Exploring Volatile Profiles in Cactus-Based Fermented Beverages: Effects of Fermentation Method
Abstract
1. Introduction
2. Materials and Methods
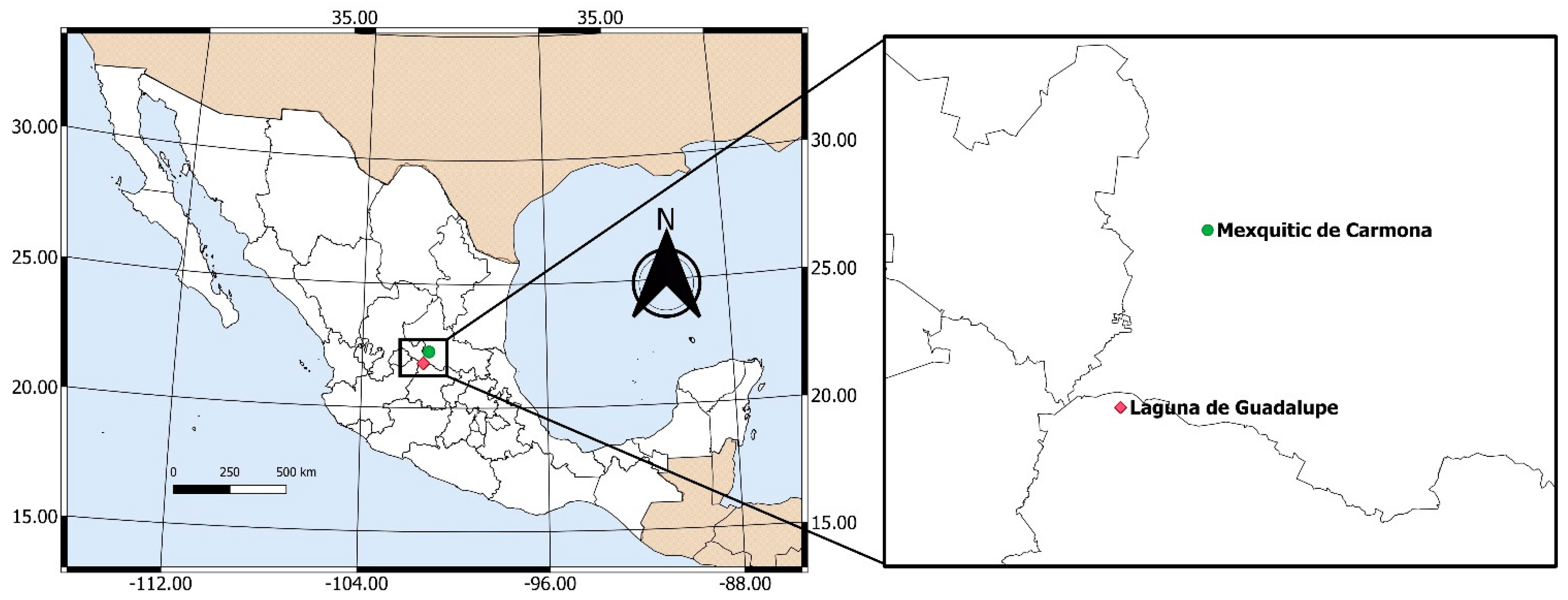
2.1. Study System and Sampling Method
2.2. Extraction of Volatile Compounds and Gas Chromatography–Mass Spectrometry Analyses
2.3. Volatile Profile Characterization
2.4. Sensory Profiling
2.5. Statistical Analysis
3. Results
3.1. Characterization of Organic Compounds
3.2. Differences in the Composition of Organic Compounds Between the Fermentation Practices
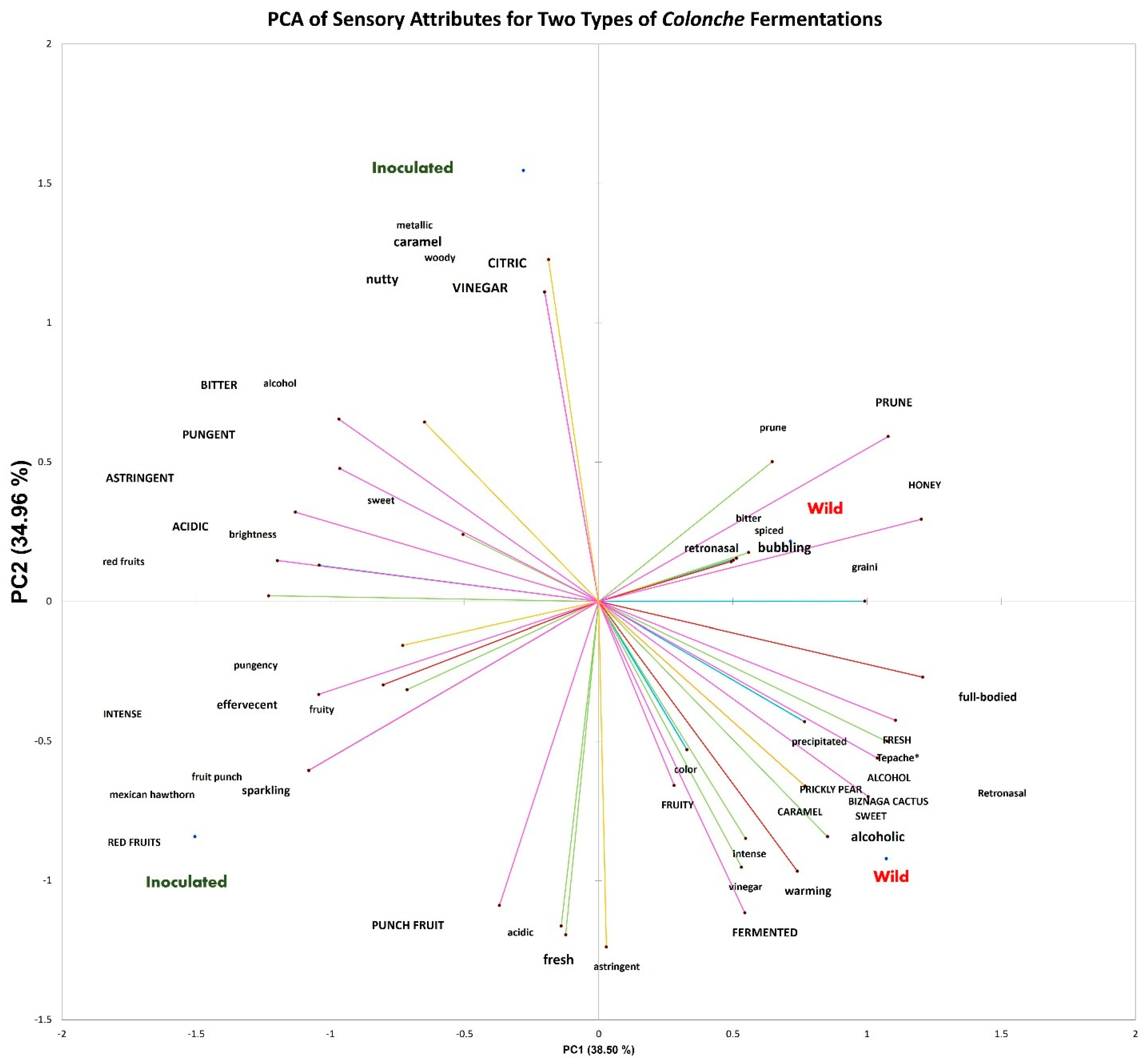
3.3. Sensory Differences Between Fermentation Practices in the Colonche Samples
4. Discussion
4.1. Fermentation Practices and Traditional Ecological Knowledge: Different Outcomes That Can Be Tasted and Smelled
4.2. Alcohols and Organic Compounds, Metabolic By-Products, and Sensory Markers
4.3. Phenolics and Spice-Linked Volatiles
4.4. Acids and Flavor Outcomes
4.5. Aldehydes and Ketones: Compounds from Raw Materials and Fermentation Processes
4.6. Esters, Fruity, and Floral Complexity in Colonche Samples
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FP | Flash Profile |
| TMFB | Traditional Mexican Fermented Beverage |
| VOCs | volatile organic compounds |
| LAB | lactic acid bacteria |
| AAB | acetic acid bacteria |
| WC | wild fermented Colonche |
| InC | inoculated Colonche |
| LLE | liquid–liquid extraction |
References
- Tamang, J.P.; Cotter, P.D.; Endo, A.; Han, N.S.; Kort, R.; Liu, S.Q.; Hutkins, R. Fermented foods in a global age: East meets West. Compr. Rev. Food Sci. Food Saf. 2020, 19, 184–217. [Google Scholar] [CrossRef] [PubMed]
- Rubio, M.T.; Lappe, P.; Wacher, C.; Ulloa, M. Estudio microbiano y químico de la fermentación de soluciones de piloncillo inoculadas con tibicos. Rev. Latinoam. De Microbiol. 1993, 35, 19–31. [Google Scholar]
- Tamang, J.P.; Thapa, N.; Tamang, B.; Rai, A.; Chettri, R. Microorganisms in fermented foods and beverages. In Health Benefits of Fermented Foods and Beverages; CRC Press: Boca Raton, FL, USA, 2015; Volume 7, pp. 1–10. [Google Scholar]
- Tamang, J.P. (Ed.) Health Benefits of Fermented Foods and Beverages; CRC Press by Taylor & Francis: Boca Raton, FL, USA, 2015; pp. 1–111. [Google Scholar]
- Tamang, J.P.; Jeyaram, K.; Rai, A.K.; Mukherjee, P.K. Diversity of beneficial microorganisms and their functionalities in community-specific ethnic fermented foods of the Eastern Himalayas. Food Res. Int. 2021, 148, 110633. [Google Scholar] [CrossRef] [PubMed]
