Improved Cellulolytic Activity of Alternaria citri: Optimization and EMS Treatment for Enhanced Cellulase Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Microorganism and Culture Conditions
2.2. Screening of A. citri Cellulases
2.3. Chemical Mutagenesis Using EMS
2.4. Screening and Selection of Mutant Strains
2.4.1. Endoglucanase Assay
2.4.2. Exoglucanase Assay
2.4.3. β-Glucosidase
2.5. Optimization of Physical Factors for Cellulase Production
2.6. Optimization of Nutritional Requirements for Cellulase Production
2.7. Cellulase Purification from Wild A. citri and Mutant A. citri 305
2.8. Determination of Protein Concentration
2.9. Enzyme Characterization from Wild A. citri and Mutant A. citri 305
2.9.1. Effects of Temperature, pH, Metal Ions, and Additives on Purified Cellulase Activity
2.9.2. Enzyme Kinetics of Purified Cellulases from Wild A. citri and Mutant A. citri 305
2.10. Statistical Analysis
3. Results and Discussion
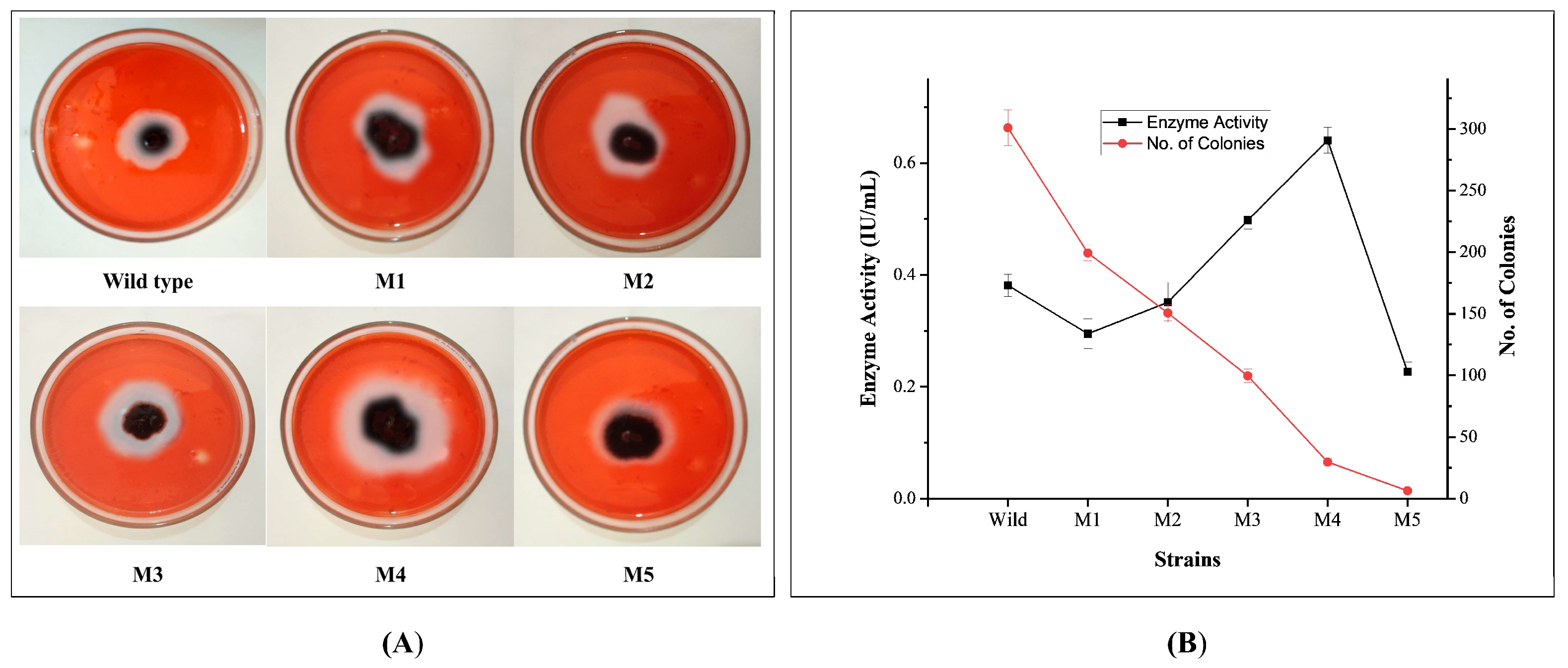
3.1. Effects of EMS Mutagenesis and Cellulolytic Screening of Mutants
3.2. Optimization of Physical Parameters for Cellulase Production by Wild A. citri and Mutant A. citri 305
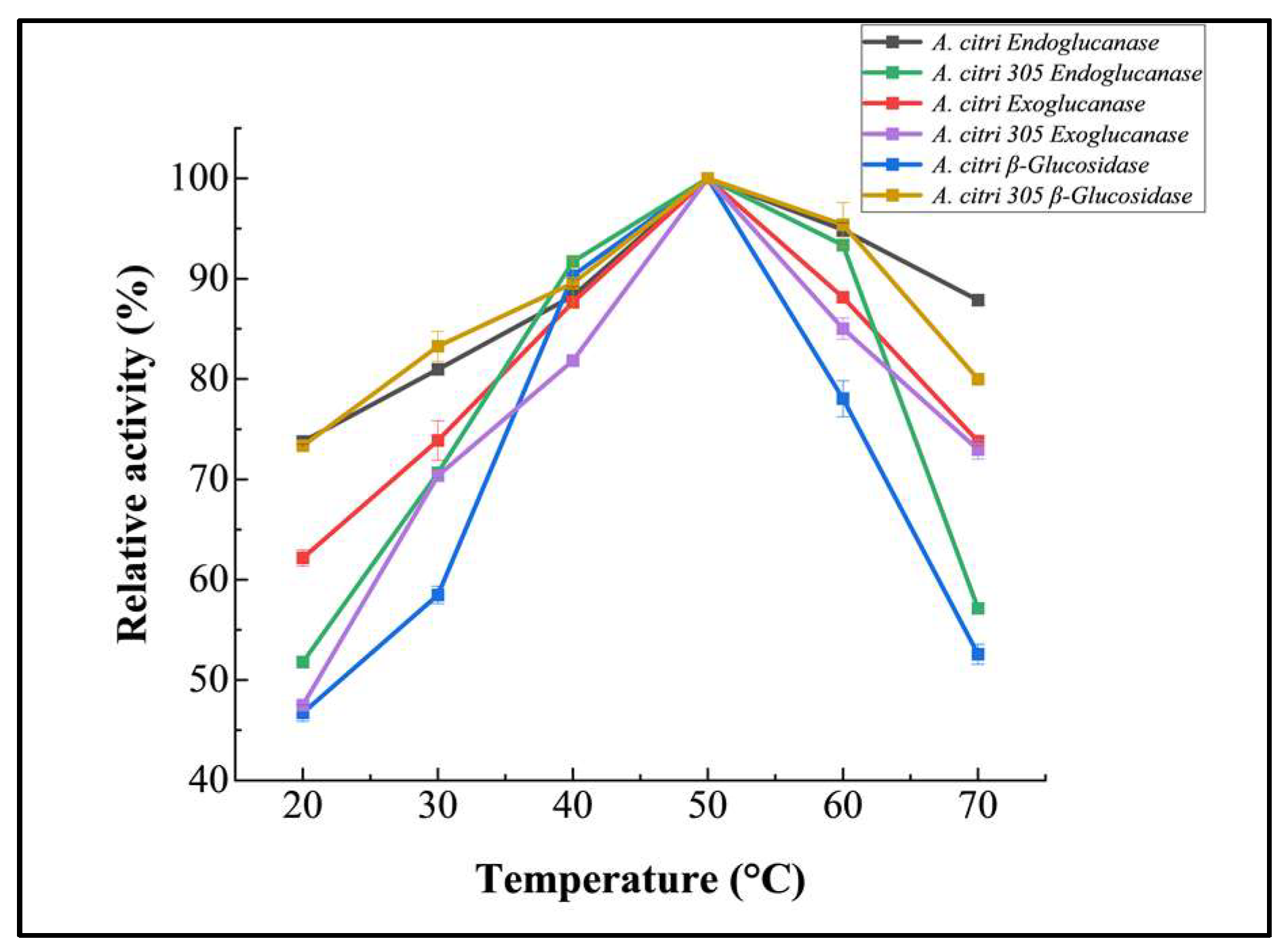
3.2.1. Temperature Optimization
3.2.2. Optimization of pH
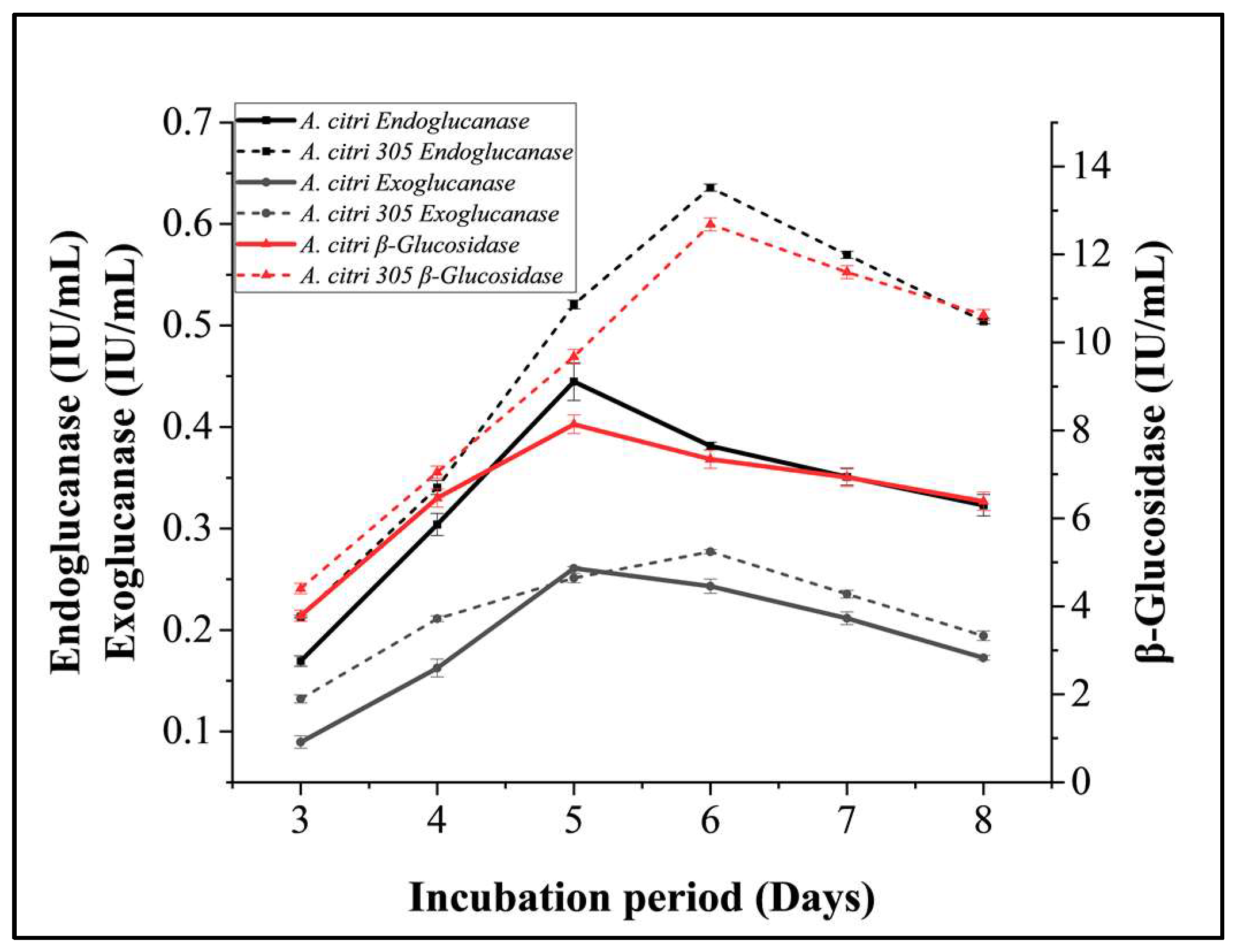
3.2.3. Incubation Period Optimization
3.3. Optimization of Nutritional Requirements for Cellulase Production by A. citri and A. citri 305
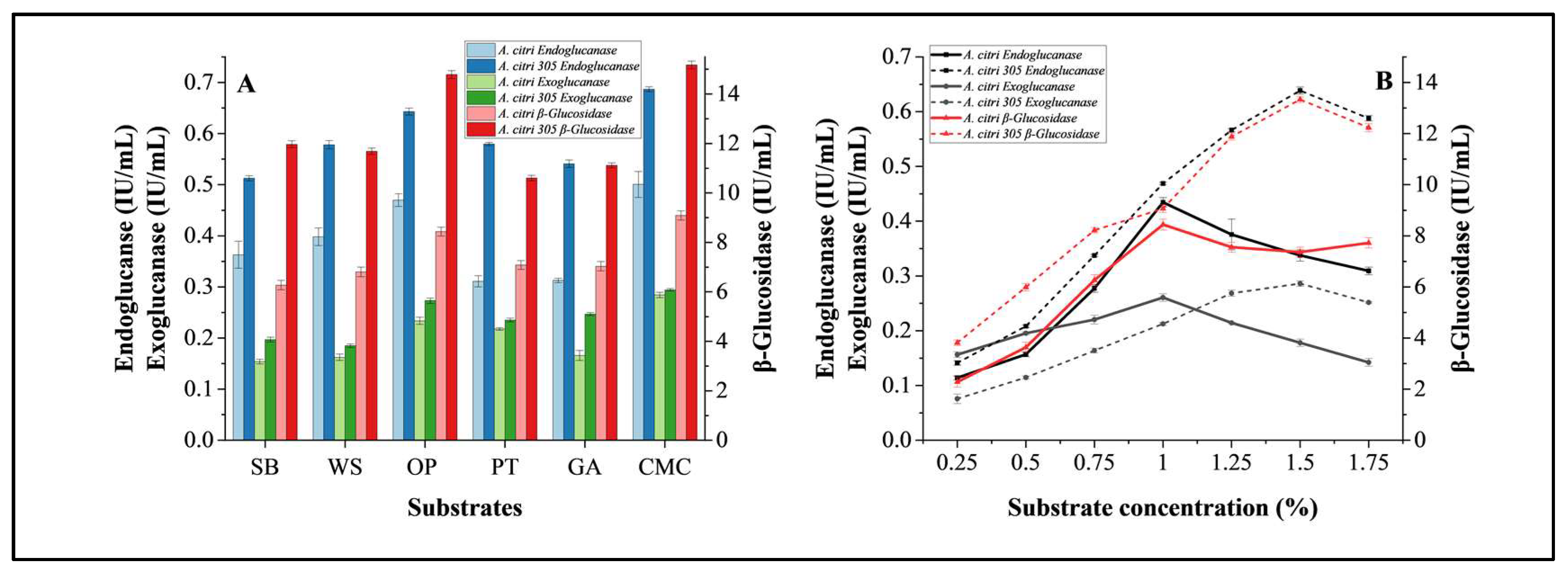
3.3.1. Substrate Optimization
3.3.2. Carbon Source Optimization
3.3.3. Nitrogen Source Optimization
3.4. Cellulase Purification from Wild A. citri and Mutant Strain A. citri 305
3.5. Characterization of Purified Cellulases from Wild A. citri and Mutant Strain A. citri 305
3.5.1. Effect of Temperature on the Activity of Purified Cellulases
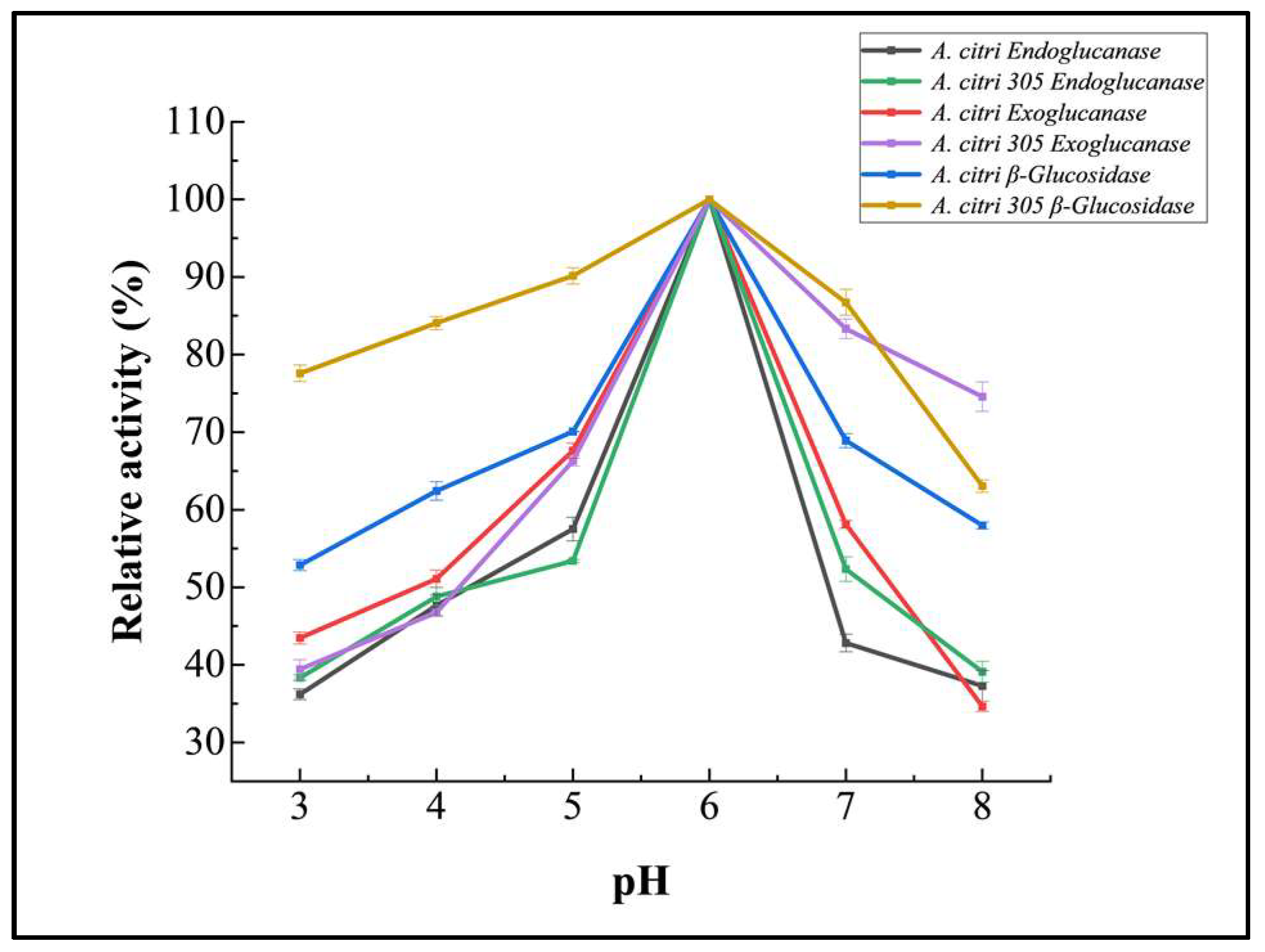
3.5.2. Effect of pH on Purified Cellulase Activity
3.5.3. Effects of Metal Ions on Purified Cellulase Activity
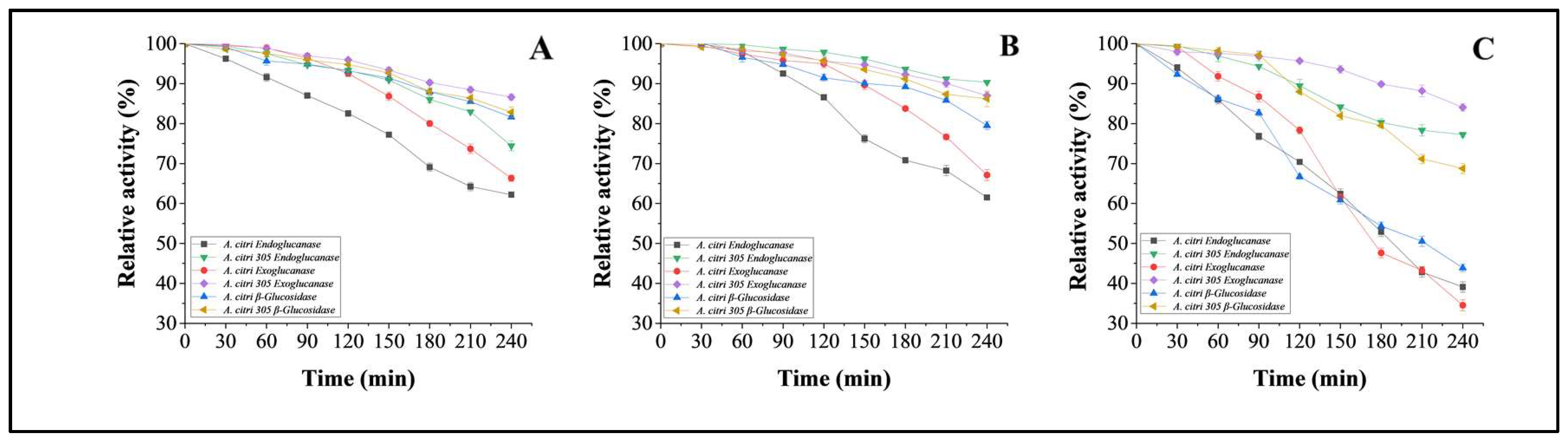
3.5.4. Thermo-Stability Studies on Cellulases from Wild A. citri and Mutant Strain A. citri 305
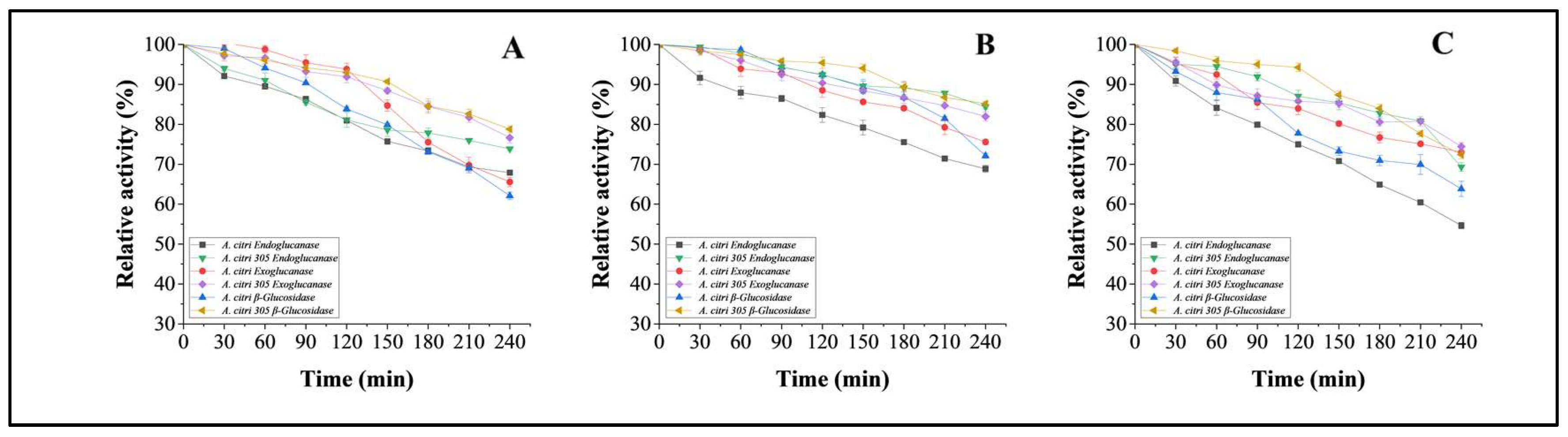
3.5.5. pH Stability Studies on Purified Cellulases from Wild A. citri and Mutant Strain of A. citri 305
3.6. Kinetics Analysis of Purified Cellulase
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| EMS | Ethyl methanesulfonate |
| CMC | Carboxymethyl cellulose |
| OP | Orange peel |
| SB | Sugarcane bagasse |
| WS | Wheat straw |
References
- Ahmed, S.; Riaz, S.; Jamil, A. Molecular Cloning of Fungal Xylanases: An Overview. Appl. Microbiol. Biotechnol. 2009, 84, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Mustafa, G.; Arshad, M.; Rajoka, M.I. Fungal Biomass Protein Production from Trichoderma harzianum Using Rice Polishing. BioMed Res. Int. 2017, 2017, 6232793. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Jabeen, A.; Jamil, A. Xylanase from Trichoderma harzianum: Enzyme Characterization and gene isolation. J. Chem. Soc. Pak. 2007, 29, 176–182. [Google Scholar]
- Ahmed, S.; Imdad, S.S.; Jamil, A. Comparative Study for the Kinetics of Extracellular Xylanases from Trichoderma harzianum and Chaetomium thermophilum. Electron. J. Biotechnol. 2012, 15, 3. [Google Scholar] [CrossRef]
