Early Fermentation Dynamics and Aerobic Stability of Maize Silage Improved by Dual-Strain Lactic Acid Bacteria Inoculation
Abstract
1. Introduction
2. Materials and Methods
2.1. Crop Material, Microbial Inoculants, and Silage Preparation
- Control (C)—water application only;
- LBL—a 50:50 combination of Lentilactobacillus buchneri (DSM22501) and Lactococcus lactis (DSM11037), SiloSolve® FC (Novonesis, Lyngby, Denmark) applied at rate 150,000 CFU g−1 forage.
2.2. Silages Sampling, Chemical and Microbiological Analyses
2.3. Calculations for Corrected Dry Matter (DMc) Concentration and Dry Matter Loss
2.4. Aerobic Stability Evaluation of the Silages
2.5. Statistical Analyses
3. Results
3.1. Characterization of Maize Silage Stored in Mini-Silos and Opened at 2, 4, 8, 16, and 32 Days
3.1.1. Fermentation Profile of the Silages upon Opening
3.1.2. Nutritional Composition of Silages upon Opening
3.1.3. Microbial Profile of the Silages upon Opening
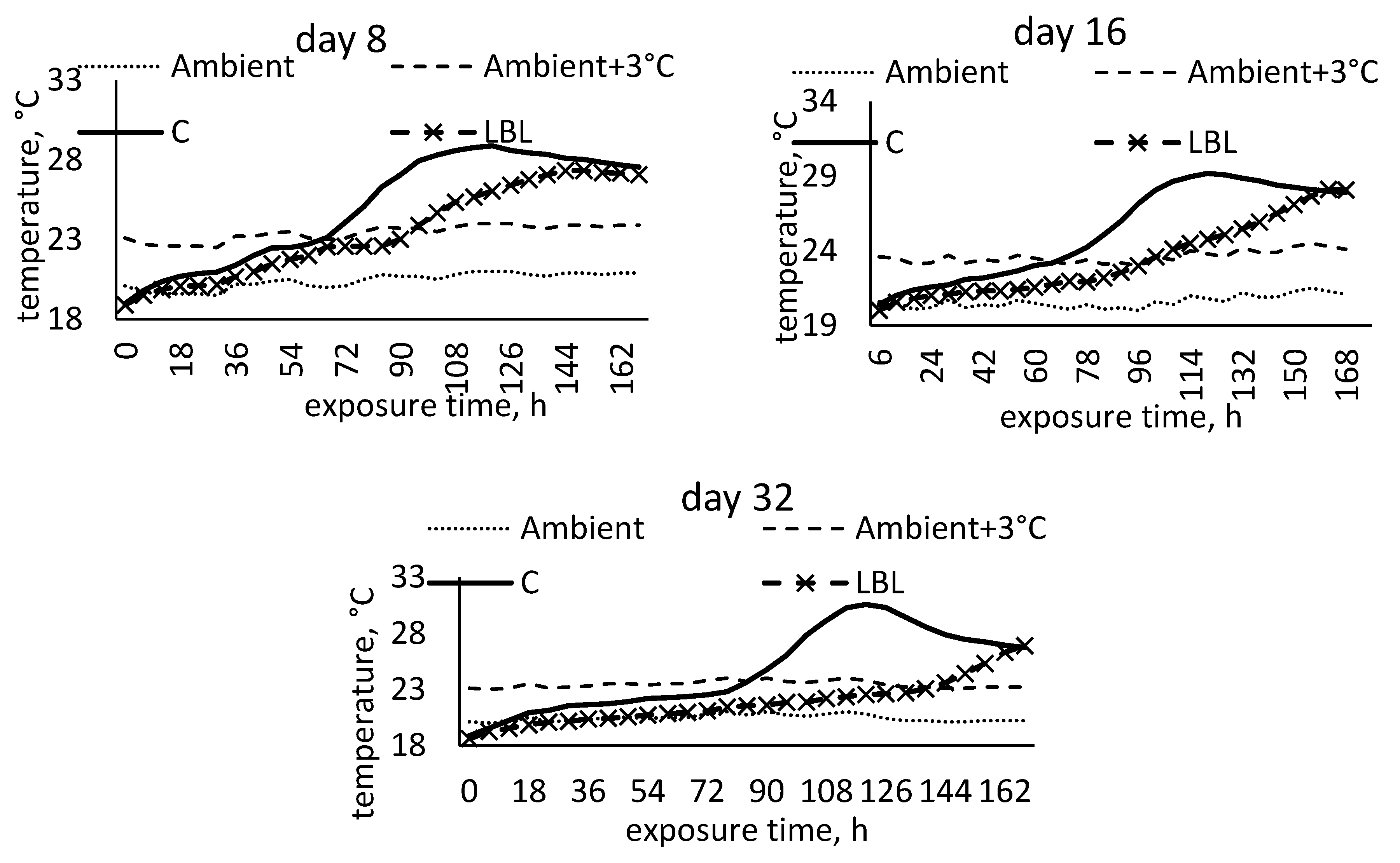
3.1.4. Characteristics of Maize Silage Aerobic Stability
4. Discussion
4.1. Characteristics of Forage Prior to Ensiling
4.1.1. Nutritional Composition and Fermentation Characteristics of Silages upon Opening
4.1.2. Microbial and Aerobic Stability Characteristics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, L.L.; Yuan, X.J.; Li, J.F.; Dong, Z.H.; Shao, T. Dynamics of microbial community and fermentation quality during ensiling of sterile and nonsterile alfalfa with or without Lactobacillus plantarum inoculant. Bioresour. Technol. 2019, 275, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, B.F.; Sales, G.F.C.; Schwan, R.F.; Ávila, C.S. Criteria for lactic acid bacteria screening to enhance silage quality. J. Appl. Microbiol. 2021, 130, 341–355. [Google Scholar] [CrossRef] [PubMed]
- Fabiszewska, A.U.; Zielińska, K.J.; Wróbel, B. Trends in designing microbial silage quality by biotechnological methods using lactic acid bacteria inoculants: A minireview. World J. Microbiol. Biotechnol. 2019, 35, 76. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, J.; Dong, Z.; Chen, L.; Yuan, X.; Shao, T. The effects of lactic acid bacteria strains isolated from various substrates on the fermentation quality of common vetch (Vicia sativa L.) in Tibet. Grass Forage Sci. 2018, 73, 639–647. [Google Scholar] [CrossRef]
- Guo, X.; Xu, D.; Li, F.; Bai, J.; Su, R. Current approaches on the roles of lactic acid bacteria in crop silage. Microb. Biotechnol. 2023, 16, 67–87. [Google Scholar] [CrossRef]
- Muck, R.E.; Nadeau, E.M.G.; McAllister, T.A.; Contreras-Govea, F.E.; Santos, M.C.; Kung, L., Jr. Silage review: Recent advances and future uses of silage additives. J. Dairy Sci. 2018, 101, 3980–4000. [Google Scholar] [CrossRef]
- Liu, E.Y.; Wang, S.; Wang, S.; Khan, N.A.; Zhou, X.; Tang, S.; Zhou, C.H.; Tan, Z.; Lui, Y. Bacterial inoculants and enzymes based silage cocktails boost the ensiling quality of biomasses from reed, corn and rice straw. Chem. Biol. Technol. Agric. 2024, 11, 29. [Google Scholar] [CrossRef]
- Ranjit, N.K.; Kung, L., Jr. The effect of Lactobacillus buchneri, Lactobacillus plantarum, or a chemical preservative on the fermentation and aerobic stability of corn silage. J. Dairy Sci. 2000, 83, 526–535. [Google Scholar] [CrossRef]
- Kleinschmit, D.H.; Kung, L., Jr. A meta-analysis of the effects of Lactobacillus buchneri on the fermentation and aerobic stability of corn and grass and small-grain silages. J. Dairy Sci. 2006, 89, 4005–4013. [Google Scholar] [CrossRef]
- Arriola, K.G.; Queiroz, O.C.M.; Romero, J.J.; Casper, D.; Muniz, E.; Hamie, J.; Adesogan, A.T. Effect of microbial inoculants on the quality and aerobic stability of bermudagrass round-bale haylage. J. Dairy Sci. 2015, 98, 478–485. [Google Scholar] [CrossRef]
- Broberg, A.; Jacobsson, K.; Strom, K.; Schnurer, J. Metabolite profiles of lactic acid bacteria in grass silage. Appl. Environ. Microbiol. 2007, 73, 5547–5552. [Google Scholar] [CrossRef] [PubMed]
- Schnürer, J.; Magnusson, J. Antifungal lactic acid bacteria as biopreservatives. Trends Food Sci. Technol. 2005, 16, 70–78. [Google Scholar] [CrossRef]
