Genomic and Fermentation Characterization of Kluyveromyces marxianus and Saccharomyces cerevisiae in Root Extract-Based Low-Alcohol Beverage
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Preparation
2.2. DNA Extraction, WGS Analysis, and Gene Annotation
2.3. Effects of Different Carbon and Nitrogen Sources on Alcohol Production
2.4. Effects of Different Temperatures and Cultivation Times on Alcohol Production
2.5. Fermentation of Root Extracts
2.6. Determination of Alcohol Production and Culture Growth
2.7. Analysis of Volatile Compounds in Fermented Beverages
2.8. Statically Analysis
3. Results
3.1. Genomic Characterization
3.2. Gene Annotation
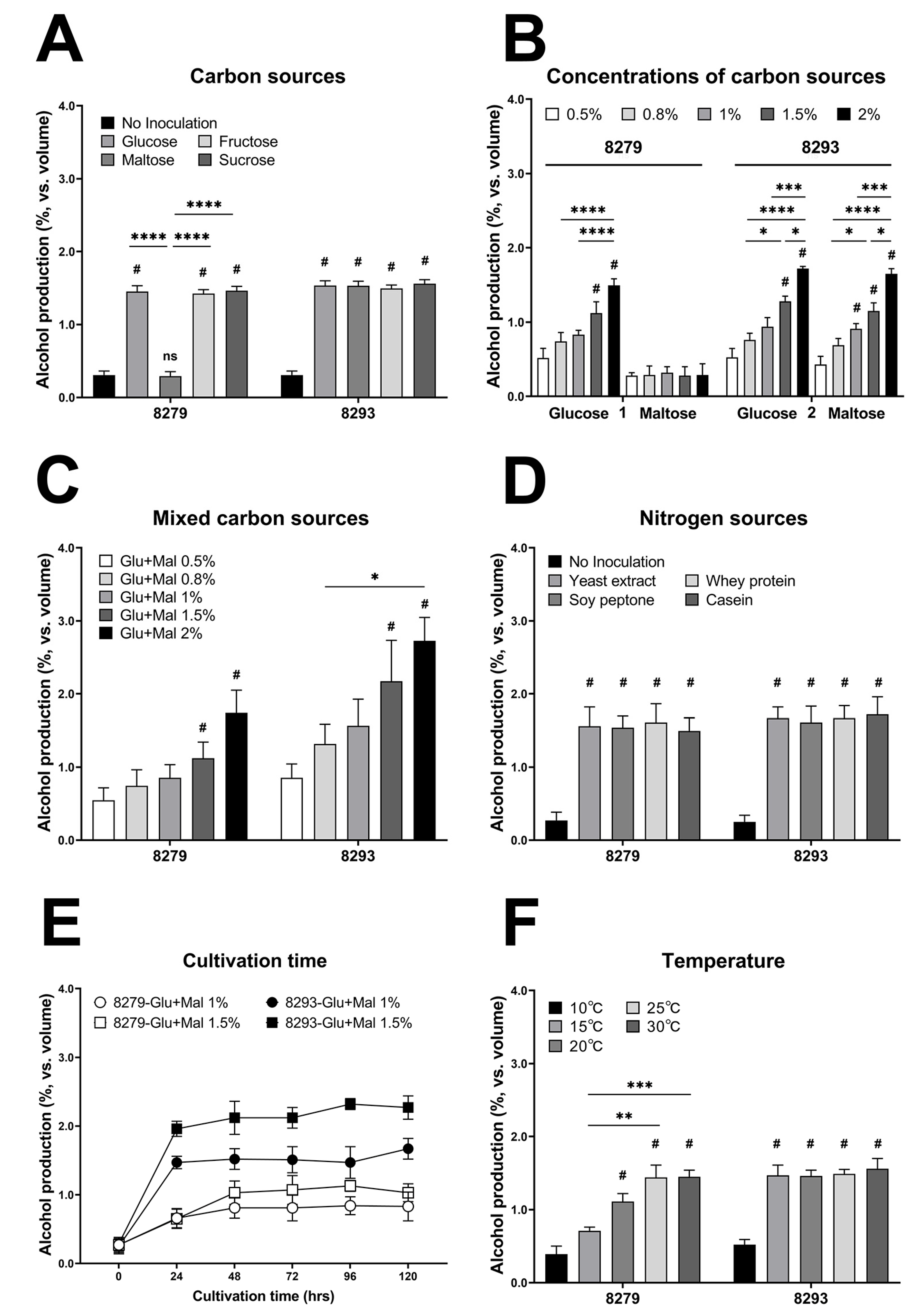
3.3. Effects of Carbon and Nitrogen Sources on Alcohol Production
3.4. Effects of Cultivation Temperature and Time on Alcohol Production
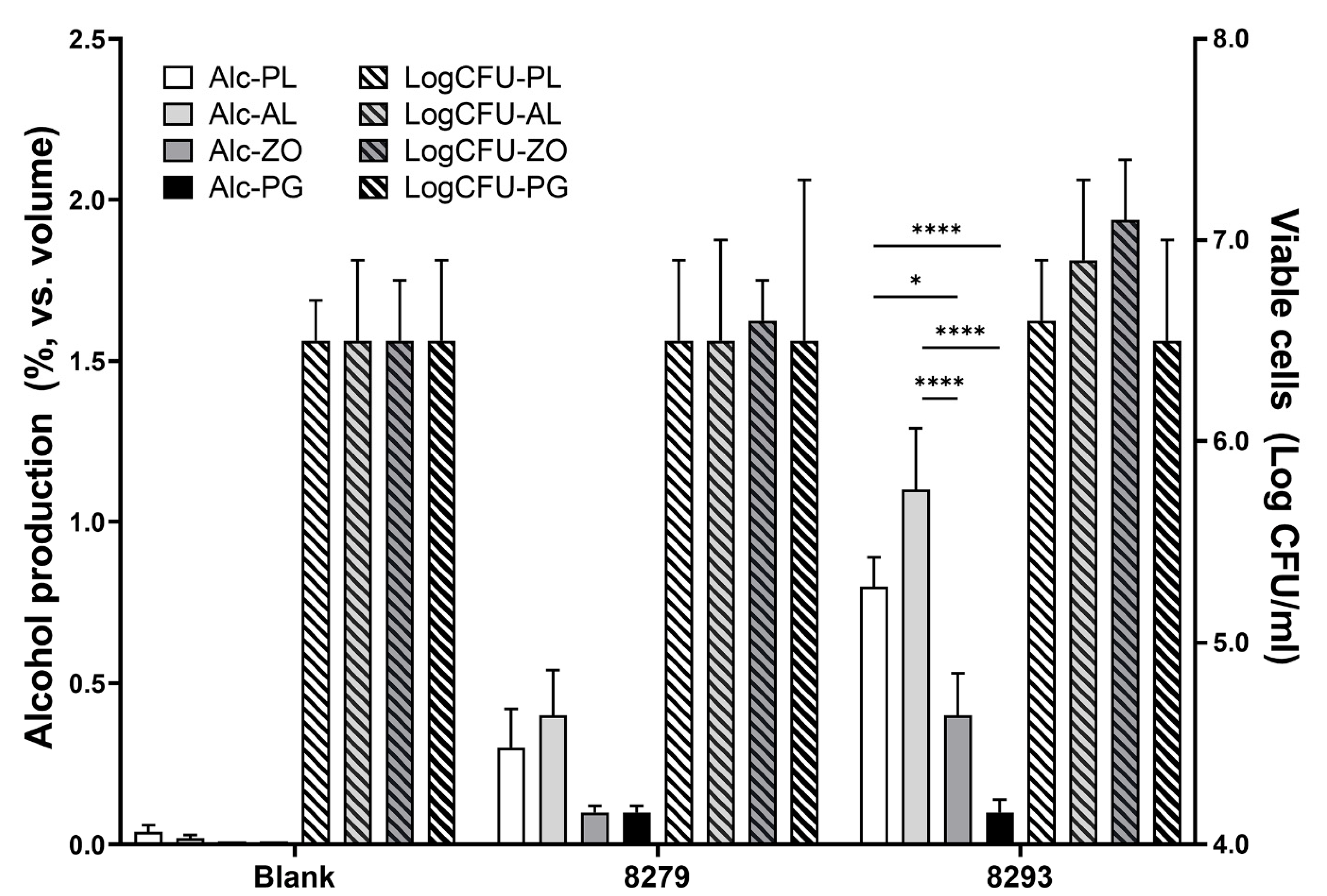
3.5. Fermentation Characteristics of Beverages Using Root Extracts
3.6. Volatile Compound Profiles of Fermented Beverages
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| KM | Kluyveromyces marxianus |
| SC | Saccharomyces cerevisiae |
| LAB | lactic acid bacteria |
| WGS | whole-genome sequencing |
| PL | Pueraria lobata |
| AL | Arctium lappa |
| ZO | Zingiber officinale |
| PG | Platycodon grandiflorus |
| CFU | colony-forming unit |
| GC | guanine–cytosine |
| NAB | non-alcoholic beverage |
References
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Hutkins, R. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Tamang, J.P.; Cotter, P.D.; Endo, A.; Han, N.S.; Kort, R.; Liu, S.Q.; Mayo, B.; Westerik, N.; Hutkins, R. Fermented foods in a global age: East meets West. Compr. Rev. Food Sci. Food Saf. 2020, 19, 184–217. [Google Scholar] [CrossRef] [PubMed]
- Rezac, S.; Kok, C.R.; Heermann, M.; Hutkins, R. Fermented foods as a dietary source of live organisms. Front. Microbiol. 2018, 9, 1785. [Google Scholar] [CrossRef]
- Dimidi, E.; Cox, S.R.; Rossi, M.; Whelan, K. Fermented foods: Definitions and characteristics, impact on the gut microbiota and effects on gastrointestinal health and disease. Nutrients 2019, 11, 1806. [Google Scholar] [CrossRef]
