Enhancing Mead Aroma Using Non-Saccharomyces Yeast β-Glucosidase Producers Isolated from Honey: A Case Study in the Upper Turi Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Enumeration of Yeasts in Honey Samples
2.2. Selection of ß-Glucosidase-Producing Yeasts
2.3. Mead Production with ß-Glucosidase-Producing Yeast
2.4. Physicochemical Analyses of the Mead
2.5. Determination of Volatile Compounds in Mead
2.6. Statistical Analysis
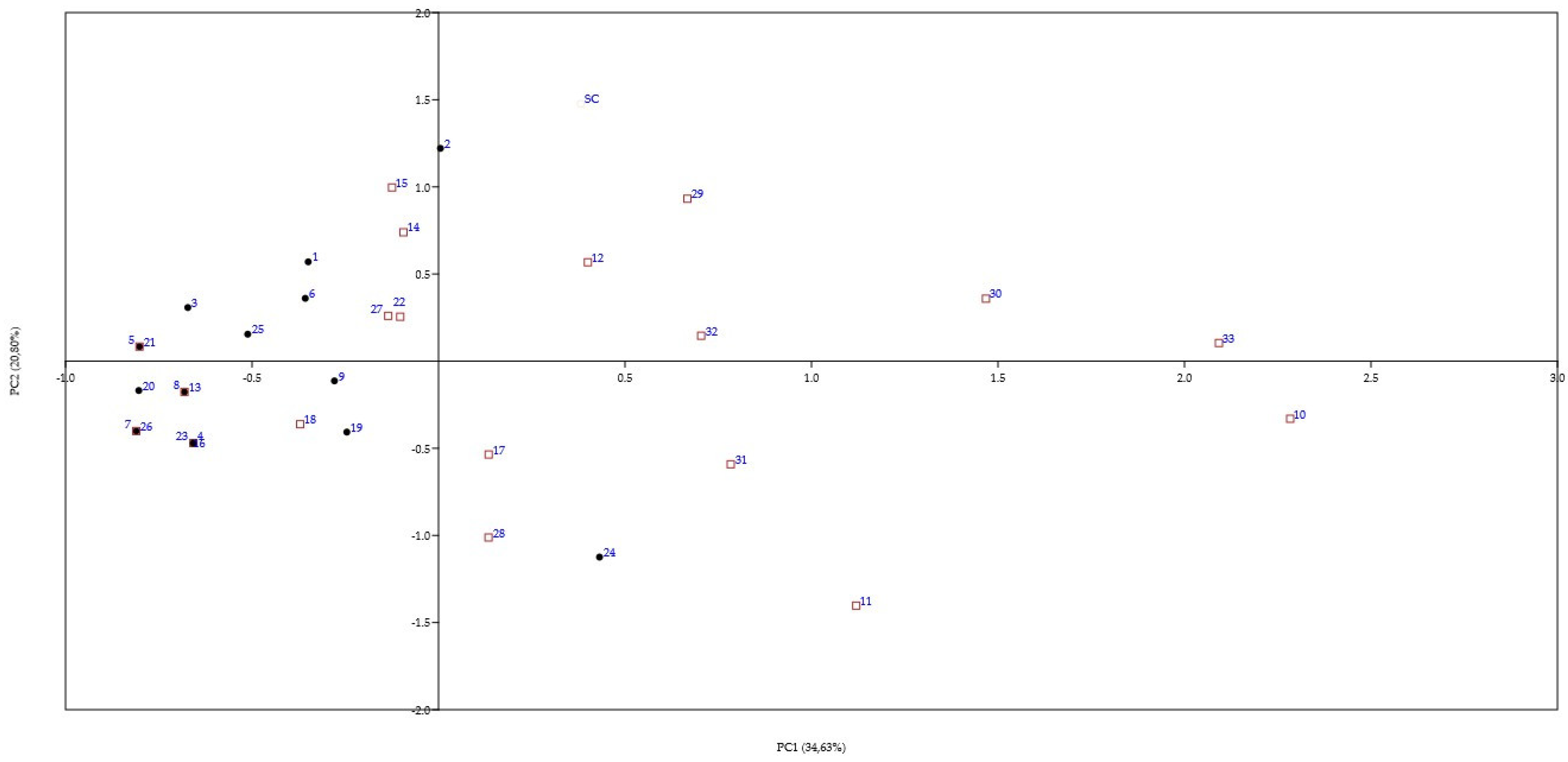
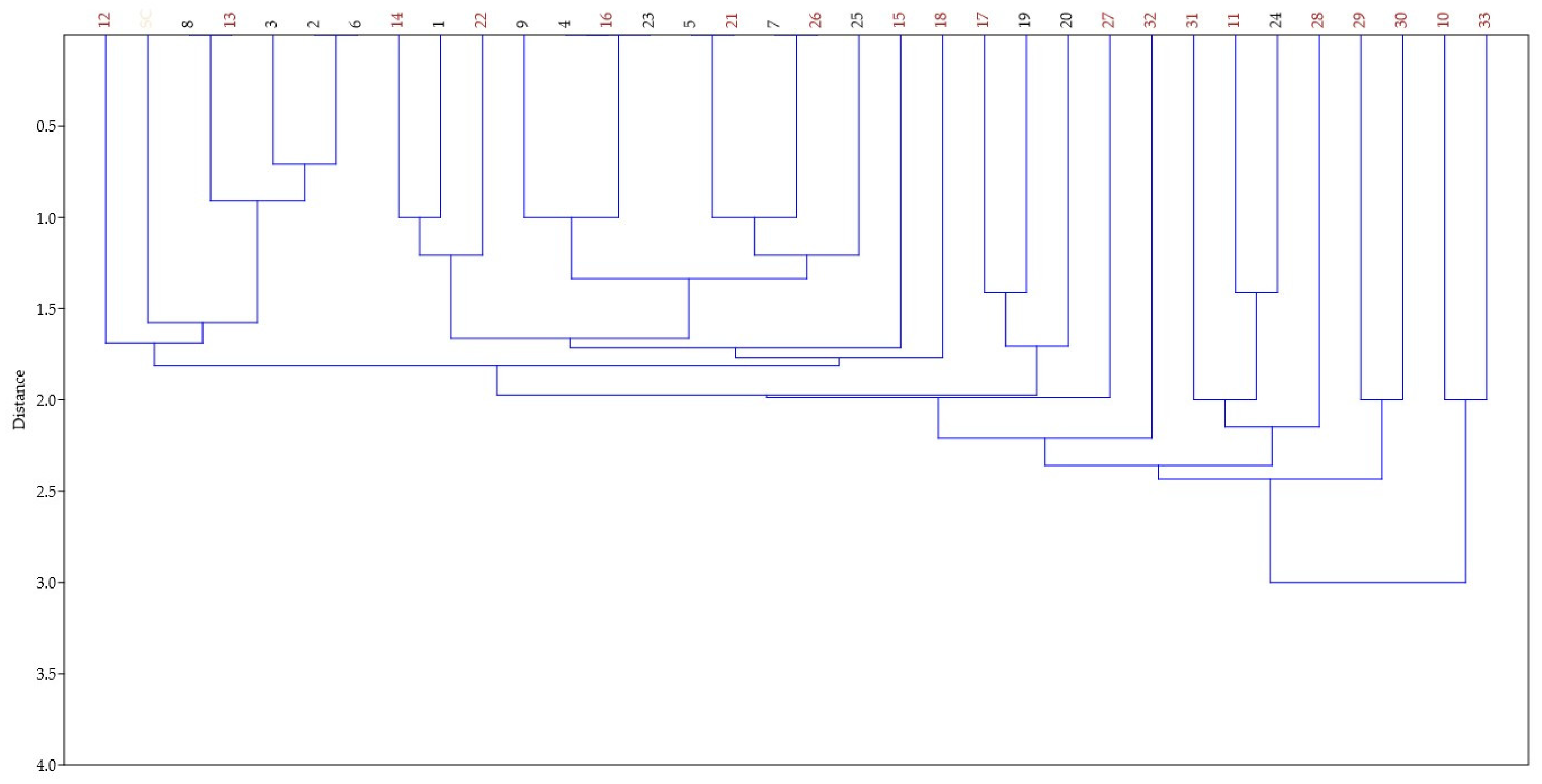
3. Results
3.1. Quantification of Yeasts in Honey Samples
3.2. Selection of ß-Glucosidase-Producing Yeasts
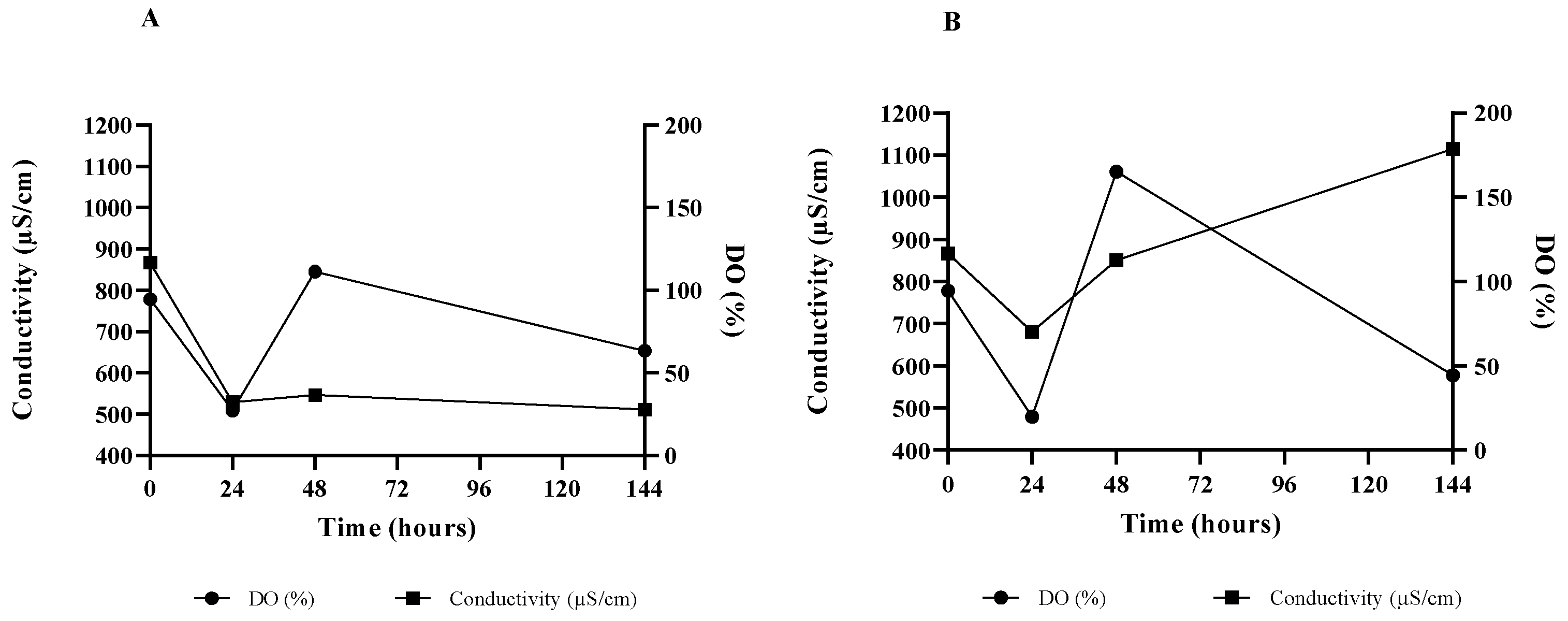
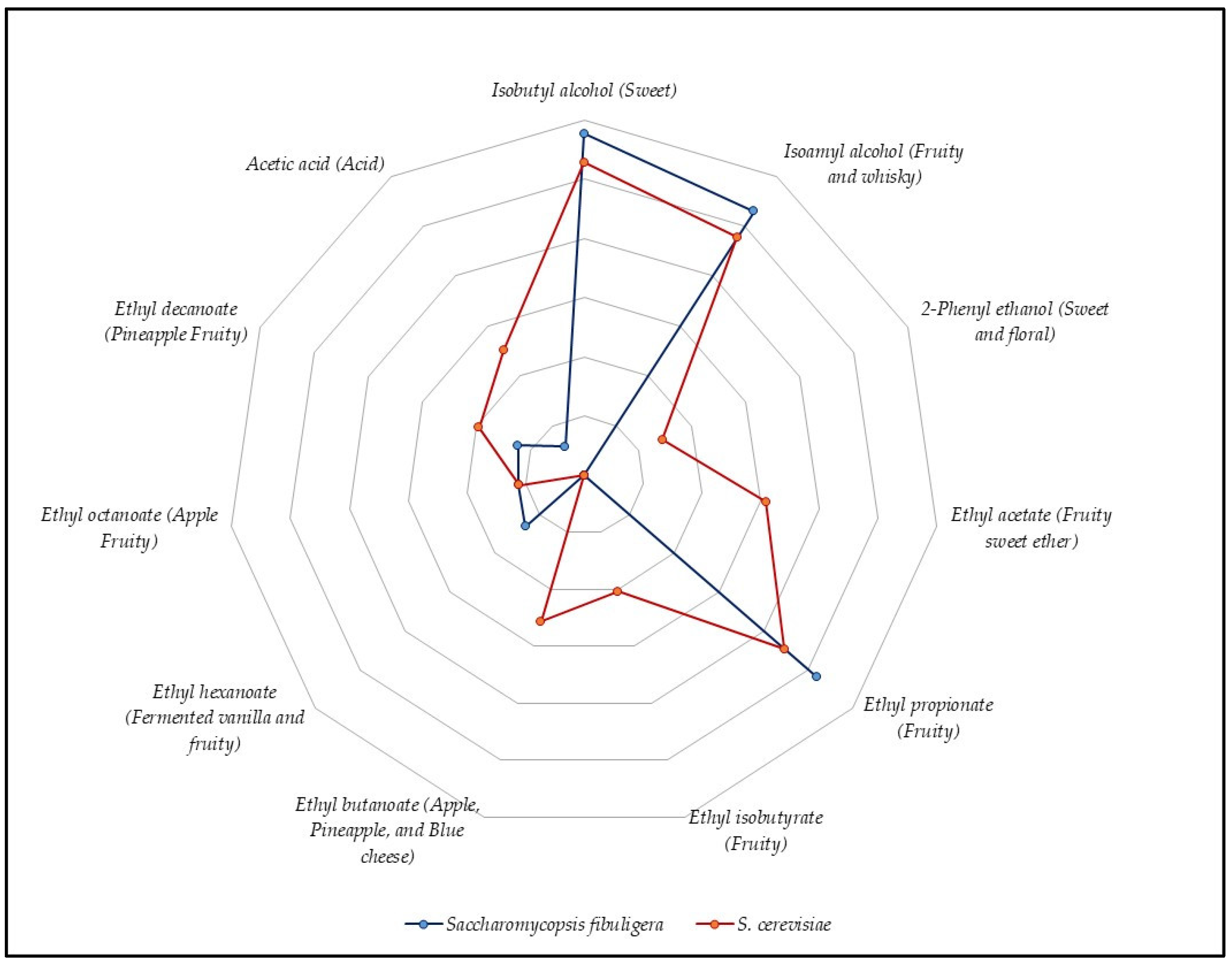
3.3. Mead Production with ß-Glucosidase-Producing Yeast
4. Discussion
4.1. Quantification of Molds and Yeasts in Honey Samples
4.2. Selection of ß-Glucosidase-Producing Yeasts
4.3. Mead Production with ß-Glucosidase-Producing Yeast
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| GC-MS | Gas Chromatography-Mass Spectrometry |
| YGP | Yeast Glucose Peptone |
| IAL | Instituto Adolfo Lutz |
| EtOH | Ethanol |
| SPME | Solid Phase Microextraction |
| CFU | Colony Forming Units |
| DO | Dissolved Oxygen |
References
- Marcolin, L.C.; Lima, L.R.; de Oliveira Arias, J.L.; Berrio, A.C.B.; Kupski, L.; Barbosa, S.C.; Primel, E.G. Meliponinae and Apis mellifera Honey in Southern Brazil: Physicochemical Characterization and Determination of Pesticides. Food Chem. 2021, 363, 130175. [Google Scholar] [CrossRef]
- Seraglio, S.K.T.; Schulz, M.; Brugnerotto, P.; Silva, B.; Gonzaga, L.V.; Fett, R.; Costa, A.C.O. Quality, Composition and Health-Protective Properties of Citrus Honey: A Review. Food Res. Int. 2021, 143, 110268. [Google Scholar] [CrossRef]
- Hosny, I.; Ghani, S.; Aziz, N. Nutrient Composition and Microbiological Quality of Three Unifloral Honeys with Emphasis on Processing of Honey Probiotic Youghurt. Glob. Vet. 2009, 3, 107–112. [Google Scholar]
- Snowdon, J.A.; Cliver, D.O. Microorganisms in Honey. Int. J. Food Microbiol. 1996, 31, 1–26. [Google Scholar] [CrossRef]
- Lorenzini, M.; Simonato, B.; Slaghenaufi, D.; Ugliano, M.; Zapparoli, G. Assessment of Yeasts for Apple Juice Fermentation and Production of Cider Volatile Compounds. LWT 2019, 99, 224–230. [Google Scholar] [CrossRef]
- Pérez, G.; Fariña, L.; Barquet, M.; Boido, E.; Gaggero, C.; Dellacassa, E.; Carrau, F. A Quick Screening Method to Identify β-Glucosidase Activity in Native Wine Yeast Strains: Application of Esculin Glycerol Agar (EGA) Medium. World J. Microbiol. Biotechnol. 2011, 27, 47–55. [Google Scholar] [CrossRef]