- Tamang, J.P. History and culture of Indian ethnic fermented foods and beverages. In Ethnic Fermented Foods and Beverages of India: Science History and Culture; Springer: Singapore, 2020; pp. 1–40. [Google Scholar]
- Tamang, J.P.; Thapa, N.; Bhalla, T.C.; Savitri. Ethnic fermented foods and beverages of India. In Ethnic Fermented Foods and Alcoholic Beverages of Asia; Springer: New Delhi, India, 2016; pp. 17–72. [Google Scholar]
- Tamang, J.P.; Watanabe, K.; Holzapfel, W.H. Diversity of microorganisms in global fermented foods and beverages. Front. Microbiol. 2016, 7, 377. [Google Scholar] [CrossRef]
- Tamang, J.P. Diversity of fermented beverages and alcoholic drinks. In Fermented Foods and Beverages of the World; CRC Press: Boca Raton, FL, USA, 2010; pp. 85–125. [Google Scholar]
- Mukisa, I.M.; Byaruhanga, Y.B.; Muyanja, C.M.; Langsrud, T.; Narvhus, J.A. Production of organic flavor compounds by dominant lactic acid bacteria and yeasts from Obushera, a traditional sorghum malt fermented beverage. Food Sci. Nutr. 2017, 5, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Katz, S.E. The Art of Fermentation: An In-Depth Exploration of Essential Concepts and Processes from Around the World; Chelsea Green Publishing: Chelsea, VT, USA, 2012; pp. 1–15. [Google Scholar]
- Achi, O.K. The potential for upgrading traditional fermented foods through biotechnology. Afr. J. Biotechnol. 2005, 4, 375–380. [Google Scholar]
- Achi, O.K.; Akomas, N.S. Comparative assessment of fermentation techniques in the processing of fufu, a traditional fermented cassava product. Pak. J. Nutr. 2006, 5, 224–229. [Google Scholar]
- Parente, E.; Cogan, T.M.; Powell, I.B. Starter cultures: General aspects. In Cheese; Academic Press: Cambridge, MA, USA, 2017; pp. 201–226. [Google Scholar]
- Routray, W.; Mishra, H.N. Scientific and technical aspects of yogurt aroma and taste: A review. Compr. Rev. Food Sci. Food Saf. 2011, 10, 208–220. [Google Scholar] [CrossRef]
- Bordiga, M.; Nollet, L.M. (Eds.) Food Aroma Evolution: During Food Processing, Cooking, and Aging; CRC Press: Boca Raton, FL, USA, 2019; pp. 1–155. [Google Scholar]
- Shutz, H.G. Appropriateness as a measure of cognitive-contextual aspects of food acceptance. In Measurement of Food Preferences; Macfie, H.J.H., Thomson, D.M.H., Eds.; Blackie Academic and Professional: London, UK, 1994; pp. 25–49. [Google Scholar]
