Flavonoid Profiling of Aglianico and Cabernet Sauvignon Cultivars from Campania, Sicily, and Molise, Three Regions of Southern Italy
Abstract
1. Introduction
2. Materials and Methods
2.1. Vineyard Characteristics
2.2. Winemaking Trials
2.3. Grapes and Wines Analysis
2.3.1. Grape Extraction
2.3.2. Base Parameters of Grapes and Wines
2.3.3. Spectrophotometric Analysis
2.4. High-Performance Liquid Chromatography Analyses of Anthocyanins and Acetaldehyde
2.5. Statistical Analysis
3. Results and Discussion
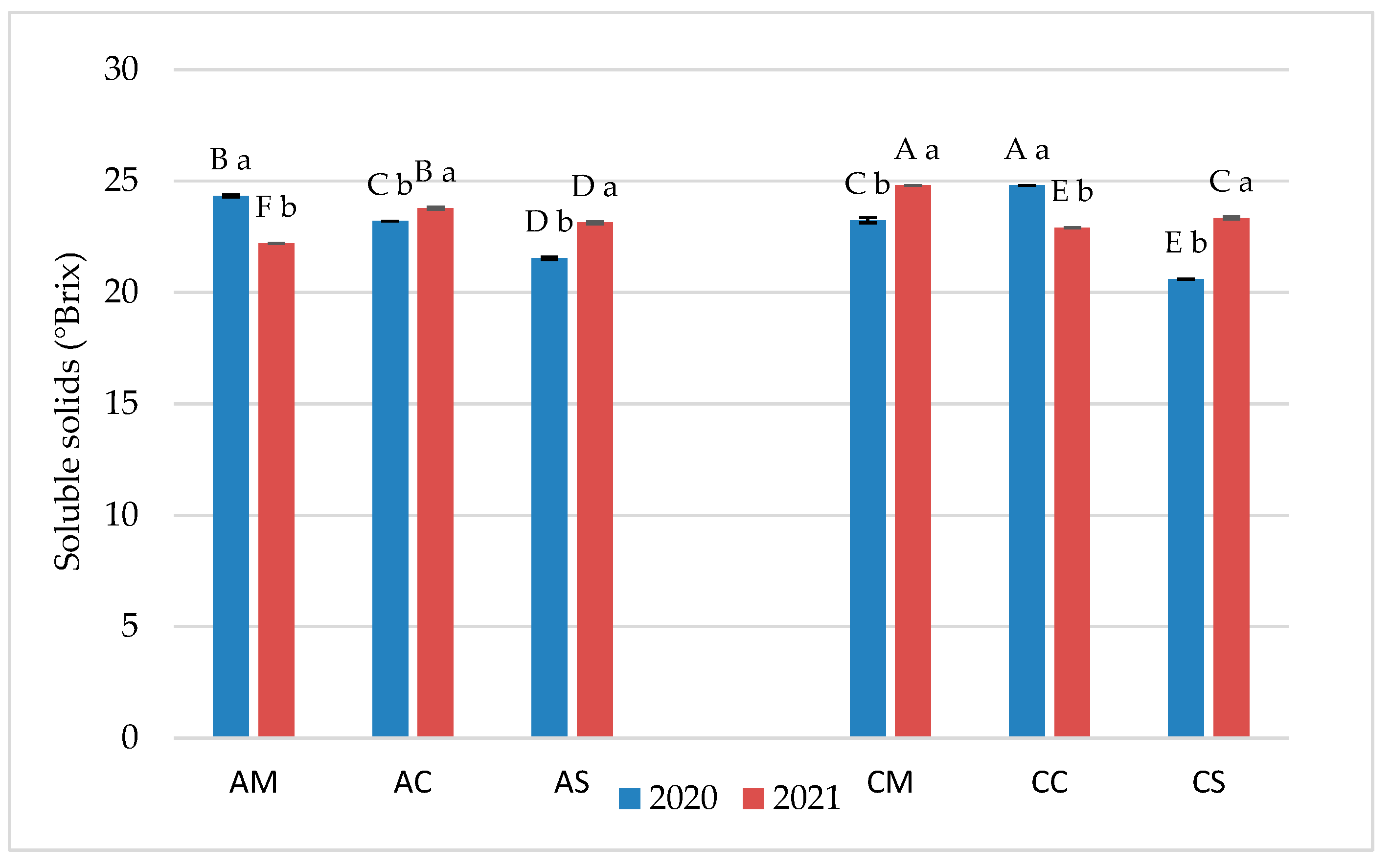
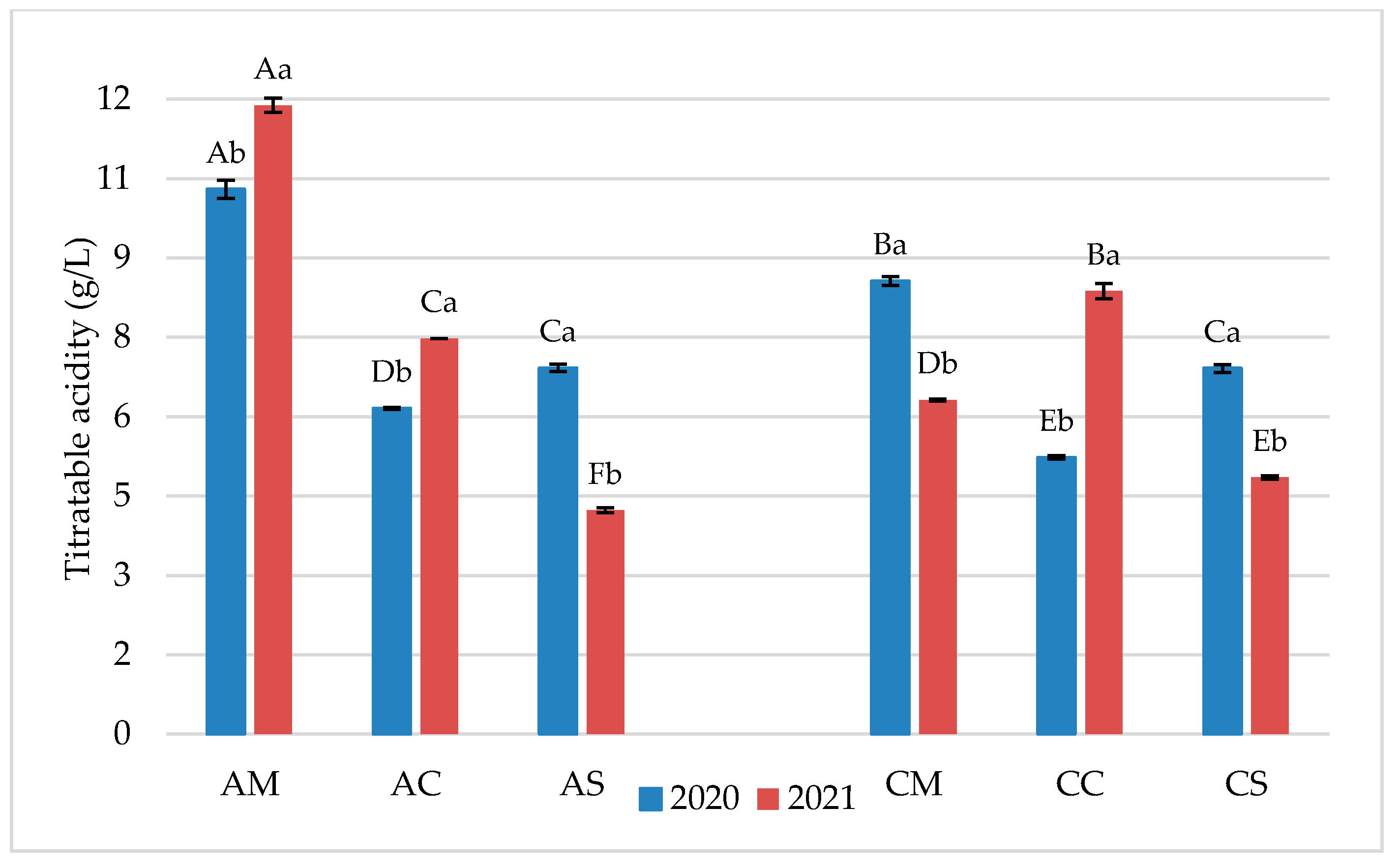
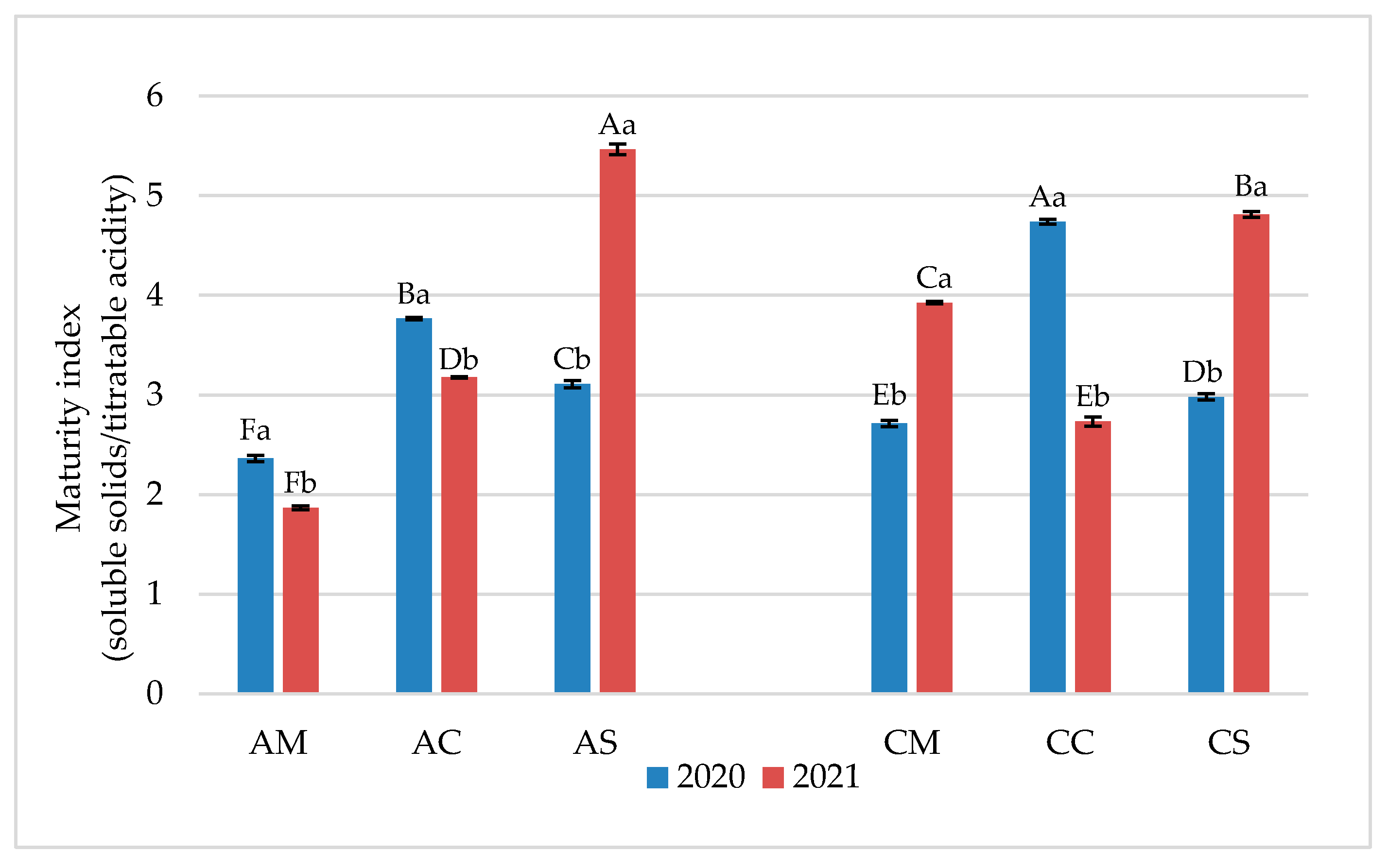
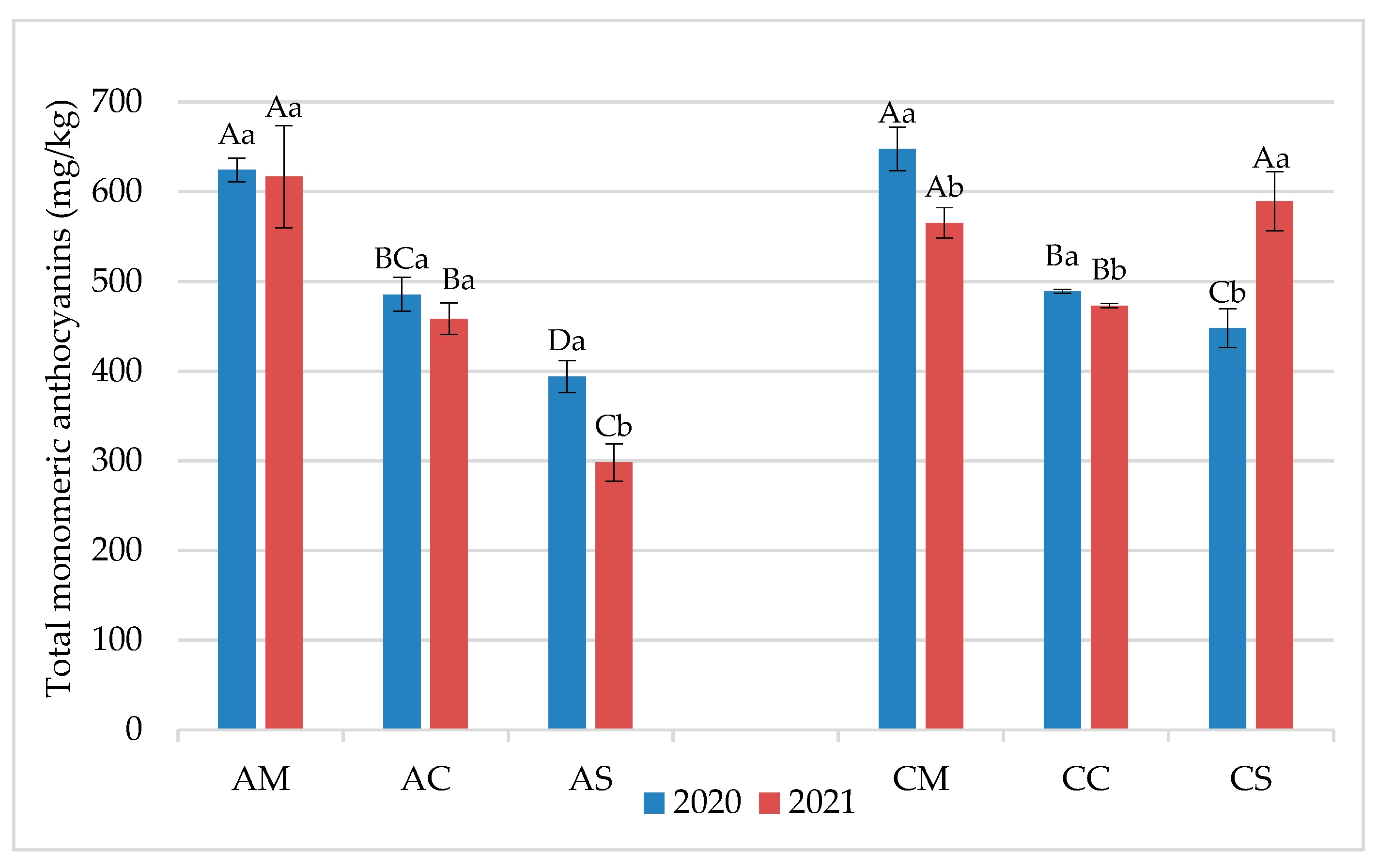
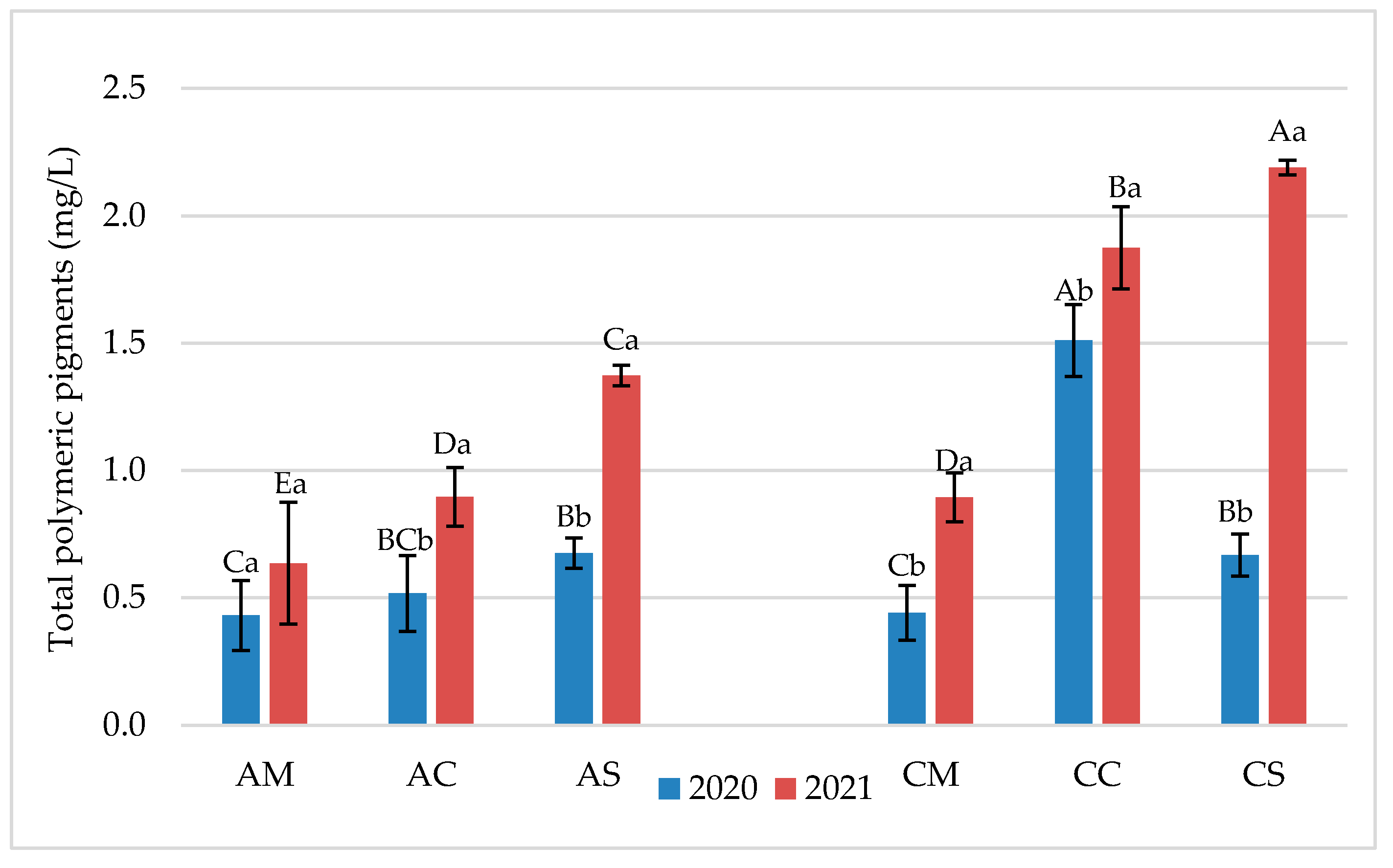
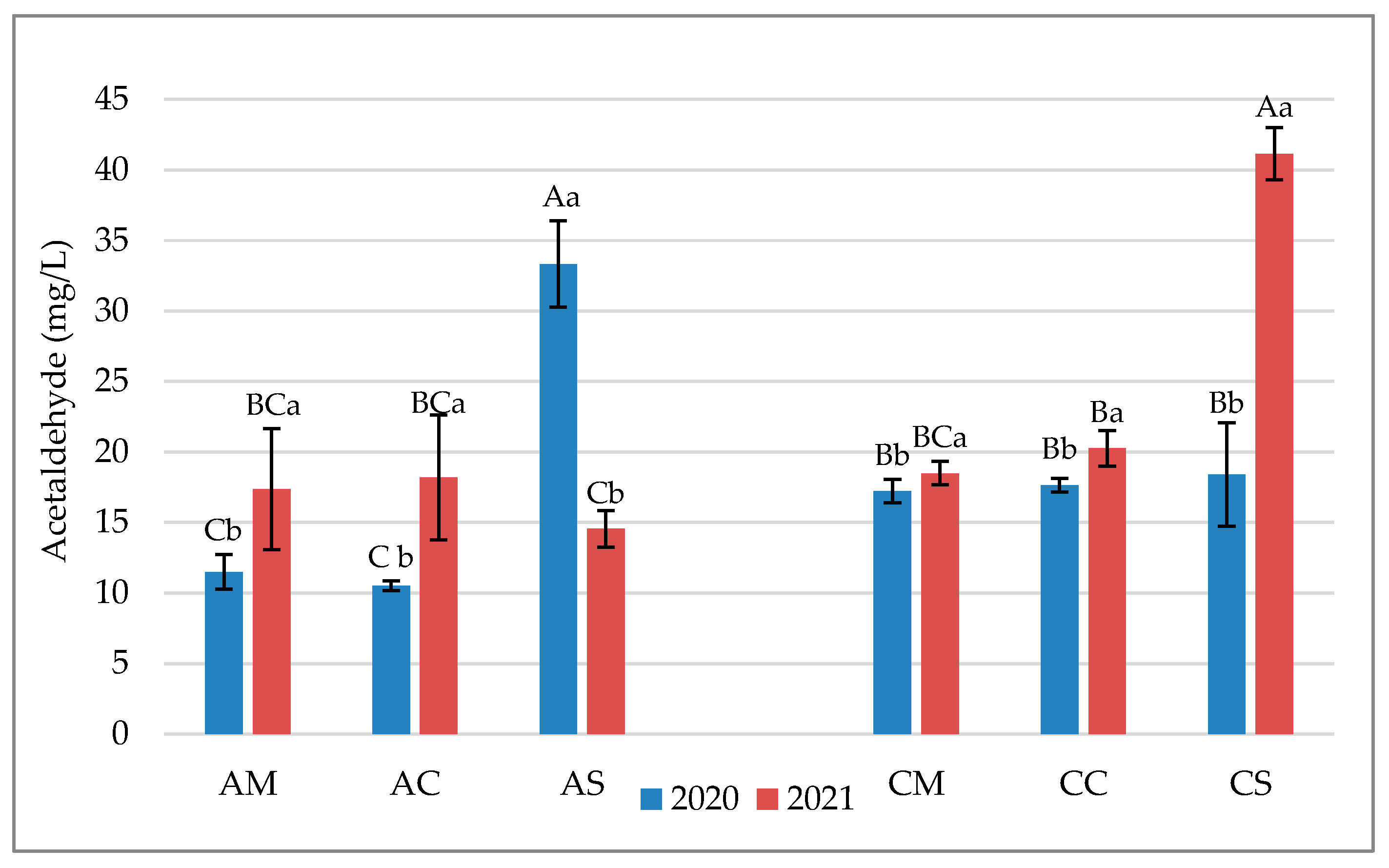
3.1. Chemical Parameters in Grapes and Wines
3.2. Flavonoid Profile of Grapes and Wines
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Iorizzo, M.; Sicilia, A.; Nicolosi, E.; Forino, M.; Picariello, L.; Lo Piero, A.R.; Vitale, A.; Monaco, E.; Ferlito, F.; Succi, M.; et al. Investigating the Impact of Pedoclimatic Conditions on the Oenological Performance of Two Red Cultivars Grown throughout Southern Italy. Front. Plant Sci. 2023, 14, 1250208. [Google Scholar] [CrossRef] [PubMed]
- Iorizzo, M.; Bagnoli, D.; Vergalito, F.; Testa, B.; Tremonte, P.; Succi, M.; Pannella, G.; Letizia, F.; Albanese, G.; Lombardi, S.J.; et al. Diversity of Fungal Communities on Cabernet and Aglianico Grapes from Vineyards Located in Southern Italy. Front. Microbiol. 2024, 1, 1399968. [Google Scholar] [CrossRef] [PubMed]
- Mansour, G.; Ghanem, C.; Mercenaro, L.; Nassif, N.; Hassoun, G.; Caro, A.D. Effects of Altitude on the Chemical Composition of Grapes and Wine: A Review. OENO One 2022, 56, 227–239. [Google Scholar] [CrossRef]
- Bonfante, A.; Brillante, L. Terroir Analysis and Its Complexity: This Article Is Published in Cooperation with Terclim 2022 (XIVth International Terroir Congress and 2nd ClimWine Symposium), 3–8 July 2022, Bordeaux, France. OENO One 2022, 56, 375–388. [Google Scholar] [CrossRef]
- Testa, B.; Coppola, F.; Iorizzo, M.; Di Renzo, M.; Coppola, R.; Succi, M. Preliminary Characterisation of Metschnikowia Pulcherrima to Be Used as a Starter Culture in Red Winemaking. Beverages 2024, 10, 88. [Google Scholar] [CrossRef]