- Gaensly, F.; Carla, B.; Almeida, G.; Picheth, G.; Maria, T.; Bonfim, B. Autochthonous Yeasts with β -Glucosidase Activity Increase Resveratrol Concentration during the Alcoholic Fermentation of Vitis labrusca Grape Must. J. Funct. Foods 2015, 19, 288–295. [Google Scholar] [CrossRef]
- Fia, G.; Giovani, G.; Rosi, I. Study of β-Glucosidase Production by Wine-Related Yeasts during Alcoholic Fermentation. A New Rapid Fluorimetric Method to Determine Enzymatic Activity. J. Appl. Microbiol. 2005, 99, 509–517. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, C.; Li, J.; Xu, Y. Different Influences of β-Glucosidases on Volatile Compounds and Anthocyanins of Cabernet Gernischt and Possible Reason. Food Chem. 2013, 140, 245–254. [Google Scholar] [CrossRef]
- Pontoh, J.; Low, N.H. Purification and Characterization of β-Glucosidase from Honey Bees (Apis mellifera). Insect Biochem. Mol. Biol. 2002, 32, 679–690. [Google Scholar] [CrossRef]
- Bi, P.; Sun, W.; Li, S.; Liu, X.; Tian, Y.; Long, F.; Zhang, Z.; Guo, J. Characterization of the Effect of Non-Saccharomyces Cerevisiaes on the Non-Volatile Constituents and Volatile Profiles of Low-Alcoholic Pomegranate Beverages. Food Biosci. 2024, 59, 103870. [Google Scholar] [CrossRef]
- Prestianni, R.; Matraxia, M.; Naselli, V.; Pirrone, A.; Badalamenti, N.; Ingrassia, M.; Gaglio, R.; Settanni, L.; Columba, P.; Maggio, A.; et al. Use of Sequentially Inoculation of Saccharomyces Cerevisiae and Hanseniaspora Uvarum Strains Isolated from Honey By-Products to Improve and Stabilize the Quality of Mead Produced in Sicily. Food Microbiol. 2022, 107, 104064. [Google Scholar] [CrossRef]
- APHA. Compendium of Methods for the Microbiological Examination of Foods, 4th ed.; American Public Health Association, Ed.; American Public Health Association: Washington, DC, USA, 2001; Volume 4, ISBN 087553175X. [Google Scholar]
- Kurtzman, C.P.; Fell, J.W.; Boekhout, T.; Robert, V. Methods for Isolation, Phenotypic Characterization and Maintenance of Yeasts. In The Yeasts, a Taxonomic Study; Elsevier: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Serra, J.L.; Mouchrek, A.N.; de Oliveira, A.C.; Correia, M.G.d.S.; Burgos, W.J.M.; Vandenberghe, L.P.d.S.; Neto, D.P.d.C.; Soccol, C.R.; Baeten, V.; Darnet, S.; et al. β-Glycosidase Activity Associated with the Formation of Aroma Compounds in Native Non-Saccharomyces Yeasts Isolated from Cocoa Bean Fermentation. BASE 2024, 28, 37–53. [Google Scholar] [CrossRef]
- Duarte, W.F.; Dias, D.R.; Oliveira, J.M.; Teixeira, J.A.; de Almeida e Silva, J.B.; Schwan, R.F. Characterization of Different Fruit Wines Made from Cacao, Cupuassu, Gabiroba, Jaboticaba and Umbu. LWT Food Sci. Technol. 2010, 43, 1564–1572. [Google Scholar] [CrossRef]