- Lemfack, M.C.; Nickel, J.; Dunkel, M.; Preissner, R.; Piechulla, B. mVOC: A database of microbial volatiles. Nucleic Acids Res. 2014, 42, D744–D748. [Google Scholar] [CrossRef]
- Lemfack, M.C.; Gohlke, B.O.; Toguem, S.M.T.; Preissner, S.; Piechulla, B.; Preissner, R. mVOC 2.0: A database of microbial volatiles. Nucleic Acids Res. 2018, 46, D1261–D1265. [Google Scholar] [CrossRef]
- Schmidt, R.; Cordovez, V.; De Boer, W.; Raaijmakers, J.; Garbeva, P. Volatile affairs in microbial interactions. ISME J. 2015, 9, 2329–2335. [Google Scholar] [CrossRef] [PubMed]
- McGee, H. Nose Dive: A Field Guide to the World’s Smells; Hachette: London, UK, 2020; pp. 3–37. [Google Scholar]
- Swiegers, J.H.; Bartowsky, E.J.; Henschke, P.A.; Pretorius, I. Yeast and bacterial modulation of wine aroma and flavour. Aust. J. Grape Wine Res. 2005, 11, 139–173. [Google Scholar] [CrossRef]
- Wheatley, R.E. The consequences of volatile organic compound mediated bacterial and fungal interactions. Antonie Van Leeuwenhoek 2002, 81, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Pichersky, E.; Gershenzon, J. Biochemistry of plant volatiles. Plant Physiol. 2004, 135, 1893–1902. [Google Scholar] [CrossRef]
- Soccol, C.R.; Medeiros, A.B.; Vandenberghe, L.P.; Woiciechowski, A.L. Flavor compounds produced by fungi, yeasts, and bacteria. Handb. Food Prod. Manuf. 2007, 1, 179–191. [Google Scholar]
- Sadineni, V.; Kondapalli, N.; Obulam, V.S.R. Effect of co-fermentation with Saccharomyces cerevisiae and Torulaspora delbrueckii or Metschnikowia pulcherrima on the aroma and sensory properties of mango wine. Ann. Microbiol. 2012, 62, 1353–1360. [Google Scholar] [CrossRef]
- Azzolini, M.; Tosi, E.; Lorenzini, M.; Finato, F.; Zapparoli, G. Contribution to the aroma of white wines by controlled Torulaspora delbrueckii cultures in association with Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2015, 31, 277–293. [Google Scholar] [CrossRef]
- Romano, P.; Braschi, G.; Siesto, G.; Patrignani, F.; Lanciotti, R. Role of yeasts on the sensory component of wines. Foods 2022, 11, 1921. [Google Scholar] [CrossRef]
- Romano, P.; Fiore, C.; Paraggio, M.; Caruso, M.; Capece, A. Function of yeast species and strains in wine flavour. Int. J. Food Microbiol. 2003, 86, 169–180. [Google Scholar] [CrossRef]