- Aschemann-Witzel, J.; Gantriis, R.F.; Fraga, P.; Perez-Cueto, F.J.A. Plant-based food and protein trend from a business perspective: Markets, consumers, and the challenges and opportunities in the future. Crit. Rev. Food Sci. Nutr. 2021, 61, 3119–3128. [Google Scholar] [CrossRef]
- Arena, M.P.; Capozzi, V.; Spano, G.; Fiocco, D. The potential of lactic acid bacteria to colonize biotic and abiotic surfaces and the investigation of their interactions and mechanisms. Appl. Microbiol. Biotechnol. 2017, 101, 2641–2657. [Google Scholar] [CrossRef] [PubMed]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I.S. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Styger, G.; Prior, B.; Bauer, F.F. Wine flavor and aroma. J. Ind. Microbiol. Biotechnol. 2011, 38, 1145–1159. [Google Scholar] [CrossRef]
- Lilly, M.; Lambrechts, M.G.; Pretorius, I.S. Effect of increased yeast alcohol acetyltransferase activity on flavor profiles of wine and distillates. Appl. Environ. Microbiol. 2000, 66, 744–753. [Google Scholar] [CrossRef]
- Okaru, A.O.; Lachenmeier, D.W. Defining No and Low (NoLo) alcohol products. Nutrients 2022, 14, 3873. [Google Scholar] [CrossRef]
- Martins, N.; Petropoulos, S.; Ferreira, I.C.F.R. Chemical composition and bioactive compounds of commonly consumed roots and tubers. Curr. Opin. Food Sci. 2016, 8, 36–41. [Google Scholar]
- Xiang, H.; Sun-Waterhouse, D.; Waterhouse, G.I.N.; Cui, C.; Ruan, Z. Fermentation-enabled wellness foods: A fresh perspective. Food Sci. Hum. Wellness 2019, 8, 203–243. [Google Scholar] [CrossRef]
- Zamfir, M.; Angelescu, I.-R.; Voaides, C.; Cornea, C.-P.; Boiu-Sicuia, O.; Grosu-Tudor, S.-S. Non-dairy fermented beverages produced with functional lactic acid bacteria. Microorganisms 2022, 10, 2314. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kang, H.J.; Yi, S.H.; Jung, Y.H. Antioxidant properties of kombucha made with tartary buckwheat tea and burdock tea. Prev. Nutr. Food Sci. 2023, 28, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, L.I.G.; Costa, W.K.A.; Oliveira, F.C.; Bezerril, F.F.; Eireli, L.P.A.M.; Lima, M.S.; Noronha, M.F.; Cabral, L.; Wagner, R.; Pimentel, T.C.; et al. Ginger beer derived from back-slopping: Volatile compounds, microbial communities on activation and fermentation, metabolites, and sensory characteristics. Food Chem. 2024, 435, 137640. [Google Scholar] [CrossRef]
