Synergetic Effect of Accentuated Cut Edges (ACE) and Macerating Enzymes on Aroma and Sensory Profiles of Marquette Red Wine
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and Standards
2.2. Grape Samples and Winemaking
2.3. Aroma Analysis by Gas Chromatography-Mass Spectrometry (GC-MS) with Solid-Phase Microextraction (SPME)
2.4. Training Sessions and Descriptive Sensory Evaluation
2.5. Statistical Analysis
3. Results and Discussion
3.1. Wine Aroma Profile by GC-MS
3.2. Wine Aroma Profile by Descriptive Sensory Analysis
3.3. Wine Color Attributes by Instrumental and Sensory Analysis
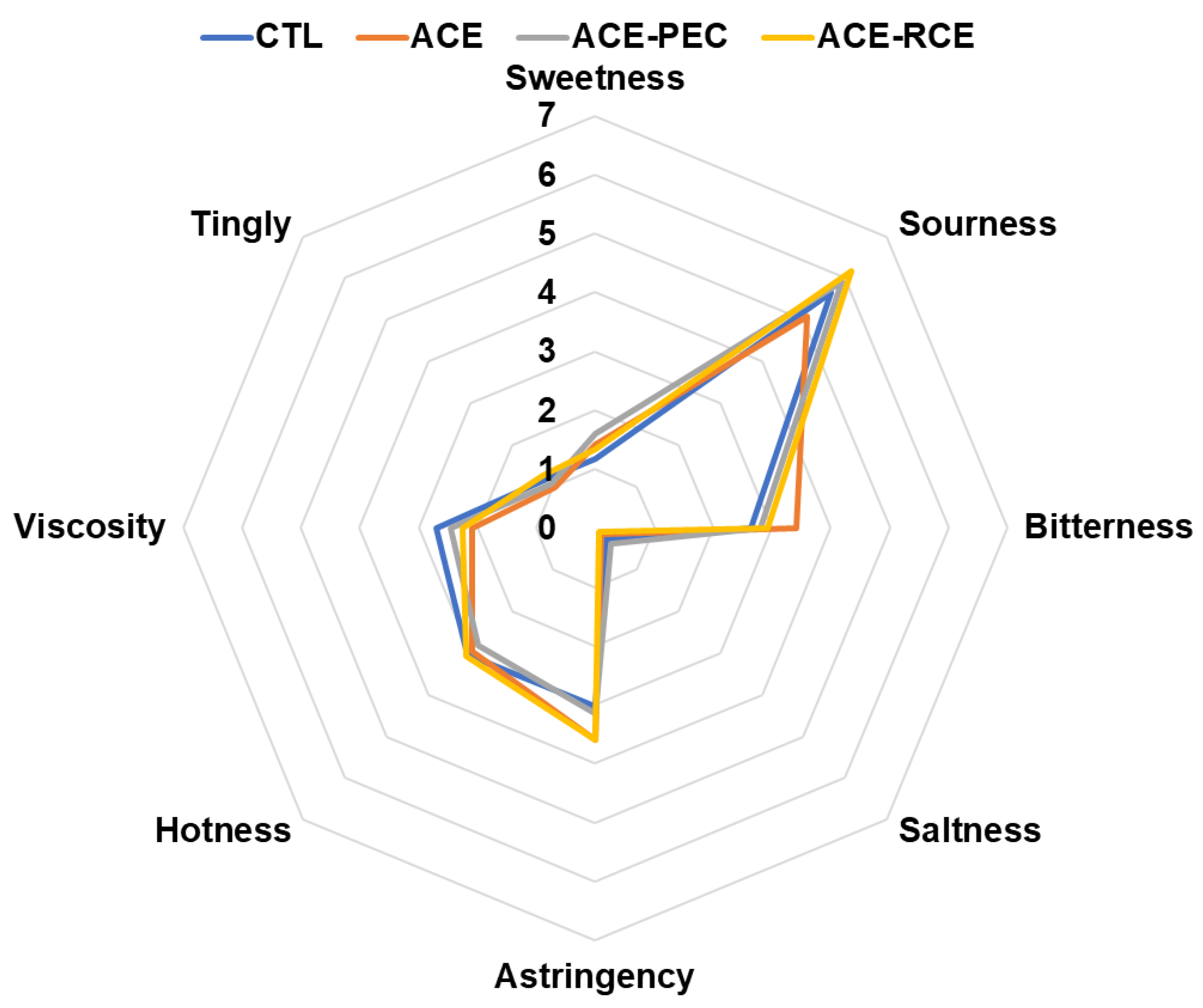
3.4. Wine Sensory Attributes of Taste and Mouthfeel
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parpinello, G.P.; Versari, A.; Chinnici, F.; Galassi, S. Relationship among Sensory Descriptors, Consumer Preference and Color Parameters of Italian Novello Red Wines. Food Res. Int. 2009, 42, 1389–1395. [Google Scholar] [CrossRef]
- Mansfield, A.K.; Schirle-Keller, J.-P.; Reineccius, G.A. Identification of Odor-Impact Compounds in Red Table Wines Produced from Frontenac Grapes. Am. J. Enol. Vitic. 2011, 62, 169–176. [Google Scholar] [CrossRef]
- Kassara, S.; Kennedy, J.A. Relationship between Red Wine Grade and Phenolics. 2. Tannin Composition and Size. J. Agric. Food Chem. 2011, 59, 8409–8412. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry; John Wiley & Sons, Ltd.: Chichester, UK, 2016; ISBN 9781118730720. [Google Scholar]
- Rice, S.; Lutt, N.; Koziel, J.; Dharmadhikari, M.; Fennell, A. Determination of Selected Aromas in Marquette and Frontenac Wine Using Headspace-SPME Coupled with GC-MS and Simultaneous Olfactometry. Separations 2018, 5, 20. [Google Scholar] [CrossRef]
- Hemstad, P.; Luby, J. Grapevine Plant Named “Marquette”. U.S. Patent No. US PP19,579 P3, 16 December 2008. [Google Scholar]
- Slegers, A.; Angers, P.; Ouellet, É.; Truchon, T.; Pedneault, K. Volatile Compounds from Grape Skin, Juice and Wine from Five Interspecific Hybrid Grape Cultivars Grown in Québec (Canada) for Wine Production. Molecules 2015, 20, 10980–11016. [Google Scholar] [CrossRef]
- Rice, S.; Maurer, D.L.; Fennell, A.; Dharmadhikari, M.; Koziel, J.A. Evaluation of Volatile Metabolites Emitted In-Vivo from Cold-Hardy Grapes during Ripening Using SPME and GC-MS: A Proof-of-Concept. Molecules 2019, 24, 536. [Google Scholar] [CrossRef]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Microbial Modulation of Aromatic Esters in Wine: Current Knowledge and Future Prospects. Food Chem. 2010, 121, 1–16. [Google Scholar] [CrossRef]
