Sci. Biotechnol. 2021, 12, 54. [Google Scholar] [CrossRef]
- Zheng, J.; Li, S.; He, J.; Liu, H.; Huang, Y.; Jiang, X.; Zhao, X.; Li, J.; Feng, B.; Che, L.; et al. A Gestational Pectin Diet Could Improve the Health of Multiparous Sows by Modulating the Gut Microbiota and Cytokine Level during Late Pregnancy. Animals 2024, 14, 1559. [Google Scholar] [CrossRef]
- Chen, M.; Zhao, Y.; Li, S.; Chang, Z.; Liu, H.; Zhang, D.; Wang, S.; Zhang, X.; Wang, J. Maternal Malic Acid May Ameliorate Oxidative Stress and Inflammation in Sows through Modulating Gut Microbiota and Host Metabolic Profiles during Late Pregnancy. Antioxidants 2024, 13, 253. [Google Scholar] [CrossRef] [PubMed]
- Yi, X.; Cai, R.; Shaoyong, W.; Wang, G.; Yan, W.; He, Z.; Li, R.; Chao, M.; Zhao, T.; Deng, L.; et al. Melatonin promotes gut anti-oxidative status in perinatal rat by remodeling the gut microbiome. Redox Biol. 2023, 65, 102829. [Google Scholar] [CrossRef]
- Hong, K.J.; Lee, C.H.; Kim, S.W. Aspergillus oryzae GB-107 fermentation improves nutritional quality of food soybeans and feed soybean meals. J. Med. Food 2004, 7, 430–435. [Google Scholar] [CrossRef]
- The General Administration of Customs of the People’s Republic of China. Available online: http://www.customs.gov.cn//customs/302249/zfxxgk/2799825/302274/302275/4794420/index.html (accessed on 1 January 2025).
- Lambo, M.T.; Ma, H.; Zhang, H.; Song, P.; Mao, H.; Cui, G.; Dai, B.; Li, Y.; Zhang, Y. Mechanism of action, benefits, and research gap in fermented soybean meal utilization as a high-quality protein source for livestock and poultry. Anim. Nutr. 2024, 16, 130–146. [Google Scholar] [CrossRef]
- Akhtar, N.; Cai, H.Y.; Kiarie, E.G.; Li, J. A novel Bacillus sp. with rapid growth property and high enzyme activity that allows efficient fermentation of soybean meal for improving digestibility in growing pigs. J. Appl. Microbiol. 2022, 133, 3–17. [Google Scholar] [CrossRef]
- Chen, Y.C.; Yu, Y.H. Bacillus licheniformis-fermented products improve growth performance and the fecal microbiota community in broilers. Poult. Sci. 2020, 99, 1432–1443. [Google Scholar] [CrossRef]
- Qi, N.; Zhan, X.; Milmine, J.; Sahar, M.; Chang, K.H.; Li, J. Isolation and characterization of a novel hydrolase-producing probiotic Bacillus licheniformis and its application in the fermentation of soybean meal. Front. Nutr. 2023, 10, 1123422. [Google Scholar] [CrossRef]
- Zhou, T.; Han, S.; Li, Z.; He, P. Purification and Quantification of Kunitz Trypsin Inhibitor in Soybean Using Two-Dimensional Liquid Chromatography. Food Anal. Methods 2017, 10, 3350–3360. [Google Scholar] [CrossRef]