- Benatti, A.L.T.; Polizeli, M.L.T. M Lignocellulolytic Biocatalysts: The Main Players Involved in Multiple Biotechnological Processes for Biomass Valorization. Microorganisms 2023, 11, 162. [Google Scholar] [CrossRef]
- Boondaeng, A.; Keabpimai, J.; Trakunjae, C.; Vaithanomsat, P.; Srichola, P.; Niyomvong, N. Cellulase Production under Solid-State Fermentation by Aspergillus Sp. IN5: Parameter Optimization and Application. Heliyon 2024, 10, e26601. [Google Scholar] [CrossRef]
- Korsa, G.; Konwarh, R.; Masi, C.; Ayele, A.; Haile, S. Microbial Cellulase Production and Its Potential Application for Textile Industries. Ann. Microbiol. 2023, 73, 13. [Google Scholar] [CrossRef]
- Malik, W.A.; Javed, S. Enhancement of Cellulase Production by Cellulolytic Bacteria SB125 in Submerged Fermentation Medium and Biochemical Characterization of the Enzyme. Int. J. Biol. Macromol. 2024, 263, 130415. [Google Scholar] [CrossRef]
- Liu, S.; Ahmed, S.; Zhang, C.; Liu, T.; Shao, C.; Fang, Y. Diversity and antimicrobial activity of culturable fungi associated with sea anemone Anthopleura xanthogrammica. Electron. J. Biotechnol. 2020, 44, 41–46. [Google Scholar] [CrossRef]
- Iqbal, S.; Atiq, M.; Sahi, S.T.; Akbar, N.; Rajput, N.A. Progressive Alterations in Mineral Contents in Citrus Genotypes toward Alternaria citri Causing Brown Spot of Citrus. PLoS ONE 2024, 19, e0306031. [Google Scholar] [CrossRef]
- Li, H.; Zhang, M.; Zhang, Y.; Xu, X.; Zhao, Y.; Jiang, X.; Zhang, R.; Gui, Z. Characterization of Cellulose-Degrading Bacteria Isolated from Silkworm Excrement and Optimization of Its Cellulase Production. Polymers 2023, 15, 4142. [Google Scholar] [CrossRef] [PubMed]
- Jakob, M.; Mahendran, A.R.; Gindl-Altmutter, W.; Bliem, P.; Konnerth, J.; Mueller, U.; Veigel, S. The Strength and Stiffness of Oriented Wood and Cellulose-Fibre Materials: A Review. Prog. Mater. Sci. 2022, 125, 100916. [Google Scholar] [CrossRef]
- Doan, C.T.; Tran, T.N.; Pham, T.P.; Tran, T.T.T.; Truong, B.P.; Nguyen, T.T.; Nguyen, T.M.; Bui, T.Q.H.; Nguyen, A.D.; Wang, S.-L. Production, Purification, and Characterization of a Cellulase from Paenibacillus Elgii. Polymers 2024, 16, 2037. [Google Scholar] [CrossRef] [PubMed]
- Pata, U.K. Linking Renewable Energy, Globalization, Agriculture, CO2 Emissions and Ecological Footprint in BRIC Countries: A Sustainability Perspective. Renew. Energy 2021, 173, 197–208. [Google Scholar] [CrossRef]
- Sharma, A.; Tewari, R.; Rana, S.S.; Soni, R.; Soni, S.K. Cellulases: Classification, Methods of Determination and Industrial Applications. Appl. Biochem. Biotechnol. 2016, 179, 1346–1380. [Google Scholar] [CrossRef]
- Boro, M.; Verma, A.K. Optimization of Cellulase Production by Cohnella Xylanilytica RU-14 Using Statistical Methods. Appl. Biochem. Biotechnol. 2024, 196, 2757–2770. [Google Scholar] [CrossRef]
- Abdelhamed, E.A.; Madian, H.R.; Salem, A.A.; Sidkey, N. Bioethanol Production from Hydrolysis and Fermentation of Rice Straw Using a Combination of Microbial Isolates. Egypt. J. Chem. 2024, 67, 167–180. [Google Scholar] [CrossRef]
- Kaur, G.; Taggar, M.S.; Kalia, A.; Krishania, M.; Singh, A. Fungal Secretomes of Aspergillus terreus Repertoires Cultivated on Native and Acid/Alkali Treated Paddy Straw for Cellulase and Xylanase Production. BioEnergy Res. 2024, 17, 145–159. [Google Scholar] [CrossRef]