- Gil, M.; Cabellos, J.M.; Arroyo, T.; Prodanov, M. Characterization of the Volatile Fraction of Young Wines from the Denomination of Origin “Vinos de Madrid” (Spain). Anal. Chim. Acta 2006, 563, 145–153. [Google Scholar] [CrossRef]
- He, F.; Liang, N.N.; Mu, L.; Pan, Q.H.; Wang, J.; Reeves, M.J.; Duan, C.Q. Anthocyanins and Their Variation in Red Wines II. Anthocyanin Derived Pigments and Their Color Evolution. Molecules 2012, 17, 1483–1519. [Google Scholar] [CrossRef]
- Bautista-Ortin, A.B.; Martinez-Cutillas, A.; Ros-Garcia, J.M.; Lopez-Roca, J.M.; Gomez-Plaza, E. Improving Colour Extraction and Stability in Red Wines: The Use of Maceration Enzymes and Enological Tannins. Int. J. Food Sci. Technol. 2005, 40, 867–878. [Google Scholar] [CrossRef]
- Doco, T.; Williams, P.; Cheynier, V. Effect of Flash Release and Pectinolytic Enzyme Treatments on Wine Polysaccharide Composition. J. Agric. Food Chem. 2007, 55, 6643–6649. [Google Scholar] [CrossRef] [PubMed]
- Parley, A.; Vanhanen, L.; Heatherbell, D. Effects of Pre-Fermentation Enzyme Maceration on Extraction and Colour Stability in Pinot Noir Wine. Aust. J. Grape Wine Res. 2001, 7, 146–152. [Google Scholar] [CrossRef]
- Mercurio, M.D.; Dambergs, R.G.; Cozzolino, D.; Herderich, M.J.; Smith, P.A. Relationship between Red Wine Grades and Phenolics. 1. Tannin and Total Phenolics Concentrations. J. Agric. Food Chem. 2010, 58, 12313–12319. [Google Scholar] [CrossRef]
- Peleg, H.; Gacon, K.; Schlich, P.; Noble, A.C. Bitterness and Astringency of Flavan-3-Ol Monomers, Dimers and Trimers. J. Sci. Food Agric. 1999, 79, 1123–1128. [Google Scholar] [CrossRef]
- Sparrow, A.M.; Holt, H.E.; Pearson, W.; Dambergs, R.G.; Close, D.C. Accentuated Cut Edges (ACE): Effects of Skin Fragmentation on the Composition and Sensory Attributes of Pinot Noir Wines. Am. J. Enol. Vitic. 2016, 67, 169–178. [Google Scholar] [CrossRef]
- Kang, W.; Bindon, K.A.; Wang, X.; Muhlack, R.A.; Smith, P.A.; Niimi, J.; Bastian, S.E.P. Chemical and Sensory Impacts of Accentuated Cut Edges (ACE) Grape Must Polyphenol Extraction Technique on Shiraz Wines. Foods 2020, 9, 1027. [Google Scholar] [CrossRef]
- Li, S.; Bindon, K.; Bastian, S.E.P.; Jiranek, V.; Wilkinson, K.L. Use of Winemaking Supplements to Modify the Composition and Sensory Properties of Shiraz Wine. J. Agric. Food Chem. 2017, 65, 1353–1364. [Google Scholar] [CrossRef]
- Kuhlman, B.; Hansen, J.; Jørgensen, B.; du Toit, W.; Moore, J.P. The Effect of Enzyme Treatment on Polyphenol and Cell Wall Polysaccharide Extraction from the Grape Berry and Subsequent Sensory Attributes in Cabernet Sauvignon Wines. Food Chem. 2022, 385, 132645. [Google Scholar] [CrossRef]