- Khemariya, P.; Singh, S.; Nath, G.; Gulati, A.K. Probiotic Lactococcus lactis: A Review. Turkish JAF Sci. Technol. 2017, 5, 556–562. [Google Scholar] [CrossRef]
- Tabacco, E.; Piano, S.; Cavallarin, L.; Bernardes, T.F.; Borreani, G. Clostridia Spore Formation during Aerobic Deterioration of Maize and Sorghum Silages as Influenced by Lactobacillus buchneri and Lactobacillus plantarum Inoculants. J. Appl. Microbiol. 2009, 107, 1632–1641. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Weinberg, Z.G.; Ogunade, I.M.; Cervantes, A.A.P.; Arriola, K.G.; Jiang, Y.; Kim, D.; Li, X.; Gonçalves, M.C.M.; Vyas, D.; et al. Meta-Analysis of Effects of Inoculation with Homofermentative and Facultative Heterofermentative Lactic Acid Bacteria on Silage Fermentation, Aerobic Stability, and the Performance of Dairy Cows. J. Dairy Sci. 2017, 100, 4587–4603. [Google Scholar] [CrossRef]
- Arriola, K.G.; Oliveira, A.S.; Jiang, Y.; Kim, D.; Silva, H.M.; Kim, S.C.; Amaro, F.X.; Ogunade, I.M.; Sultana, H.; Pech Cervantes, A.A.; et al. Meta-Analysis of Effects of Inoculation with Lactobacillus buchneri, with or without Other Bacteria, on Silage Fermentation, Aerobic Stability, and Performance of Dairy Cows. J. Dairy Sci. 2021, 104, 7653–7670. [Google Scholar] [CrossRef]
- Samelis, J.; Giannou, E.; Pappa, E.C.; Bogović-Matijašić, B.; Lianou, A.; Parapouli, M.; Drainas, C. Behavior of Artificial Listerial Contamination in Model Greek Graviera Cheeses Manufactured with the Indigenous Nisin A-Producing Strain Lactococcus lactis subsp. Cremoris M104 as Costarter Culture. J. Food Saf. 2017, 37, e12326. [Google Scholar] [CrossRef]
- Diepersloot, E.C.; Pupo, M.R.; Ghizzi, L.G.; Gusmão, J.O.; Heinzen, C., Jr.; McCary, C.L.; Wallau, M.O.; Ferraretto, L.F. Effects of Microbial Inoculation and Storage Length on Fermentation Profile and Nutrient Composition of Whole-Plant Sorghum Silage of Different Varieties. Front. Microbiol. 2021, 12, 660567. [Google Scholar] [CrossRef]
- Zhao, X.; Sun, Y.; Chang, Z.; Yao, B.; Han, Z.; Wang, T.; Shang, N.; Wang, R. Innovative Lactic Acid Production Techniques Driving Advances in Silage Fermentation. Fermentation 2024, 10, 533. [Google Scholar] [CrossRef]
- Ni, K.; Wang, F.; Zhu, B.; Yang, J.; Zhou, G.; Pan, Y.; Tao, Y.; Zhong, J. Effects of lactic acid bacteria and molasses additives on the microbial community and fermentation quality of soybean silage. Bioresour. Technol. 2017, 238, 706–715. [Google Scholar] [CrossRef]
- Xia, G.H.; Zhang, M.Z.; Huang, Y.; Chen, C.; Yang, F.Y.; Hao, J. Heat-resistant lactic acid bacteria inoculants modulated the bacterial microbiota and fermentation quality of whole plant maize silage after long-term storage in the subtropical area. Anim. Feed. Sci. Technol. 2024, 310, 115931. [Google Scholar] [CrossRef]
- Oude Elferink, S.J.W.H.; Krooneman, J.; Gottschal, J.C.; Spolestra, S.F.; Faber, F.; Driehuis, F. Anaerobic conversion of lactic acid to acetic acid and 1,2-Propanediol by Lactobacillus buchneri. Appl. Environ. Microbiol. 2001, 67, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Nishino, N.; Yoshida, M.; Shiota, H.; Sakaguchi, E. Accumulation of 1,2-propanediol and enhancement of aerobic stability in whole crop maize silage inoculated with Lactobacillus buchneri. J. Appl. Microbiol. 2003, 94, 800–807. [Google Scholar] [CrossRef]
- Hidrichsen, I.K.; Augustson, E.U.; Lund, B.; Jensen, M.M.; Raun, M.; Jatkauskas, J.; Vrotniakiene, V.; Ohlsoon, C. Characterization of different lactic acid bacteria in terms of their oxygen cosuming capacity, aerobic stability and pathogen inhibition. In Proceedings of the 16th International Silage Conference, Hameelin, Finland, 2–4 July 2012; Kuoppala, K., Rinne, M., Vanhatalo, A., Eds.; MTT Agrifood Research Finland University of Helsinki: Helsinki, Finland, 2012; pp. 105–106. [Google Scholar]
- ISO 15214:1998; Microbiology of Food and Animal Feeding Stuffs—Horizontal Method for the Enumeration of Mesophilic Lactic Acid Bacteria—Colony-Count Technique at 30 Degrees C. ISO: Geneva, Switzerland, 1998. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:15214:ed-1:v1:en (accessed on 20 April 2017).