- Ascencio, J.J.; Magalhães, L.S.; Ferreira, F.B.; Heinz, O.; Ferraz, A.; Chandel, A.K. Surfactant-Enhanced Enzymatic Hydrolysis of Eucalyptus Kraft Pulp: The Interrelationship Between Lignin Reduction and Sugar Recovery. Catalysts 2025, 15, 47. [Google Scholar] [CrossRef]
- Feng, J.; Techapun, C.; Phimolsiripol, Y.; Phongthai, S.; Khemacheewakul, J.; Taesuwan, S.; Mahakuntha, C.; Porninta, K.; Htike, S.L.; Kumar, A. Utilization of Agricultural Wastes for Co-Production of Xylitol, Ethanol, and Phenylacetylcarbinol: A Review. Bioresour. Technol. 2024, 392, 129926. [Google Scholar] [CrossRef]
- Rosales-Calderon, O.; Trajano, H.L.; Duff, S.J.B. Stability of Commercial Glucanase and β -Glucosidase Preparations under Hydrolysis Conditions. PeerJ 2014, 2, e402. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.-R.; Kim, Y.-S.; Park, C.-S.; Lee, J.-K.; Kim, Y.-S.; Oh, D.-K. Characterization of a Recombinant β-Glucosidase from the Thermophilic Bacterium Caldicellulosiruptor saccharolyticus. J. Biosci. Bioeng. 2009, 108, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, A.; Ahmed, S.; Mehmood, T.; Cruz-Reyes, J.; Jamil, A.; Nawaz, S. Cloning, Expression, and Characterization of a Metalloprotease from Thermophilic Bacterium Streptomyces thermovulgaris. Biology 2024, 13, 619. [Google Scholar] [CrossRef] [PubMed]
- Jalak, J.; Kurašin, M.; Teugjas, H.; Väljamäe, P. Endo-Exo Synergism in Cellulose Hydrolysis Revisited. J. Biol. Chem. 2012, 287, 28802–28815. [Google Scholar] [CrossRef]
- Gan, J.; Iqbal, H.M.N.; Show, P.L.; Rahdar, A.; Bilal, M. Upgrading Recalcitrant Lignocellulosic Biomass Hydrolysis by Immobilized Cellulolytic Enzyme–Based Nanobiocatalytic Systems: A Review. Biomass Convers. Biorefinery 2024, 14, 4485–4509. [Google Scholar] [CrossRef]
- Ahmed, S.; Bashir, A.; Saleem, H.; Saadia, M.; Jamil, A. Production and purification of cellulose-degrading enzymes from a filamentous fungus Trichoderma harzianum. Pak. J. Bot. 2009, 41, 1411–1419. [Google Scholar]
- Boro, M.; Verma, A.K.; Chettri, D.; Yata, V.K.; Verma, A.K. Strategies Involved in Biofuel Production from Agro-Based Lignocellulose Biomass. Environ. Technol. Innov. 2022, 28, 102679. [Google Scholar] [CrossRef]
- Da Silva, M.F.; Flaibam, B.; De Mélo, A.H.F.; Sampaio, U.; Clerici, M.T.P.S.; Goldbeck, R. Optimization of Enzymatic Hydrolysis of Cellulose Extracted from Bamboo Culm for Bioethanol Production by Saccharomyces cerevisiae Modified via CRISPR/Cas9. Food Res. Int. 2024, 192, 114768. [Google Scholar] [CrossRef]
- Singh, A.; Bajar, S.; Devi, A.; Pant, D. An Overview on the Recent Developments in Fungal Cellulase Production and Their Industrial Applications. Bioresour. Technol. Rep. 2021, 14, 100652. [Google Scholar] [CrossRef]
- Reis, C.E.R.; Libardi Junior, N.; Bento, H.B.S.; Carvalho, A.K.F.D.; Vandenberghe, L.P.D.S.; Soccol, C.R.; Aminabhavi, T.M.; Chandel, A.K. Process Strategies to Reduce Cellulase Enzyme Loading for Renewable Sugar Production in Biorefineries. Chem. Eng. J. 2023, 451, 138690. [Google Scholar] [CrossRef]
- Chukwuma, O.B.; Rafatullah, M.; Tajarudin, H.A.; Ismail, N. Lignocellulolytic Enzymes in Biotechnological and Industrial Processes: A Review. Sustainability 2020, 12, 7282. [Google Scholar] [CrossRef]
- Faheina Junior, G.S.; Sousa, K.A.; Zilli, J.E.; Vergara, C.; Pinto, G.A.S.; Santiago-Aguiar, R.S. Enhanced Cellulase Production by Talaromyces Amestolkiae CMIAT055 Using Banana Pseudostem. Waste Biomass Valorization 2022, 13, 3535–3546. [Google Scholar] [CrossRef]