- Nurgel, C.; Erten, H.; Canbas, A.; Cabaroğlu, T.; Selli, S. Contribution by Saccharomyces cerevisiae yeasts to fermentation and flavour compounds in wines from cv. Kalecik karasi grape. J. Inst. Brew. 2002, 108, 68–72. [Google Scholar] [CrossRef]
- Demuyter, C.; Lollier, M.; Legras, J.L.; Le Jeune, C. Predominance of Saccharomyces uvarum during spontaneous alcoholic fermentation, for three consecutive years, in an Alsatian winery. J. Appl. Microbiol. 2004, 97, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Linares, C.; Álvarez-Ríos, G.D.; Figueredo-Urbina, C.J.; Islas, L.A.; Lappe-Oliveras, P.; Nabhan, G.P.; Casas, A. Traditional fermented beverages of Mexico: A biocultural unseen foodscape. Foods 2021, 10, 2390. [Google Scholar] [CrossRef]
- Ojeda-Linares, C.I.; Vallejo, M.; Lappe-Oliveras, P.; Casas, A. Traditional management of microorganisms in fermented beverages from cactus fruits in Mexico: An ethnobiological approach. J. Ethnobiol. Ethnomedicine 2020, 16, 1. [Google Scholar] [CrossRef] [PubMed]
- Ojeda-Linares, C.I.; Solís-García, I.A.; Casas, A. Constructing micro-landscapes: Management and selection practices on microbial communities in a traditional fermented beverage. Front. Ecol. Evol. 2022, 10, 821268. [Google Scholar] [CrossRef]
- NIST2011.L; NIST Chemistry WebBook. National Institute of Standards and Technology. U.S. Department of Commerce: Gaithersburg, MD, USA, 2020. Available online: https://webbook.nist.gov/chemistry/ (accessed on 1 February 2025).
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 5 online ed.; Texensis Publishing: Gruver, TX, USA, 2017. [Google Scholar]
- National Library of Medicine, National Center for Biotechnology Information. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 10 October 2023).
- Väkeväinen, K.; Hernández, J.; Simontaival, A.I.; Severiano-Pérez, P.; Díaz-Ruiz, G.; von Wright, A.; Plumed-Ferrer, C. Effect of different starter cultures on the sensory properties and microbiological quality of Atole agrio, a fermented maize product. Food Control 2020, 109, 106907. [Google Scholar] [CrossRef]
- Arnés, E.; Severiano-Pérez, P.; Astier, M. Sensory profile and acceptance of maize tortillas by rural and urban consumers in Mexico. J. Sci. Food Agric. 2022, 102, 2300–2308. [Google Scholar] [CrossRef]
- ISO 8589:2007 (E); Sensory Analysis: General Guidance for the Design of Test Rooms. ISO, European Committee for Standardization: Geneva, Switzerland, 2007.