- Jatkauskas, J.; Vrotniakiene, V.; Eisner, I.; Witt, K.L.; do Amaral, R.C. Comparison of the Chemical and Microbial Composition and Aerobic Stability of High-Moisture Barley Grain Ensiled with Either Chemical or Viable Lactic Acid Bacteria Application. Fermentation 2024, 10, 62. [Google Scholar] [CrossRef]
- Weissbach, F.E.; Strubelt, C. Correcting the dry matter content of maize silages as a substrate for biogas production. Landtech. Net63 2008, 2, 82–83. Available online: https://www.landtechnik-online.eu/ojs-2.4.5/index.php/landtechnik/article/view/2008-2-082-083/1237 (accessed on 19 June 2016).
- Moran, J.; Weinberg, Z.G.; Ashbell, G. A comparison of two methods for the evaluation of the aerobic stability of whole crop wheat silage. In Proceedings of the 11th International Silage Conference, Aberystwyth, UK, 8–11 September 1996; Jones, D.I.H., Jones, R., Dewhurst, R., Merry, R., Haigh, P.H., Eds.; University of Wales: Aberystwyth, UK, 1996; pp. 162–163. [Google Scholar]
- SAS Institute Inc. SAS/STAT® 9.4 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2014. [Google Scholar]
- Nussio, L. Silage Production and Utilisation. In Proceedings of the 14th International Silage Conference, a Satellite Workshop of the 20th International Grassland Congress, Belfast, Northern Ireland, 3–6 July 2005; Park, R.S., Stronge, M.D., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2005; pp. 97–107. Available online: http://books.google.fr/books?hl=fr&lr=&id=rxDTDWJ4jOoC (accessed on 15 February 2018).
- Li, Y.; Du, S.; Sun, L.; Cheng, Q.; Hao, J.; Lu, Q.; Ge, G.; Wang, Z.J.; Jia, Y. Effects of lactic acid bacteria and molasses additives on dynamic fermentation quality and microbial community of native grass silage. Front. Microbiol. 2022, 13, 1–13. [Google Scholar] [CrossRef]
- DLG Testing Guidelines for the Award und Use of the DLG Quality Mark for Ensiling Agents, Prepared Under the Auspices of the DLG Commission for Ensiling Agents, Schwarz, F.J.; München, T.U.; Thalmann, A., Eds; p. 60. 2018. Available online: http://www.dlg.org/siliermittel.html (accessed on 27 April 2022).
- He, L.; Lv, H.; Xing, Y.; Chen, X.; Zhang, Q. Intrinsic tannins affect ensiling characteristics and proteolysis of Neolamarckia cadamba leaf silage by largely altering bacterial community. Bioresour. Technol. 2020, 311, 123496. [Google Scholar] [CrossRef]
- McDonald, P.; Edwards, R.A.; Greenhalgh, J.F.D.; Morgan, C.A.; Sinclair, L.A.; Wilkinson, R.G. Animal Nutrition, 7th ed.; Pearson Education Limited: Edinburgh Gate, Harlow, UK, 2010; p. 711. Available online: https://www.up.lublin.pl/files/animal/ANIM%20NUTR/animal-nutrition_mcdonald_et_al.pdf (accessed on 4 May 2011).
- Ozkose, E.; Akyol, I.; Kar, B.; Comlekcioglu, U.; Ekinci, M.S. Expression of fungal cellulose gene in Lactococcus lactis to construct novel recombinant silage inoculants. Folia Microbiol. 2009, 54, 335–342. [Google Scholar] [CrossRef]
- Nkosi, B.D.; Langa, A.R.C.; Thomas, A.R.C.; Meeske, R. Effects of bacterial silage inoculants on whole-crop maize silage fermentation and silage digestibility in rams. S. Afr. J. Anim. Sci. 2011, 41, 350–359. [Google Scholar] [CrossRef]
- Cai, Y.; Du, Z.; Yamasaki, S.; Nguluve, D.; Tinga, B.; Macome, F.; Oya, T. Community of natural lactic acid bacteria and silage fermentation of corn stover and sugarcane tops in Africa. Asian-Australas. J. Anim. Sci. 2020, 33, 1252–1264. [Google Scholar] [CrossRef]
- Ranjit, N.K.; Taylor, C.C.; Kung, L., Jr. Effect of Lactobacillus buchneri 40788 on the fermentation, aerobic stability and nutritive value of maize silage. Grass Forage Sci. 2002, 57, 73–81. [Google Scholar] [CrossRef]
- McDonald, P.; Henderson, A.R.; Heron, S.J.E. The Biochemistry of Silage, 2nd ed.; Chalcombe Publications: Marlow, UK, 1991; 340p, Available online: http://books.google.com/books?id=oUcjAQAAMAAJ (accessed on 15 March 2015).
- Xu, Z.; He, H.; Zhang, S.; Kong, J. Effects of inoculants Lactobacillus brevis and Lactobacillus parafarraginis on the fermentation characteristics and microbial communities of corn stover silage. Sci. Rep. 2017, 7, 13614. [Google Scholar] [CrossRef] [PubMed]
- Nazar, M.; Wang, S.; Zhao, J.; Dong, Z.; Li, J.; Kaka, N.A.; Shao, T. Abundance and diversity of epiphytic microbiota on forage crops and their fermentation characteristic during the ensiling of sterile sudan grass. World J. Microbiol. Biotechnol. 2021, 37, 27. [Google Scholar] [CrossRef]
- Weinberg, Z.G.; Chen, Y. Effects of Storage Period on the Composition of Whole Crop Wheat and Corn Silages. Anim. Feed Sci. Technol. 2013, 185, 196–200. [Google Scholar] [CrossRef]
- Okoye, C.O.; Wang, Y.; Gao, L.; Wu, Y.; Li, X.; Sun, J.; Jiang, J. The performance of lactic acid bacteria in silage production: A review of modern biotechnology for silage improvement. Microbiol. Res. 2023, 266, 127212. [Google Scholar] [CrossRef]
- Basso, F.C.; Bernardes, T.F.; Roth, A.P.D.T.P.; Lodo, B.N.; Berchielli, T.T.; Reis, R.A. Fermentation and aerobic stability of corn silage inoculated with Lactobacillus buchneri. Rev. Bras. Zootec. 2012, 41, 1789–1794. [Google Scholar] [CrossRef]
- Salvo, P.A.R.; Basso, F.C.; Rabelo, C.H.S.; Oliveira, A.A.; Sader, A.P.; Casagrade, D.R.; Berchielli, T.T.; Reis, R.A. Characteristics of corn silages inoculated with Lactobacillus buchneri and L. plantarum. Arch. Zootec. 2013, 62, 239. [Google Scholar] [CrossRef]
- Amaral, R.C.; Carvalho, B.F.; Costa, D.M.; Morenz, M.J.F.; Schwan, R.F.; da Silva Ávila, C.L. Novel lactic acid bacteria strains enhance the conservation of elephant grass silage cv. BRS Capiaçu. Anim. Feed Sci. Technol. 2020, 264, 114472. [Google Scholar] [CrossRef]
- Honig, H.; Thaysen, J. 10 years testing of silage additives by DLG—A comprehensive data evaluation. In Proceedings of the 13th International Silage Conference, Auchincruive, UK, 11–13 September 2002; Gechie, L.M., Thomas, C., Eds.; Scottish Agricultural College (SAC): Auchincruive, UK, 2002; pp. 232–233. [Google Scholar]
- Auerbach, H.; Weiss, K.; Theobald, P.; Nadeau, E. Effects of inoculant type on dry matter losses, fermentation pattern, yeast count and aerobic stability of green rye silages. In Proceedings of the 12 BOKU-Symposium Tierernährung, Vienna, Austria, 11 April 2013; Mair, C., Kraft, M., Wetscherek, W., Schedle, K., Eds.; Institut fur Tierernahrung, Tierische Lebensmittel: Wien, Austria, 2013; pp. 179–185. Available online: https://boku.ac.at/fileadmin/data/H03000/H97000/H97600/Symptagungsbaende/BOKU_Symposium_2013.pdf (accessed on 19 August 2018).