- Fatima, N.; Ahmad, I.; Shakir, H.A.; Khan, M.; Franco, M.; Irfan, M. Production and characterization of cellulases from Aspergillus niger under static fermentation. Cellul. Chem. Technol. 2024, 58, 705–712. [Google Scholar] [CrossRef]
- Santos, G.B.; de Sousa Francisco Filho, Á.; da Silva Rodrigues, J.R.; de Souza, R.R. Cellulase Production by Aspergillus niger Using Urban Lignocellulosic Waste as Substrate: Evaluation of Different Cultivation Strategies. J. Environ. Manag. 2022, 305, 114431. [Google Scholar] [CrossRef] [PubMed]
- Touijer, H.; Benchemsi, N.; Irfan, M.; Tramice, A.; Slighoua, M.; Mothana, R.A.; Alanzi, A.R.; Dalila, B.; Bekkari, H. Statistical Optimization and Purification of Cellulase Enzyme Production from Trichosporon insectorum. Fermentation 2024, 10, 453. [Google Scholar] [CrossRef]
- Infanzón-Rodríguez, M.I.; del Moral, S.; Gómez-Rodríguez, J.; Faife-Pérez, E.; Aguilar-Uscanga, M.G. Second-Generation Bioethanol Production and Cellulases of Aspergillus niger ITV02 Using Sugarcane Bagasse as Substrate. BioEnergy Res. 2024, 17, 160–172. [Google Scholar] [CrossRef]
- Chavan, S.; Shete, A.; Dharne, M.S. Strain Improvement for Cellulolytic Enzymes for Effective Saccharification of Lignocellulosic Biomass by Mutant of Penicillium funiculosum NCIM 1228. Syst. Microbiol. Biomanufacturing 2024, 4, 716–730. [Google Scholar] [CrossRef]
- Liu, F.; Wang, Z.; Manglekar, R.R.; Geng, A. Enhanced Cellulase Production through Random Mutagenesis of Talaromyces pinophilus OPC4-1 and Fermentation Optimization. Process Biochem. 2020, 90, 12–22. [Google Scholar] [CrossRef]
- Asghar, A.; Afzaal, M.; Saeed, F.; Ahmed, A.; Ateeq, H.; Shah, Y.A.; Islam, F.; Hussain, M.; Akram, N.; Shah, M.A. Valorization and Food Applications of Okara (Soybean Residue): A Concurrent Review. Food Sci. Nutr. 2023, 11, 3631–3640. [Google Scholar] [CrossRef]
- Ahmed, S.; Ahmad, F.; Hashmi, A.S. Production of Microbial biomass protein by sequential culture fermentation of Arachniotus sp. and Candida utilis. Pak. J. Bot. 2010, 42, 1225–1234. [Google Scholar]
- Miranda-Sosa, A.; Del Moral, S.; Infanzón-Rodriguez, M.I.; Aguilar-Uscanga, M.G. Reappraisal of Different Agro-Industrial Waste for the Optimization of Cellulase Production from Aspergillus niger ITV02 in a Liquid Medium Using a Box–Benkhen Design. 3 Biotech 2024, 14, 278. [Google Scholar] [CrossRef] [PubMed]
- Dixit, M.; Shukla, P. Multi-Efficient Endoglucanase from Aspergillus niger MPS25 and Its Potential Applications in Saccharification of Wheat Straw and Waste Paper Deinking. Chemosphere 2023, 313, 137298. [Google Scholar] [CrossRef] [PubMed]
- Adsul, M.G.; Dixit, P.; Saini, J.K.; Gupta, R.P.; Ramakumar, S.S.V.; Mathur, A.S. Morphologically Favorable Mutant of Trichoderma reesei for Low Viscosity Cellulase Production. Biotechnol. Bioeng. 2022, 119, 2167–2181. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.-Q.; Li, C.; Lin, Y.; Wu, S.-S.; Gan, L.-H.; Liu, J.; Yang, S.-L.; Zeng, X.-H.; Lin, L. Cellulase Production and Efficient Saccharification of Biomass by a New Mutant Trichoderma afroharzianum MEA-12. Biotechnol. Biofuels 2021, 14, 219. [Google Scholar] [CrossRef]