- Mansfield, A.K.; Vickers, Z.M. Characterization of the Aroma of Red Frontenac Table Wines by Descriptive Analysis. Am. J. Enol. Vitic. 2009, 60, 435–441. [Google Scholar] [CrossRef]
- Cheng, Y.; Watrelot, A.A. Synergetic Effect of Accentuated Cut Edges (ACE) and Macerating Enzymes on the Phenolic Composition of Marquette Red Wines. Food Res. Int. 2024, 195, 114968. [Google Scholar] [CrossRef]
- Cai, L.; Rice, S.; Koziel, J.; Dharmadhikari, M. Development of an Automated Method for Selected Aromas of Red Wines from Cold-Hardy Grapes Using Solid-Phase Microextraction and Gas Chromatography-Mass Spectrometry-Olfactometry. Separations 2017, 4, 24. [Google Scholar] [CrossRef]
- Gómez García-Carpintero, E.; Gómez Gallego, M.A.; Sánchez-Palomo, E.; González Viñas, M.A. Impact of Alternative Technique to Ageing Using Oak Chips in Alcoholic or in Malolactic Fermentation on Volatile and Sensory Composition of Red Wines. Food Chem. 2012, 134, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.; Pereira, V.; Pontes, M.; Albuquerque, F.; Marques, J.C. Acetic Acid and Ethyl Acetate in Madeira Wines: Evolution with Ageing and Assessment of the Odour Rejection Threshold. Ciência e Técnica Vitivinícola 2017, 32, 1–11. [Google Scholar] [CrossRef]
- Antalick, G.; Suklje, K.; Blackman, J.W.; Meeks, C. Influence of Grape Composition on Red Wine Ester Profile: Comparison between Cabernet Sauvignon and Shiraz Cultivars from Australian Warm Climate. J. Agric. Food Chem. 2015, 63, 4664–4672. [Google Scholar] [CrossRef]
- Cortés-Diéguez, S.; Rodriguez-Solana, R.; Domínguez, J.M.; Díaz, E. Impact Odorants and Sensory Profile of Young Red Wines from Four Galician (NW of Spain) Traditional Cultivars. J. Inst. Brew. 2015, 121, 628–635. [Google Scholar] [CrossRef]
- Pedneault, K.; Dorais, M.; Angers, P. Flavor of Cold-Hardy Grapes: Impact of Berry Maturity and Environmental Conditions. J. Agric. Food Chem. 2013, 61, 10418–10438. [Google Scholar] [CrossRef]
- Rollero, S.; Zietsman, A.J.J.; Buffetto, F.; Schückel, J.; Ortiz-Julien, A.; Divol, B. Kluyveromyces Marxianus Secretes a Pectinase in Shiraz Grape Must That Impacts Technological Properties and Aroma Profile of Wine. J. Agric. Food Chem. 2018, 66, 11739–11747. [Google Scholar] [CrossRef]
- Espejo, F. Role of Commercial Enzymes in Wine Production: A Critical Review of Recent Research. J. Food Sci. Technol. 2021, 58, 9–21. [Google Scholar] [CrossRef]