- Becker, R.A.; Chambers, J.M.; Wilks, A.R. The New S Language; Wadsworth & Brooks/Cole: Pacific Grove, CA, USA, 1988. [Google Scholar]
- Wei, T.; Simko, V.; Levy, M.; Xie, Y.; Jin, Y.; Zemla, J. Package ‘corrplot’. Statistician 2017, 56, e24. [Google Scholar]
- Oksanen, J.; Kindt, R.; Legendre, P.; O’Hara, B.; Stevens, M.H.H.; Oksanen, M.J.; Suggests, M.A.S.S. The vegan package. Community Ecol. Package 2007, 10, 719. [Google Scholar]
- Chen, C.; Huang, K.; Yu, H.; Tian, H. The diversity of microbial communities in Chinese milk fan and their effects on volatile organic compound profiles. J. Dairy Sci. 2021, 104, 2581–2593. [Google Scholar] [CrossRef]
- Orji, M.U.; Mbata, T.I.; Aniche, G.N.; Ahonkhai, I. The use of starter cultures to produce ‘Pito’, a Nigerian fermented alcoholic beverage. World J. Microbiol. Biotechnol. 2003, 19, 733–736. [Google Scholar] [CrossRef]
- Zorba, M.; Hancioglu, O.; Genc, M.; Karapinar, M.; Ova, G. The use of starter cultures in the fermentation of boza, a traditional Turkish beverage. Process Biochem. 2003, 38, 1405–1411. [Google Scholar] [CrossRef]
- Lévi-Strauss, C. The culinary triangle. In Food and Culture, 3rd ed.; Routledge: London, UK, 2012; pp. 54–61. [Google Scholar]
- Mosciano, G.; Fasano, M.; Cassidy, J.; Connelly, K.; Mazeiko, P.; Montenegro, A.; Sadurai, S. Organoleptic characteristics of flavor materials. Perfum. Flavorist 1993, 18, 39. [Google Scholar]
- Scognamiglio, J.; Jones, L.; Letizia, C.S.; Api, A.M. Fragrance material review on phenylethyl alcohol. Food Chem. Toxicol. 2012, 50, S224–S239. [Google Scholar] [CrossRef] [PubMed]
- Budić-Leto, I.; Humar, I.; Zdunić, G.; Hribar, J.; Zlatić, E. Volatile Compounds in Prošek Dessert Wines Produced from White and Red Grapes. Czech J. Food Sci. 2015, 33, 354–360. [Google Scholar] [CrossRef]
- Azah, R.N.; Roshanida, A.R.; Norzita, N. Production of 3-Methyl-1-Butanol by yeast wild strain. Int. J. Biol. Biomol. Agric. Food Biotechnol. Eng. 2014, 8, 412–415. [Google Scholar]
- Escalante, A.; Elena Rodríguez, M.; Martínez, A.; López-Munguía, A.; Bolívar, F.; Gosset, G. Characterization of bacterial diversity in Pulque, a traditional Mexican alcoholic fermented beverage, as determined by 16S rDNA analysis. FEMS Microbiol. Lett. 2004, 235, 273–279. [Google Scholar] [CrossRef]
- De León-Rodríguez, A.; Escalante-Minakata, P.; Jiménez-García, M.I.; Ordoñez-Acevedo, L.G.; Flores Flores, J.L.; Barba de la Rosa, A.P. Characterization of volatile compounds from ethnic agave alcoholic beverages by gas chromatography-mass spectrometry. Food Technol. Biotechnol. 2008, 46, 448–455. [Google Scholar]
- Dysvik, A.; La Rosa, S.L.; Liland, K.H.; Myhrer, K.S.; Østlie, H.M.; De Rouck, G.; Wicklund, T. Co-fermentation involving Saccharomyces cerevisiae and Lactobacillus species tolerant to brewing-related stress factors for controlled and rapid production of sour beer. Front. Microbiol. 2020, 11, 279. [Google Scholar] [CrossRef]
- Gutiérrez-Sarmiento, W.; Peña-Ocaña, B.A.; Lam-Gutiérrez, A.; Guzmán-Albores, J.M.; Jasso-Chávez, R.; Ruíz-Valdiviezo, V.M. Microbial community structure, physicochemical characteristics and predictive functionalities of the Mexican tepache fermented beverage. Microbiol. Res. 2022, 260, 127045. [Google Scholar] [CrossRef]
- Escalante, A.; López Soto, D.R.; Velázquez Gutiérrez, J.E.; Giles-Gómez, M.; Bolívar, F.; López-Munguía, A. Pulque, a traditional Mexican alcoholic fermented beverage: Historical, microbiological, and technical aspects. Front. Microbiol. 2016, 7, 1026. [Google Scholar] [CrossRef]