- Testa, B.; Coppola, F.; Letizia, F.; Albanese, G.; Karaulli, J.; Ruci, M.; Pistillo, M.; Germinara, G.S.; Messia, M.C.; Succi, M.; et al. Versatility of Saccharomyces Cerevisiae 41CM in the Brewery Sector: Use as a Starter for “Ale” and “Lager” Craft Beer Production. Processes 2022, 10, 2495. [Google Scholar] [CrossRef]
- Karaulli, J.; Xhaferaj, N.; Coppola, F.; Testa, B.; Letizia, F.; Kyçyk, O.; Kongoli, R.; Ruci, M.; Lamçe, F.; Sulaj, K.; et al. Bioprospecting of Metschnikowia Pulcherrima Strains, Isolated from a Vineyard Ecosystem, as Novel Starter Cultures for Craft Beer Production. Fermentation 2024, 10, 513. [Google Scholar] [CrossRef]
- Definition of Vitivinicultural “Terroir”|OIV. Available online: https://www.oiv.int/node/3362 (accessed on 21 March 2025).
- Bonfante, A.; Monaco, E.; Langella, G.; Mercogliano, P.; Bucchignani, E.; Manna, P.; Terribile, F. A Dynamic Viticultural Zoning to Explore the Resilience of Terroir Concept under Climate Change. Sci. Total Environ. 2018, 624, 294–308. [Google Scholar] [CrossRef]
- van Leeuwen, C. 9–Terroir: The Effect of the Physical Environment on Vine Growth, Grape Ripening, and Wine Sensory Attributes. In Managing Wine Quality, 2nd ed.; Reynolds, A.G., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2022; pp. 341–393. ISBN 978-0-08-102067-8. [Google Scholar]
- Cameron, B. Phenology and Terroir Heard Through the Grapevine. In Phenology: An Integrative Environmental Science; Springer: Cham, Switzerland, 2025; pp. 573–593. ISBN 978-3-031-75026-7. [Google Scholar]
- Rouxinol, M.I.; Martins, M.R.; Barroso, J.M.; Rato, A.E. Wine Grapes Ripening: A Review on Climate Effect and Analytical Approach to Increase Wine Quality. Appl. Biosci. 2023, 2, 347–372. [Google Scholar] [CrossRef]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine Polyphenol Content and Its Influence on Wine Quality and Properties: A Review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef]
- Silva, A.; Silva, V.; Igrejas, G.; Aires, A.; Falco, V.; Valentão, P.; Poeta, P. Phenolic Compounds Classification and Their Distribution in Winemaking By-Products. Eur. Food Res. Technol. 2022, 249, 207–239. [Google Scholar] [CrossRef]