- Sadoudi, M.; Tourdot-maréchal, R.; Rousseaux, S.; Steyer, D.; Gallardo-chacón, J.; Ballester, J.; Vichi, S.; Guérin-schneider, R.; Caixach, J. Yeast e Yeast Interactions Revealed by Aromatic profile Analysis of Sauvignon Blanc Wine Fermented by Single or Co-Culture of Non-Saccharomyces and Saccharomyces Yeasts. YFMIC 2012, 32, 243–253. [Google Scholar] [CrossRef]
- Instituto Adolfo Lutz. Métodos Físico-Químicos para Análise de Alimentos, 5th ed.; Zenebon, O., Pascuet, N.S., Tiglea, P., Eds.; Paulista State University (Unesp): São Paulo, Brazil, 2008. [Google Scholar]
- Torres Neto, A.B.; Epifânio Da Silva, M.; Silva, B.; Swarnakar, R.; Luiz, F.; Da Silva, H. Cinética e Caracterização Físico-Química do Fermentado do Pseudofruto do Caju (Anacardium occidentale L.). Quim. Nova 2006, 29, 489–492. [Google Scholar] [CrossRef]
- Bayoï, J.R.; Etoa, F.X. Physicochemical Changes Occurring during Long-Time Fermentation of the Indigenous Alcoholic Sorghum-Based Beverages Brewed in Northern Cameroon. Beverages 2021, 7, 39. [Google Scholar] [CrossRef]
- Colombié, S.; Latrille, E.; Sablayrolles, J.M. Interest of On-Line Monitoring Electrical Conductivity during Wine Fermentation. Eur. Food Res. Technol. 2008, 226, 1553–1557. [Google Scholar] [CrossRef]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar] [CrossRef]
- Estado do Rio Grande do Norte. Decreto N° 30860, de 25 Agosto de 2021. Regulamento Que Dispõe Sobre a Criação, o Comércio, o Transporte de Abelhas sem Ferrão (Meliponídeas) no Estado do Rio Grande do Norte, Estabelece os Requisitos Sanitários de Produção/Processamento e o Padrão de Identidade e Qualidade do Mel. Diário Oficial do Estado. Rio Grande do Norte, RN, Brazil. Available online: https://www.normasbrasil.com.br/norma/decreto-30860-2021-rn_419347.html (accessed on 6 April 2025).
- Estado do Paraná. Portaria N° 63, de 10 Março de 2017. Regulamento Técnico de Identidade e Qualidade de Méis de Abelhas sem Ferrão Para o Estado do Paraná. Diário Oficial do Estado. Paraná, PR, Brazil. Available online: https://www.agricultura.pr.gov.br/sites/default/arquivos_restritos/files/documento/2021-06/portaria_adapar_63-2017_regulamento_tecnico_mel_asf_pr.pdf (accessed on 6 April 2025).
- Estado de São Paulo. Resolução SAA N° 52 de 03 de Outubro de 2017. Regulamento Técnico de Identidade e Padrão do mel Elaborado Pelas Abelhas da Subfamília Meliponinae (Hymenoptera, Apidae). Diário Oficial do Estado. São Paulo, SP, Brazil. Available online: https://www.defesa.agricultura.sp.gov.br/legislacoes/resolucao-saa-52-de-03-10-2017,1114.html (accessed on 6 April 2025).
- Estado de Santa Catarina. Portaria SAR N° 37 de 04 de Novembro de 2020. Norma Interna Regulamentadora do Mel de Abelhas Sem Ferrão No Estado de Santa Catarina. Diário Oficial do Estado. Santa Catarina, SC, Brazil. Available online: https://www.cidasc.sc.gov.br/inspecao/files/2020/11/Portaria-SAR-n%C2%BA-37-Mel-de-Abelha-sem-Ferr%C3%A3o.pdf (accessed on 6 April 2025).