- Hidayati, F.L.N.; Cahyanto, M.N. Enhancement of Indigenous Fungal Cellulase Production by Gamma Rays. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2021; Volume 1192, p. 012004. [Google Scholar]
- Türkoğlu, A.; Haliloğlu, K.; Tosun, M.; Bujak, H.; Eren, B.; Demirel, F.; Szulc, P.; Karagöz, H.; Selwet, M.; Özkan, G.; et al. Ethyl Methanesulfonate (EMS) Mutagen Toxicity-Induced DNA Damage, Cytosine Methylation Alteration, and iPBS-Retrotransposon Polymorphisms in Wheat (Triticum aestivum L.). Agronomy 2023, 13, 1767. [Google Scholar] [CrossRef]
- Sega, G.A. A Review of the Genetic Effects of Ethyl Methanesulfonate. Mutat. Res. Genet. Toxicol. 1984, 134, 113–142. [Google Scholar] [CrossRef]
- Xu, L.; Wang, W.; Wu, J.; Shin, J.H.; Wang, P.; Unarta, I.C.; Chong, J.; Wang, Y.; Wang, D. Mechanism of DNA Alkylation-Induced Transcriptional Stalling, Lesion Bypass, and Mutagenesis. Proc. Natl. Acad. Sci. USA 2017, 114, E7082–E7091. [Google Scholar] [CrossRef]
- Sangkharak, K.; Vangsirikul, P.; Janthachat, S. Strain Improvement and Optimization for Enhanced Production of Cellulase in Cellulomonas Sp. TSU-03. Afr. J. Microbiol. Res. 2012, 6, 1079–1084. [Google Scholar] [CrossRef]
- El-Khamisi, E.F.; Soliman, E.A.M.; El-Sayed, G.M.; Nour, S.A.; Abdel-Monem, M.O.; Hassan, M.G. Optimization, Gene Cloning, Expression, and Molecular Docking Insights for Enhanced Cellulase Enzyme Production by Bacillus amyloliquefaciens Strain Elh1. Microb. Cell Factories 2024, 23, 191. [Google Scholar] [CrossRef]
- Deep, S.; Sharma, P.; Behera, N. Optimization of Extracellular Cellulase Enzyme Production from Alternaria brassicicola. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 127. [Google Scholar]
- Cherkupally, R.; Amballa, H.; Bhoomi, N.R. In Vitro Screening for Enzymatic Activity of Trichoderma Species for Biocontrol Potential. Ann. Plant Sci. 2017, 6, 1784–1789. [Google Scholar] [CrossRef]
- Haq, I.; Ali, S.; Saleem, A.; Javed, M. Mutagenesis of Bacillus licheniformis through Ethyl Methanesulfonate for Alpha Amylase Production. Pak. J. Bot. 2009, 41, 1489–1498. [Google Scholar]
- Infanzón-Rodríguez, M.I.; Ragazzo-Sánchez, J.A.; Del Moral, S.; Calderón-Santoyo, M.; Aguilar-Uscanga, M.G. Enzymatic Hydrolysis of Lignocellulosic Biomass Using Native Cellulase Produced by Aspergillus niger ITV02 under Liquid State Fermentation. Biotechnol. Appl. Biochem. 2022, 69, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Mandels, M.; Weber, J. The Production of Cellulases. In Cellulases and Their Applications; Advances in Chemistry; American Chemical Society: Washington, DC, USA, 1969; Volume 95, pp. 391–414. [Google Scholar] [CrossRef]
- Reczey, K.; Szengyel, Z.; Eklund, R.; Zacchi, G. Cellulase Production by T. reesei. Bioresour. Technol. 1996, 57, 25–30. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Lu, S.; Liu, X.; Zhang, H.; Wang, W. Neg1 Overexpression in Trichoderma harzianum T4 Enhanced Its Ability to Produce Spores and Antagonistic Activity against Phytopathogenic Fungi. Arch. Microbiol. 2024, 206, 365. [Google Scholar] [CrossRef]
- Kamande, S.M.; Omwenga, G.I.; Ngugi, M.P. Production of Cellulases by Xylaria Sp. and Nemania Sp. Using Lignocellulose Substrates for Bioethanol Production from Maize Cobs. Heliyon 2024, 10, e36802. [Google Scholar] [CrossRef]