- Drewnowski, A.; Gomez-Carneros, C. Bitter taste, phytonutrients, and the consumer: A review. Am. J. Clin. Nutr. 2000, 72, 1424–1435. [Google Scholar] [CrossRef]
- Hittinger, C.T.; Steele, J.L.; Ryder, D.S. Diverse yeasts for diverse fermented beverages and foods. Curr. Opin. Biotechnol. 2018, 49, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Qu, J.; Wang, L.; Zhang, J. Genome sequencing, assembly, and characterization of Pichia fermentans Z9Y-3 as a non-Saccharomyces yeast with aroma enhancing potential. Food Biosci. 2023, 53, 102701. [Google Scholar] [CrossRef]
- Lu, Z.; Guo, L.; Chen, X.; Lu, Q.; Wu, Y.; Chen, D.; Wu, R.; Chen, Y. Omics sequencing of Saccharomyces cerevisiae strain with improved capacity for ethanol production. Fermentation 2023, 9, 483. [Google Scholar] [CrossRef]
- Lucchini, S.; Thompson, A.; Hinton, J.C. Microarrays for microbiologists. Microbiology 2011, 147, 1403–1414. [Google Scholar] [CrossRef]
- Aziz, R.K.; Bartels, D.; Best, A.A.; DeJongh, M.; Disz, T.; Edwards, R.A.; Formsma, K.; Gerdes, S.; Glass, E.M.; Kubal, M.; et al. The RAST Server: Rapid annotations using subsystems technology. BMC Genom. 2008, 9, 75. [Google Scholar] [CrossRef] [PubMed]
- Huerta-Cepas, J.; Forslund, K.; Coelho, L.P.; Szklarczyk, D.; Jensen, L.J.; von Mering, C.; Bork, P. Fast genome-wide functional annotation through orthology assignment by eggNOG-mapper. Mol. Biol. Evol. 2017, 34, 2115–2122. [Google Scholar] [CrossRef] [PubMed]
- Cloninger, L. Alcohol determination of malt-based beverages by rapid distillation. J. Am. Soc. Brew. Chem. 2018, 76, 21–23. [Google Scholar] [CrossRef]
- Bruner, J.; Marcus, A.; Fox, G. Dry-hop creep potential of various Saccharomyces yeast species and strains. Fermentation 2021, 7, 66. [Google Scholar] [CrossRef]