- Lu, H.-C.; Han, X.; Tian, M.-B.; Shi, N.; Li, M.-Y.; Duan, C.-Q.; He, F.; Wang, J. Integrated Metabolome and Transcriptome Analyses Reveal Insights into How Macro-Terroir Affects Polyphenols of Cabernet Sauvignon Grapes. Sci. Hortic. 2025, 341, 113996. [Google Scholar] [CrossRef]
- Cheynier, V.V.; Duenas-Paton, M.; Salas, E.; Maury, C.; Souquet, J.M.; Manchado-Sarni, P.; Fulcrand, H. Structure and Properties of Wine Pigments and Tannins. Am. J. Enol. Vitic. 2006, 57, 298–305. [Google Scholar] [CrossRef]
- El Rayess, Y.; Nehme, N.; Azzi-Achkouty, S.; Julien, S.G. Wine Phenolic Compounds: Chemistry, Functionality and Health Benefits. Antioxidants 2024, 13, 1312. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T.; Almikhlafi, M.A.; Alghamdi, S.Q.; Alruwaili, A.S.; Hossain, M.S.; et al. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef]
- Testa, B.; Coppola, F.; Succi, M.; Iorizzo, M. Biotechnological Strategies for Ethanol Reduction in Wine. Fermentation 2025, 11, 159. [Google Scholar] [CrossRef]
- Deng, Y.; Lu, S. Biosynthesis and Regulation of Phenylpropanoids in Plants. Crit. Rev. Plant Sci. 2017, 36, 257–290. [Google Scholar] [CrossRef]
- Rienth, M.; Vigneron, N.; Darriet, P.; Sweetman, C.; Burbidge, C.; Bonghi, C.; Walker, R.P.; Famiani, F.; Castellarin, S.D. Grape Berry Secondary Metabolites and Their Modulation by Abiotic Factors in a Climate Change Scenario–A Review. Front. Plant Sci. 2021, 12, 643258. [Google Scholar] [CrossRef]
- Eder, R.; Pajovic Scepanovic, R.; Raicevic, D.; Popović, T.; Korntheuer, K.; Wendelin, S.; Forneck, A.; Philipp, C. Study of the Effects of Climatic Conditions on the Phenolic Content and Antioxidant Activity of Austrian and Montenegrin Red Wines. OENO One 2023, 57, 68–85. [Google Scholar] [CrossRef]
- Cosme, F.; Vilela, A.; Moreira, L.; Moura, C.; Enríquez, J.A.P.; Filipe-Ribeiro, L.; Nunes, F.M. Terroir Effect on the Phenolic Composition and Chromatic Characteristics of Mencía/Jaen Monovarietal Wines: Bierzo D.O. (Spain) and Dão D.O. (Portugal). Molecules 2020, 25, 6008. [Google Scholar] [CrossRef]
- Costa, E.; Cosme, F.; Rivero-Perez, M.; Jordão, A.; González-SanJosé, M.L. Influence of Wine Region Provenance on Phenolic Composition, Antioxidant Capacity and Radical Scavenger Activity of Traditional Portuguese Red Grape Varieties. Eur. Food Res. Technol. 2015, 241, 61–73. [Google Scholar] [CrossRef]
