Abstract
Persimmon waste, accounting for up to 30% of annual harvests, presents economic and environmental challenges due to limited processing options. This study introduces a staged fermentation process to transform persimmon into high-quality wine using a Saccharomyces cerevisiae consortium (EC/Aq/F33 = 4/4/7) followed by Monascus purpureus. The process decouples ethanol production and esterification to avoid nutrient competition: an 8-day yeast phase (21.11 °Brix, 0.29 g kg−1 yeast, 19.90 °C, pH 3.8) yields 12.12% (v/v) ethanol, followed by M. purpureus (1.56% inoculation, 22.6 °C, pH 4.0) on day 8 for 10 days, producing 3.56 g L−1 esters. This results in 32 volatile compounds (e.g., phenethyl acetate), achieving a sensory score of 90.9/100–43% higher in aromatic diversity than single-strain fermentation. By shortening fermentation time by 40% compared with traditional methods (>25 days) and enhancing flavor complexity, this scalable approach offers a sustainable solution for upcycling persimmon and other tannin-rich perishables, supporting eco-friendly food production.
1. Introduction
Persimmon (Diospyros kaki Thunb.), a traditional Chinese fruit rich in bioactive compounds, such as polyphenols, vitamins, and dietary fibers, is prized for its antioxidant and lipid-regulating health benefits [1,2]. Despite its nutritional value, thousands of tons of persimmon fruits are discarded annually due to high production volumes, stringent quality standards, and consumer expectations [3]. This waste not only exacerbates economic losses in mountainous regions but also contributes to environmental pollution through organic decomposition [4]. Fermentation into fruit wine presents a viable solution for valorizing surplus persimmons, particularly as the global demand for low-alcohol, flavorful beverages grows [5,6]. However, persimmon wine remains understudied, with limited data on the optimal fermentation conditions, microbial consortia, and aroma profiles, which hinders its industrial potential.
Persimmon wine production traditionally uses single-strain Saccharomyces cerevisiae inoculations, which suffer from critical drawbacks [7]. These drawbacks include low ethanol conversion efficiency (<8% v/v), which is nearly 50% lower than blueberry wine yields, prolonged fermentation cycles (>15 days), and insufficient synthesis of flavor-enhancing esters (e.g., ethyl acetate <0.5 g L−1) [8,9]. These inefficiencies are compounded by persimmon’s natural deficiency in organic acids (e.g., malic and citric acid), which disrupts pH regulation during fermentation, further suppressing yeast metabolic activity and yielding bland flavor profiles [10,11]. While microbial consortia and co-culture strategies have improved fermentation dynamics in other fruit wines (e.g., apple and grape wines) by enhancing ethanol tolerance and ester diversity [12], their application to persimmon wine remains unexplored, particularly in the context of its high-tannin and low-acid matrix.
Recent advances in fruit wine biotechnology highlight the potential of mixed-yeast consortia to overcome single-strain limitations. Synergistic interactions between S. cerevisiae, Lactobacillus plantarum, and Pichia kluyveri—selected for their complementary metabolic traits such as acid tolerance and ester diversity—have increased ethanol yields (up to 14% v/v) and the flavor complexity in apple and grape wine [13,14]. Similarly, the filamentous fungus Monascus purpureus, traditionally used in rice wine for pigment production, has demonstrated esterification capabilities via extracellular enzymes (e.g., lipases, acyltransferases) that catalyze fatty acid conversion into fruity esters [15,16]. However, co-inoculating Monascus and yeast early in fermentation triggers nutrient competition, suppressing ethanol and ester yields [17,18]. While co-culture strategies have improved fermentation dynamics in grape wines, early-phase competition between yeast and filamentous fungi (e.g., Aspergillus oryzae) often suppresses target metabolite yields. Staged inoculation, as demonstrated in sake production, could resolve this bottleneck by temporally separating ethanol synthesis and flavor maturation [19]. Additionally, Monascus purpureus exhibits broader substrate specificity in esterification compared with Aspergillus species [20], making it a promising candidate for tannin-rich fruit matrices like persimmon.
In this study, we hypothesize that a three-yeast consortium combined with staged inoculation of Monascus purpureus will overcome the limitations of single-strain fermentation by optimizing microbial synergy and minimizing nutrient competition. To validate this hypothesis, we pursue three objectives: (1) Screen and assemble a yeast consortium with rapid fermentation kinetics, achieving an ethanol yield >90% of the theoretical maximum within the yeast-dominated phase (8 days), followed by ester enhancement and cross-strain compatibility, prioritizing strains with complementary metabolic traits. (2) We hypothesize that inoculating M. purpureus after an 8-day yeast phase will bypass early competition, enhancing ester yields over a 10-day secondary phase. (3) Track dynamic flavor compound profiles using GC–MS to correlate microbial metabolic shifts (e.g., yeast ester synthesis, Monascus-derived enzymes) with aroma development, establishing a process optimization framework. By integrating the microbial consortia design with staged fermentation strategies, this work advances a dual-phase fermentation model for persimmon wine, addressing both efficiency (ethanol yield, fermentation time) and quality (flavor complexity), while providing actionable insights for upcycling perishable fruits into high-value products.
2. Materials and Methods
2.1. Materials and Reagents
Persimmons from Jinan, China, were analyzed for traits critical to fermentation: total sugar content (13.47 ± 0.35 °Brix, measured according to GB/T 5009.7-2016 [21], China), moisture content (78.2% w/w, determined according to GB 5009.3-2016 [22], China), pH (3.8 ± 0.1, determined by potentiometric measurement following GB/T 10468-1989 [23], China), and tannin content (32.0 ± 1.2 mg kg−1, determined by the Folin–Ciocalteu method as NY/T 1600-2008 [24], China). These parameters influence microbial activity and flavor during fermentation.
Three brewing-grade yeast strains were used: Saccharomyces cerevisiae EC (Shanghai Kangxi Food Co., Ltd., Shanghai, China), Saccharomyces cerevisiae Aq (Angel Yeast Co., Ltd., Yichang, China) and Saccharomyces cerevisiae F33 (LAFFORT Oenologie, Floirac, France). The esterifying Monascus purpureus strain ZH-3, with an esterification capacity of 74.6 mg g−1 100 h−1, was provided by Shandong Zhonghui Bio-technology Co., Ltd. (Binzhou, China).
Glucose, yeast extract, agar powder, and analytical-grade chemicals (e.g., sodium hydroxide, dichloromethane, sulfuric acid) were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China).