- Han, X.; Qin, Q.; Li, C.; Zhao, X.; Song, F.; An, M.; Chen, Y.; Wang, X.; Huang, W.; Zhan, J.; et al. Application of Non-Saccharomyces Yeasts with High β-Glucosidase Activity to Enhance Terpene-Related Floral Flavor in Craft Beer. Food Chem. 2023, 404, 134726. [Google Scholar] [CrossRef]
- Mesaik, M.A.; Dastagir, N.; Uddin, N.; Rehman, K.; Azim, M.K. Characterization of Immunomodulatory Activities of Honey Glycoproteins and Glycopeptides. J. Agric. Food Chem. 2015, 63, 177–184. [Google Scholar] [CrossRef]
- Giannattasio, S.; Guaragnella, N.; Ždralević, M.; Marra, E. Molecular Mechanisms of Saccharomyces Cerevisiae Stress Adaptation and Programmed Cell Death in Response to Acetic Acid. Front Microbiol 2013, 4, 33. [Google Scholar] [CrossRef]
- Lima, J.; Arouche, M.; Pereira, L.; Alves, L.; Costa, F.; Silva, G.A. Condições Higiênico-Sanitárias do Mel Produzido por Apis Melífera no Estado do Maranhão. Rev. Eletrônica Fac. Ceres 2015, 4, 1–9. [Google Scholar] [CrossRef]
- Ribeiro, R.; Starikoff, K.R. Avaliação da Qualidade Físico-Química e Microbiológica de Mel Comercializado. Rev. Ciências Agrovet. 2019, 18, 111–118. [Google Scholar] [CrossRef][Green Version]
- Good, A.P.; Gauthier, M.-P.L.; Vannette, R.L.; Fukami, T. Honey Bees Avoid Nectar Colonized by Three Bacterial Species, but Not by a Yeast Species, Isolated from the Bee Gut. PLoS ONE 2014, 9, e86494. [Google Scholar] [CrossRef]
- Ribeiro, M.H.M.; da Luz, C.F.P.; de Albuquerque, P.M.C. Palynology as a Tool for Distinguishing Geopropolis Samples from Stingless Bee Species in the Maranhense Amazon, Brazil. J. Apic. Res. 2019, 58, 16–36. [Google Scholar] [CrossRef]
- Holanda, C.A.; Marques Brandão, C.; Lima Souza, J.; De Souza Ribeiro, M.N.; Coêlho Alves, L.M.; Pires Costa, M.C. Quality and Estimative of Time-Consuming of Tiúba Honey (Melipona Fasciculata Smith) Produced in Cerrado Region from Maranhão State, Brasil. Rev. Bras. Pesqui. Alimentos 2015, 6, 53. [Google Scholar] [CrossRef]
- Evangelista-Rodrigues, A.; Silva, E.; da Mônica, S.; Beserra, E.M.F.; Rodrigues, M.L. Análise Físico-Química dos Méis das Abelhas Apis mellifera e Melipona Scutellaris Produzidos em Regiões Distintas no Estado da Paraíba. Ciência Rural. 2005, 35, 1166–1171. [Google Scholar] [CrossRef]
- Guimarães, T.M.; Moriel, D.G.; Machado, I.P.; Picheth, C.M.T.F.; Bonfim, T.M.B. Isolation and Characterization of Saccharomyces Cerevisiae Strains of Winery Interest. Rev. Bras. Ciências Farm. 2006, 42, 119–126. [Google Scholar] [CrossRef]