- Xing, R.-R.; He, F.; Xiao, H.-L.; Duan, C.-Q.; Pan, Q.-H. Accumulation Pattern of Flavonoids in Cabernet Sauvignon Grapes Grown in a Low-Latitude and High-Altitude Region. S. Afr. J. Enol. Vitic. 2016, 36, 32–43. [Google Scholar] [CrossRef][Green Version]
- Chen, X.; Wang, Z.; Li, Y.; Liu, Q.; Yuan, C. Survey of the Phenolic Content and Antioxidant Properties of Wines from Five Regions of China According to Variety and Vintage. LWT 2022, 169, 114004. [Google Scholar] [CrossRef]
- Merkytė, V.; Longo, E.; Windisch, G.; Boselli, E. Phenolic Compounds as Markers of Wine Quality and Authenticity. Foods 2020, 9, 1785. [Google Scholar] [CrossRef]
- Urvieta, R.; Buscema, F.; Bottini, R.; Coste, B.; Fontana, A. Phenolic and Sensory Profiles Discriminate Geographical Indications for Malbec Wines from Different Regions of Mendoza, Argentina. Food Chem. 2018, 265, 120–127. [Google Scholar] [CrossRef]
- Kumšta, M.; Pavloušek, P.; Kárník, P. Use of Anthocyanin Profiles When Differentiating Individual Varietal Wines and Terroirs. Food Technol. Biotechnol. 2014, 52, 383–390. [Google Scholar] [CrossRef]
- Wolkovich, E.; García de Cortázar-Atauri, I.; Morales-Castilla, I.; Nicholas, K.; Lacombe, T. From Pinot to Xinomavro in the World’s Future Wine-Growing Regions. Nat. Clim. Change 2018, 8, 29–37. [Google Scholar] [CrossRef]
- Caccavello, G.; Giaccone, M.; Scognamiglio, P.; Mataffo, A.; Teobaldelli, M.; Basile, B. Vegetative, Yield, and Berry Quality Response of Aglianico to Shoot-Trimming Applied at Three Stages of Berry Ripening. Am. J. Enol. Vitic. 2019, 70, 351–359. [Google Scholar] [CrossRef]
- Ryan, J.-M.; Revilla, E. Anthocyanin Composition of Cabernet Sauvignon and Tempranillo Grapes at Different Stages of Ripening. J. Agric. Food Chem. 2003, 51, 3372–3378. [Google Scholar] [CrossRef]
- Ferlito, F.; Nicolosi, E.; Sicilia, A.; Villano, C.; Aversano, R.; Lo Piero, A.R. Physiological and Productive Responses of Two Vitis vinifera L. Cultivars across Three Sites in Central-South Italy. Horticulturae 2023, 9, 1321. [Google Scholar] [CrossRef]