Statistical analysis was performed using Design-Expert 12 (Stat-Ease Inc., Minneapolis, MN, USA). A second-order polynomial regression model was fitted to the experimental data, and its significance was validated via multifactor analysis of variance (ANOVA). Model adequacy was assessed using the coefficient of determination (R2), adjusted R2, and lack-of-fit test. Significance of individual terms (linear, quadratic, interaction) was determined at p < 0.05.
For single-factor experiments, one-way ANOVA followed by Duncan’s multiple range test (implemented in SPSS 20.0, IBM Corp., Armonk, NY, USA) was used to compare means across treatments. Differences were deemed statistically significant at p < 0.05.
Graphical representations, including 3D response surfaces, contour plots, and regression diagnostics, were generated using OriginPro 2016 (OriginLab Corp., Northampton, MA, USA).
2.2. Persimmon Juice Preparation
Persimmons were frozen at −20 °C for a minimum of 15 days using a commercial-grade freezer (Model BC/BD-369GHPT, Haier Group Corporation, Qingdao, China). The frozen persimmons were then slowly thawed at room temperature for approximately 24 h to effectively reduce tannin content and bitterness. The thawed persimmons were then washed with deionized water and homogenized into pulp.
Gelation occurred during pulp homogenization due to the high pectin content of the persimmon pulp (21.60 g kg−1). To enhance juice yield, the pulp underwent enzymatic digestion using pectinase (EC 3.2.1.15). The detailed optimization process is shown in the Supplementary Information, including single-factor optimization of pectinase addition, enzymatic temperature and duration (Figure S1a–c), and subsequent three-factor, three-level orthogonal array (Table S1) for the identification of the optimal pretreatment conditions of persimmon juice preparation. Optimal conditions: pectinase (EC 3.2.1.15) was added at 1.6 g kg−1, and enzymatic digestion was conducted at 40 °C for 3.5 h to maximize juice yield (70.83%).
2.3. Strain Activation and Inoculum Preparation
Yeast: Strains EC, Aq, and F33 were rehydrated in sterile 2% (w/v) glucose (1:10, w/v) at 36~38 °C for 20~30 min until activation (OD600 ≥ 0.8). Activated yeast was inoculated into YPD medium (10 g L−1 yeast extract, 20 g L−1 peptone, 20 g L−1 glucose) and cultured at 30 °C (150 rpm, 48 h).
Monascus purpureus: ZH-3 spores were cultured on PDA slants (30 °C, 7–10 days), then transferred to liquid medium (30 g L−1 glucose, 5 g L−1 peptone, 3 g L−1 yeast extract), and incubated at 32 °C (200 rpm, 40–45 h).
2.4. Single-Factor Optimization of Mixed Yeast Fermentation for Alcohol Production
Alcohol content was quantified via steam distillation following the national standard protocol (GB/T 15038-2006 [25], China), and residual sugar concentration was determined by Fehling’s reagent titration using an SGD-IV automatic reducing sugar determinator (Institute of Biology, Shandong Academy of Sciences, Jinan). All experiments were performed in biological triplicate (independent fermentations) to ensure reproducibility.
2.4.1. Determination of Optimal Yeast Composition Ratio
The experimental design was established using Design-Expert software 13 to determine the optimal mixed yeast ratio of EC, Aq, and F33. The fermentation rate was evaluated based on CO2 weight loss, while the residual sugar content was measured to assess the mixed yeast consortium’s substrate utilization efficiency. The fermentation performance of the mixed yeast cultures was systematically evaluated through integrating data on CO2 weight loss and residual sugar levels. We comprehensively analyzed these parameters to determine the optimal mixed yeast ratio for enhanced fermentation performance.
2.4.2. Optimization of Yeast Inoculation Ratio
The raw persimmon juice retained a total sugar content of 13.22 ± 0.18 °Brix, and then the initial sugar concentration of persimmon juice was adjusted to 20 °Brix using sucrose, and the pH was stabilized at 3.8 using a 0.1 M citrate buffer. Mixed yeast strains (EC/Aq/F33, 4:4:7 ratio) were inoculated at ratios of 0.2, 0.25, 0.3, 0.35, and 0.4 g kg−1 (yeast biomass: juice). Fermentation was conducted in anaerobic conditions at 22 °C for 8 days. Alcohol content and residual sugar were analyzed daily to track fermentation kinetics.
2.4.3. Effect of Initial Sugar Concentration
Using the optimal yeast ratio (0.3 g kg−1) and pH 3.8, the raw persimmon juice retained a total sugar content of 13.42 ± 0.13 °Brix and was adjusted to 20, 21, 22, 23, and 24 °Brix by sucrose supplementation. Fermentation proceeded at 22 °C for 8 days. Alcohol yield (%, v/v) and residual sugar (g L−1) were measured post fermentation.
2.4.4. Effect of pH
With fixed yeast inoculation ratio (0.3 g kg−1) and initial sugar concentration (20 °Brix), the pH was adjusted to 3.4, 3.6, 3.8, 4.0, and 4.2 using 0.1 M citrate buffer. Fermentation lasted 8 days at 22 °C. Final alcohol content and residual sugar were quantified to determine pH effects.
2.4.5. Effect of Temperature
Fermentation was tested at 18 °C, 20 °C, 22 °C, 24 °C, and 26 °C under fixed conditions (0.3 g kg−1 yeast, pH 3.8, 20 °Brix). Alcohol content and residual sugar were measured after 8 days to identify temperature-dependent metabolic efficiency.
2.4.6. Effect of Fermentation Duration
Fermentation time was tested at 0, 2, 4, 6, 8, and 10 days (22 °C, 0.3 g kg−1, pH 3.8, 20 °Brix). Alcohol content and residual sugar levels were analyzed at each timepoint to profile fermentation kinetics and determine the optimal duration.
2.5. Multivariate Optimization of Mixed Yeast Fermentation Using Box–Behnken Design
A three-factor, three-level Box–Behnken design (BBD) was employed to optimize the experimental conditions, utilizing 17 runs with three independent variables, including (A) initial sugar concentration (21, 22, 23 °Brix), (B) yeast inoculation ratio (0.25, 0.30, 0.35 g kg−1), and (C) fermentation temperature (18, 20, 22 °C). Alcohol content (%) and residual sugar (g L−1) were selected as dual response variables to maximize fermentation efficiency (e.g., maximizing alcohol while minimizing residual sugar). A second-order polynomial model was fitted to the data, and optimal conditions were derived using response surface methodology. The design matrix and results are detailed in Table S2.
2.6. Single-Factor Optimization of Monascus purpureus Fermentation for Esterification
To assess ethanol inhibition on M. purpureus, ZH-3 was cultured in persimmon juice containing 8–12% (v/v) ethanol. Total ester concentration was quantified in triplicate via saponification titration (GB/T 15038-2006, China). Sensory evaluation was performed by a trained panel (see Sensory Evaluation Methodology below).