- Alcántara-Hernández, R.J.; Rodríguez-Álvarez, J.A.; Valenzuela-Encinas, C.; Gutiérrez-Miceli, F.A.; Castañón-González, H.; Marsch, R.; Dendooven, L. The bacterial community in ‘taberna’a traditional beverage of Southern Mexico. Lett. Appl. Microbiol. 2010, 51, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Hazelwood, L.A.; Daran, J.M.; Van Maris, A.J.; Pronk, J.T.; Dickinson, J.R. The Ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef] [PubMed]
- Styger, G.; Jacobson, D.; Bauer, F.F. Identifying genes that impact on aroma profiles produced by Saccharomyces cerevisiae and the production of higher alcohols. Appl. Microbiol. Biotechnol. 2011, 91, 713–730. [Google Scholar] [CrossRef] [PubMed]
- Maicas, S. The role of yeasts in fermentation processes. Microorganisms 2020, 8, 1142. [Google Scholar] [CrossRef]
- Carpena, M.; Fraga-Corral, M.; Otero, P.; Nogueira, R.A.; Garcia-Oliveira, P.; Prieto, M.A.; Simal-Gandara, J. Secondary aroma: Influence of wine microorganisms in their aroma profile. Foods 2020, 10, 51. [Google Scholar] [CrossRef]
- Jeromel, A.; Korenika, A.-M.J.; Tomaz, I. 6—An Influence of Different Yeast Species on Wine Aroma Composition. In Fermented Beverages; Grumezescu, A.M., Holban, A.M.B.T.-F.B., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 171–285. ISBN 978-0-12-815271-3. [Google Scholar]
- Rodríguez-Lerma, G.K.; Gutiérrez-Moreno, K.; Cárdenas-Manríquez, M.; Botello-Álvarez, E.; Jiménez-Islas, H.; Rico-Martínez, R.; Navarrete-Bolaños, J.L. Microbial ecology studies of spontaneous fermentation: Starter culture selection for prickly pear wine production. J. Food Sci. 2011, 76, M346–M352. [Google Scholar] [CrossRef]
- Navarrete-Bolaños, J.L.; Fato-Aldeco, E.; Gutiérrez-Moreno, K.; Botello-Álvarez, J.E.; Jiménez-Islas, H.; Rico-Martínez, R. A strategy to design efficient fermentation processes for traditional beverages production: Prickly pear wine. J. Food Sci. 2013, 78, M1560–M1568. [Google Scholar] [CrossRef]
- Idowu, S.; Adekoya, A.E.; Igiehon, O.O.; Idowu, A.T. Clove (Syzygium aromaticum) spices: A review on their bioactivities, current use, and potential application in dairy products. J. Food Meas. Charact. 2021, 15, 3419–3435. [Google Scholar] [CrossRef]
- Nabavi, S.F.; Di Lorenzo, A.; Izadi, M.; Sobarzo-Sánchez, E.; Daglia, M.; Nabavi, S.M. Antibacterial effects of cinnamon: From farm to food, cosmetic and pharmaceutical industries. Nutrients 2015, 7, 7729–7748. [Google Scholar] [CrossRef]
- Apablaza, E.; Sáenz, C.; Prat, L.; Ubeda, C. Comprehensive characterization and volatile profile of cactus pear fruits from six different species and cultivars. ACS Food Sci. Technol. 2021, 1, 928–936. [Google Scholar] [CrossRef]
- Sepúlveda, L.; Laredo-Alcalá, E.; Buenrostro-Figueroa, J.J.; Ascacio-Valdés, J.A.; Genisheva, Z.; Aguilar, C.; Teixeira, J. Ellagic acid production using polyphenols from orange peel waste by submerged fermentation. Electron. J. Biotechnol. 2020, 43, 1–7. [Google Scholar] [CrossRef]
- Sauer, M.; Porro, D.; Mattanovich, D.; Branduardi, P. Microbial production of organic acids: Expanding the markets. Trends Biotechnol. 2008, 26, 100–108. [Google Scholar] [CrossRef]
- Yoshida, S.; Yokoyama, A. Identification and characterization of genes related to the production of organic acids in yeast. J. Biosci. Bioeng. 2012, 113, 556–561. [Google Scholar] [CrossRef] [PubMed]
- De Vuyst, L.; Leroy, F. Functional role of yeasts, lactic acid bacteria and acetic acid bacteria in cocoa fermentation processes. FEMS Microbiol. Rev. 2020, 44, 432–453. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Guo, W.; Ai, L.; Chen, C.; Tian, H. Unraveling the difference in aroma characteristics of Huangjiu from Shaoxing region fermented with different brewing water, using descriptive sensory analysis, comprehensive two-dimensional gas chromatography–quadrupole mass spectrometry and multivariate data analysis. Food Chem. 2022, 372, 131227. [Google Scholar]