- Fonseca, G.G.; de Carvalho, N.M.B.; Gombert, A.K. Growth of the yeast Kluyveromyces marxianus CBS 6556 on different sugar combinations as sole carbon and energy source. Appl. Microbiol. Biotechnol. 2013, 97, 5055–5067. [Google Scholar] [CrossRef]
- Lo, S.-C.; Yang, C.-Y.; Mathew, D.C.; Huang, C.-C. Growth and autolysis of the kefir yeast Kluyveromyces marxianus in lactate culture. Sci. Rep. 2021, 11, 14552. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Kim, D.H.; Park, H.D. Effects of protectant and rehydration conditions on the survival rate and malolactic fermentation efficiency of freeze-dried Lactobacillus plantarum JH287. Appl. Microbiol. Biotechnol. 2016, 100, 7853–7863. [Google Scholar] [CrossRef]
- Choi, K.T.; Park, C.W.; Lee, S.H.; Lee, Y.N.; Oh, J.Y.; Choi, J.S.; Choe, D.; Lee, S.B. Effects of co-fermentation by Saccharomyces cerevisiae and Hanseniaspora uvarum yeasts on the volatile aromatic compound and sensory quality of distilled soju. J. Korean Soc. Food Sci. Nutr. 2024, 53, 639–647. [Google Scholar] [CrossRef]
- Heux, S.; Cachon, R.; Dequin, S. Physiology of the yeast Kluyveromyces marxianus during batch and chemostat cultures with glucose as the sole carbon source. FEMS Yeast Res. 2006, 6, 469–476. [Google Scholar]
- Wang, D.; Wang, L.; Hou, L.; Deng, X.; Gao, Q.; Gao, N. Metabolic engineering of Saccharomyces cerevisiae for accumulating pyruvic acid. Ann. Microbiol. 2015, 65, 2323–2331. [Google Scholar] [CrossRef]
- Wang, X.; Bali, M.; Medintz, I.; Michels, C.A. Intracellular maltose is sufficient to induce MAL gene expression in Saccharomyces cerevisiae. Eukaryot. Cell 2002, 1, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Hellborg, L. Yeast diversity in the brewing industry. In Beer in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 77–88. [Google Scholar]
- Sene, L.; Tavares, B.; de Almeida Felipe, M.G.; dos Santos, J.C.; Pereira, F.M.; Tominc, G.C.; da Cunha, M.A.A. Ethanol production by Kluyveromyces marxianus ATCC 36907: Fermentation features and mathematical modeling. Biocatal. Agric. Biotechnol. 2023, 51, 102789. [Google Scholar] [CrossRef]