2.6.1. Effect of Monascus purpureus Inoculation Ratio
Persimmon juice (initial sugar concentration: 20°Brix, pH: 3.8) was fermented at 24 °C for 8 days. Lyophilized Monascus purpureus was inoculated at 1%, 2%, 3%, 4%, and 5% (biomass: juice). Total ester concentration (g L−1) was measured post fermentation to identify the optimal ratio for ester biosynthesis.
2.6.2. Effect of Initial Sugar Concentration
Under fixed conditions (3% M. purpureus, pH: 3.8), the initial sugar concentration of persimmon juice was adjusted to 12, 16, 20, 24, and 28 °Brix using sucrose. Fermentation proceeded at 24 °C for 8 days. Total ester concentration (g L−1) was analyzed to assess sugar-driven esterification efficiency.
2.6.3. Effect of pH
Persimmon juice (3% M. purpureus, 20 °Brix) was adjusted to pH 3.0, 3.4, 3.8, 4.2, and 4.6 using 0.1 M citrate buffe. Fermentation lasted 8 days at 24 °C. Total ester concentration (g L−1) was quantified to determine the pH optimum.
2.6.4. Effect of Temperature
Persimmon juice (3% M. purpureus, pH: 3.8, 20 °Brix) was fermented at 20 °C, 24 °C, 28 °C, 32 °C, and 36 °C for 8 days. Total ester concentration (g L−1) was measured to identify the temperature effects on ester accumulation.
2.6.5. Effect of Fermentation Duration
Using optimized parameters (3% M. purpureus, pH 3.8, 20 °Brix, 24 °C), fermentation was monitored over 0, 2, 4, 6, 8, 10, and 12 days. Total ester concentration was analyzed at 48 h intervals to profile biosynthesis kinetics and determine peak yield timing.
2.7. Multivariate Optimization of Monascus purpureus Fermentation Using Box–Behnken Design
A three-factor, three-level Box–Behnken design was employed to optimize (A) inoculation ratio (0, 1.5, 3.0%), (B) temperature (20, 22, 24 °C), and (C) pH (3.8, 4.0, 4.2). Response variables: total ester concentration (g L−1)—quantified via saponification titration. Sensory evaluation score was assessed by a trained panel (see below). Statistical analysis: a second-order polynomial model was fitted to the data, and optimal conditions were derived using response surface methodology (RSM). The design matrix and results are detailed in Table S3.
2.8. Sensory Evaluation and Aroma Profiling
Sensory evaluation was conducted by a trained panel (n = 10) using a 9-point hedonic scale (Table S4, Supporting Information). The criteria were adapted from ISO 11035:1994 [26] (sensory attribute ranking) and OIV wine evaluation guidelines, with modifications to prioritize persimmon-specific attributes such as fruit note balance, sweetness–acidity harmony, and aftertaste duration. Each category (Color, Aroma, Taste, Aftertaste) was weighted to reflect its contribution to the final score (90.9/100), which exceeds industry benchmarks for fruit wines [27,28]. Panelists underwent calibration sessions to ensure consistency with these standards.
Aroma profiling of persimmon wine was performed using solid-phase microextraction (SPME) coupled with gas chromatography–mass spectrometry (GC–MS). Volatile compounds were extracted by exposing an SPME fiber to the wine’s headspace for 40 min at ambient temperature. After adsorption, the fiber was thermally desorbed in the GC injection port at 280 °C for 5 min to release the captured analytes for subsequent analysis.
The GC–MS system (GC-MS-QP2007, Shimadzu, Kyoto, Japan) was equipped with a DB-Wax capillary column (30 m × 0.25 mm × 0.25 μm, Agilent Technologies, Santa Clara, CA, USA) and operated with helium as the carrier gas at a constant flow rate of 1 mL min−1. The oven temperature program was optimized to resolve complex volatile mixtures: initial hold at 40 °C for 10 min, followed by a ramp to 100 °C at 3 °C min−1, then to 180 °C at 4 °C min−1, and finally to 220 °C at 6 °C min−1, with a 15 min hold at the final temperature.
For mass spectrometric detection, the system operated in electron ionization (EI) mode at 70 eV, scanning a mass range of 35~450 m z−1 to ensure broad coverage of volatile and semi-volatile compounds. Critical components of the MS setup—the transfer line and ion source—were maintained at 280 °C to prevent condensation of analytes and ensure consistent ionization efficiency.
2.9. Staged Fermentation Protocol
The yeast phase was conducted with a Saccharomyces cerevisiae consortium (EC/Aq/F33 = 4/4/7) at 0.29 g kg−1, 21.11 °Brix, 19.90 °C, and pH 3.8 for 8 days under anaerobic conditions. On day 8, when ethanol reached 12.12% (v/v) and residual sugar dropped to <2 g L−1, Monascus purpureus ZH-3 was inoculated at 1.6% (equivalent to 16 g Kg−1 w/w). The Monascus phase proceeded at 22.6 °C and pH 4.0 for 10 days, totaling 18 days. The pH was adjusted from 3.8 to 4.0 using 0.1 M citrate buffer prior to M. purpureus inoculation to optimize esterase activity.
3. Results
3.1. Synergistic Effects of Mixed Yeast Composition Ratios
The compositional ratio of S. cerevisiae (EC), S. cerevisiae (Aq), and S. cerevisiae (F33) significantly influenced the fermentation efficiency (Table 1). Single-strain fermentations (ratios of 1:0:0, 0:1:0, or 0:0:1) exhibited a suboptimal performance, with minimal CO2 production (6.20–6.39 g) and elevated residual sugar (1.51–1.65 g L−1), reflecting limited substrate conversion. In contrast, the 4/4/7 ratio (EC: Aq: F33) achieved maximal CO2 production (10.99 ± 0.34 g) and minimal residual sugar (0.62 ± 0.01 g L−1), representing a 76.3% increase in CO2 yield and a 61.8% reduction in residual sugar compared with the best-performing single strain (EC, 1:0:0).

Table 1.
Alcohol production efficiency of Saccharomyces cerevisiae consortia (EC/Aq/F33) at varying composition ratios.
Metabolic partitioning and synergy: Deviations from the 4/4/7 ratio (e.g., 3/1/1 or 1/1/1) reduced efficiency (p < 0.05, Duncan’s test), underscoring the necessity of strain-specific metabolic roles. EC conferred ethanol tolerance (>10% v/v), Aq enhanced glucose uptake via upregulated hexose transporters, and F33 facilitated polysaccharide degradation through β-glucosidase and pectinase activity. Our 4/4/7 ratio uniquely optimized persimmon juice fermentation.