- Auerbach, H.; Nadeau, E. Effects of Additive Type on Fermentation and Aerobic Stability and Its Interaction with Air Exposure on Silage Nutritive Value. Agronomy 2020, 10, 1229. [Google Scholar] [CrossRef]
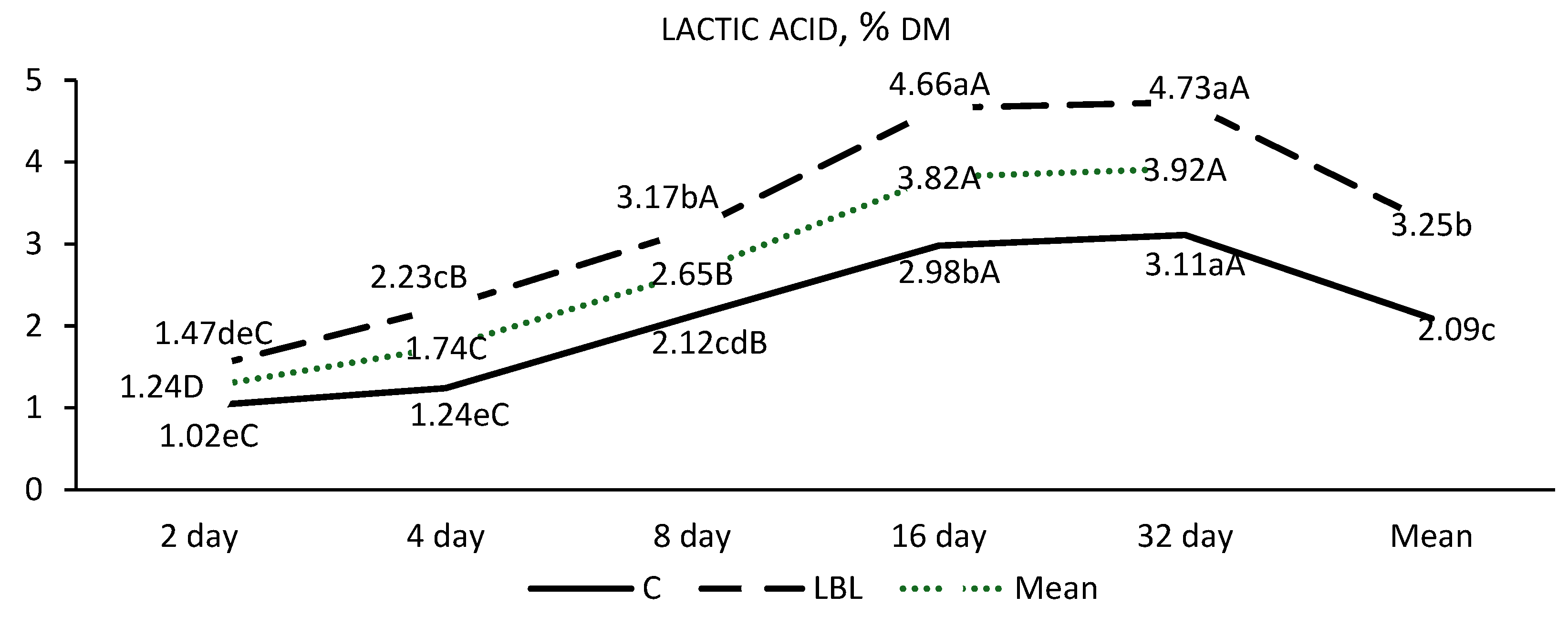
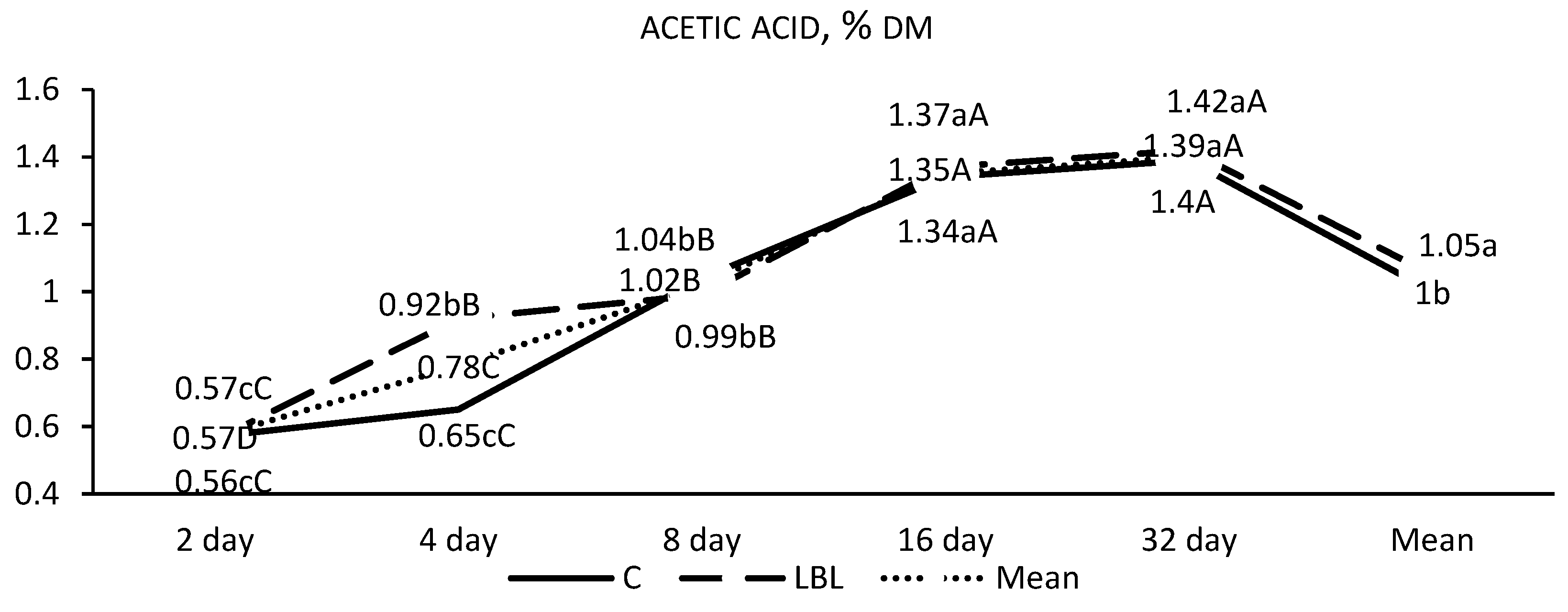
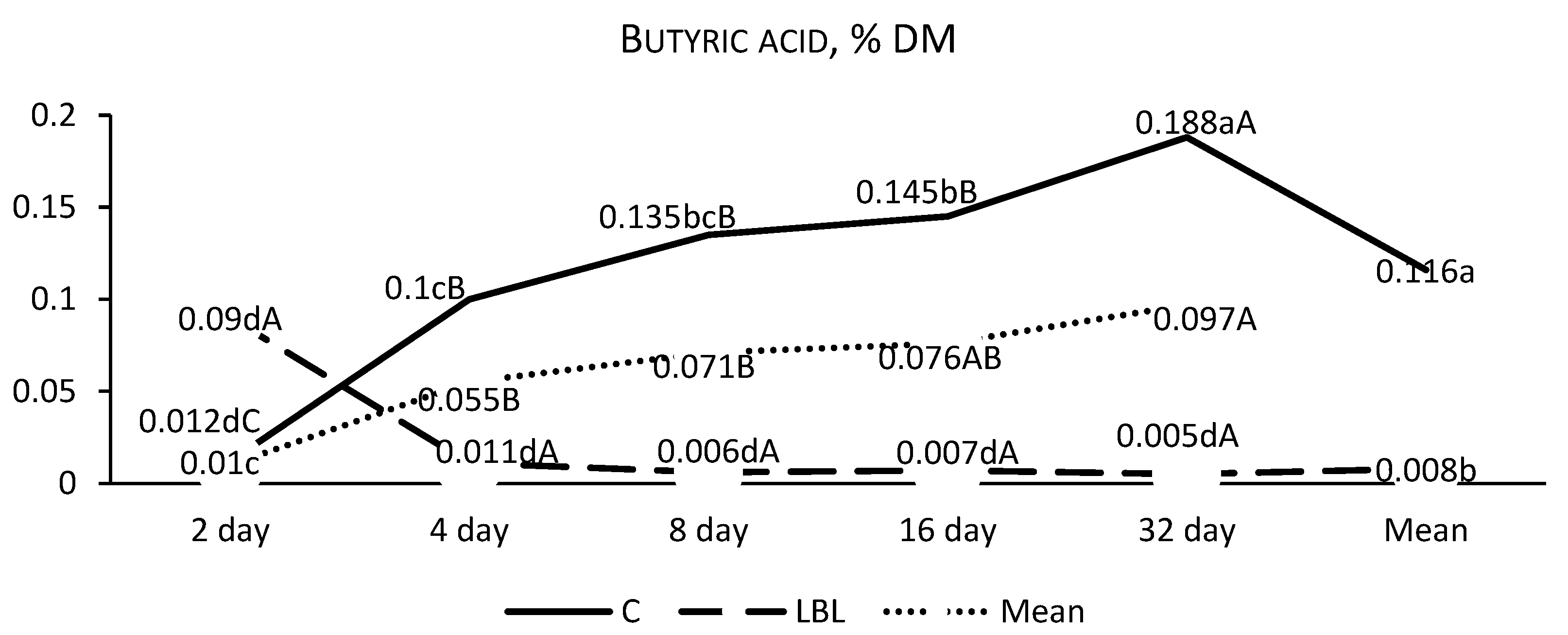
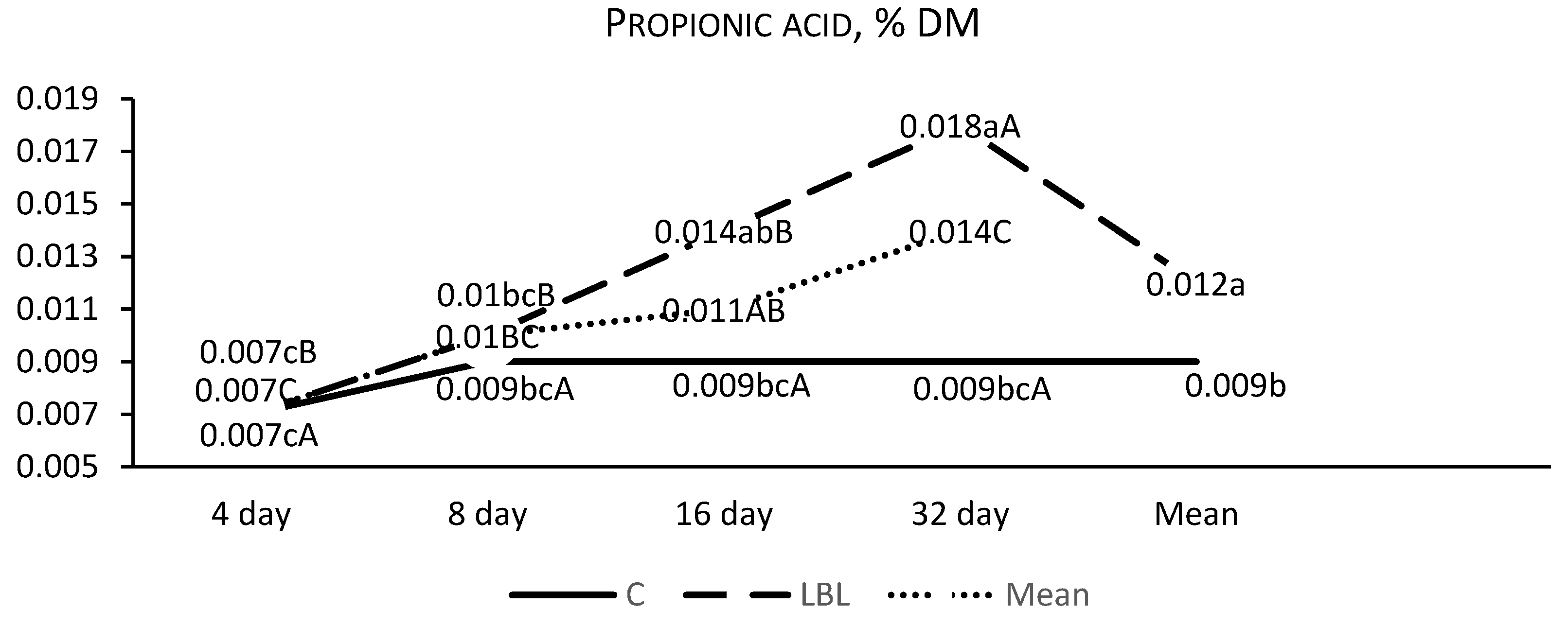
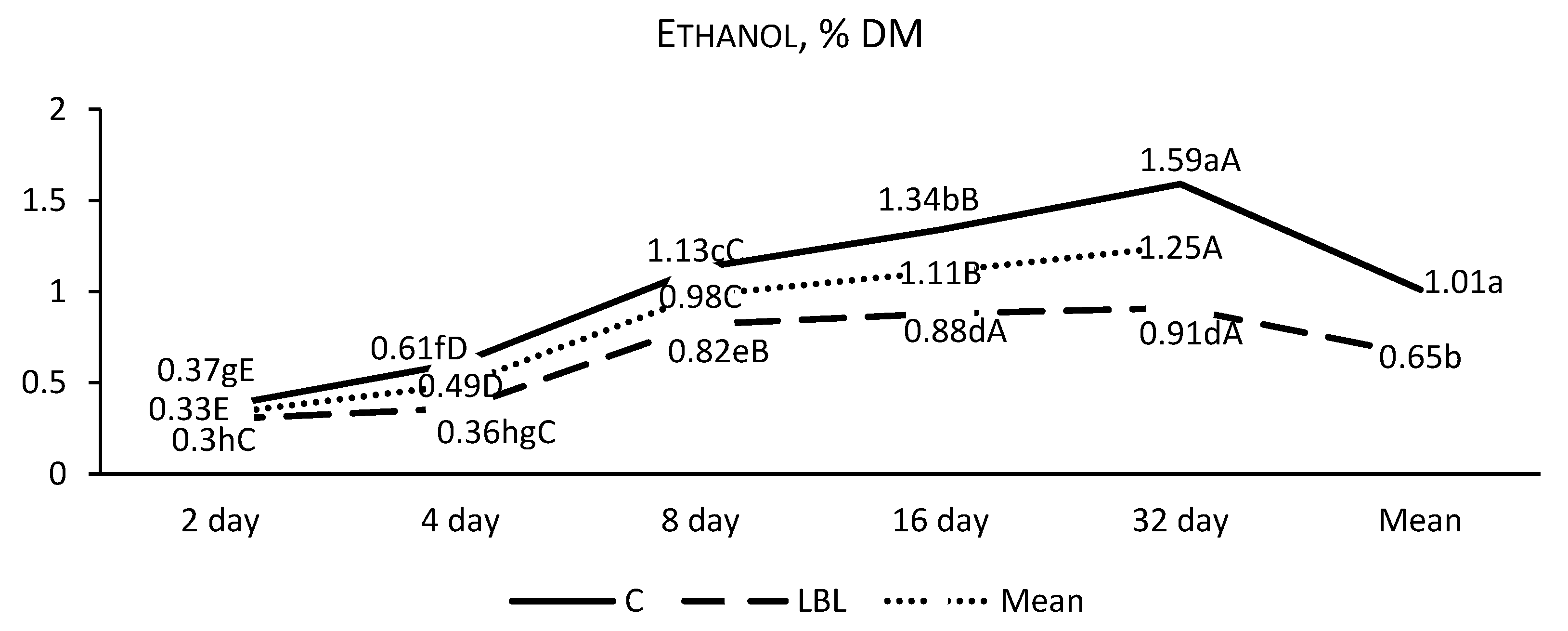
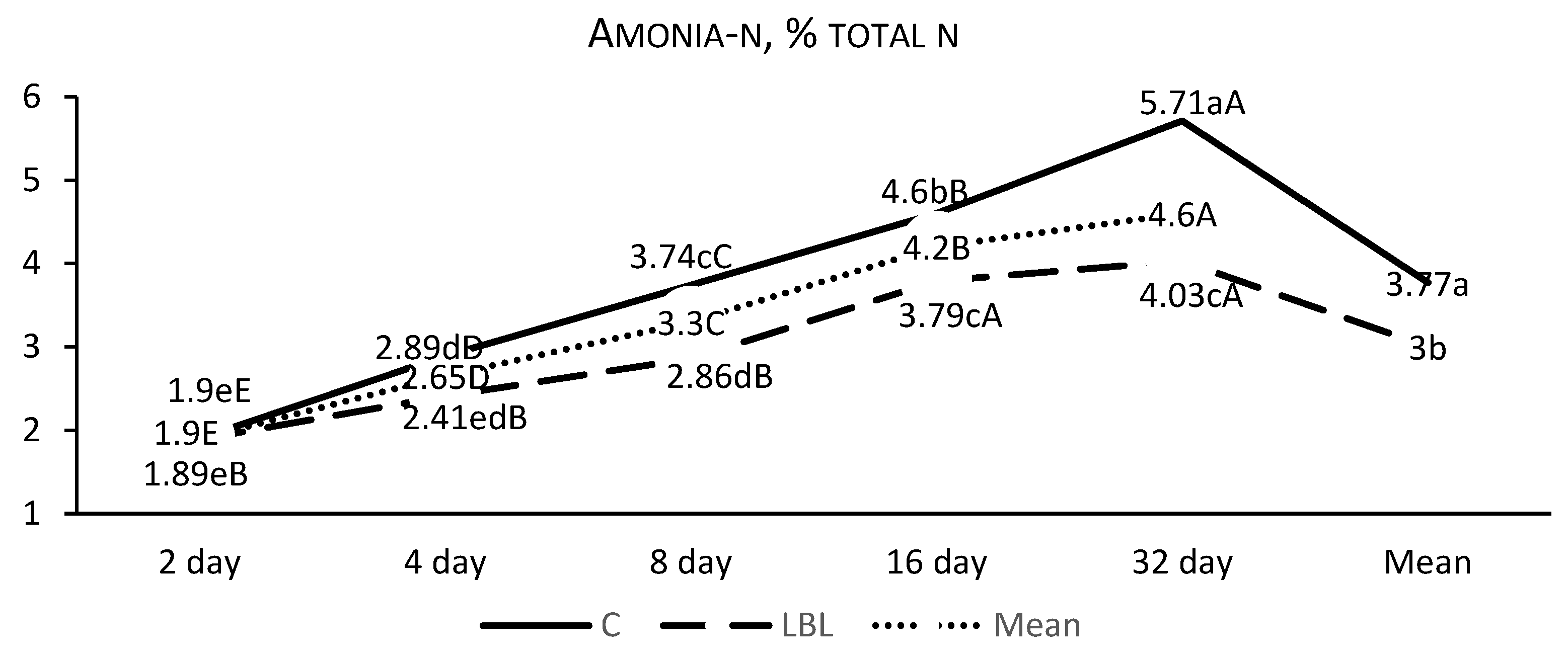
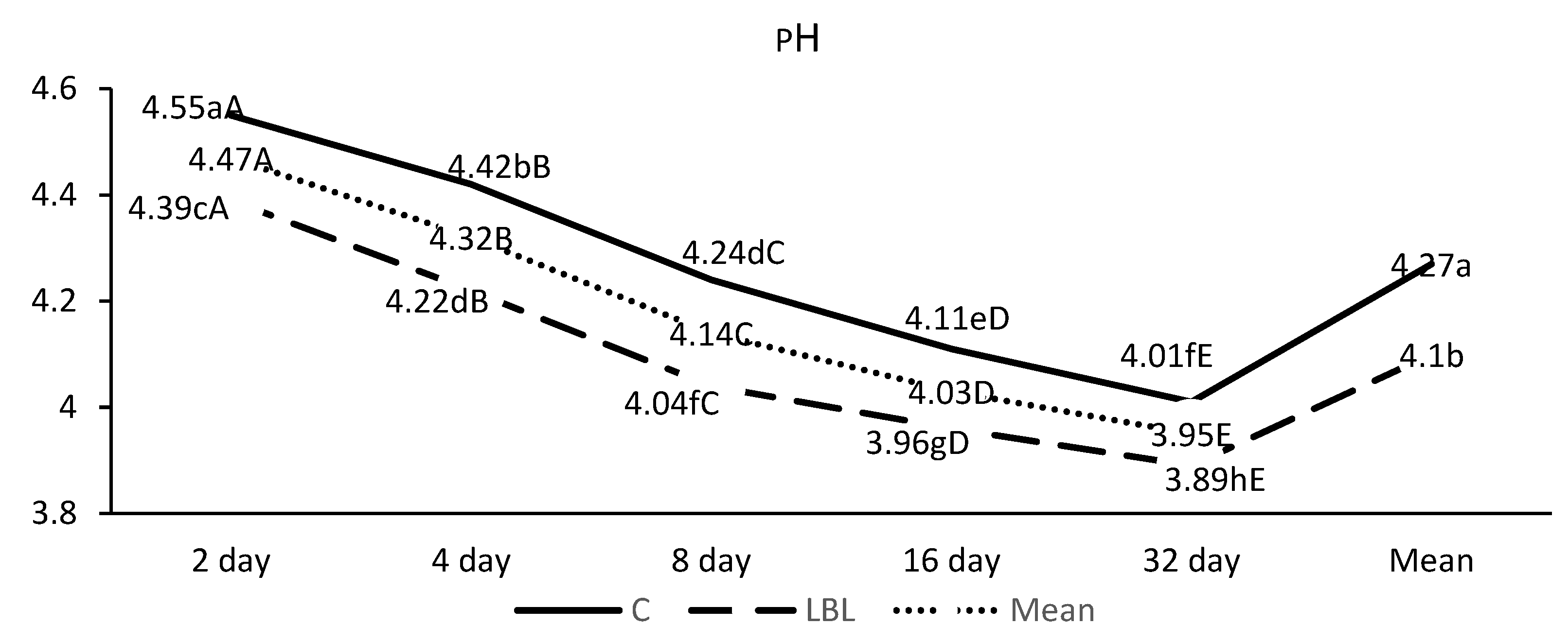
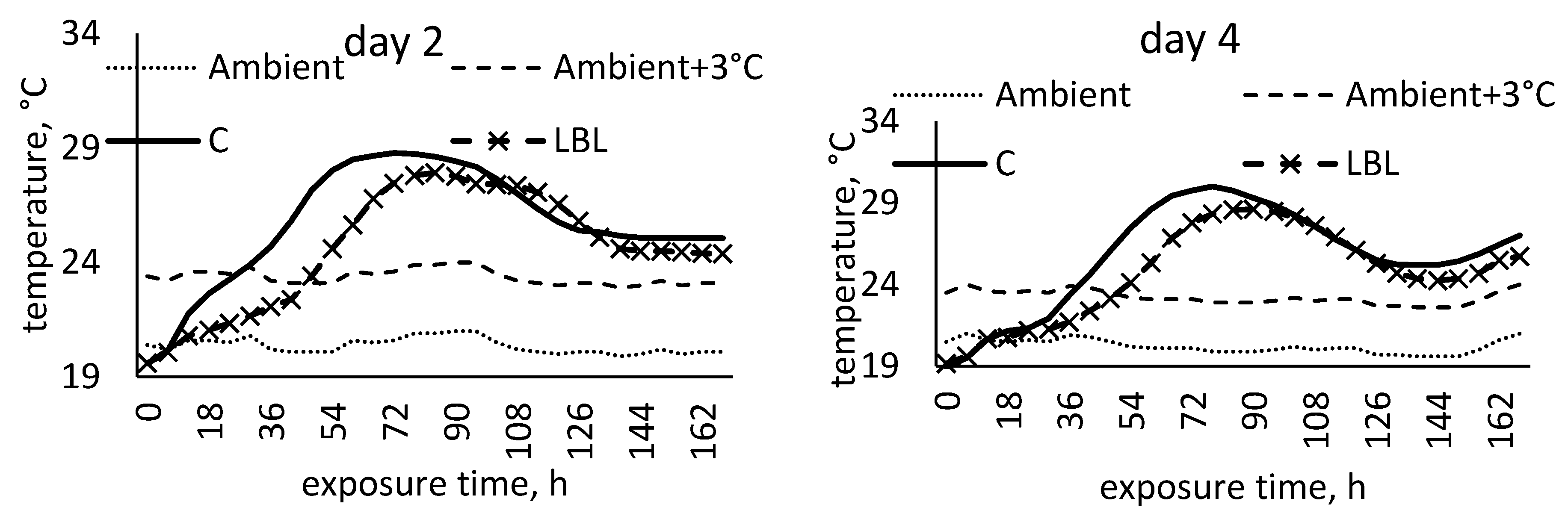
| Item | Average | SD |
|---|---|---|
| DM, g kg−1 | 343.9 | 05.60 |
| Crude protein, g kg−1 DM | 67.5 | 06.75 |
| Ether extract g kg−1 DM | 24.2 | 01.2 |
| Crude fibre, gkg−1 DM | 205.9 | 12.35 |
| NFE, g kg−1 DM | 603.7 | 10.04 |
| Crude ash, g kg−1 DM | 98.63 | 01.71 |
| WSC, g kg−1 DM | 87.7 | 03.22 |
| ADF, g kg−1 DM | 250.3 | 13.53 |
| NDF, g kg−1 DM | 397.2 | 23.68 |
| pH | 6.04 | 0.018 |
| Nitrate, mg kg−1 DM | 447.76 | 15.525 |
| Buffer capacity, mequiv100 g−1 DM | 19.46 | 2.056 |
| Yeasts, log10CFU g−1 FM | 5.75 | 0.076 |
| LAB, log10CFU g−1 FM | 4.97 | 0.087 |
| Moulds, log10CFU g−1 FM | 5.52 | 0.049 |
| Treatment | Expected Counts | Actual Counts | ± % |
|---|---|---|---|
| C (water) | 0 | <1.0 × 10 | |