- Wang, X.; Fan, W.; Xu, Y. Comparison on aroma compounds in Chinese soy sauce and strong aroma type liquors by gas chromatography–olfactometry, chemical quantitative and odor activity values analysis. Eur. Food Res. Technol. 2014, 239, 813–825. [Google Scholar] [CrossRef]
- Xu, W.; Jiang, J.; Xu, Q.; Zhong, M. Drinking tastes of Chinese rice wine under different heating temperatures analyzed by gas chromatography–mass spectrometry and tribology tests. J. Texture Stud. 2021, 52, 124–136. [Google Scholar] [CrossRef]
- Fan, X.; Liu, G.; Qiao, Y.; Zhang, Y.; Leng, C.; Chen, H.; Feng, Z. Characterization of volatile compounds by SPME-GC-MS during the ripening of Kedong Sufu, a typical Chinese traditional bacteria-fermented soybean product. J. Food Sci. 2019, 84, 2441–2448. [Google Scholar] [CrossRef]
| Volatile Compound | tR b | Colonche Samples | ||
|---|---|---|---|---|
| Wild a | Inoculated a | |||
| 1,3-butanediol | 8.95 | 0.53 ± 0.54 | 0.28 ± 0.31 | |
| Alcohols | (E)-2-hexenol | 10.39 | 0.22 ± 0.57 | 0.27 ± 0.76 |
| 1-butanol | 5.24 | 0.29 ± 0.28 | 0.19 ± 0.26 | |
| 3-methyl,1-butanol *** | 6.30 | 4.29 ± 4.64 | 1.39 ± 2.80 | |
| 2,3-butanediol | 8.55 | 0.65 ± 1.00 | 0.31 ± 0.67 | |
| 1-hexanol,2-ethyl | 13.61 | 0.08 ± 0.11 | 0.00 ± 0.01 | |
| 1-Propanol,2-methyl *** | 6.28 | 1.02 ± 1.42 | 0.23 ± 0.45 | |
| Isoprenol | 8.75 | 0.02 ± 0.03 | 0.00 ± 0.01 | |
| 3-Heptanol,4-methyl ** | 10.39 | 0.16 ± 0.31 | 0.00 ± 0.01 | |
| Phenylethyl alcohol | 14.91 | 0.22± 0.25 | 0.12 ± 0.13 | |
| Phenols | Phenol, 2,4-bis(1,1-dimethylethyl) | 32.83 | 0.72 ± 0.89 | 0.42 ± 0.74 |
| Decane-2-methyl | 8.55 | 0.10 ± 0.13 | Nd | |
| Dodecane *** | 12.50 | 0.10 ± 0.10 | 0.02 ± 0.06 | |
| Dodecane, 2,6,10-trimethyl | 6.10 | 0.10 ± 0.22 | 0.00 ± 0.01 | |
| Alkanes | Dodecane, 2,4-dimethyl | 12.04 | 0.14 ± 0.13 | 0.06 ± 0.10 |
| Heptadecane | 19.08 | 0.10 ± 0.25 | 0.02 ± 0.02 | |
| Hexadecane | 19.48 | 0.04 ± 0.06 | 0.03 ± 0.05 | |
| Pentadecane | 13.83 | 0.10 ± 0.08 | 0.07 ± 0.08 | |
| Hexadecane, 2,6,10,14,-tetramethyl | 12.35 | 0.09 ± 0.15 | 0.0 ± 0.00 | |
| Hexadecane, 4-methyl | 19.00 | 0.40 ± 1.08 | 0.03 ± 0.05 | |
| Tridecane, 3-methyl | 24.45 | 0.01 ± 0.02 | Nd | |
| Tridecane, 5-methyl | 18.12 | 0.04 ± 0.05 | 0.01 ± 0.02 | |
| Alkens | 1-Dodecene | 12.00 | 0.04 ± 0.12 | 0.00 ± 0.01 |
| 2-Undecene, 5-methyl *** | 4.62 | 0.46 ± 0.48 | 0.23 ± 0.45 | |
| 1-Eicosense | 28.98 | 0.04 ± 0.09 | 0.01 ± 0.02 | |
| 10-Eicosene, (E) | 30.45 | 0.03 ± 0.05 | 0.01 ± 0.01 | |
| Aldehydes | Hexanal | 24.19 | 0.07 ± 0.07 | 0.01 ± 0.02 |
| (E)-2-hexenal | 18.30 | 3.09 ± 7.67 | 0.64 ± 1.14 | |
| 1,3-Dimethylbenzene | 4.98 | 0.24 ± 0.39 | 0.27 ± 0.39 | |
| Benzaldehyde, 4-methyl | 48.80 | 0.06 ± 0.07 | 0.05 ± 0.13 | |
| Aroma compounds | Benzyl-alcohol | 27.01 | 0.15 ± 0.23 | 0.20 ± 0.35 |
| Phenylethyl alcohol | 32.84 | 4.08 ± 3.43 | 1.90 ± 3.36 | |
| prop-2-enyl 2-phenylacetate | 4.50 | 0.38 ± 0.79 | 0.06 ± 0.11 | |
| Ethylbenzene *** | 4.87 | 0.36 ± 0.37 | 0.18 ± 0.30 | |
| Acetic acid | 12.47 | 0.04 ± 0.71 | 2.64 ± 5.37 | |
| Carboxylic acids | Hexanoic acid | 6.70 | 0.07 ± 0.08 | 1.20 ± 2.10 |
| Decanoic acid | 18.41 | 0.04 ± 0.07 | 0.07 ± 0.19 | |
| Octanoic acid ** | 33.51 | 0.77 ± 1.76 | 0.06 ± 0.13 | |
| Esters | Isoamyl lactate | 16.38 | 0.07 ± 0.10 | 0.00 ± 0.02 |
| ethyl (E)-octadec-9-enoate | 40.37 | 0.15 ± 0.34 | Nd | |
| Ethyl linolelaidate | 47.40 | 0.20 ± 0.20 | 0.13 ± 0.19 | |
| Butanedioic acid | 7.97 | 1.68 ± 2.15 | 0.77 ± 1.11 | |
| Decanoic acid, ethyl ester | 18.61 | 0.50 ± 0.65 | 0.73 ± 2.06 | |