3.2. Single-Factor Optimization of Mixed Yeast Fermentation for Alcohol Production
3.2.1. Yeast Inoculation Ratio
The optimal inoculation ratio of 0.3 g kg−1 yielded a peak alcohol content (10.31~10.90% v/v) and minimal residual sugar (1.15–1.17 g L−1; Figure 1a). Suboptimal ratios (0.20–0.25 g kg−1) reduced alcohol synthesis (8.18–9.56% v/v) due to insufficient biomass, while higher ratios (0.35–0.40 g kg−1) decreased efficiency (alcohol: 9.13–10.22% v/v; residual sugar: 1.12–1.19 g L−1), likely due to nutrient competition or ethanol feedback inhibition. The 0.3 g kg−1 ratio balanced yeast activity and substrate availability, maximizing ethanol flux while minimizing the metabolic byproducts.
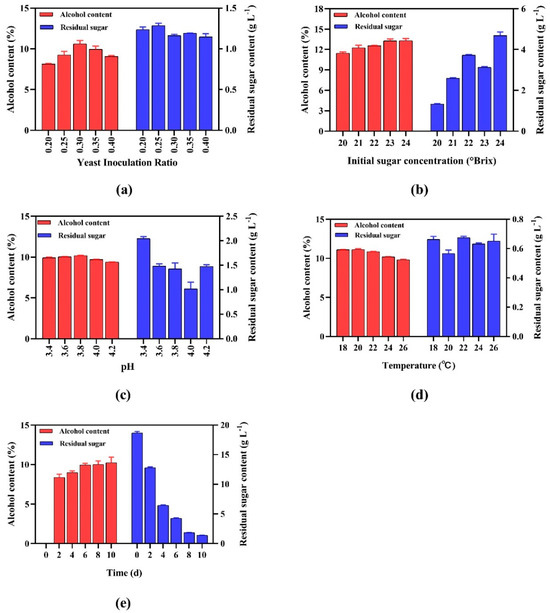
Figure 1.
Single-factor optimization of mixed yeast (Saccharomyces cerevisiae EC-Aq-F33) fermentation parameters for alcohol production: (a) inoculation ratio, (b) initial sugar concentration, (c) pH, (d) temperature, (e) duration.
3.2.2. Initial Sugar Concentration
Fermentation efficiency peaked at 23 °Brix, yielding 13.25–13.56% v/v alcohol and 3.09–3.14 g L−1 residual sugar (Figure 1b). However, alcohol production declined slightly beyond 23 °Brix, as evidenced by the 12.95–13.48% v/v alcohol at 24°Brix, coinciding with a sharp rise in residual sugar (4.55–4.88 g L−1). This result indicates substrate inhibition via osmotic stress or ethanol toxicity, highlighting persimmon-specific metabolic thresholds due to its distinct polysaccharide and tannin profiles.
3.2.3. pH Dependence
A pH of 3.8 maximized alcohol synthesis, yielding ethanol concentrations of 10.15–10.22% (v/v) while maintaining moderate residual sugar levels (1.31–1.55 g L−1; Figure 1c). However, at pH values exceeding 3.8, ethanol production decreased, accompanied by variations in residual sugar. At pH 4.0, residual sugar reached its lowest level (0.89–1.16 g L−1), yet alcohol synthesis declined to 9.67–9.74%, indicating a trade-off between substrate utilization and ethanol yield. At pH 4.2, ethanol production further decreased to 9.38–9.40%, with residual sugar levels (1.43–1.50 g L−1) similar to or slightly higher than those at pH 3.8. The decrease in ethanol production as pH increases beyond 3.8 can be attributed to the sensitivity of key fermentative enzymes, such as pyruvate decarboxylase and alcohol dehydrogenase, to pH changes [29]. At pH 4.0, despite efficient sugar consumption (evidenced by low residual sugar), the reduced ethanol yield suggests a shift in metabolic flux toward alternative products, such as glycerol or organic acids, rather than ethanol [30]. At pH 4.2, both sugar consumption and ethanol production are diminished, likely due to overall enzymatic inhibition under less favorable pH conditions. Thus, pH 3.8 represents an optimal balance where both substrate utilization and ethanol yield are maximized.
3.2.4. Temperature Effects
Overall, 20 °C emerged as the optimal temperature, yielding 11.08–11.24% v/v alcohol and 0.54–0.58 g L−1 residual sugar (Figure 1d). Higher temperatures progressively impaired ethanol synthesis (26 °C: 9.74–9.90% v/v) and increased residual sugar variability (0.60–0.70 g L−1), likely due to thermal stress disrupting membrane integrity and glycolytic enzymes [31].
3.2.5. Fermentation Duration
Alcohol production plateaued at 8 days (9.50–10.33% v/v), with residual sugar sharply decreasing from ~18.8 g L−1 to ~1.83 g L−1 (92% reduction; Figure 1e). Prolonged fermentation (>8 days) yielded negligible gains (day 10: 9.36–10.64% v/v alcohol; 1.36–1.42 g L−1 residual sugar), indicating metabolic stagnation from nutrient depletion.
3.3. Multivariate Optimization of Mixed Yeast Fermentation Using Box–Behnken Design
The Box–Behnken design model for residual sugar minimization, as shown in Table 2, revealed significant effects of key factors and interactions. The main effects of initial sugar concentration (A, p = 0.0001) and temperature (C, p = 0.0022) are highly significant (p < 0.01), indicating strong influences on residual sugar levels. In contrast, the inoculation ratio (B, p = 0.0789) is not significant (p > 0.05), suggesting a limited individual impact within the tested range. The interaction between initial sugar concentration and temperature (AC, p = 0.0047) is highly significant, demonstrating a synergistic effect on residual sugar reduction. However, interactions AB (p = 0.2293) and BC (p = 0.9825) are not significant, indicating negligible combined effects of these factor pairs. Among quadratic terms, only temperature (C2, p = 0.0416) shows a significant non-linear effect (p < 0.05), while initial sugar concentration (A2, p = 0.3841) and inoculation ratio (B2, p = 0.1034) exhibit minimal quadratic contributions. The model’s R2 value of 0.9434 indicates that 94.34% of the variability in residual sugar is explained, and the non-significant lack of fit (p = 0.1265) confirms the model’s adequacy for predicting residual sugar outcomes.

Table 2.
ANOVA for Box–Behnken design model of mixed-yeast fermentation: Residual sugar minimization.