- Kadoguchi, E.A.; Velasco, J.; Silva, S.S.D.; Ingle, A.P.; Segato, F.; Chandel, A.K. Optimization of Cellulase Production from Agri-Industrial Residues by Aspergillus terreus NIH2624. Processes 2024, 12, 2169. [Google Scholar] [CrossRef]
- Saroj, P.; P, M.; Narasimhulu, K. Biochemical Characterization of Thermostable Carboxymethyl Cellulase and β-Glucosidase from Aspergillus fumigatus JCM 10253. Appl. Biochem. Biotechnol. 2022, 194, 2503–2527. [Google Scholar] [CrossRef]
- Gad, S.; Ayakar, S.; Adivarekar, R. Formulation and Characterization of Bacterial Consortium for Efficient Lignocellulosic Waste Degradation. J. Environ. Chem. Eng. 2024, 12, 112619. [Google Scholar] [CrossRef]
- Kumar, A.K.; Parikh, B.S.; Singh, S.P.; Shah, D. Use of Combined UV and Chemical Mutagenesis Treatment of Aspergillus terreus D34 for Hyper-Production of Cellulose-Degrading Enzymes and Enzymatic Hydrolysis of Mild-Alkali Pretreated Rice Straw. Bioresour. Bioprocess. 2015, 2, 35. [Google Scholar] [CrossRef]
- Bhardwaj, S.; Mishra, A.; Ghosh, D.; Sinha, A.K. Magnetically Separable Spent Coffee Grounds as a Potential Novel Support for the Covalent Immobilization of β-glucosidase for Cellobiose Hydrolysis. J. Chem. Technol. Biotechnol. 2024, 99, 1984–1995. [Google Scholar] [CrossRef]
- Msango, K.; Gouda, M.N.R.; Ramakrishnan, B.; Kumar, A.; Subramanian, S. Variation and Functional Profile of Gut Bacteria in the Scarab Beetle, Anomala dimidiata, under a Cellulose-Enriched Microenvironment. Sci. Rep. 2024, 14, 22400. [Google Scholar] [CrossRef] [PubMed]
- Ghose, T.K. Measurement of Cellulase Activities. Pure Appl. Chem. 1987, 59, 257–268. [Google Scholar] [CrossRef]
- Sun, J.; Wang, W.; Hao, J. A Novel GH1 β-Glucosidase from an Arctic Bacterium: Characterization and Secretory Expression in Bacillus subtilis. Process Biochem. 2024, 140, 108–116. [Google Scholar] [CrossRef]
- Prakash, S.; Kumari, H.; Pasrija, R.; Kumar, A. Unraveling the Intricacies of Carbon Catabolic Repression and Glucose in Fungal Pathogenesis. Hum. Fungal Dis. 2025, 1, 169–182. [Google Scholar] [CrossRef]
- Ten Kate, G.A.; Sanders, P.; Dijkhuizen, L.; Van Leeuwen, S.S. Kinetics and Products of Thermotoga maritima β-Glucosidase with Lactose and Cellobiose. Appl. Microbiol. Biotechnol. 2024, 108, 349. [Google Scholar] [CrossRef]
- Gawade, D.B.; Perane, R.R.; Deokar, C.D.; Raghuwanshi, K.S.; Suryawanshi, A.P. Effect of Physiological Factors on Production of Cellulolytic Enzymes by Rhizoctonia bataticola. Indian Phytopathol. 2018, 71, 555–561. [Google Scholar] [CrossRef]
- Sulyman, A.O.; Igunnu, A.; Malomo, S.O. Isolation, Purification and Characterization of Cellulase Produced by Aspergillus niger Cultured on Arachis Hypogaea Shells. Heliyon 2020, 6, e05668. [Google Scholar] [CrossRef]
- Sarsan, S.; Merugu, R. Role of Bioprocess Parameters to Improve Cellulase Production: Part II. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2019; pp. 77–97. [Google Scholar] [CrossRef]
- Gaur, R.; Tiwari, S. Isolation, Production, Purification and Characterization of an Organic-Solvent-Thermostable Alkalophilic Cellulase from Bacillus vallismortis RG-07. BMC Biotechnol. 2015, 15, 19. [Google Scholar] [CrossRef]
- Ahmed, K.; Munawar, S.; Naz, S. Purification and Characterization of cellulase from Aspergillus. Chem. Bio. Environ. Sci. 2015, 27, 4341–4344. Available online: https://api.semanticscholar.org/CorpusID:212459652 (accessed on 12 October 2024).