| Dodecanoic acid | 29.49 | 0.02 ± 0.04 | 0.00 ± 0.02 | |
| Ethyl 9-hexadecenoate | 10.26 | 1.23 ± 2.96 | 0.07 ± 0.15 | |
| Ethyl dl-2-hydroxycaproate | 40.29 | 0.30 ± 0.60 | 0.06 ± 0.09 | |
| Oleic acid | 48.62 | 0.56 ± 1.61 | Nd | |
| Hexadecanoic acid | 43.76 | 1.03 ± 1.81 | 0.10 ± 0.15 | |
| Linoleic acid | 47.60 | 0.52 ± 0.87 | 0.07 ± 0.22 | |
| Vinyl hexanoate | 26.19 | 0.20 ± 0.26 | 0.02 ± 0.05 | |
| Propanoic acid, 2-hydroxy-, ethyl ester, (S) | 9.37 | 5.69 ± 9.15 | 2.34 ± 3.83 | |
| 3-Hydroxy-2-butanone | 7.12 | 0.40 ± 0.43 | 0.19 ± 0.43 | |
| Ketones | 4-Hydroxy-4-methyl-2-pentanone | 24.34 | 0.06 ± 0.06 | 0.05 ± 0.06 |
| 7-Tridecanone | 11.25 | 0.05 ± 0.10 | Nd | |
| Lactones | Butyrolactone | 18.23 | 0.07 ± 0.16 | 0.01 ± 0.02 |
| Df | Sum of Sqs | F | Pr | Sig | |
|---|---|---|---|---|---|
| Fermentation type | 1 | 40.2 | 15.34 | 9.56 × 10−5 | *** |
| Volatile | 54 | 675.1 | 4.76 | <2 × 10−16 | *** |
| Residual | 1044 | 2736.7 |
| Df | Sum of Sqs | R2 | F | Sig | |
|---|---|---|---|---|---|
| Model | 1 | 0.528 | 0.10 | 2.04 | 0.025 * |
| Residual | 18 | 4.62 | 0.89 | ||
| Total | 19 | 5.15 | 1.00 |
| Attributes | Colonche | ||
|---|---|---|---|
| Wild | Inoculated | ||
| Appearance | Color | + | + |
| Brightness | + | + | |
| Grainy | + | + | |
| Precipitated | + | + | |
| Bubbling | + | − | |
| Odor | Acidic | + | + |
| Alcoholic | + | + | |
| Sweet | + | + | |
| Prune | + | + | |
| Fruity | + | + | |
| Tepache | + | + | |
| Fresh | + | + | |
| Bitter | + | + | |
| Vinegar | + | + | |
| Intense | + | + | |
| Red fruits | − | + | |
| Caramel | − | + | |
| Spiced | + | − | |
| Tejocote | − | + | |
| Fruit punch | − | + | |
| Woody | − | + | |
| Nutty | − | + | |
| Mouthfeel | Full-bodied | + | + |
| Warming | + | + | |
| Viscous | − | + | |
| Retronasal | + | − | |
| Sparkling | + | − | |
| Effervescent | − | + | |
| Flavor | Alcohol | + | + |
| Sweet | + | + | |
| Honey | + | + | |
| Caramel | + | + | |
| Prune | + | + | |
| Fruity | + | + | |
| Acidic | + | + | |
| Fermented | + | + | |
| Fresh | + | − | |
| Prickly pear fruit | + | − | |
| Cacti/Biznaga | + | − | |
| Punch fruit | + | + | |
| Astringent | + | + | |
| Intense | + | + | |
| Red fruits | − | + | |
| Vinegar | − | + | |
| Bitter | − | + | |
| Pungent | + | + | |
| Citric | − | + | |
| Aftertaste | Astringent | + | + |
| Metallic | + | + | |
| Pungency | + | + | |
| Total | 50 | 38 | 43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ojeda-Linares, C.; Casas, A.; Severiano-Pérez, P.; Sandoval-Velasco, M.; García-Rodríguez, Y.M.; Espinosa-García, F.J. Exploring Volatile Profiles in Cactus-Based Fermented Beverages: Effects of Fermentation Method. Fermentation 2025, 11, 275. https://doi.org/10.3390/fermentation11050275
Ojeda-Linares C, Casas A, Severiano-Pérez P, Sandoval-Velasco M, García-Rodríguez YM, Espinosa-García FJ. Exploring Volatile Profiles in Cactus-Based Fermented Beverages: Effects of Fermentation Method. Fermentation. 2025; 11(5):275. https://doi.org/10.3390/fermentation11050275
Chicago/Turabian StyleOjeda-Linares, César, Alejandro Casas, Patricia Severiano-Pérez, Marcela Sandoval-Velasco, Yolanda M. García-Rodríguez, and Francisco J. Espinosa-García. 2025. "Exploring Volatile Profiles in Cactus-Based Fermented Beverages: Effects of Fermentation Method" Fermentation 11, no. 5: 275. https://doi.org/10.3390/fermentation11050275
APA StyleOjeda-Linares, C., Casas, A., Severiano-Pérez, P., Sandoval-Velasco, M., García-Rodríguez, Y. M., & Espinosa-García, F. J. (2025). Exploring Volatile Profiles in Cactus-Based Fermented Beverages: Effects of Fermentation Method. Fermentation, 11(5), 275. https://doi.org/10.3390/fermentation11050275