- Lan, Y.; Wang, J.; Aubie, E.; Crombleholme, M.; Reynolds, A. Effects of Frozen Materials Other Than Grapes on Red Wine Aroma Compounds. Impacts of Harvest Technologies. Am. J. Enol. Vitic. 2022, 73, 134–147. [Google Scholar] [CrossRef]
- Petronilho, S.; Lopez, R.; Ferreira, V.; Coimbra, M.A.; Rocha, S.M. Revealing the Usefulness of Aroma Networks to Explain Wine Aroma Properties: A Case Study of Portuguese Wines. Molecules 2020, 25, 272. [Google Scholar] [CrossRef]
- Manns, D.C.; Coquard Lenerz, C.T.M.; Mansfield, A.K. Impact of Processing Parameters on the Phenolic Profile of Wines Produced from Hybrid Red Grapes Maréchal Foch, Corot Noir, and Marquette. J. Food Sci. 2013, 78, C696–C702. [Google Scholar] [CrossRef] [PubMed]
- Di Profio, F.; Reynolds, A.G.; Kasimos, A. Canopy Management and Enzyme Impacts on Merlot, Cabernet Franc, and Cabernet Sauvignon. II. Wine Composition and Quality. Am. J. Enol. Vitic. 2011, 62, 152–168. [Google Scholar] [CrossRef]
- Fan, S.; Liu, C.; Li, Y.; Zhang, Y. Visual Representation of Red Wine Color: Methodology, Comparison and Applications. Foods 2023, 12, 924. [Google Scholar] [CrossRef] [PubMed]
- Pedneault, K.; Provost, C. Fungus Resistant Grape Varieties as a Suitable Alternative for Organic Wine Production: Benefits, Limits, and Challenges. Sci. Hortic. 2016, 208, 57–77. [Google Scholar] [CrossRef]
- Ducasse, M.A.; Canal-Llauberes, R.M.; de Lumley, M.; Williams, P.; Souquet, J.M.; Fulcrand, H.; Doco, T.; Cheynier, V. Effect of Macerating Enzyme Treatment on the Polyphenol and Polysaccharide Composition of Red Wines. Food Chem. 2010, 118, 369–376. [Google Scholar] [CrossRef]
- Vidal, S.; Francis, L.; Williams, P.; Kwiatkowski, M.; Gawel, R.; Cheynier, V.; Waters, E. The Mouth-Feel Properties of Polysaccharides and Anthocyanins in a Wine like Medium. Food Chem. 2004, 85, 519–525. [Google Scholar] [CrossRef]
- Vidal, S.; Courcoux, P.; Francis, L.; Kwiatkowski, M.; Gawel, R.; Williams, P.; Waters, E.; Cheynier, V. Use of an Experimental Design Approach for Evaluation of Key Wine Components on Mouth-Feel Perception. Food Qual. Prefer. 2004, 15, 209–217. [Google Scholar] [CrossRef]
- Quijada-Morín, N.; Williams, P.; Rivas-Gonzalo, J.C.; Doco, T.; Escribano-Bailón, M.T. Polyphenolic, Polysaccharide and Oligosaccharide Composition of Tempranillo Red Wines and Their Relationship with the Perceived Astringency. Food Chem. 2014, 154, 44–51. [Google Scholar] [CrossRef]

| Standard Attributes | Preparation |
|---|---|
| Aroma (prepared in black wine glasses) | |
| Grape jelly | Hy-Vee® Concord Grape Jelly—25 g |