| LBL * | 1.5 × 108 | 1.6 × 108 | +6.7% |
| Items | C | LBL | Mean | SE |
|---|---|---|---|---|
| DM, % | ||||
| 2 days | 34.0 aA | 34.0 aA | 34.0 A | 0.116 |
| 4 days | 33.8 aA | 33.8 aA | 33.8 A | 0.116 |
| 8 days | 32.5 cB | 33.1 bB | 32.8 B | 0.116 |
| 16 days | 31.9 dC | 32.9 bcB | 32.4 C | 0.116 |
| 32 days | 31.6 dC | 32.8 bcB | 32.2 C | 0.116 |
| Mean | 32.8 b | 33.3 a | ||
| DMc, % | ||||
| 2 days | 34.3 aA | 34.3 aA | 34.3 A | 0.076 |
| 4 days | 34.3 aA | 34.3 aA | 34.3 A | 0.076 |
| 8 days | 33.4 cdB | 33.8 abcA | 33.6 B | 0.076 |
| 16 days | 32.9 deB | 33.8 bcA | 33.3 BC | 0.076 |
| 32 days | 32.7 eB | 33.7 cdA | 33.4 C | 0.076 |
| Mean | 33.5 b | 34.0 a | ||
| CP, % DM | ||||
| 2 days | 6.43 aA | 6.64 aA | 6.53 A | 0.110 |
| 4 days | 6.13 aA | 6.64 aA | 6.38 AB | 0.110 |
| 8 days | 5.42 bB | 6.65 aA | 6.03 B | 0.110 |
| 16 days | 5.05 bcB | 5.44 bB | 5.25 C | 0.110 |
| 32 days | 4.75 cB | 5.41 bB | 5.08 C | 0.110 |
| Mean | 5.56 b | 6.16 a | ||
| NDF, % DM | ||||
| 2 days | 41.32 aA | 41.46 aA | 41.40 | 0.481 |
| 4 days | 41.92 aA | 41.51 aA | 41.72 | 0.481 |
| 8 days | 42.32 aA | 42.18 aA | 42.25 | 0.481 |
| 16 days | 42.17 aA | 41.66 aA | 41.91 | 0.481 |
| 32 days | 41.85 aA | 41.30 aA | 41.58 | 0.481 |
| Mean | 41.91 a | 41.62 a | ||
| ADF, | ||||