The derived second-order polynomial equation (Equation (1)) predicted residual sugar as follows:
Optimization via Design-Expert software identified the optimal glucose metabolism rate at conditions of initial sugar 21.11 °Brix, inoculation ratio 0.29 g kg−1, and temperature 19.90 °C (desirability = 1.0), demonstrating that moderate sugar and sub-20 °C temperatures mitigate metabolic stress while sustaining ethanol flux, a finding with critical implications for energy-efficient persimmon wine production.
3.4. Single-Factor Optimization of Monascus purpureus Fermentation for Esterification
3.4.1. Inoculation Ratio
Figure 2a illustrates the relationship between esterifying Monascus purpureus inoculation ratio and total ester concentration. Total esters increased with inoculation levels up to 3 g kg−1, reaching a maximum mean value of 3.18 ± 0.05 g L−1 (n = 3), followed by a decline at higher ratios (4–5 g kg−1). This biphasic trend suggests that moderate inoculation enhances ester synthesis, likely by providing sufficient microbial activity to drive esterification reactions. However, exceeding 3 g kg−1 may saturate the system’s metabolic capacity, potentially due to substrate limitations (e.g., precursor acids or alcohols) or nutrient competition, thereby suppressing ester production [31].
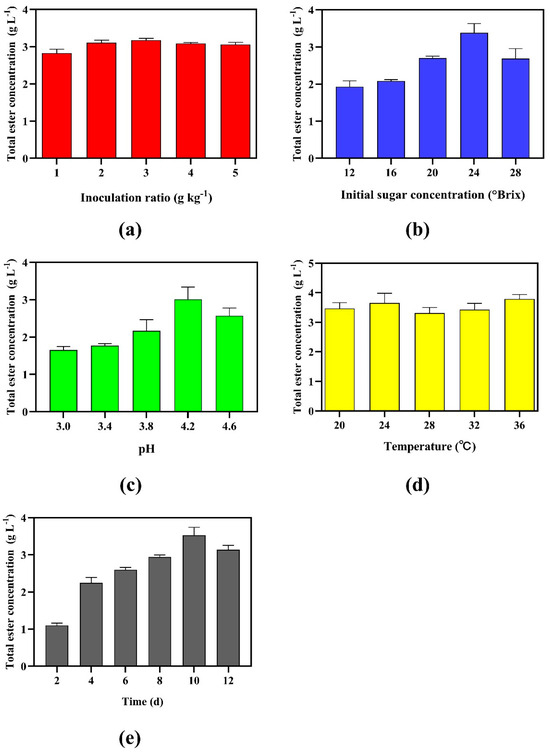
Figure 2.
Single-factor optimization of Monascus purpureus fermentation parameters for ester biosynthesis: (a) inoculation ratio, (b) initial sugar concentration, (c) pH, (d) temperature, (e) duration.
3.4.2. Initial Sugar Concentration
As illustrated in Figure 2b, the total ester concentration exhibited a progressive increase with rising initial sugar concentrations, climbing from 1.93 g L−1 at 12 °Brix to a peak of 3.38 g L−1 at 24 °Brix. This trend implies that elevated sugar concentrations enhance yeast-derived enzymatic activity and substrate availability, promoting ester synthesis. The highest individual value (3.59 g L−1) at 24 °Brix underscores the robustness of this condition. However, exceeding 24 °Brix resulted in a marked decline (2.69 g L−1 at 28 °Brix), likely due to osmotic stress or substrate inhibition, which suppress microbial viability.
3.4.3. pH Dependence
Figure 2c demonstrates the influence of pH on total ester concentration. Ester production increased sharply from 1.65 ± 0.10 g L−1 at pH 3.0 to 3.21 ± 0.32 g L−1 at pH 4.2, followed by a decline at pH 4.6 (2.57 ± 0.21 g L−1). This trend highlights pH as the most influential factor on ester variability compared with inoculation ratios or temperature. The peak at pH 4.2 aligns with the optimal activity of ester-synthesizing enzymes (e.g., esterase and lipase), which are often most efficient in slightly acidic environments.
3.4.4. Temperature Effects
Figure 2d depicts the effect of fermentation temperature on total ester concentration. Ester production increased from 20 °C to 24 °C, peaking at 3.65 ± 0.33 g L−1, followed by a decline at 28 °C (3.31 ± 0.18 g L−1) and 32 °C (3.43 ± 0.21 g L−1). Unexpectedly, a secondary rise occurred at 36 °C (3.79 ± 0.15 g L−1), suggesting metabolic adaptability or thermotolerant enzyme isoforms.
3.4.5. Fermentation Duration
Figure 2e shows the biphasic relationship between fermentation time and ester concentration. Ester levels rose progressively from 1.10 g L−1 at day 2 to a peak of 3.53 g L−1 by day 10, followed by a decline to 3.14 g L−1 at day 12. This ascending phase reflects the cumulative activity, while the decline likely stems from enzymatic ester degradation or precursor depletion.
3.5. Multivariate Optimization of Monascus purpureus Fermentation Using Box–Behnken Design
The quadratic regression model for sensory scores (Equation (2)) was statistically significant (F = 9.37, p = 0.0038), explaining 92.3% of the variability (R2 = 0.923) in the multi-strain fermentation system. The ANOVA results (Table 3) identified significant factors influencing sensory scores: Monascus Purpureus inoculation (A, p = 0.0067), the interaction between inoculation and pH (AC, p = 0.0202), and the quadratic terms of inoculation (A2, p = 0.0106), temperature (B2, p = 0.0010), and pH (C2, p = 0.0407). The quadratic effects of temperature (B2, coefficient = −2.99) and inoculation (A2, coefficient = −1.91) exhibited the strongest negative impacts, suggesting non-linear metabolic thresholds in esterification dynamics.

Table 3.
ANOVA for Box–Behnken design model of Monascus purpureus fermentation: sensory score optimization.
The regression equation (Equation (2)) is:
This model highlights that sensory scores are driven by inoculation ratio (A) and its synergistic interaction with pH (AC). Notably, neither temperature (B, p = 0.9760) nor pH (C, p = 0.2874) alone had significant linear effects, emphasizing the importance of multifactorial optimization. The non-significant lack of fit (p = 0.9741) confirmed the model’s robustness.
Figure 3a illustrates that the peak sensory scores (90–92) occurred at 1.2–1.8% inoculation combined with pH 3.8–4.0. This optimal range balances microbial activity and enzymatic efficiency, enhancing flavor compound synthesis (e.g., ethyl hexanoate, isoamyl acetate). The acidic pH likely promotes alcohol-to-ester conversion, while moderate inoculation avoids metabolic saturation.