- Nicolosi, E.; Sicilia, A.; Lo Piero, A.; Ferlito, F. Assessment of the Plasticity Level of Two Vitis vinifera L. Cultivars in Three Environments of Central and Southern Italy as an Indicator of the Impact of Climate Change. In Proceedings of the EHC2024: International Symposium on Viticulture and Winemaking between Tradition and Innovation 1418, Bucharest, Romania, 12 May 2024; pp. 331–340. [Google Scholar]
- Sicilia, A.; Villano, C.; Aversano, R.; Di Serio, E.; Nicolosi, E.; Ferlito, F.; Lo Piero, A.R. Study of Red Vine Phenotypic Plasticity across Central-Southern Italy Sites: An Integrated Analysis of the Transcriptome and Weather Indices through WGCNA. Front. Plant Sci. 2024, 15, 1498649. [Google Scholar] [CrossRef] [PubMed]
- Genovese, A.; Basile, B.; Lamorte, S.A.; Lisanti, M.T.; Corrado, G.; Lecce, L.; Strollo, D.; Moio, L.; Gambuti, A. Influence of Berry Ripening Stages over Phenolics and Volatile Compounds in Aged Aglianico Wine. Horticulturae 2021, 7, 184. [Google Scholar] [CrossRef]
- Muñoz, F.; Urvieta, R.; Buscema, F.; Rasse, M.; Fontana, A.; Berli, F. Phenolic Characterization of Cabernet Sauvignon Wines from Different Geographical Indications of Mendoza, Argentina: Effects of Plant Material and Environment. Front. Sustain. Food Syst. 2021, 5, 700642. [Google Scholar] [CrossRef]
- Nicolosi, E.; Sicilia, A.; Ferlito, F.; Bonfante, A.; Monaco, E.; Lo Piero, A.R. Phenotypic Plasticity in Bud Fruitfulness Expressed in Two Distinct Wine Grape Cultivars Grown under Three Different Pedoclimatic Conditions. Agriculture 2022, 12, 1660. [Google Scholar] [CrossRef]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants: BBCH Monograph; Open Agrar Repositorium: Quedlinburg, Germany, 2018. [Google Scholar] [CrossRef]
- Lorenz, D.H.; Eichhorn, K.W.; Bleiholder, H.; Klose, R.; Meier, U.; Weber, E. Growth Stages of the Grapevine: Phenological Growth Stages of the Grapevine (Vitis vinifera L. ssp. Vinifera)? Codes and Descriptions According to the Extended BBCH Scale? Aust. J. Grape Wine Res. 2008, 1, 100–103. [Google Scholar] [CrossRef]
- Compendium of International Methods of Wine and Must Analysis|OIV. Available online: https://www.oiv.int/standards/compendium-of-international-methods-of-wine-and-must-analysis (accessed on 24 March 2025).