- Chen, L.; Zhao, Z.; Yu, W.; Zheng, L.; Li, L.; Gu, W.; Xu, H.; Wei, B.; Yan, X. Nutritional quality improvement of soybean meal by Bacillus velezensis and Lactobacillus plantarum during two-stage solid- state fermentation. AMB Express 2021, 11, 23. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Hou, Y.; Xu, H.; Huang, L.; Dabbour, M.; Mintah, B.K.; He, R.; Ma, H. Effect of solid-state fermentation by three different Bacillus species on composition and protein structure of soybean meal. J. Sci. Food Agric. 2022, 102, 557–566. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Shen, X.; Chen, R.; Peng, Y.; Cai, Y.; Zeng, S.; Liu, D.; Yang, J.; Zhuang, W.; et al. Solid-state fermentation through synthetic microbiome: An effective strategy for converting Chinese distillers’ grains into functional protein feed. Int. J. Food Microbiol. 2025, 435, 111154. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Lu, J.; Cai, G. Quality improvement of soybean meal by yeast fermentation based on the degradation of anti-nutritional factors and accumulation of beneficial metabolites. J. Sci. Food Agric. 2024, 104, 1441–1449. [Google Scholar] [CrossRef]
- Yan, H.; Jin, J.Q.; Yang, P.; Yu, B.; He, J.; Mao, X.B.; Yu, J.; Chen, D.W. Fermented soybean meal increases nutrient digestibility via the improvement of intestinal function, anti-oxidative capacity and immune function of weaned pigs. Animal 2022, 16, 100557. [Google Scholar] [CrossRef]
- Tan, K.; Bian, Z.; Liang, H.; Hu, W.; Xia, M.; Han, S.; Chen, B. Enzymolytic soybean meal-impact on growth performance, nutrient digestibility, antioxidative capacity, and intestinal health of weaned piglets. Front. Vet. Sci. 2024, 11, 1381823. [Google Scholar] [CrossRef]
- Luo, W.; Yin, X.; Yao, J.; Cheng, J.; Zhang, J.; Xu, W.; Mu, Y.; Xu, J. Fermented Soybean Meal Affects the Reproductive Performance and Oxidative Status of Sows, and the Growth of Piglets. Animals 2021, 11, 597. [Google Scholar] [CrossRef]
- Huang, J.; Dai, Y.; Zhang, Y.; Liu, G.; Peng, F.; Xie, M.; Xiong, T. Dynamics of bacterial community, metabolites profile and physicochemical characteristics during solid-state fermentation of soybean meal and corn mixed substrates inoculated with Bacillus pumilus and Limosilactobacillus fermentum. J. Sci. Food Agric. 2023, 103, 5588–5599. [Google Scholar] [CrossRef]
- Doppler, M.; Kluger, B.; Bueschl, C.; Schneider, C.; Krska, R.; Delcambre, S.; Hiller, K.; Lemmens, M.; Schuhmacher, R. Stable Isotope-Assisted Evaluation of Different Extraction Solvents for Untargeted Metabolomics of Plants. Int. J. Mol. Sci. 2016, 17, 1017. [Google Scholar] [CrossRef] [PubMed]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carrière, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Jin, M.; Kalainy, S.; Baskota, N.; Chiang, D.; Deehan, E.C.; McDougall, C.; Tandon, P.; Martínez, I.; Cervera, C.; Walter, J.; et al. Faecal microbiota from patients with cirrhosis has a low capacity to ferment non-digestible carbohydrates into short-chain fatty acids. Liver Int. 2019, 39, 1437–1447. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Wang, C.; Zhang, Q.; Yang, H.; Deehan, E.C.; Zong, X.; Wang, Y.; Jin, M. Dynamics of microbial communities during inulin fermentation associated with the temporal response in SCFA production. Carbohydr. Polym. 2022, 298, 120057. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.A.; Bosch, M.W.; Boer, H.; Verstegen, M.W.A.; Tamminga, S. An in vitro batch culture method to assess potential fermentability of feed ingredients for monogastric diets. Anim. Feed. Sci. Technol. 2005, 123–124, 445–462. [Google Scholar] [CrossRef]