- Jin, X.; Song, J.; Ma, J.; Liu, G.-Q. Thermostable β-Xylosidase from Aspergillus fumigatus: Purification, Characterization and Potential Application in Lignocellulose Bioethanol Production. Renew. Energy 2020, 155, 1425–1431. [Google Scholar] [CrossRef]
- Taha, A.S.J.; Taha, A.J.; Faisal, Z.G. Purification and Kinetic Study on Cellulase Produced by Local Trichoderma viride. Nat. Sci. 2015, 13, 87–90. [Google Scholar]
- Regmi, S.; Choi, Y.S.; Kim, Y.K.; Khan, M.M.; Lee, S.H.; Cho, S.S.; Jin, Y.-Y.; Lee, D.Y.; Yoo, J.C.; Suh, J.-W. Endoglucanase Produced by Bacillus subtilis Strain CBS31: Biochemical Characterization, Thermodynamic Study, Enzymatic Hydrolysis, and Bio-Industrial Applications. Biotechnol. Bioprocess Eng. 2020, 25, 104–116. [Google Scholar] [CrossRef]
- Ribeiro, O.; Magalhães, F.; Aguiar, T.Q.; Wiebe, M.G.; Penttilä, M.; Domingues, L. Random and Direct Mutagenesis to Enhance Protein Secretion in Ashbya gossypii. Bioengineered 2013, 4, 322–331. [Google Scholar] [CrossRef]
- Chen, L.; Duan, L.; Sun, M.; Yang, Z.; Li, H.; Hu, K.; Yang, H.; Liu, L. Current Trends and Insights on EMS Mutagenesis Application to Studies on Plant Abiotic Stress Tolerance and Development. Front. Plant Sci. 2023, 13, 1052569. [Google Scholar] [CrossRef]
- Fonseca, R.; Capel, C.; Nieto-Canseco, R.; Ortiz-Atienza, A.; Bretones, S.; López-Fábregas, J.D.; Quevedo-Colmena, A.S.; Lebrón, R.; Barragán-Lozano, T.; Villalobos-Ramírez, V.; et al. A Tomato EMS-Mutagenized Population Provides New Valuable Resources for Gene Discovery and Breeding of Developmental Traits. Plants 2022, 11, 2453. [Google Scholar] [CrossRef]
- Wang, H.; Zhai, L.; Geng, A. Enhanced Cellulase and Reducing Sugar Production by a New Mutant Strain Trichoderma harzianum EUA20. J. Biosci. Bioeng. 2020, 129, 242–249. [Google Scholar] [CrossRef]
- Sharma, A.K.; Bhati, N. Enhancement of Lipase Production by Ethyl Methane Sulfonate Mutagenesis of Soil Fungal Isolate. Plant Sci. Today 2019, 6, 600–606. [Google Scholar]
- Aljammas, H.A.; Yazji, S.; Azizieh, A. Enhancement of Protease Production from Rhizomucor miehei by Mutagenesis with Ethyl Methanesulfonate, Ultraviolet, and Microwaves—A Preliminary Study. Bioresour. Technol. Rep. 2022, 20, 101287. [Google Scholar] [CrossRef]
- Elakkiya, P.; Muralikrishnan, V. Cellulase Production and Purification of Mutant Strain Trichoderma viride. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 720–727. [Google Scholar]
- Pramanik, S.K.; Mahmud, S.; Paul, G.K.; Jabin, T.; Naher, K.; Uddin, M.S.; Zaman, S.; Saleh, M.A. Fermentation Optimization of Cellulase Production from Sugarcane Bagasse by Bacillus pseudomycoides and Molecular Modeling Study of Cellulase. Curr. Res. Microb. Sci. 2021, 2, 100013. [Google Scholar] [CrossRef] [PubMed]
- Nehad, E.A.; Yoness, M.F.; Reem, A.A. Optimization and Purification of Cellulase Produced by Penicillium decumbens and Its Application. Egypt. Pharm. J. 2019, 18, 391–402. [Google Scholar]
- Acharya, P.B.; Acharya, D.K.; Modi, H.A. Optimization for Cellulase Production by Aspergillus niger Using Saw Dust as Substrate. Afr. J. Biotechnol. 2008, 7, 4147–4152. [Google Scholar]
- Latifian, M.; Hamidi-Esfahani, Z.; Barzegar, M. Evaluation of Culture Conditions for Cellulase Production by Two Trichoderma reesei Mutants under Solid-State Fermentation Conditions. Bioresour. Technol. 2007, 98, 3634–3637. [Google Scholar] [CrossRef]
- Abd Elrsoul, R.; Bakhiet, S.E.A. Optimization of Factors Influencing Cellulase Production by Some Indigenous Isolated Fungal Species. Jordan J. Biol. Sci. 2018, 11, 31–36. [Google Scholar]
- Dutt, D.; Kumar, A. Optimization of Cellulase Production under Solid-State Fermentation by Aspergillus flavus (AT-2) and Aspergillus niger (AT-3) and Its Impact on Stickies and Ink Particle Size of Sorted Office Paper. Cellul. Chem. Technol. 2014, 48, 285–298. [Google Scholar]
- Ng, I.-S.; Chen, P.T.; Ju, Y.-M.; Tsai, S.-W. Novel Cellulase Screening and Optimal Production from the Wood Decaying Xylariaceae: Daldinia Species. Appl. Biochem. Biotechnol. 2024, 196, 1–11. [Google Scholar] [CrossRef]
- Sethi, S.; Datta, A.; Gupta, B.L.; Gupta, S. Optimization of Cellulase Production from Bacteria Isolated from Soil. ISRN Biotechnol. 2013, 2013, 985685. [Google Scholar] [CrossRef]
- Kaur, H.P.; Joshi, D. Optimization of Cellulase Produced by Fungus Isolated from Water. World J. Pharm. Sci. 2015, 4, 521–534. [Google Scholar]
- Gautam, S.P.; Bundela, P.S.; Pandey, A.K.; Awasthi, M.K.; Sarsaiya, S. Optimization of the Medium for the Production of Cellulase by the Trichoderma viride Using Submerged Fermentation. Int. J. Environ. Sci. 2010, 1, 656–665. [Google Scholar]
- Vijayaraghavan, P.; Vincent, S.P.; Dhillon, G.S. Solid-Substrate Bioprocessing of Cow Dung for the Production of Carboxymethyl Cellulase by Bacillus halodurans IND18. Waste Manag. 2016, 48, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Saleem, A.; El-Said, A.H.M.; Maghraby, T.A.; Hussein, M.A. Pathogenicity and Pectinase Activity of Some Facultative Mycoparasites Isolated from Vicia Faba Diseased Leaves in Relation to Photosynthetic Pigments of Plant. J. Plant Pathol. Microbiol. 2012, 3, 141. [Google Scholar]
- Ghori, M.I.; Ahmed, S.; Malana, M.A.; Jamil, A. Corn stover-enhanced cellulase production by Aspergillus niger NRRL 567. Afr. J. Biotechnol 2011, 10, 5878–5886. [Google Scholar]
- Dar, M.A.; Pawar, K.D.; Rajput, B.P.; Rahi, P.; Pandit, R.S. Purification of a Cellulase from Cellulolytic Gut Bacterium, Bacillus tequilensis G9 and Its Evaluation for Valorization of Agro-Wastes into Added Value Byproducts. Biocatal. Agric. Biotechnol. 2019, 20, 101219. [Google Scholar] [CrossRef]
- Elsababty, Z.E.; Abdel-Aziz, S.H.; Ibrahim, A.M.; Guirgis, A.A.; Dawwam, G.E. Purification, Biochemical Characterization, and Molecular Cloning of Cellulase from Bacillus licheniformis Strain Z9 Isolated from Soil. J. Genet. Eng. Biotechnol. 2022, 20, 34. [Google Scholar] [CrossRef]
- Kim, B.-K.; Lee, B.-H.; Lee, Y.-J.; Jin, I.-H.; Chung, C.-H.; Lee, J.-W. Purification and Characterization of Carboxymethylcellulase Isolated from a Marine Bacterium, Bacillus subtilis Subsp. Subtilis A-53. Enzym. Microb. Technol. 2009, 44, 411–416. [Google Scholar] [CrossRef]