- Gambuti, A.; Han, G.; Peterson, A.L.; Waterhouse, A.L. Sulfur Dioxide and Glutathione Alter the Outcome of Microoxygenation. Am. J. Enol. Vitic. 2015, 66, 411–423. [Google Scholar] [CrossRef][Green Version]
- Harbertson, J.; Picciotto, E.; Adams, D. Measurement of Polymeric Pigments in Grape Berry Extracts and Wines Using a Protein Precipitation Assay Combined with Bisulfite Bleaching. Am. J. Enol. Vitic. 2003, 54, 301–306. [Google Scholar] [CrossRef]
- Sadras, V.O.; Petrie, P.R. Climate Shifts in South-Eastern Australia: Early Maturity of Chardonnay, Shiraz and Cabernet Sauvignon Is Associated with Early Onset Rather than Faster Ripening. Aust. J. Grape Wine Res. 2011, 17, 199–205. [Google Scholar] [CrossRef]
- Greer, D.; Weedon, M. Temperature-Dependent Responses of the Berry Developmental Processes of Three Grapevine (Vitis vinifera) Cultivars. N. Z. J. Crop Hortic. Sci. 2014, 42, 233–246. [Google Scholar] [CrossRef]
- de Rességuier, L.; Inchboard, L.; Parker, A.K.; Petitjean, T.; van Leeuwen, C. Drivers of Grape Berry Sugar Accumulation in Field Conditions at Local Scale: This Article Is Published in Cooperation with the XVth International Terroir Congress, 18–22 November 2024, Mendoza, Argentina. Guest Editors: Federico Berli, Jorge Prieto and Martín Fanzone. OENO One 2024, 58. [Google Scholar] [CrossRef]
- Rebucci, B.; Poni, S.; Intrieri, C.; Magnanini, E.; Lakso, A. Effects of Manipulated Grape Berry Transpiration on Post-veraison Sugar Accumulation. Aust. J. Grape Wine Res. 1997, 3, 57–65. [Google Scholar] [CrossRef]
- Parker, A.K.; de Cortázar-Atauri, I.G.; Gény, L.; Spring, J.-L.; Destrac, A.; Schultz, H.; Molitor, D.; Lacombe, T.; Graça, A.; Monamy, C. Temperature-Based Grapevine Sugar Ripeness Modelling for a Wide Range of Vitis vinifera L. Cultivars. Agric. For. Meteorol. 2020, 285, 107902. [Google Scholar] [CrossRef]
- Gli Indicatori del Clima in Italia nel 2021–Anno XVII. Available online: https://www.isprambiente.gov.it/it/pubblicazioni/stato-dellambiente/gli-indicatori-del-clima-in-italia-nel-2021-2013-anno-xvii (accessed on 24 March 2025).
- Tarara, J.M.; Lee, J.; Spayd, S.E.; Scagel, C.F. Berry Temperature and Solar Radiation Alter Acylation, Proportion, and Concentration of Anthocyanin in Merlot Grapes. Am. J. Enol. Vitic. 2008, 59, 235–247. [Google Scholar] [CrossRef]
- Mira de Orduña, R. Climate Change Associated Effects on Grape and Wine Quality and Production. Food Res. Int. 2010, 43, 1844–1855. [Google Scholar] [CrossRef]
- Meléndez, E.; Ortiz, M.C.; Sarabia, L.A.; Íñiguez, M.; Puras, P. Modelling Phenolic and Technological Maturities of Grapes by Means of the Multivariate Relation between Organoleptic and Physicochemical Properties. Anal. Chim. Acta 2013, 761, 53–61. [Google Scholar] [CrossRef]
- Adelsheim, D.; Busch, C.; Catena, L.; Champy, B.; Coetzee, J.; Coia, L.; Croser, B.; Draper, P.; Durbourdieu, D.; Frank, F.; et al. Climate Change: Field Reports from Leading Winemakers. J. Wine Econ. 2016, 11, 5–47. [Google Scholar] [CrossRef]
- van Leeuwen, C.; Destrac-Irvine, A.; Dubernet, M.; Duchêne, E.; Gowdy, M.; Marguerit, E.; Pieri, P.; Parker, A.; de Rességuier, L.; Ollat, N. An Update on the Impact of Climate Change in Viticulture and Potential Adaptations. Agronomy 2019, 9, 514. [Google Scholar] [CrossRef]
- Plantevin, M.; Merpault, Y.; Lecourt, J.; Destrac-Irvine, A.; Dijsktra, L.; van Leeuwen, C. Characterization of Varietal Effects on the Acidity and pH of Grape Berries for Selection of Varieties Better Adapted to Climate Change. Front. Plant Sci. 2024, 15, 1439114. [Google Scholar] [CrossRef]
- Jordão, A.M.; Vilela, A.; Cosme, F. From Sugar of Grape to Alcohol of Wine: Sensorial Impact of Alcohol in Wine. Beverages 2015, 1, 292–310. [Google Scholar] [CrossRef]
- Amerine, M.; Roessler, E.; Ough, C. Acids and the Acid Taste. I. The Effect of pH and Titratable Acidity. Am. J. Enol. Vitic. 1965, 16, 29–37. [Google Scholar] [CrossRef]
- Lattey, K.A.; Bramley, B.; Francis, I. Consumer Acceptability, Sensory Properties and Expert Quality Judgements of Australian Cabernet Sauvignon and Shiraz Wines. Aust. J. Grape Wine Res. 2010, 16, 189–202. [Google Scholar] [CrossRef]
- Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Santos, J.A. An Overview of Climate Change Impacts on European Viticulture. Food Energy Secur. 2012, 1, 94–110. [Google Scholar] [CrossRef]
- Chidi, B.S.; Rossouw, D.; Buica, A.S.; Bauer, F.F. Determining the Impact of Industrial Wine Yeast Strains on Organic Acid Production Under White and Red Wine-like Fermentation Conditions. S. Afr. J. Enol. Vitic. 2015, 36, 316–327. [Google Scholar] [CrossRef]
- Wu, W.; Zhao, N. Metabolomics of Lactic Acid Bacteria. In Lactic Acid Bacteria: Omics and Functional Evaluation; Chen, W., Ed.; Springer Nature: Singapore, 2019; pp. 167–182. ISBN 978-981-13-7832-4. [Google Scholar]
- Capozzi, V.; Tufariello, M.; De Simone, N.; Fragasso, M.; Grieco, F. Biodiversity of Oenological Lactic Acid Bacteria: Species- and Strain-Dependent Plus/Minus Effects on Wine Quality and Safety. Fermentation 2021, 7, 24. [Google Scholar] [CrossRef]
- Maximum Acceptable Limits|OIV. Available online: https://www.oiv.int/it/standards/international-code-of-oenological-practices/annexes/maximum-acceptable-limits (accessed on 24 March 2025).