- Lee, J.H.; Son, H.S.; Jeong, J.H.; Noh, J.M.; Kim, J.M.; Choi, H.S.; Lee, Y.H. Quality characteristics of Takju, Yakju, and Spirit made from Phellinus linteus and ginger. Culin. Sci. Hosp. Res. 2015, 21, 103–119. [Google Scholar]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Karimi, E.; Ashkani, S. Changes in nutritional metabolites of young ginger (Zingiber officinale Roscoe) in response to elevated carbon dioxide. Molecules 2014, 19, 16693–16706. [Google Scholar] [CrossRef] [PubMed]
- Petkova, N.; Hambarlyiska, I.; Tumbarski, Y.; Vrancheva, R.; Raeva, M.; Ivanov, I. Phytochemical composition and antimicrobial properties of burdock (Arctium lappa L.) roots extracts. Biointerface Res. Appl. Chem. 2022, 12, 2826–2842. [Google Scholar]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A. Gingerols and shogaols: Important nutraceutical principles from ginger. Phytochemistry 2015, 117, 554–568. [Google Scholar] [CrossRef]
- Rojas, I.B.; Smith, P.A.; Bartowsky, E.J. Influence of choice of yeasts on volatile fermentation-derived compounds, colour and phenolics composition in Cabernet Sauvignon wine. World J. Microbiol. Biotechnol. 2012, 28, 3311–3321. [Google Scholar] [CrossRef]
- Braga, C.M.; Zielinski, A.A.F.; da Silva, K.M.; de Souza, F.K.F.; Pietrowski, G.A.M.; Couto, M.; Granato, D.; Wosiacki, G.; Nogueira, A. Classification of juices and fermented beverages made from unripe, ripe and senescent apples based on the aromatic profile using chemometrics. Food Chem. 2013, 141, 967–974. [Google Scholar] [CrossRef]
- Steingass, C.B.; Glock, M.P.; Lieb, V.M.; Carle, R. Light-induced alterations of pineapple (Ananas comosus [L.] Merr.) juice volatiles during accelerated ageing and mass spectrometric studies into their precursors. Food Res. Int. 2017, 100, 366–374. [Google Scholar] [CrossRef]
- George, J.; Pramanik, I.; Sanewski, G.; Nguyen, T.; Pun, S.; Edwards, D.; Currie, M.; Møller, S.; Hardner, C.; Lyons, P.; et al. Relationship between key aroma compounds and sensory attributes of Australian grown commercial pineapple cultivars. J. Agric. Food Chem. 2025, 73, 5839–5849. [Google Scholar] [CrossRef]