- Sharafi, S.; Rasooli, I.; Beheshti-Maal, K. Isolation, Characterization and Optimization of Indigenous Acetic Acid Bacteria and Evaluation of Their Preservation Methods. Iran. J. Microbiol. 2010, 2, 38–45. [Google Scholar]
- da Silva, R.O.; Batistote, M.; Cereda, M.P. Alcoholic Fermentation by the Wild Yeasts under Thermal, Osmotic and Ethanol Stress. Braz. Arch. Biol. Technol. 2013, 56, 161–169. [Google Scholar] [CrossRef]
- Chi, Z.; Chi, Z.; Liu, G.; Wang, F.; Ju, L.; Zhang, T. Saccharomycopsis Fibuligera and Its Applications in Biotechnology. Biotechnol. Adv. 2009, 27, 423–431. [Google Scholar] [CrossRef]
- Tang, H.; Hou, J.; Shen, Y.; Xu, L.; Yang, H.; Fang, X.; Bao, X. High β-Glucosidase Secretion in Saccharomyces Cerevisiae Improves the Efficiency of Cellulase Hydrolysis and Ethanol Production in Simultaneous Saccharification and Fermentation. J. Microbiol. Biotechnol. 2013, 23, 1577–1585. [Google Scholar] [CrossRef]
- Carvalho, C.M.; Meirinho, S. Choupina Yeast Species Associated with Honey: Different Identification Methods. Arch. Zootec. 2010, 59, 8–13. [Google Scholar] [CrossRef]
- da Fonseca Meireles, S.; dos Santos, S.F.; Rafael, M.S.; da Mota, A.J.; da Silva, C.G.N. Yeasts from the Nests of Two Amazonian Stingless Bees: Screening and PCR-RFLP Molecular Analysis. Symbiosis 2022, 87, 153–163. [Google Scholar] [CrossRef]
- da Silva, R.N.A.; Magalhães-Guedes, K.T.; de Oliveira Alves, R.M.; Souza, A.C.; Schwan, R.F.; Umsza-Guez, M.A. Yeast Diversity in Honey and Pollen Samples from Stingless Bees in the State of Bahia, Brazil: Use of the MALDI-TOF MS/Genbank Proteomic Technique. Microorganisms 2024, 12, 678. [Google Scholar] [CrossRef]
- Marques, L.J.P.; Muniz, F.H.; Lopes, G.d.S.; Silva, J.M. Levantamento da Flora Apícola em Santa Luzia do Paruá, Sudoeste da Amazônia, Maranhão. Acta Bot. Bras. 2011, 25, 141–149. [Google Scholar] [CrossRef]
- Saksinchai, S.; Suzuki, M.; Chantawannakul, P.; Ohkuma, M.; Lumyong, S. A Novel Ascosporogenous Yeast Species, Zygosaccharomyces Siamensis, and the Sugar Tolerant Yeasts Associated with Raw Honey Collected in Thailand. Fungal Divers. 2012, 52, 123–139. [Google Scholar] [CrossRef]
- Silva, M.S.; Marina Arruda, L.; Lanna Xavier, P.; Ximena Díaz Ramírez, M.; Augusto da Silveira, F.; Cristiano Santana, W.; Henrique Alves da Silva, P.; Gomes Fietto, L.; Renon Eller, M. Selection of Yeasts from Bee Products for Alcoholic Beverage Production. Food Microbiol. 2020, 51, 323–334. [Google Scholar] [CrossRef]
- Pereira, A.P.; Mendes-Ferreira, A.; Dias, L.G.; Oliveira, J.M.; Estevinho, L.M.; Mendes-Faia, A. Volatile Composition and Sensory Properties of Mead. Microorganisms 2019, 7, 404. [Google Scholar] [CrossRef]