- Muccillo, L.; Gambuti, A.; Frusciante, L.; Iorizzo, M.; Moio, L.; Raieta, K.; Rinaldi, A.; Colantuoni, V.; Aversano, R. Biochemical Features of Native Red Wines and Genetic Diversity of the Corresponding Grape Varieties from Campania Region. Food Chem. 2014, 143, 506–513. [Google Scholar] [CrossRef]
- Peña-Neira, A. Chapter 18–Management of Astringency in Red Wines. In Red Wine Technology; Morata, A., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 257–272. ISBN 978-0-12-814399-5. [Google Scholar]
- Mori, K.; Goto-Yamamoto, N.; Kitayama, M.; Hashizume, K. Loss of Anthocyanins in Red-Wine Grape under High Temperature. J. Exp. Bot. 2007, 58, 1935–1945. [Google Scholar] [CrossRef]
- Gaiotti, F.; Pastore, C.; Filippetti, I.; Lovat, L.; Belfiore, N.; Tomasi, D. Low Night Temperature at Veraison Enhances the Accumulation of Anthocyanins in Corvina Grapes (Vitis vinifera L.). Sci. Rep. 2018, 8, 8719. [Google Scholar] [CrossRef] [PubMed]
- Bambina, P.; Gancel, A.-L.; Corona, O.; Jourdes, M.; Teissedre, P.-L. Soil Effect on Proanthocyanidins Composition of Red and White Wines Obtained from Nero d’Avola and Grillo Vitis vinifera L. Cultivars. Food Chem. 2024, 443, 138521. [Google Scholar] [CrossRef]
- Bambina, P.; Vitaggio, C.; Pollon, M.; Papa, G.L.; Conte, P.; Cinquanta, L.; Corona, O. Soil Effect on Phenolic and Volatile Composition of Nero d’Avola Red Wines as Revealed by a Chromatography-Based Targeted Metabolomic Approach. J. Food Compos. Anal. 2024, 131, 106278. [Google Scholar] [CrossRef]
- Braidot, E.; Zancani, M.; Petrussa, E.; Peresson, C.; Bertolini, A.; Patui, S.; Macrì, F.; Vianello, A. Transport and Accumulation of Flavonoids in Grapevine (Vitis vinifera L.). Plant Signal. Behav. 2008, 3, 626–632. [Google Scholar] [CrossRef]
- He, F.; Mu, L.; Yan, G.-L.; Liang, N.-N.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Biosynthesis of Anthocyanins and Their Regulation in Colored Grapes. Molecules 2010, 15, 9057–9091. [Google Scholar] [CrossRef] [PubMed]
- Soubeyrand, E.; Basteau, C.; Hilbert, G.; van Leeuwen, C.; Delrot, S.; Gomès, E. Nitrogen Supply Affects Anthocyanin Biosynthetic and Regulatory Genes in Grapevine Cv. Cabernet-Sauvignon Berries. Phytochemistry 2014, 103, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Li, B.; Chen, H.; Song, C.; Meng, J.; Xi, Z.; Zhang, Z. Iron Supply Affects Anthocyanin Content and Related Gene Expression in Berries of Vitis vinifera Cv. Cabernet Sauvignon. Molecules 2017, 22, 283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-K.; Lan, Y.-B.; Huang, Y.; Zhao, X.; Duan, C.-Q. Targeted Metabolomics of Anthocyanin Derivatives during Prolonged Wine Aging: Evolution, Color Contribution and Aging Prediction. Food Chem. 2021, 339, 127795. [Google Scholar] [CrossRef] [PubMed]
- Delić, K.; Milinčić, D.D.; Pešić, M.B.; Lević, S.; Nedović, V.A.; Gancel, A.-L.; Jourdes, M.; Teissedre, P.-L. Grape, Wine and Pomace Anthocyanins: Winemaking Biochemical Transformations, Application and Potential Benefits. OENO One 2024, 58. [Google Scholar] [CrossRef]
- Waterhouse, A.; Zhu, J. A Quarter Century of Wine Pigment Discovery. J. Sci. Food Agric. 2019, 100, 5093–5101. [Google Scholar] [CrossRef]
- Coppola, F.; Picariello, L.; Forino, M.; Moio, L.; Gambuti, A. Comparison of Three Accelerated Oxidation Tests Applied to Red Wines with Different Chemical Composition. Molecules 2021, 26, 815. [Google Scholar] [CrossRef]
- Marrufo-Curtido, A.; Ferreira, V.; Escudero, A. Factors That Affect the Accumulation of Strecker Aldehydes in Standardized Wines: The Importance of pH in Oxidation. Molecules 2022, 27, 3056. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.L.; Lizama, V.; Álvarez, I.; Nieuwoudt, H.; García, M.J.; Aleixandre, J.L.; du Toit, W.J. Effect of Acetaldehyde Addition on the Phenolic Substances and Volatile Compounds of Red Tempranillo Wines. Aust. J. Grape Wine Res. 2016, 22, 205–214. [Google Scholar] [CrossRef]
- Gambuti, A.; Picariello, L.; Moio, L.; Waterhouse, A. Cabernet Sauvignon Aging Stability Altered by Micro-Oxygenation. Am. J. Enol. Vitic. 2019, 70, 323–331. [Google Scholar] [CrossRef]




| Years | Samples | pH | Titratable Acidity (g/L) | Volatile Acidity (g/L) | Alcohol (% v/v) | L-Malic Acid (g/L) | L-Lactic Acid (g/L) | Citric Acid (mg/L) |
|---|---|---|---|---|---|---|---|---|
| 2020 | AM | 3.30 ± 0.01 d | 7.28 ± 0.14 a | 0.34 ± 0.04 bc | 13.8 ± 0.1 a | 0.21 ± 0.06 c | 1.05 ± 0.05 a | 0.25 ± 0.03 c |