| Strawberry | Smucker’s® Jam Seedless Strawberry Jam—25 g |
| Plum | Smucker’s® Jam Red Plum—25 g |
| Black cherry | Tillen Farms® Bada Bing cherries—2 pieces (cut-off) |
| Blackcurrant | Le Nez Du Vin Mater aroma kit, Blackcurrant—2 drops dilute with 4 mL purified drinking water (Great Value®) |
| Honey | Fischer’s® Clover Honey—25 g |
| Dry fig | Great Value® Dried Figs—2 pieces (cut-off) |
| Candy fruit | Trader Joe’s Dried Baby Sweet Pineapple—2 pieces (cut-off) |
| Green apple | Freshly cut-off Granny Smith Apples |
| Cider | Redd’s® Hard Apple Fruit Beer, 5% ABV—4 mL |
| Grass | Freshly tall fescue collected from local lawn |
| Floral | Le Nez Du Vin Mater aroma kit, Rose—2 drops dilute with 4 mL purified drinking water |
| Woody | House Blend French Oak Chips—5 pieces with 8 mL purified drinking water |
| Raisin | Great Value® Sun-dried Raisins—5 pieces (cut-off) |
| Alcohol | 13% v/v ethanol (diluted from Everclear® Grain Alcohol, 75.5% Alc/vol, 151 proof) in purified drinking water—30 mL |
| Acetone | Onyx Professional® 100% Acetone Nail Polish Remover (dilution factor = 5) |
| Taste (prepared in clear plastic portion cup) | |
| Sweetness | Sucrose (C&H® pure cane sugar) in purified drinking water (40 g/L) |
| Sourness | Tartaric acid (Presque Isle Wine Cellars®) in purified drinking water (1.5 g/L) |
| Bitterness | Caffeine (Sigma-Aldrich®, anhydrous, 99%) in purified drinking water (1 g/L) |
| Saltness | Table salt (Morton®) in purified drinking water (4 g/L) |
| Mouthfeel (prepared in clear plastic portion cup) | |
| Astringency | Alum (Tone’s®) in purified drinking water (1 g/L) |
| Hotness | 13% v/v ethanol (diluted from Everclear® Grain Alcohol, 75.5% Alc/vol, 151 proof) in purified drinking water |
| Viscosity | Starch (Great Value®, corn starch) in purified drinking water (boiled, 5 g/L) |
| Tingly | LaCroix® Sparkling water, pure |
| CTL | ACE | ACE-PEC | ACE-RCE | p-Value | |
|---|---|---|---|---|---|
| Ethyl esters | |||||
| Ethyl acetate | 0.90 ± 0.09 | 1.10 ± 0.18 | 0.86 ± 0.05 | 0.97 ± 0.3 | ns |
| Ethyl butanoate | 0.06 ± 0.00 ab | 0.07 ± 0.01 a | 0.06 ± 0.00 b | 0.05 ± 0.01 b | 0.0206 |
| Ethyl hexanoate | 0.52 ± 0.09 | 0.56 ± 0.11 | 0.41 ± 0.05 | 0.38 ± 0.02 | ns |
| Ethyl octanoate | 0.64 ± 0.37 | 0.67 ± 0.27 | 0.39 ± 0.10 | 0.43 ± 0.14 | ns |
| Ethyl decanoate | 0.08 ± 0.04 | 0.08 ± 0.02 | 0.07 ± 0.01 | 0.08 ± 0.04 | ns |
| Ethyl lactate | 0.12 ± 0.02 | 0.12 ± 0.02 | 0.14 ± 0.00 | 0.13 ± 0.03 | ns |
| Ethyl 2-methylpropanoate | 0.03 ± 0.00 ab | 0.03 ± 0.00 a | 0.03 ± 0.00 b | 0.02 ± 0.00 b | 0.0167 |