| 2 days | 24.53 aA | 24.41 aA | 24.471 A | 0.523 |
| 4 days | 24.87 aA | 23.9 aA | 24.38 A | 0.523 |
| 8 days | 24.18 aA | 23.52 aA | 23.85 A | 0.523 |
| 16 days | 24.12 aA | 22.58 aA | 23.35 A | 0.523 |
| 32 days | 23.93 aA | 22.6 aA | 23.26 A | 0.523 |
| Mean | 24.33 a | 23.40 b | ||
| DM losses, % | ||||
| 2 days | 0.58 eC | 0.61 eB | 0.60 C | 0.193 |
| 4 days | 0.94 deC | 0.66 eB | 0.80 C | 0.193 |
| 8 days | 3.97 bB | 2.21 cdA | 3.09 B | 0.193 |
| 16 days | 5.46 aA | 2.44 cA | 3.95 A | 0.193 |
| 32 days | 6.10 aA | 2.66 bcA | 4.38 A | 0.193 |
| Mean | 3.41 a | 1.72 b |
| Items | C | LBL | Mean | SE |
|---|---|---|---|---|
| Yeast, log10CFU g−1 FM | ||||
| 2 days | 5.08 aA | 4.32 cA | 4.70 A | 0.051 |
| 4 days | 4.72 bB | 3.63 deB | 4.17 B | 0.051 |
| 8 days | 4.63 bcC | 2.47 fBC | 3.55 C | 0.051 |
| 16 days | 3.93 dD | 1.34 gD | 2.63 D | 0.051 |
| 32 days | 3.32 eE | 1.06 gD | 2.19 E | 0.051 |
| Mean | 4.34 a | 2.56 b | ||
| Mold, log10CFU g−1 FM | ||||
| 2 days | 3.49 aA | 2.52 cdA | 3.09 A | 0.063 |
| 4 days | 3.12 bB | 2.41 dA | 2.77 B | 0.063 |
| 8 days | 2.86 bcB | 1.97 eB | 2.42 C | 0.063 |
| 16 days | 2.71 cdB | 1.78 efB | 2.25 CD | 0.063 |
| 32 days | 2.67 cdB | 1.45 fC | 2.06 D | 0.063 |
| Mean | 2.97 a | 2.03 b |
| Items | C | LBL | Mean | SE |