- Holt, S.; Miks, M.H.; de Carvalho, B.T.; Foulquié-Moreno, M.R.; Thevelein, J.M. The molecular biology of fruity and floral aromas in beer and other alcoholic beverages. FEMS Microbiol. Rev. 2019, 43, 193–222. [Google Scholar] [CrossRef] [PubMed]

| Contents | LRCC8279 | LRCC8293 |
|---|---|---|
| Species | Kluyveromyces marxianus | Saccharomyces cerevisiae |
| Genome size (bp) | 11,008,819 | 14,689,445 |
| GC content (%) | 40.1 | 38.2 |
| Number of genes | 5317 | 7414 |
| Number of CDSs | 5122 | 7059 |
| Number of rRNAs | 186 | 349 |
| Number of tRNAs | 9 | 7 |
| Genes | EC No. | Product | 8279 | 8293 | |
|---|---|---|---|---|---|
| Glucose | Hxk | 2.7.1.1 | Hexokinase | + | + |
| Pgi | 5.3.1.9 | glucose-6-phosphate isomerase | − | + | |
| pkf1,2 | 2.7.1.11 | ATP-dependent 6-phosphofructokinase | + | + | |
| Pyk | 2.7.1.40 | pyruvate kinase | + | + | |
| Maltose | Maa | 2.3.1.79 | maltose O-acetyltransferase | + | + |
| mal13 | − | maltose fermentation regulatory | − | + | |
| mal31 | − | maltose permease | − | + | |
| mal61 | − | maltose permease | − | + | |
| mal63 | − | maltose fermentation regulatory | − | + | |
| ypr196w | − | maltose fermentation regulatory | − | + | |
| Sucrose | Suc | 3.2.1.26 | Invertase | + | + |
| Msc | − | monosaccharide transporter | + | + | |
| Glk | 2.7.1.2 | Glucokinase | + | + | |
| hxk2 | 2.7.1.1 | hexokinase-2 | + | + | |
| Fructose | frk1 | 2.7.1.4 | fructokinase 1 | + | + |
| fba1 | 4.1.2.13 | fructose-bisphosphate aldolase | + | + | |
| fbp1 | 3.1.3.11 | fructose-1,6-bisphosphatase | + | + | |
| Alcohol production | pdc1 | 4.1.1.1 | pyruvate decarboxylase | + | + |
| adh1 | 1.1.1.1 | alcohol dehydrogenase 1 | + | + | |
| adh2 | 1.1.1.1 | alcohol dehydrogenase 2 | + | + | |
| adh6 | 1.1.1.1 | aldehyde dehydrogenase 6 | + | + | |
| Gpd | 1.1.1.8 | glycerol-3-phosphate dehydrogenase | + | + | |
| Nde | 1.6.5.3 | NADH dehydrogenase | + | + | |
| Ndi | 1.6.5.3 | NADH dehydrogenase | + | + |
| Sensory Characteristics | Volatile Compounds Identified (GC-MS Peak Area, ×106) | |||||||
|---|---|---|---|---|---|---|---|---|
| PL-82791 | PL-82931 | AL-82791 | AL-82931 | ZO-82791 | ZO-82931 | PG-82791 | PG-82931 | |
| Ethereal | 1.89 ± 0.56 c | 49.66 ± 2.25 b | 78.37 ± 1.74 a | 2.54 ± 0.54 c | 82.27 ± 11.99 a | 2.55 ± 0.45 c | 85.48 ± 5.25 a | 2.23 ± 0.14 c |