- Nguyen, N.K.; Deehan, E.C.; Zhang, Z.; Jin, M.; Baskota, N.; Perez-Muñoz, M.E.; Cole, J.; Tuncil, Y.E.; Seethaler, B.; Wang, T.; et al. Gut microbiota modulation with long-chain corn bran arabinoxylan in adults with overweight and obesity is linked to an individualized temporal increase in fecal propionate. Microbiome 2020, 8, 118. [Google Scholar] [CrossRef]
- Best, D.J.; Roberts, D.E. The upper tail probabilities of Spearman’s Rho. Appl. Stat. 1975, 24, 377–379. [Google Scholar] [CrossRef]
- Hao, Y.N.; Wang, Z.G.; He, R.; Ju, X.R.; Yuan, J. Quality improvement of rapeseed meal based on static-state fermented with mixed microorganisms. Sci. Agric. Sin. 2020, 53, 2066–2077. [Google Scholar]
- Guo, M.M.; Cao, X.; Zhang, K.; Yang, Y.X.; Wang, X.L.; Chen, Y.L. Effects and metabolites of soybean meal fermented by compound microbes. Chin. J. Anim. Nutr. 2022, 34, 659–670. [Google Scholar]
- Cho, J.H.; Min, B.J.; Chen, Y.J.; Yoo, J.S.; Wang, Q.; Kim, J.D.; Kim, I.H. Evaluation of FSP (Fermented Soy Protein) to Replace Soybean Meal in Weaned Pigs: Growth Performance, Blood Urea Nitrogen and Total Protein Concentrations in Serum and Nutrient Digestibility. Asian Australas. J. Anim. Sci. 2007, 20, 1874–1879. [Google Scholar] [CrossRef]
- Kiers, J.L.; Meijer, J.C.; Nout, M.J.; Rombouts, F.M.; Nabuurs, M.J.; van der Meulen, J. Effect of fermented soya beans on diarrhoea and feed efficiency in weaned piglets. J. Appl. Microbiol. 2003, 95, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.; Jung, H.; Karuppasamy, S.; Lee, J.Y.; Kang, K.D.; Hwang, K.Y.; Seong, S.I.; Suh, J.G. Anti-hyperlipidemic effect of soybean extract fermented by Bacillus subtilis MORI in db/db mice. Lab. Anim. Res. 2012, 28, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Vanbelle, M.; Teller, E.; Focant, M. Probiotics in animal nutrition: A review. Arch. Tierernahr. 1990, 40, 543–567. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, B.; Liu, G.; Shi, H.; Wang, J. Effect of Bacillus subtilis and Lactobacillus plantarum on solid-state fermentation of soybean meal. J. Sci. Food Agric. 2023, 103, 6070–6079. [Google Scholar] [CrossRef]
- Dai, C.H.; Ma, H.L.; He, R.H.; Huang, L.R.; Zhu, S.Y.; Ding, Q.Z.; Luo, L. Improvement of nutritional value and bioactivity of soybean meal by solid-state fermentation with Bacillus subtilis. LWT-Food Sci. Technol. 2017, 86, 1–7. [Google Scholar] [CrossRef]
- Wongputtisin, P.; Khanongnuch, C.; Khongbantad, W.; Niamsup, P.; Lumyong, S. Screening and selection of Bacillus spp. for fermented corticate soybean meal production. J. Appl. Microbiol. 2012, 113, 798–806. [Google Scholar] [CrossRef]
- Missotten, J.A.; Michiels, J.; Degroote, J.; De Smet, S. Fermented liquid feed for pigs: An ancient technique for the future. J. Anim. Sci. Biotechnol. 2015, 6, 4. [Google Scholar] [CrossRef]