|---|---|---|---|---|
| AST, hours | ||||
| 2 days | 32.40 gB | 49.20 fgC | 40.80 C | 0.583 |
| 4 days | 42.00 fgB | 55.20 efC | 48.60 C | 0.583 |
| 8 days | 69.60 deA | 98.40 bcB | 84.00 B | 0.583 |
| 16 days | 78.00 dA | 109.20 bB | 94.20 B | 0.583 |
| 32 days | 88.00 cdA | 146.40 aA | 117.60 A | 0.583 |
| Mean | 62.16 b | 91.92 a | ||
| pH value | ||||
| 2 days | 8.49 aA | 8.09 bcA | 8.30 A | 0.153 |
| 4 days | 8.30 abB | 7.83 cdA | 8.10 A | 0.153 |
| 8 days | 7.90 cdB | 6.90 eB | 7.39 B | 0.153 |
| 16 days | 7.80 cdB | 6.70 eB | 7.28 B | 0.153 |
| 32 days | 7.58 dB | 4.77 fC | 6.22 C | 0.153 |
| Mean | 8.01 a | 6.90 b | ||
| Yeast, log10CFU/g FM | ||||
| 2 days | 7.88 cdB | 7.50 efA | 7.69 AB | 0.155 |
| 4 days | 7.64 deB | 7.37 efA | 7.50 C | 0.155 |
| 8 days | 8.00 bcA | 7.25 fA | 7.62 BC | 0.155 |
| 16 days | 8.28 abA | 7.39 fA | 7.83 A | 0.155 |
| 32 days | 8.30 aA | 7.24 fA | 7.77 AB | 0.155 |
| Mean | 8.02 a | 7.35 b | ||
| Mold, log10CFU/g FM | ||||
| 2 days | 6.69 efD | 6.47 efBC | 6.58 D | 0.151 |
| 4 days | 7.18 cdC | 6.79 deBC | 6.99 BC | 0.151 |
| 8 days | 7.46 cC | 6.25 fB | 6.85 CD | 0.151 |
| 16 days | 8.69 aA | 7.37 cA | 8.03 A | 0.151 |
| 32 days | 8.16 bB | 6.30 efB | 7.23 B | 0.151 |