- Asha, B.M. Purification and Characterization of a Thermophilic Cellulase from a Novel Cellulolytic Strain, Paenibacillus barcinonensis. J. Microbiol. Biotechnol. 2012, 22, 1501–1509. [Google Scholar] [CrossRef]
- Banerjee, S.; Maiti, T.K.; Roy, R.N. Production, Purification, and Characterization of Cellulase from Acinetobacter junii GAC 16.2, a Novel Cellulolytic Gut Isolate of Gryllotalpa Africana, and Its Effects on Cotton Fiber and Sawdust. Ann. Microbiol. 2020, 70, 28. [Google Scholar] [CrossRef]
- Steiner, E.; Margesin, R. Production and Partial Characterization of a Crude Cold-Active Cellulase (CMCase) from Bacillus mycoides AR20-61 Isolated from an Alpine Forest Site. Ann. Microbiol. 2020, 70, 67. [Google Scholar] [CrossRef]
- Mohapatra, S.; Padhy, S.; Mohapatra, P.K.D.; Thatoi, H.N. Enhanced Reducing Sugar Production by Saccharification of Lignocellulosic Biomass, Pennisetum Species through Cellulase from a Newly Isolated Aspergillus fumigatus. Bioresour. Technol. 2018, 253, 262–272. [Google Scholar] [CrossRef]
- Das, S.; Bhattacharya, A.; Haldar, S.; Ganguly, A.; Gu, S.; Ting, Y.P.; Chatterjee, P.K. Optimization of Enzymatic Saccharification of Water Hyacinth Biomass for Bio-Ethanol: Comparison between Artificial Neural Network and Response Surface Methodology. Sustain. Mater. Technol. 2015, 3, 17–28. [Google Scholar] [CrossRef]
- Okonkwo, I.F. Effect of Metal Ions and Enzyme Inhibitor on the Activity of Cellulase Enzyme of Aspergillus flavus. Int. J. Environ. Agric. Biotechnol. 2019, 4, 727–734. [Google Scholar] [CrossRef]
- Pino, M.S.; Michelin, M.; Rodríguez-Jasso, R.M.; Oliva-Taravilla, A.; Teixeira, J.A.; Ruiz, H.A. Hot Compressed Water Pretreatment and Surfactant Effect on Enzymatic Hydrolysis Using Agave Bagasse. Energies 2021, 14, 4746. [Google Scholar] [CrossRef]
- Singh, B. Purification and Characterization of a Protease-Resistant Phytase of Aspergillus oryzae SBS50 Whose Properties Make It Exceptionally Useful as a Feed Supplement. Int. J. Biol. Macromol. 2017, 103, 458–466. [Google Scholar] [CrossRef]
- Abhishek Walia, A.W.; Preeti Mehta, P.M.; Anjali Chauhan, A.C.; Saurabh Kulshrestha, S.K.; Shirkot, C.K. Purification and Characterization of Cellulase-Free Low Molecular Weight Endo β-1, 4 Xylanase from an Alkalophilic Cellulosimicrobium cellulans CKMX1 Isolated from Mushroom Compost. World J. Microbiol. Biotechnol. 2014, 30, 2597–2608. [Google Scholar] [CrossRef]




| Cellulases | Parameters | Culture Supernatant | Partially Purified Enzyme | ||
| A. citri | A. citri 305 | A. citri | A. citri 305 | ||
| TP (mg/mL) | 1.07 ± 0.02 | 1.55 ± 0.01 | 0.35 ± 0.01 | 0.17 ± 0.02 | |
| Endoglucanase | TA (U) | 0.43 ± 0.03 | 0.65 ± 0.04 | 0.27 ± 0.01 | 0.28 ± 0.01 |
| SA (U/mg) | 0.40 ± 0.03 | 0.42 ± 0.026 | 0.85 ± 0.03 | 1.65 ± 0.18 | |
| PF | 1 | 1 | 2.12 | 3.95 | |
| R (%) | 100 | 100 | 62.79 | 43.07 | |
| Exoglucanase | TA (U) | 0.26 ± 0.02 | 0.27 ± 0.05 | 0.09 ± 0.003 | 0.10 ± 0.01 |
| SA (U/mg) | 0.24 ± 0.02 | 0.18 ± 0.034 | 0.28 ± 0.02 | 0.58 ± 0.06 | |
| PF | 1 | 1 | 1.17 | 3.34 | |
| R (%) | 100 | 100 | 34.61 | 37.03 | |
| β-glucosidase | TA (U) | 8.59 ± 1.44 | 12.85 ± 0.91 | 3.88 ± 0.31 | 1.49 ± 0.26 |
| SA (U/mg) | 8.02 ± 1.35 | 8.29 ± 0.588 | 12.26 ± 1.05 | 8.76 ± 1.79 | |
| PF | 1 | 1 | 1.52 | 1.05 | |
| R (%) | 100 | 100 | 45.16 | 11.59 | |
| Metal Ions (10%) | Relative Activity | Relative Activity | Relative Activity | |||
|---|---|---|---|---|---|---|
| (%) | (%) | (%) | ||||
| β-glucosidase | Endoglucanase | Exoglucanase | ||||
| A. citri | A. citri 305 | A. citri | A. citri 305 | A. citri | A. citri 305 | |
| Control | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| NaNO3 | 104 ± 1 | 103 ± 1 | 96 ± 1 | 96 ± 1 | 126 ± 1 | 104 ± 3 |
| FeSO4 | 127 ± 1 | 139 ± 3 | 203 ± 1 | 137 ± 1 | 275 ± 1 | 285 ± 2 |
| MgSO4 | 107 ± 1 | 135 ± 2 | 119 ± 2 | 113 ± 1 | 135 ± 2 | 205 ± 2 |
| ZnSO4 | 120 ± 1 | 108 ± 2 | 131 ± 2 | 112 ± 1 | 156 ± 0 | 195 ± 2 |
| KH2PO4 | 79 ± 3 | 90 ± 4 | 96 ± 1 | 94 ± 0 | 115 ± 1 | 118 ± 0 |
| CoCl2 | 53 ± 1 | 73 ± 3 | 70 ± 1 | 73 ± 1 | 95 ± 1 | 78 ± 3 |
| Additives (5 mM) | β-glucosidase | Endoglucanase | Exoglucanase | |||
| (%) | (%) | (%) | ||||
| A. citri | A. citri 305 | A. citri | A. citri 305 | A. citri | A. citri 305 | |
| Control | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 | 100 ± 0 |
| SDS | 56 ± 2 | 96 ± 1 | 63 ± 1 | 60 ± 1 | 41 ± 2 | 54 ± 1 |
| Tween 80 | 107 ± 2 | 108 ± 0 | 118 ± 2 | 112 ± 1 | 126 ± 2 | 165 ± 1 |
| EDTA | 67 ± 3 | 97 ± 1 | 77 ± 1 | 83 ± 1 | 78 ± 2 | 90 ± 1 |
| Urea | 68 ± 3 | 59 ± 2 | 96 ± 3 | 75 ± 0 | 86 ± 2 | 64 ± 4 |
| Cellulases | Strains | Km | Vmax | Efficiency |
|---|---|---|---|---|
| (mg/mL) | (mg/mL/min) | (/min) | ||
| Endoglucanase | A. citri | 0.492 ± 0.004 | 0.039 ± 0.000 | 0.078 ± 0.001 |
| A. citri 305 | 0.295 ± 0.006 | 0.060 ± 0.000 | 0.202 ± 0.004 | |
| Exoglucanase | A. citri | 0.278 ± 0.011 | 0.023 ± 0.000 | 0.084 ± 0.003 |
| A. citri 305 | 0.241 ± 0.007 | 0.023 ± 0.000 | 0.094 ± 0.004 | |
| β-glucosidase | A. citri | 0.048 ± 0.001 | 0.754 ± 0.011 | 15.683 ± 0.099 |
| A. citri 305 | 0.031 ± 0.001 | 1.128 ± 0.004 | 36.765 ± 1.598 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, S.; Andaleeb, H.; Aslam, A.; Raza, J.A.; Waseem, S.M.Y.; Javaid, A.; Talib, C. Improved Cellulolytic Activity of Alternaria citri: Optimization and EMS Treatment for Enhanced Cellulase Production. Fermentation 2025, 11, 274. https://doi.org/10.3390/fermentation11050274
Ahmed S, Andaleeb H, Aslam A, Raza JA, Waseem SMY, Javaid A, Talib C. Improved Cellulolytic Activity of Alternaria citri: Optimization and EMS Treatment for Enhanced Cellulase Production. Fermentation. 2025; 11(5):274. https://doi.org/10.3390/fermentation11050274
Chicago/Turabian StyleAhmed, Sibtain, Hina Andaleeb, Aqsa Aslam, Junaid Ahmad Raza, Sheikh Muhammad Yahya Waseem, Atayyaba Javaid, and Chand Talib. 2025. "Improved Cellulolytic Activity of Alternaria citri: Optimization and EMS Treatment for Enhanced Cellulase Production" Fermentation 11, no. 5: 274. https://doi.org/10.3390/fermentation11050274
APA StyleAhmed, S., Andaleeb, H., Aslam, A., Raza, J. A., Waseem, S. M. Y., Javaid, A., & Talib, C. (2025). Improved Cellulolytic Activity of Alternaria citri: Optimization and EMS Treatment for Enhanced Cellulase Production. Fermentation, 11(5), 274. https://doi.org/10.3390/fermentation11050274