- Li, C.; Wang, Y.; Sha, S.; Yin, H.; Zhang, H.; Wang, Y.; Zhao, B.; Song, F. Analysis of the Tendency for the Electronic Conductivity to Change during Alcoholic Fermentation. Sci. Rep. 2019, 9, 5512. [Google Scholar] [CrossRef] [PubMed]
- David, M.H.; Kirsop, B.H. Yeast Growth in Relation to the Dissolved Oxygen and Sterol Content of Wort. J. Inst. Brew. 1973, 79, 20–25. [Google Scholar] [CrossRef]
- Verbelen, P.J.; Dekoninck, T.M.L.; Saerens, S.M.G.; Van Mulders, S.E.; Thevelein, J.M.; Delvaux, F.R. Impact of Pitching Rate on Yeast Fermentation Performance and Beer Flavour. Appl. Microbiol. Biotechnol. 2009, 82, 155–167. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, E.; Beauvoit, B. Role of the Non-respiratory Pathways in the Utilization of Molecular Oxygen by Saccharomyces cerevisiae. Yeast 2003, 20, 1115–1144. [Google Scholar] [CrossRef]
| N° of Samples | Municipalities | Bee Type | Specie Bee | Yeast (CFU/ g) |
|---|---|---|---|---|
| 1 | Viana | Tiúba | Melipona fasciculata | 928 |
| 2 | Newton Bello | Africanized | Apis mellifera | 88 |
| 3 | Nova Olinda | Africanized | Apis mellifera | 548 |
| 4 | Santa Luzia | Tiúba | Melipona fasciculata | 62 |
| 5 | Santa Luzia | Africanized | Apis mellifera | 57 |
| 6 | Santa Luzia | Africanized | Apis mellifera | 117 |
| 7 | Viana | Africanized | Apis mellifera | 0 |
| 8 | Viana | Tiúba | Melipona fasciculata | 22 |
| 9 | Nova Olinda | Tiúba | Melipona fasciculata | 138 |
| 10 | Santa Luzia | Tiúba | Melipona fasciculata | 7 |
| 11 | Viana | Africanized | Apis mellifera | 94 |
| 12 | Viana | Africanized | Apis mellifera | 308 |
| 13 | Nova Olinda | Tiúba | Melipona fasciculata | 195 |
| 14 | Santa Luzia | Tiúba | Melipona fasciculata | 102 |
| 15 | Santa Luzia | Africanized | Apis mellifera | 170 |
| 16 | Santa Luzia | Tiúba | Melipona fasciculata | 37 |
| 17 | Santa Luzia | Tiúba | Melipona fasciculata | 36 |
| 18 | Santa Luzia | Africanized | Apis mellifera | 0 |
| 19 | Maranhãozinho | Africanized | Apis mellifera | 316 |
| 20 | Maranhãozinho | Africanized | Apis mellifera | 272 |
| 21 | Nunes Freire | Africanized | Apis mellifera | 164 |
| 22 | Nunes Freire | Africanized | Apis mellifera | 174 |
| 23 | Nunes Freire | Africanized | Apis mellifera | 594 |
| 24 | Viana | Africanized | Apis mellifera | 1 |
| 25 | Viana | Tiúba | Melipona fasciculata | 5 |
| 26 | Viana | Africanized | Apis mellifera | 13 |
| 27 | Viana | Africanized | Apis mellifera | 0 |
| 28 | Viana | Tiúba | Melipona fasciculata | 9 |
| 29 | Newton Bello | Africanized | Apis mellifera | 0 |
| 30 | Nova Olinda | Africanized | Apis mellifera | 104 |
| 31 | Santa Luzia | Tiúba | Melipona fasciculata | 1 |
| 32 | Viana | Africanized | Apis mellifera | 0 |
| 33 | Viana | Tiúba | Melipona fasciculata | 0 |
| 34 | Viana | Tiúba | Melipona fasciculata | 0 |