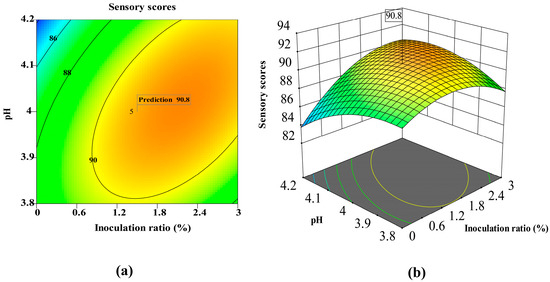
Figure 3.
Response surface methodology (RSM) analysis of sensory score: interaction between Monascus purpureus inoculation ratio and pH: (a) contour plot (b) 3D surface plot.
A sharp decline in sensory scores at pH > 4.0 (Figure 3b) suggests disrupted esterification, possibly due to microbial dysbiosis or enzyme denaturation. Similarly, Figure 4a,b shows reduced scores at temperatures <21 °C or >23 °C, with maxima at 22 °C and pH 4.0. Although temperature (B) and pH (C) alone were non-significant, their interaction (BC, p = 0.087) implies thermos–pH synergism in flavor development—a novel insight for persimmon wine, where the thermal lability of tannins may modulate microbial metabolism.
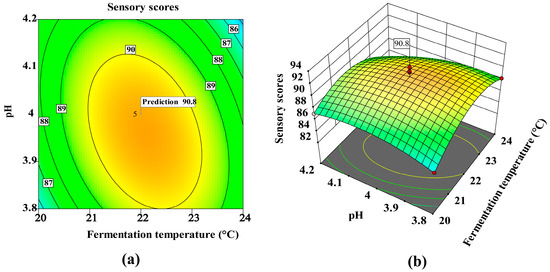
Figure 4.
Response surface methodology (RSM) analysis of sensory score: interaction between fermentation temperature and pH: (a) contour plot, (b) 3D surface plot.
The model predicted optimal conditions as 1.56% Monascus Purpureus inoculation, 22.57 °C fermentation temperature, and a pH of 3.97, yielding a sensory score of 90.70, alcohol content of 12.12%, and total esters of 3.56 g L−1. To validate these predictions, an experimental verification was conducted using conditions A = 1.60%, B = 22.6 °C, and C = 4.0. Experimental validation under near-optimal conditions confirmed these results, with an average sensory score of 90.9, demonstrating the model’s accuracy.
3.6. Sensory Evaluation and Aroma Profile of Persimmon Wine
Table 4 demonstrates that multi-strain fermentation (MSF)—employing a S. cerevisiae (EC/Aq/F33 = 4/4/7) consortium coupled with M. purpureus—significantly outperformed single-yeast (Aq, EC, F33) and mixed-yeast (EC–Aq–F33) fermentations in aromatic diversity and compositional balance. The MSF technique generated 32 distinct volatile compounds, surpassing the mixed-yeast system (27) and individual strains (Aq: 20, EC: 21, F33: 19) (Table S5). Notably, phenylethyl alcohol, a key floral compound, dominated the MSF profile at 73.08%—nearly double the concentration in mixed-yeast fermentation (47.06%) and 3.2-fold higher than single-yeast fermentation (Aq: 33.62%, EC: 28.64%, F33: 42.31%).

Table 4.
Comparative analysis of volatile compounds in persimmon wine: multi-strain fermentation (MSF) vs. single/mixed yeast.
MSF further demonstrated enhanced flavor complexity through balanced ester production, including phenethyl acetate (1.72%) and ethyl octanoate (1.08%), the latter contributing a brandy-like nuance absent in single-strain systems. MSF uniquely synthesized decanal (0.05%), a citrus-derived aldehyde, and elevated the level of esters such as ethyl decanoate (0.75%) and 2,3-butanediol (0.16%), which synergistically softened potential alcohol harshness with creamy and sweet undertones. Additionally, MSF suppressed off-flavor compounds like 2,4-di-tert-butylphenol (3.17% vs. 6.07% in Aq) while integrating subtle floral terpenes (e.g., citronellol, 0.19%). This orchestrated balance of esters, alcohols, and aldehydes highlights MSF’s ability to craft a multidimensional sensory profile unachievable through conventional fermentation.
4. Discussion
Persimmon valorization is hindered by their high tannin content, rapid spoilage, and inefficient conventional fermentation methods, which often produce suboptimal ethanol yields (<8% v/v) and monotonous flavor profiles [32,33]. This study introduces a two-stage fermentation strategy combining a specialized Saccharomyces cerevisiae consortium (EC/Aq/F33, 4:4:7 ratio) and Monascus purpureus ZH-3 to address these limitations. The sequential design decouples ethanol production from esterification, reducing total processing time by 40% (18 days) while enhancing aromatic complexity. During the initial 8-day yeast phase (19.90 °C, pH 3.8), strain-specific metabolic roles—EC (ethanol tolerance), Aq (glucose assimilation), and F33 (polysaccharide degradation)—enabled efficient sugar conversion, yielding 12.12% (v/v) ethanol with residual sugar <2 g L−1. The subsequent inoculation of M. purpureus (day 8) avoided substrate competition [34,35], leveraging its esterase activity to synthesize 3.56 g L−1 esters, including phenethyl acetate (1.72%) and ethyl octanoate (1.08%).
Thermal (19.90 °C to 22.6 °C) and pH (3.8 to 4.0) adjustments during the 10-day Monascus phase optimized enzymatic performance, aligning with M. purpureus lipase activity optima (25–30 °C) and enhancing substrate affinity for persimmon tannin–fatty acid complexes [36]. This sequential approach generated a multifaceted volatile profile (32 compounds), exceeding that of single-strain fermentations (19–27 compounds). Key contributors included floral geranylgeraniol (0.28%), spicy terpinen-4-ol (0.43%), and isoamyl propionate (0.14%), which mitigated ethanol harshness akin to aged spirits. Sensory analysis confirmed a superior quality (90.9/100), attributed to balanced ester–alcohol ratios, reduced astringency, and the suppression of off-flavors (e.g., 2,4-di-tert-butylphenol at 3.17% vs. 6.07% in controls) [37].
Pilot-scale trials validated process robustness (92.3% reproducibility under ±0.5 °C, ±0.1 pH control), with 70.83% pulp conversion to fermentable juice minimizing agro-waste. By resolving the metabolic conflicts and optimizing the biotic–abiotic interactions, this bioprocess offers a scalable template for tannin-rich substrates, addressing the economic and environmental challenges in perishable crop utilization [38]. Future work should explore metabolic engineering for tailored flavor modulation and lifecycle assessments to quantify circular economy benefits, advancing sustainable agro-industrial biotechnology.