| Mean | 7.64 a | 6.63 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jatkauskas, J.; Amaral, R.C.d.; Witt, K.L.; Joergensen, J.N.; Eisner, I.; Vrotniakiene, V. Early Fermentation Dynamics and Aerobic Stability of Maize Silage Improved by Dual-Strain Lactic Acid Bacteria Inoculation. Fermentation 2025, 11, 293. https://doi.org/10.3390/fermentation11050293
Jatkauskas J, Amaral RCd, Witt KL, Joergensen JN, Eisner I, Vrotniakiene V. Early Fermentation Dynamics and Aerobic Stability of Maize Silage Improved by Dual-Strain Lactic Acid Bacteria Inoculation. Fermentation. 2025; 11(5):293. https://doi.org/10.3390/fermentation11050293
Chicago/Turabian StyleJatkauskas, Jonas, Rafael Camargo do Amaral, Kristian Lybek Witt, Jens Noesgaard Joergensen, Ivan Eisner, and Vilma Vrotniakiene. 2025. "Early Fermentation Dynamics and Aerobic Stability of Maize Silage Improved by Dual-Strain Lactic Acid Bacteria Inoculation" Fermentation 11, no. 5: 293. https://doi.org/10.3390/fermentation11050293
APA StyleJatkauskas, J., Amaral, R. C. d., Witt, K. L., Joergensen, J. N., Eisner, I., & Vrotniakiene, V. (2025). Early Fermentation Dynamics and Aerobic Stability of Maize Silage Improved by Dual-Strain Lactic Acid Bacteria Inoculation. Fermentation, 11(5), 293. https://doi.org/10.3390/fermentation11050293