| 35 | Newton Bello | Africanized | Apis mellifera | 0 |
| 36 | Viana | Tiúba | Melipona fasciculata | 13 |
| 37 | Nova Olinda | Africanized | Apis mellifera | 0 |
| 38 | Nova Olinda | Africanized | Apis mellifera | 0 |
| 39 | Nova Olinda | Africanized | Apis mellifera | 0 |
| 40 | Nova Olinda | Africanized | Apis mellifera | 0 |
| 41 | Nova Olinda | Africanized | Apis mellifera | 0 |
| 42 | Nova Olinda | Africanized | Apis mellifera | 14 |
| 43 | Nunes Freire | Tiúba | Melipona fasciculata | 0 |
| 44 | Nunes Freire | Tiúba | Melipona fasciculata | 0 |
| 45 | Nunes Freire | Tiúba | Melipona fasciculata | 0 |
| 46 | Nunes Freire | Tiúba | Melipona fasciculata | 0 |
| 47 | Nunes Freire | Tiúba | Melipona fasciculata | 0 |
| 48 | Nunes Freire | Tiúba | Melipona fasciculata | 0 |
| 49 | Nunes Freire | Tiúba | Melipona fasciculata | 0 |
| 50 | Maranhãozinho | Tiúba | Melipona fasciculata | 0 |
| 51 | Maranhãozinho | Africanized | Apis mellifera | 0 |
| 52 | Maranhãozinho | Africanized | Apis mellifera | 0 |
| 53 | Maranhãozinho | Africanized | Apis mellifera | 0 |
| 54 | Maranhãozinho | Africanized | Apis mellifera | 0 |
| 55 | Maranhãozinho | Tiúba | Melipona fasciculata | 0 |
| 56 | Maranhãozinho | Tiúba | Melipona fasciculata | 0 |
| 57 | Maranhãozinho | Africanized | Apis mellifera | 0 |
| 58 | Newton Belo | Tiúba | Melipona fasciculata | 2 |
| 59 | Newton Belo | Tiúba | Melipona fasciculata | 2 |
| 60 | Newton Belo | Tiúba | Melipona fasciculata | 0 |
| 61 | Newton Belo | Tiúba | Melipona fasciculata | 0 |
| 62 | Newton Belo | Tiúba | Melipona fasciculata | 0 |
| 63 | Newton Belo | Tiúba | Melipona fasciculata | 0 |
| 64 | Newton Belo | Tiúba | Melipona fasciculata | 0 |
| 65 | Newton Belo | Tiúba | Melipona fasciculata | 0 |
| N° of Strains | Bee Specie | ß-Glycosidase Producer | Identification |
|---|---|---|---|
| 1 | M. fasciculata | - | * |
| 2 | M. fasciculata | - | * |
| 3 | M. fasciculata | - | * |
| 4 | M. fasciculata | - | * |
| 5 | M. fasciculata | - | * |
| 6 | M. fasciculata | - | Rhodoturola |
| 7 | M. fasciculata | - | * |
| 8 | M. fasciculata | - | Rhodoturola |
| 9 | M. fasciculata | - | * |
| 10 | A. mellifera | - | Kluyveromyces marxianus |
| 11 | A. mellifera | - | Kluyveromyces lactis var lactis |
| 12 | A. mellifera | - | * |
| 13 | A. mellifera | - | * |
| 14 | A. mellifera | - | * |
| 15 | A. mellifera | - | * |
| 16 | A. mellifera | - | * |
| 17 | A. mellifera | - | * |
| 18 | A. mellifera | - | Candida auringiensis |
| 19 | M. fasciculata | + | * |
| 20 | M. fasciculata | + | Saccharomycopsis fibuligera |
| 21 | A. mellifera | + | Rhodoturola |
| 22 | A. mellifera | - | Rhodoturola |
| 23 | M. fasciculata | - | * |
| 24 | M. fasciculata | - | Candida taylori |