5. Conclusions
This study established a dual-phase fermentation framework for converting persimmons into premium wine, decoupling yeast-driven ethanol synthesis (8 days) from Monascus-mediated esterification (10 days) via dynamic pH (3.8 to 4.0) and temperature (19.90 °C to 22.6 °C) control. Achieving 12.12% (v/v) ethanol and 3.56 g L−1 esters in 18 days, the process cuts fermentation time by 40% compared with traditional single-strain methods (>25 days) while delivering a sensory score of 90.9/100. This efficiency and quality stem from staged microbial collaboration, leveraging M. purpureus’s esterification versatility in a tannin-rich matrix. Beyond persimmons, this scalable template suits other perishables (e.g., hawthorn, pomegranate), offering a sustainable approach to food waste valorization and aligning with circular economy principles.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fermentation11050278/s1, Figure S1: Single-factor optimization of juice yield: (a) Pectinase addition, (b) Enzymatic temperature, and (c) Enzymatic duration; Table S1: three-factor, three-level orthogonal array of persimmon pretreatment; Table S2: Box–Behnken experimental design and results for the mixed-yeast fermentation; Table S3: Box–Behnken experimental design and results for Monascus purpureus fermentation; Table S4: Persimmon wine sensory evaluation criteria; Table S5: Total volatile components in persimmon wine fermented by different strains.
Author Contributions
X.L. contributed to conceptualization, supervision, and project administration. Q.L., Y.S., and R.X. were responsible for methodology, data curation, formal analysis, and writing the original draft. R.C. and L.L. handled investigation, software, and validation. T.Z. performed writing—review and editing. Y.D. provided resources and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Shandong Province Science and Technology Smes Innovation Ability Improvement Project under Grant [number 2023TSGC0920]; the Young Scientists Fund of the National Natural Science Foundation of China under Grant [number 32302160] and the Shandong Agriculture and Engineering University Start-Up Fund for Talented Scholars [number BSQJ202325].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Acknowledgments
Special thanks to Shandong Key Laboratory of Healthy Food Resources Exploration and Creation for their support.
Conflicts of Interest
Author Lanxiao Li is employed by the company “Yantai Gaosheng Wine Co., Ltd.” But for the purposes of this investigation, there was no financing relationship with the company; therefore, there were no conflicts of interest. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| GC–MS | Gas chromatography–mass spectrometry |
| RSM | Response surface methodology |
| SPME | Solid phase microextraction |
| MSF | Multi-strain fermentation |
References
- Matheus, J.R.V.; Andrade, C.J.; de Miyahira, R.F.; Fai, A.E.C. Persimmon (Diospyros kaki L.): Chemical properties, bioactive compounds and potential use in the development of new products-A Review. Food Rev. Int. 2020, 38, 384–401. [Google Scholar] [CrossRef]
- M. González, C.; Hernando, I.; Moraga, G. In vitro and in vivo digestion of persimmon and derived products: A review. Foods 2021, 10, 3083. [Google Scholar] [CrossRef] [PubMed]
- Gea-Botella, S.; Agulló, L.; Martí, N.; Martínez-Madrid, M.C.; Lizama, V.; Martín-Bermudo, F.; Berná, G.; Saura, D.; Valero, M. Carotenoids from persimmon juice processing. Food Res. Int. 2021, 141, 109882. [Google Scholar] [CrossRef] [PubMed]
- Hosseininejad, S.; González, C.M.; Hernando, I.; Moraga, G. Valorization of persimmon fruit through the development of new food products. Front. Food Sci. Technol. 2022, 2, 914952. [Google Scholar] [CrossRef]
- Kim, Y.M.; Lubinska-Szczygel, M.; Park, Y.S.; Deutsch, J.; Ezra, A.; Luksrikul, P.; Shafreen, R.M.B.; Gorinstein, S. Characterization of bioactivity of selective molecules in fruit wines by FTIR and NMR spectroscopies, fluorescence and docking calculations. Molecules 2023, 28, 6036. [Google Scholar] [CrossRef]
- An, X.; Wang, Z.; Li, J.; Nie, X.; Liu, K.; Zhang, Y.; Zhao, Z.; Chitrakar, B.; Ao, C. Analysis of flavor-related compounds in fermented persimmon beverages stored at different temperatures. Lwt 2022, 163, 113524. [Google Scholar] [CrossRef]
- Wei, J.; Li, Y.; Liu, Y.; Liu, S.; Yang, X.; Wang, X. Process optimization for production of persimmon wine with lower methanol. Foods 2024, 13, 748. [Google Scholar] [CrossRef]
- Lao-Martil, D.; Verhagen, K.J.; Schmitz, J.P.; Teusink, B.; Wahl, S.A.; van Riel, N.A. Kinetic modeling of Saccharomyces cerevisiae central carbon metabolism: Achievements, limitations, and opportunities. Metabolites 2022, 12, 74. [Google Scholar] [CrossRef]
- García-Barón, S.E.; Carmona-Escutia, R.P.; Herrera-López, E.J.; Leyva-Trinidad, D.A.; Gschaedler-Mathis, A. Consumers’ drivers of perception and preference of fermented food products and beverages: A systematic review. Foods 2025, 14, 713. [Google Scholar] [CrossRef]
- Niyomvong, N.; Trakunjae, C.; Boondaeng, A. Fermentation characteristics and aromatic profiles of plum wines produced with Hanseniaspora thailandica Zal1 and common wine yeasts. Molecules 2023, 28, 3009. [Google Scholar] [CrossRef]
- Choi, K.T.; Lee, S.H.; Kim, Y.J.; Choi, J.S.; Lee, S.B. Improvement of volatile aromatic compound levels and sensory quality of distilled soju derived from Saccharomyces cerevisiae and Wickerhamomyces anomalus co-fermentation. Food Chem. X 2024, 22, 101368. [Google Scholar] [CrossRef] [PubMed]
- De Gioia, M.; Russo, P.; De Simone, N.; Grieco, F.; Spano, G.; Capozzi, V.; Fragasso, M. Interactions among relevant non-Saccharomyces, Saccharomyces, and lactic acid bacteria species of the wine microbial consortium: Towards advances in antagonistic phenomena and biocontrol potential. Appl. Sci. 2022, 12, 12760. [Google Scholar] [CrossRef]