- Li, Y.; Hao, Q.; Duan, C.; Ding, Y.; Wang, Y.; Guo, X.; Liu, Y.; Guo, Y.; Zhang, Y. Integrated Microbiota and Metabolome Analysis to Assess the Effects of the Solid-State Fermentation of Corn-Soybean Meal Feed Using Compound Strains. Microorganisms 2023, 11, 1319. [Google Scholar] [CrossRef]
- Miao, X.; Niu, H.; Sun, M.; Dong, X.; Hua, M.; Su, Y.; Wang, J.; Li, D. A comparative study on the nutritional composition, protein structure and effects on gut microbiota of 5 fermented soybean products (FSPs). Food Res. Int. 2024, 183, 114199. [Google Scholar] [CrossRef]
- Wu, L.; Tang, Z.; Chen, H.; Ren, Z.; Ding, Q.; Liang, K.; Sun, Z. Mutual interaction between gut microbiota and protein/amino acid metabolism for host mucosal immunity and health. Anim. Nutr. 2021, 7, 11–16. [Google Scholar] [CrossRef]
- Mok, W.K.; Tan, Y.X.; Lee, J.; Kim, J.; Chen, W.N. A metabolomic approach to understand the solid-state fermentation of okara using Bacillus subtilis WX-17 for enhanced nutritional profile. AMB Express 2019, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Lyu, W.; Song, Y.; Chen, K.; Lv, L.; Yang, H.; Wang, W.; Xiao, Y. Anti-Inflammatory Effect of Clostridium butyricum-Derived Extracellular Vesicles in Ulcerative Colitis: Impact on Host microRNAs Expressions and Gut Microbiome Profiles. Mol. Nutr. Food Res. 2023, 67, e2200884. [Google Scholar] [CrossRef] [PubMed]
- Parrado, J.; Rodriguez-Morgado, B.; Tejada, M.; Hernandez, T.; Garcia, C. Proteomic analysis of enzyme production by Bacillus licheniformis using different feather wastes as the sole fermentation media. Enzyme Microb. Technol. 2014, 57, 1–7. [Google Scholar] [CrossRef]
- Jie, S.; Xinjun, Y.U.; Wei, W.; Yougui, Z.; Jie, J.; Zhao, W. Metabolome analysis of Saccharomyces cerevisiae in different culture patterns and growth phases. J. Zhejiang Univ. Technol. 2017, 45, 654–659. [Google Scholar]
- Niven, S.J.; Beal, J.D.; Brooks, P.H. The effect of controlled fermentation on the fate of synthetic lysine in liquid diets for pigs. Anim. Feed. Technol. 2006, 129, 304–315. [Google Scholar] [CrossRef]
- Chun-Bo, L.U.; Yin, M.; Guo-Hui, L.I.; Yun-Ying, Z.; Yu, D. Analysis and identification of main antibacterial metabolites secreted by Lactobacillus plantarum DY6. Microbiol. China 2019, 46, 2258–2271. [Google Scholar]
- Canibe, N.; Højberg, O.; Badsberg, J.H.; Jensen, B.B. Effect of feeding fermented liquid feed and fermented grain on gastrointestinal ecology and growth performance in piglets. J. Anim. Sci. 2007, 85, 2959–2971. [Google Scholar] [CrossRef]
- Choct, M.; Dersjant-Li, Y.; Mcleish, J.; Peisker, M. Soy Oligosaccharides and Soluble Non-starch Polysaccharides: A Review of Digestion, Nutritive and Anti-nutritive Effects in Pigs and Poultry. Asian Australas. J. Anim. Sci. 2010, 23, 1386–1398. [Google Scholar] [CrossRef]
- Navarro, D.; Abelilla, J.J.; Stein, H.H. Structures and characteristics of carbohydrates in diets fed to pigs: A review. J. Anim. Sci. Biotechnol. 2019, 10, 39. [Google Scholar] [CrossRef]
- Tian, Y.J.; Fan, Y.X.; Liu, J.J.; Zhao, X.Y.; Chen, W. Effect of nitrogen, carbon sources and agitation speed on acetoin production of Bacillus subtilis SF4-3. Electron. J. Biotechnol. 2016, 19, 41–49. [Google Scholar] [CrossRef]