| Floral | 81.35 ± 2.56 c | 48.45 ± 11.03 d | 378.11 ± 15.43 a | 178.67 ± 9.83 b | 394.98 ± 8.62 a | 88.29 ± 0.69 c | 123.86 ± 11.23 c | 96.34 ± 8.24 c |
| Fruity | 42.66 ± 3.65 c | 78.78 ± 3.62 b | 57.40 ± 9.64 b | 129.50 ± 11.33 a | 150.89 ± 10.26 a | 101.84 ± 13.84 ab | 53.02 ± 0.53 b | 91.88 ± 5.44 ab |
| Fermented | 25.86 ± 2.14 c | 94.38 ± 7.22 b | 72.11 ± 8.54 b | 140.99 ± 2.52 a | 90.14 ± 4.27 b | 69.59 ± 6.29 b | 62.04 ± 2.65 bc | 81.15 ± 3.22 b |
| Sour | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 6.17 ± 1.56 b | 0.00 ± 0.00 c | 14.46 ± 0.50 a | 0.00 ± 0.00 c | 3.83 ± 0.84 bc | 0.00 ± 0.00 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, E.-J.; Choi, S.-H.; Seo, M.-J.; Lee, A.-R.; Jang, C.-S.; Kwak, W.-K.; Kwak, J.-K.; Lee, J.-H.; Yoon, W.-J.; Yoon, S.-M. Genomic and Fermentation Characterization of Kluyveromyces marxianus and Saccharomyces cerevisiae in Root Extract-Based Low-Alcohol Beverage. Fermentation 2025, 11, 299. https://doi.org/10.3390/fermentation11060299
Lee E-J, Choi S-H, Seo M-J, Lee A-R, Jang C-S, Kwak W-K, Kwak J-K, Lee J-H, Yoon W-J, Yoon S-M. Genomic and Fermentation Characterization of Kluyveromyces marxianus and Saccharomyces cerevisiae in Root Extract-Based Low-Alcohol Beverage. Fermentation. 2025; 11(6):299. https://doi.org/10.3390/fermentation11060299
Chicago/Turabian StyleLee, Eun-Ju, Seung-Hyun Choi, Min-Ju Seo, A-Reum Lee, Chan-Song Jang, Woong-Kwon Kwak, Jung-Ki Kwak, Jae-Ho Lee, Won-Joo Yoon, and Seok-Min Yoon. 2025. "Genomic and Fermentation Characterization of Kluyveromyces marxianus and Saccharomyces cerevisiae in Root Extract-Based Low-Alcohol Beverage" Fermentation 11, no. 6: 299. https://doi.org/10.3390/fermentation11060299
APA StyleLee, E.-J., Choi, S.-H., Seo, M.-J., Lee, A.-R., Jang, C.-S., Kwak, W.-K., Kwak, J.-K., Lee, J.-H., Yoon, W.-J., & Yoon, S.-M. (2025). Genomic and Fermentation Characterization of Kluyveromyces marxianus and Saccharomyces cerevisiae in Root Extract-Based Low-Alcohol Beverage. Fermentation, 11(6), 299. https://doi.org/10.3390/fermentation11060299