- Du, Y.; Shim, S.; Wang, L.; Gao, X.; Fu, X. Impact of Monascus purpureus combined with Lactobacillus plantarum and Saccharomyces cerevisiae fermentation on nutritional and flavor characteristics of Pyropia yezoensis. Food Chem. 2025, 472, 142973. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Yu, X.; Hong, K. Synergistic effect enhances aromatic profile in beer brewing through mixed-culture fermentation of Pichia kluyveri and Saccharomyces cerevisiae var. diastaticus. Fermentation 2025, 11, 148. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, J.; Chen, Q.; Yang, C. Effects of main nutrient sources on improving monascus pigments and saccharifying power of Monascus purpureus in submerged fermentation. Fermentation 2023, 9, 696. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, C.; Ablimit, A.; Chen, M.; Sun, Q.; Dong, H.; Liu, W.; Huang, A.; Zhang, B.; Hu, W.; et al. Development of red grapefruit jam fermented with Monascus purpureus: Effect of naringenin on Monascus purpureus growth and metabolism. Food Biosci. 2025, 65, 106061. [Google Scholar] [CrossRef]
- Arun, K.B.; Anoopkumar, A.N.; Sindhu, R.; Binod, P.; Aneesh, E.M.; Madhavan, A.; Awasthi, M.K. Synthetic biology for sustainable food ingredients production: Recent trends. Syst. Microbiol. Biomanuf. 2023, 3, 137–149. [Google Scholar] [CrossRef]
- Cosme, F.; Inês, A.; Vilela, A. Microbial and commercial enzymes applied in the beverage production process. Fermentation 2023, 9, 385. [Google Scholar] [CrossRef]
- Lan, L.; Cao, Y.; Yuan, J.; Feng, R.; Pan, H.; Mao, X.; Ji, S.; Hu, Q.; Zhou, H. A comprehensive investigation of lipid profile during the solid-state fermentation of rice by Monascus purpureus. Foods 2025, 14, 537. [Google Scholar] [CrossRef]
- Tong, S.; Li, W.; Rao, Y.; Xiao, Y.; Yan, Y.; Guo, W.; Lü, X.; Sun, J.; Ai, L.; Ni, L. Microbiomics and metabolomics insights into the microbial regulation on the formation of flavor components in the traditional fermentation process of Chinese Hongqu aged vinegar. Food Sci. Hum. Well. 2024, 13, 2765–2778. [Google Scholar] [CrossRef]
- GB/T 5009.7-2016; National Food Safety Standard—Determination of Reducing Sugar in Foods. National Standards of the People’s Republic of China: Beijing, China, 2016.
- GB 5009.3-2016; National Food Safety Standard—Determination of Moisture Content in Foods. National Standards of the People’s Republic of China: Beijing, China, 2016.
- GB/T 10468-1989; Fruit and Vegetable Products—Determination of pH. State Technical Supervision Bureau: Beijing, China, 1989.
- NY/T 1600-2008; Determination of Tannin Content in Fruit, Vegetable and Derived Product. Spectrophotometry Method. National Standards of the People’s Republic of China: Beijing, China, 2008.
- GB/T 15038-2006; Analytical Methods of Wine and Fruit Wine. National Standards of the People’s Republic of China: Beijing, China, 2006.
- ISO 11035:1994; Sensory Analysis—Identification and Selection of Descriptors for Establishing a Sensory Profile by a Multidimensional Approach. ISO: Geneva, Switzerland, 1994.
- Gong, Z.; Wu, Z.; Yang, Q.; Liu, J.; Jiao, P.; Tang, C. Influences of lactic acid bacteria strains on the flavor profiles, metabolites and quality characteristics of red yeast rice produced by solid-state fermentation. Food Res. Int. 2024, 197, 115172. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wang, M.; Ding, Y.; Ding, Y.; Zhou, X. Flavor and quality enhancement of miiuy croaker (Miichthys miiuy) surimi by co-fermentation with Monascus purpureus and Actinomucor elegans. Food Biosci. 2025, 67, 106231. [Google Scholar] [CrossRef]
- Zhang, J.; Li, M.; Li, L.; Liu, Y.; Gu, T.; Wang, J.; Gao, M. Evaluation of differences in volatile flavor compounds between liquid-state and solid-state fermented Tartary buckwheat by Monascus purpureus. Food Chem. 2025, 464, 141662. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Xi, B.; Lu, L. Strategies to enhance production of metabolites in microbial co-culture systems. Bioresour. Technol. 2024, 406, 131049. [Google Scholar] [CrossRef]
- Anantayanon, J.; Chamkhuy, W.; Rattanaphan, N.; Panchanawaporn, S.; Laoteng, K.; Jeennor, S. Enhancing bioactive cordycepin production via precision fermentation with an engineered Aspergillus oryzae. Fermentation 2025, 11, 32. [Google Scholar] [CrossRef]
- Živković, N.M.; Čakar, U.D.; Petrović, A.V. Effects of spontaneous and inoculated fermentation on the total phenolic content and antioxidant activity of Cabernet Sauvignon wines and fermented pomace. Food Feed Res. 2024, 51, 119–129. [Google Scholar] [CrossRef]
- Zhang, J.X.; Liu, X.L.; Wang, L.; Fang, Z. Two-stage process production of microbial lipid by co-fermentation of glucose and N-acetylglucosamine from food wastes with Cryptococcus curvatus. Bioresour. Technol. 2023, 387, 129685. [Google Scholar] [CrossRef]
- Xin, Y.; Qiao, M. Towards microbial consortia in fermented foods for metabolic engineering and synthetic biology. Food Res. Int. 2025, 201, 115677. [Google Scholar] [CrossRef]
- Liang, Z.; Chen, S.; Wang, H.; Wu, Q.; Guo, W.; Ni, L.; Lv, X. Metagenomic and metabolomic profiling reveals the differences of flavor quality between Hongqu rice wines fermented with Gutian qu and Wuyi qu. Foods 2024, 13, 3114. [Google Scholar] [CrossRef]
- Jia, Y.; Wang, Z.; Liang, X.; Tu, C.; Khalifa, I.; Wang, C.; Zhu, Y.; Chen, H.; Hu, L.; Li, C. Unlocking the potential of persimmons: A comprehensive review on emerging technologies for post-harvest challenges, processing innovations, and prospective applications. Food Chem. 2024, 459, 140344. [Google Scholar] [CrossRef]
- García-García, J.C.; G-García, M.E.; Mauricio, J.C.; Moreno, J.; García-Martínez, T. Evaluation of the protein profile of a Saccharomyces cerevisiae strain immobilized in biocapsules for use in fermented foods. Foods 2024, 13, 3871. [Google Scholar] [CrossRef]
- Wang, B.; Duan, Y.; Wang, C.; Liu, C.; Wang, J.; Jia, J.; Wu, Q. Combined volatile compounds and non-targeted metabolomics analysis reveals variation in flavour characteristics, metabolic profiles and bioactivity of mulberry leaves after Monascus purpureus fermentation. J. Sci. Food Agric. 2024, 104, 3294–3305. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).