- Ma, L.; Tao, S.; Song, T.; Lyu, W.; Li, Y.; Wang, W.; Shen, Q.; Ni, Y.; Zhu, J.; Zhao, J.; et al. Clostridium butyricum and carbohydrate active enzymes contribute to the reduced fat deposition in pigs. iMeta 2024, 3, e160. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zou, H.; Li, J.; Song, T.; Lv, W.; Wang, W.; Wang, Z.; Tao, S. Impact of quorum sensing signaling molecules in gram-negative bacteria on host cells: Current understanding and future perspectives. Gut Microbes 2022, 14, 2039048. [Google Scholar] [CrossRef]
- Liu, S.; Cai, P.; You, W.; Yang, M.; Tu, Y.; Zhou, Y.; Valencak, T.G.; Xiao, Y.; Wang, Y.; Shan, T. Enhancement of gut barrier integrity by a Bacillussubtilis secreted metabolite through the GADD45AWnt/B-catenin pathway. iMeta 2025, 4, e70005. [Google Scholar] [CrossRef] [PubMed]
- Varga, G.A.; Kolver, E.S. Microbial and animal limitations to fiber digestion and utilization. J. Nutr. 1997, 127, 819s–823s. [Google Scholar] [CrossRef]
- von Engelhardt, W.; Bartels, J.; Kirschberger, S.; Meyer zu Düttingdorf, H.D.; Busche, R. Role of short-chain fatty acids in the hind gut. Vet. Q. 1998, 20 (Suppl. S3), S52–S59. [Google Scholar] [CrossRef]
- Ge, Q.; Hou, C.L.; Rao, X.H.; Zhang, A.Q.; Xiao, G.M.; Wang, L.Y.; Jin, K.N.; Sun, P.L.; Chen, L.C. In vitro fermentation characteristics of polysaccharides from coix seed and its effects on the gut microbiota. Int. J. Biol. Macromol. 2024, 262, 129994. [Google Scholar] [CrossRef] [PubMed]
- Ge, Q.; Li, H.Q.; Zheng, Z.Y.; Yang, K.; Li, P.; Xiao, Z.Q.; Xiao, G.M.; Mao, J.W. In vitro fecal fermentation characteristics of bamboo insoluble dietary fiber and its impacts on human gut microbiota. Food Res. Int. 2022, 156, 111173. [Google Scholar] [CrossRef]
- Canibe, N.; Jensen, B.B. Fermented and nonfermented liquid feed to growing pigs: Effect on aspects of gastrointestinal ecology and growth performance. J. Anim. Sci. 2003, 81, 2019–2031. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Song, R.; Zhou, J.; Jia, Y.; Lu, J. Fermented Bamboo Fiber Improves Productive Performance by Regulating Gut Microbiota and Inhibiting Chronic Inflammation of Sows and Piglets during Late Gestation and Lactation. Microbiol. Spectr. 2023, 11, e0408422. [Google Scholar] [CrossRef]
- Qiu, Y.; Tang, J.; Wang, L.; Yang, X.; Jiang, Z. Fermented Corn-Soybean Meal Improved Growth Performance and Reduced Diarrhea Incidence by Modulating Intestinal Barrier Function and Gut Microbiota in Weaned Piglets. Int. J. Mol. Sci. 2024, 25, 3199. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, Y.; Wei, S.Y.; Fu, L.L.; Wang, Y.Z.; Jin, M.L. Bioconversion of soybean meal into gut microbiota-targeting polysaccharides via fermentation by Bacillus subtilis. J. Clean. Prod. 2024, 464, 142787. [Google Scholar] [CrossRef]
- Yuan, L.; Chang, J.; Yin, Q.; Lu, M.; Di, Y.; Wang, P.; Wang, Z.; Wansg, E.; Lu, F. Fermented soybean meal improves the growth performance, nutrient digestibility, and microbial flora in piglets. Anim. Nutr. 2017, 3, 19–24. [Google Scholar] [CrossRef] [PubMed]