| 25 | M. fasciculata | - | * |
| 26 | A. mellifera | - | * |
| 27 | A. mellifera | - | * |
| 28 | A. mellifera | - | Candida auringiensis |
| 29 | A. mellifera | - | Rhodoturola |
| 30 | A. mellifera | - | Candida taylori |
| 31 | A. mellifera | - | Candida taylori |
| 32 | A. mellifera | - | * |
| 33 | A. mellifera | - | * |
| Volatile Compounds | Flavors | A/H | |
|---|---|---|---|
| Saccharomycopsis fibuligera | S. cerevisiae | ||
| Alcohols (n = 4) | |||
| Ethanol | - | - | 23.54 |
| Isobutyl alcohol | Sweet | 11.52 | 10.55 |
| 3-Methyl-1 butanol (isoamyl alcohol) | Fruity and whisky | 10.58 | 9.55 |
| 2-Phenyl ethanol | Sweet and floral | - | 2.90 |
| Esters (n = 7) | |||
| Ethyl acetate | Fruity sweet ether | - | 6.19 |
| Ethyl propionate | Fruity | 10.40 | 8.93 |
| Ethyl isobutyrate | Fruity | - | 4.08 |
| Ethyl butanoate | Apple, Pineapple, and Blue cheese | - | 5.13 |
| Ethyl hexanoate | Fermented vanilla and fruity | 2.62 | - |
| Ethyl octanoate | Apple Fruity | 2.23 | 2.22 |
| Ethyl decanoate | Pineapple Fruity | 2.44 | 3.89 |
| Organic acid (n = 1) | |||
| Acetic acid | Acid | 1.17 | 5.01 |
| Nitrogen compounds (n = 3) | |||
| 1,2-Propanediamine | Ammoniacal odor | - | 7.54 |
| Dimethylamine | - | 10.14 | - |
| Methyl hydrazine | - | 26.55 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serra, J.L.; Nojosa, A.d.S.; Carvalho, A.S.S.; Rocha, L.M.N.; Pereira, A.L.; Bastos, F.C.; Martínez-Burgos, W.J. Enhancing Mead Aroma Using Non-Saccharomyces Yeast β-Glucosidase Producers Isolated from Honey: A Case Study in the Upper Turi Region. Fermentation 2025, 11, 282. https://doi.org/10.3390/fermentation11050282
Serra JL, Nojosa AdS, Carvalho ASS, Rocha LMN, Pereira AL, Bastos FC, Martínez-Burgos WJ. Enhancing Mead Aroma Using Non-Saccharomyces Yeast β-Glucosidase Producers Isolated from Honey: A Case Study in the Upper Turi Region. Fermentation. 2025; 11(5):282. https://doi.org/10.3390/fermentation11050282
Chicago/Turabian StyleSerra, Josilene Lima, Alicinea da Silva Nojosa, Aparecida Selsiane Sousa Carvalho, Lucy Mara Nascimento Rocha, Anderson Lopes Pereira, Fernanda Carneiro Bastos, and Walter José Martínez-Burgos. 2025. "Enhancing Mead Aroma Using Non-Saccharomyces Yeast β-Glucosidase Producers Isolated from Honey: A Case Study in the Upper Turi Region" Fermentation 11, no. 5: 282. https://doi.org/10.3390/fermentation11050282
APA StyleSerra, J. L., Nojosa, A. d. S., Carvalho, A. S. S., Rocha, L. M. N., Pereira, A. L., Bastos, F. C., & Martínez-Burgos, W. J. (2025). Enhancing Mead Aroma Using Non-Saccharomyces Yeast β-Glucosidase Producers Isolated from Honey: A Case Study in the Upper Turi Region. Fermentation, 11(5), 282. https://doi.org/10.3390/fermentation11050282