| Ethyl 2-methylbutanoate | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | ns |
| Ethyl 3-methylbutanoate | 0.01 ± 0.00 ab | 0.01 ± 0.00 a | 0.01 ± 0.00 b | 0.01 ± 0.00 b | 0.0218 |
| Acetate esters | |||||
| Isoamyl acetate | 0.24 ± 0.02 | 0.25 ± 0.03 | 0.22 ± 0.04 | 0.19 ± 0.07 | ns |
| Isobutyl acetate | 0.01 ± 0 | 0.01 ± 0 | 0.01 ± 0 | 0.01 ± 0 | ns |
| Higher alcohols | |||||
| Isobutanol | 0.25 ± 0.03 | 0.27 ± 0.04 | 0.29 ± 0.01 | 0.27 ± 0.06 | ns |
| Isoamyl alcohol | 2.52 ± 0.11 | 2.81 ± 0.31 | 2.72 ± 0.06 | 2.71 ± 0.47 | ns |
| 2-phenylethanol | 0.43 ± 0.03 | 0.44 ± 0.07 | 0.42 ± 0.03 | 0.44 ± 0.05 | ns |
| 1-hexanol | 0.09 ± 0 | 0.11 ± 0.01 | 0.1 ± 0.01 | 0.1 ± 0.01 | ns |
| 1-butanol | tr | tr | tr | tr | - |
| C13-Norisoprenoid | |||||
| β-Damascenone | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | ns |
| Terpenes | |||||
| Linalool | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.01 ± 0.00 | ns |
| p-cymene | tr | tr | tr | tr | - |
| β-citronellol | tr | tr | tr | tr | - |
| Treatment | Instrumental Measurement (Published in [22]) | Sensory Analysis | ||
|---|---|---|---|---|
| Hue | Color Intensity | Hue | Color Intensity | |
| CTL | 0.6 ± 0.0 | 11.3 ± 0.1 | 1.9 ± 2.9 | 2.8 ± 2.0 B |
| ACE | 0.6 ± 0.0 | 11.4 ± 0.4 | 2.4 ± 3.7 | 3.0 ± 2.8 B |
| ACE-PEC | 0.6 ± 0.0 | 12.4 ± 0.6 | 3.0 ± 4.7 | 4.8 ± 3.5 A |
| ACE-RCE | 0.6 ± 0.0 | 11.3 ± 0.6 | 2.5 ± 3.8 | 4.8 ± 3.1 A |
| p-value | ||||
| Treatment | 0.7613 | 0.0612 | 0.1338 | 0.0009 |
| Panelist | - | - | <0.0001 | <0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Y.; Watrelot, A.A. Synergetic Effect of Accentuated Cut Edges (ACE) and Macerating Enzymes on Aroma and Sensory Profiles of Marquette Red Wine. Fermentation 2024, 10, 624. https://doi.org/10.3390/fermentation10120624
Cheng Y, Watrelot AA. Synergetic Effect of Accentuated Cut Edges (ACE) and Macerating Enzymes on Aroma and Sensory Profiles of Marquette Red Wine. Fermentation. 2024; 10(12):624. https://doi.org/10.3390/fermentation10120624
Chicago/Turabian StyleCheng, Yiliang, and Aude A. Watrelot. 2024. "Synergetic Effect of Accentuated Cut Edges (ACE) and Macerating Enzymes on Aroma and Sensory Profiles of Marquette Red Wine" Fermentation 10, no. 12: 624. https://doi.org/10.3390/fermentation10120624
APA StyleCheng, Y., & Watrelot, A. A. (2024). Synergetic Effect of Accentuated Cut Edges (ACE) and Macerating Enzymes on Aroma and Sensory Profiles of Marquette Red Wine. Fermentation, 10(12), 624. https://doi.org/10.3390/fermentation10120624