| Item | SBM | FSBM |
|---|---|---|
| pH | 6.38 ± 0.01 a | 5.24 ± 0.02 b |
| Crude protein (%) | 44.47 ± 0.94 b | 52.65 ± 0.35 a |
| Ash (%) | 5.30 ± 0.02 b | 6.28 ± 0.05 a |
| Crude fat (%) | 1.90 ± 0.02 a | 0.90 ± 0.02 b |
| Crude fiber (%) | 4.12 ± 0.07 a | 2.71 ± 0.25 b |
| NDF (%) | 11.43 ± 0.29 a | 7.29 ± 0.14 b |
| ADF (%) | 8.62 ± 0.35 a | 0.83 ± 0.05 b |
| Acid soluble protein (%) | 2.08 ± 0.07 b | 12.69 ± 0.23 a |
| Starch (%) | 0.88 ± 0.02 a | 0.53 ± 0.00 b |
| Amylose (%) | 0.79 ± 0.01 b | 2.71 ± 0.11 a |
| Reducing sugar (%) | 0.58 ± 0.04 b | 3.93 ± 0.10 a |
| Non-starch polysaccharides (%) | 28.61 ± 0.15 a | 21.74 ± 0.45 b |
| Calcium (%) | 0.34 ± 0.02 | 0.31 ± 0.02 |
| Phosphorus (%) | 0.60 ± 0.02 b | 0.82 ± 0.02 a |
| Lactate (%) | 1.03 ± 0.04 b | 3.56 ± 0.08 a |
| Essential amino acid (%) | ||
| Threonine | 1.89 ± 0.01 b | 1.97 ± 0.01 a |
| Valine | 1.89 ± 0.00 a | 1.75 ± 0.03 b |
| Methionine | 0.64 ± 0.00 b | 0.69 ± 0.01 a |
| Isoleucine | 1.64 ± 0.00 a | 1.34 ± 0.03 b |
| Leucine | 3.54 ± 0.01 b | 5.15 ± 0.09 a |
| Phenylalanine | 2.14 ± 0.04 | 2.69 ± 0.25 |
| Lysine | 2.45 ± 0.03 b | 3.33 ± 0.13 a |
| Histidine | 1.12 ± 0.02 a | 0.82 ± 0.00 b |
| Arginine | 3.22 ± 0.03 b | 3.44 ± 0.06 a |
| Tryptophan | 0.24 ± 0.00 b | 0.66 ± 0.01 a |
| Non-essential amino acid (%) | ||
| Serine | 1.45 ± 0.01 b | 4.07 ± 0.13 a |
| Glutamate | 10.00 ± 0.03 b | 10.52 ± 0.11 a |
| Glycine | 2.27 ± 0.00 b | 2.40 ± 0.07 a |
| Alanine | 1.45 ± 0.01 b | 2.79 ± 0.02 a |
| Cystine | 0.65 ± 0.01 b | 0.70 ± 0.00 a |
| Aspartate | 2.66 ± 0.06 a | 2.50 ± 0.03 b |
| Proline | 1.23 ± 0.02 b | 1.68 ± 0.02 a |
| Tyrosine | 1.55 ± 0.01 b | 1.90 ± 0.02 a |
| The total amino acids (%) | 40.03 ± 0.07 b | 48.41 ± 0.36 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Fu, L.; Chen, Y.; Yang, H.; Xiao, Y.; Ren, Y.; Wang, C. Integrated Metabolome and Microbiome Analysis Reveals the Regulatory Effects of Fermented Soybean Meal on the Gut Microbiota of Late Gestation. Fermentation 2025, 11, 315. https://doi.org/10.3390/fermentation11060315
Li Y, Fu L, Chen Y, Yang H, Xiao Y, Ren Y, Wang C. Integrated Metabolome and Microbiome Analysis Reveals the Regulatory Effects of Fermented Soybean Meal on the Gut Microbiota of Late Gestation. Fermentation. 2025; 11(6):315. https://doi.org/10.3390/fermentation11060315
Chicago/Turabian StyleLi, Yantao, Lele Fu, Yushi Chen, Hua Yang, Yingping Xiao, Ying Ren, and Cheng Wang. 2025. "Integrated Metabolome and Microbiome Analysis Reveals the Regulatory Effects of Fermented Soybean Meal on the Gut Microbiota of Late Gestation" Fermentation 11, no. 6: 315. https://doi.org/10.3390/fermentation11060315
APA StyleLi, Y., Fu, L., Chen, Y., Yang, H., Xiao, Y., Ren, Y., & Wang, C. (2025). Integrated Metabolome and Microbiome Analysis Reveals the Regulatory Effects of Fermented Soybean Meal on the Gut Microbiota of Late Gestation. Fermentation, 11(6), 315. https://doi.org/10.3390/fermentation11060315