| AC | 3.63 ± 0.02 c | 5.55 ± 0.16 b | 0.27 ± 0.02 c | 13.2 ± 0.1 b | 0.24 ± 0.06 bc | 0.54 ± 0.03 d | 0.37 ± 0.06 a | |
| AS | 3.80 ± 0.01 a | 5.23 ± 0.13 bc | 0.53 ± 0.05 a | 12.4 ± 0.2 c | 0.51 ± 0.07 a | 1.05 ± 0.03 a | 0.33 ± 0.01 abc | |
| CM | 3.70 ± 0.05 bc | 4.85 ± 0.17 c | 0.35 ± 0.05 bc | 13.5 ± 0.2 ab | 0.24 ± 0.06 bc | 0.94 ± 0.04 ab | 0.35 ± 0.01 ab | |
| CC | 3.80 ± 0.05 ab | 5.00 ± 0.20 c | 0.40 ± 0.05 abc | 14.0 ± 0.2 a | 0.33 ± 0.05 bc | 0.83 ± 0.05 bc | 0.26 ± 0.01 c | |
| CS | 3.70 ± 0.03 bc | 5.15 ± 0.13 bc | 0.41 ± 0.06 ab | 11.5 ± 0.2 d | 0.39 ± 0.01 ab | 0.74 ± 0.03 c | 0.27 ± 0.04 bc | |
| 2021 | AM | 3.28 ± 0.02 c | 9.30 ± 0.20 a | 0.24 ± 0.04 d | 12.7 ± 0.1 de | 2.46 ± 0.06 a | 0.43 ± 0.07 bc | 0.44 ± 0.01 c |
| AC | 3.32 ± 0.02 c | 6.38 ± 0.12 b | 0.39 ± 0.04 c | 13.5 ± 0.3 b | 1.32 ± 0.06 c | 0.29 ± 0.08 c | 0.53 ± 0.02 b | |
| AS | 3.78 ± 0.02 a | 4.42 ± 0.17 d | 0.49 ± 0.03 b | 13.4 ± 0.1 bc | 1.06 ± 0.04 d | 0.63 ± 0.09 ab | 0.37 ± 0.01 cd | |
| CM | 3.45 ± 0.05 b | 5.40 ± 0.10 c | 0.34 ± 0.04 c | 14.5 ± 0.1 a | 1.95 ± 0.05 b | 0.29 ± 0.09 c | 0.32 ± 0.02 d | |
| CC | 3.54 ± 0.04 b | 5.10 ± 0.10 c | 0.59 ± 0.01 a | 12.2 ± 0.2 e | 0.84 ± 0.04 e | 0.67 ± 0.03 a | 0.61 ± 0.03 a | |
| CS | 3.53 ± 0.05 b | 4.54 ± 0.24 d | 0.51 ± 0.02 ab | 12.9 ± 0.1 cd | 0.65 ± 0.02 f | 0.46 ± 0.06 abc | 0.41 ± 0.03 c |
| Years | Anthocyanins | AM | AC | AS | CM | CC | CS |
|---|---|---|---|---|---|---|---|
| 2020 | Delf-3mg | 12.62 ± 4.37 Aa | 3.30 ± 0.22 Bb | 1.57 ± 0.42 Ba | 8.87 ± 2.52 Ab | 2.91 ± 0.40 Bb | 4.50 ± 0.81 Ba |
| Cyan-3mg | 0.43 ± 0.09 ABb | 0.21 ± 0.04 Cb | 0.21 ± 0.17 Cb | 0.55 ± 0.15 Aa | 0.35 ± 0.03 ABCa | 0.24 ± 0.06 BCb | |
| Pet-3mg | 16.96 ± 4.59 Aa | 5.51 ± 0.21 Cb | 3.22 ± 0.45 Ca | 11.54 ± 2.35 Bb | 6.91 ± 0.87 Ca | 6.24 ± 1.40 Ca | |
| Peon-3mg | 3.82 ± 1.11 Bb | 8.50 ± 0.88 Aa | 2.54 ± 0.25 Ca | 0.91 ± 0.28 Db | 0.82 ± 0.09 Db | 2.56 ± 0.32 Ca | |
| Malv-3mg | 145.10 ± 26.50 Aa | 89.27 ± 6.05 Bb | 101.74 ± 9.34 Ba | 101.65 ± 16.27 Ba | 88.06 ± 9.26 Ba | 86.41 ± 19.16 Ba | |
| Malv-Ac | 14.18 ± 2.30 Ca | 5.78 ± 0.32 Da | 9.57 ± 1.18 CDa | 35.31 ± 7.22 ABa | 41.56 ± 4.27 Aa | 28.02 ± 5.65 Ba | |
| Malv-Cum | 15.28 ± 2.49 Aa | 8.86 ± 0.77 BCDb | 9.64 ± 1.25 BCa | 7.92 ± 1.33 CDa | 11.19 ± 1.40 Ba | 6.56 ± 1.57 Da | |
| 2021 | Delf-3mg | 13.01 ± 4.09 Ba | 4.647 ± 0.14 Ca | 1.28 ± 0.33 Da | 17.21 ± 1.41 Aa | 4.93 ± 0.42 Ca | 3.89 ± 0.09 CDa |
| Cyan-3mg | 0.86 ± 0.27 Aa | 0.523 ± 0.09 BCa | 0.46 ± 0.04 BCa | 0.58 ± 0.07 Ba | 0.38 ± 0.18 BCa | 0.32 ± 0.04 Ca | |
| Pet-3mg | 14.73 ± 1.08 Aa | 8.538 ± 0.68 Ba | 2.94 ± 0.12 Da | 14.43 ± 1.19 Aa | 5.74 ± 0.31 Cb | 3.85 ± 0.11 Db | |
| Peon-3mg | 5.97 ± 0.24 Aa | 5.174 ± 0.17 Bb | 2.20 ± 0.07 Cb | 5.60 ± 0.51 ABa | 2.35 ± 0.10 Ca | 2.35 ± 0.07 Ca | |
| Malv-3mg | 125.64 ± 8.13 Aa | 104.879 ± 10.19 Ba | 84.32 ± 3.06 Cb | 104.94 ± 8.36 Ba | 56.67 ± 3.05 Db | 44.09 ± 1.13 Eb | |
| Malv-Ac | 16.79 ± 17.96 BCa | 4.485 ± 0.68 Cb | 8.68 ± 0.49 Ca | 35.99 ± 2.64 Aa | 22.93 ± 0.93 Bb | 15.01 ± 0.37 BCb | |
| Malv-Cum | 11.36 ± 2.59 Ab | 12.080 ± 1.98 Aa | 4.21 ± 0.34 Cb | 7.29 ± 0.51 Ba | 3.40 ± 0.28 Cb | 2.03 ± 0.12 Cb |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coppola, F.; Gambuti, A.; Testa, B.; Succi, M.; Luciano, A.; Picariello, L.; Iorizzo, M. Flavonoid Profiling of Aglianico and Cabernet Sauvignon Cultivars from Campania, Sicily, and Molise, Three Regions of Southern Italy. Fermentation 2025, 11, 283. https://doi.org/10.3390/fermentation11050283
Coppola F, Gambuti A, Testa B, Succi M, Luciano A, Picariello L, Iorizzo M. Flavonoid Profiling of Aglianico and Cabernet Sauvignon Cultivars from Campania, Sicily, and Molise, Three Regions of Southern Italy. Fermentation. 2025; 11(5):283. https://doi.org/10.3390/fermentation11050283
Chicago/Turabian StyleCoppola, Francesca, Angelita Gambuti, Bruno Testa, Mariantonietta Succi, Alessandra Luciano, Luigi Picariello, and Massimo Iorizzo. 2025. "Flavonoid Profiling of Aglianico and Cabernet Sauvignon Cultivars from Campania, Sicily, and Molise, Three Regions of Southern Italy" Fermentation 11, no. 5: 283. https://doi.org/10.3390/fermentation11050283
APA StyleCoppola, F., Gambuti, A., Testa, B., Succi, M., Luciano, A., Picariello, L., & Iorizzo, M. (2025). Flavonoid Profiling of Aglianico and Cabernet Sauvignon Cultivars from Campania, Sicily, and Molise, Three Regions of Southern Italy. Fermentation, 11(5), 283. https://doi.org/10.3390/fermentation11050283








