Enhanced Antioxidant Properties of Saccharomyces-Fermented Defatted Tenebrio molitor Larvae Extract: A Sustainable Alternative Protein Source
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
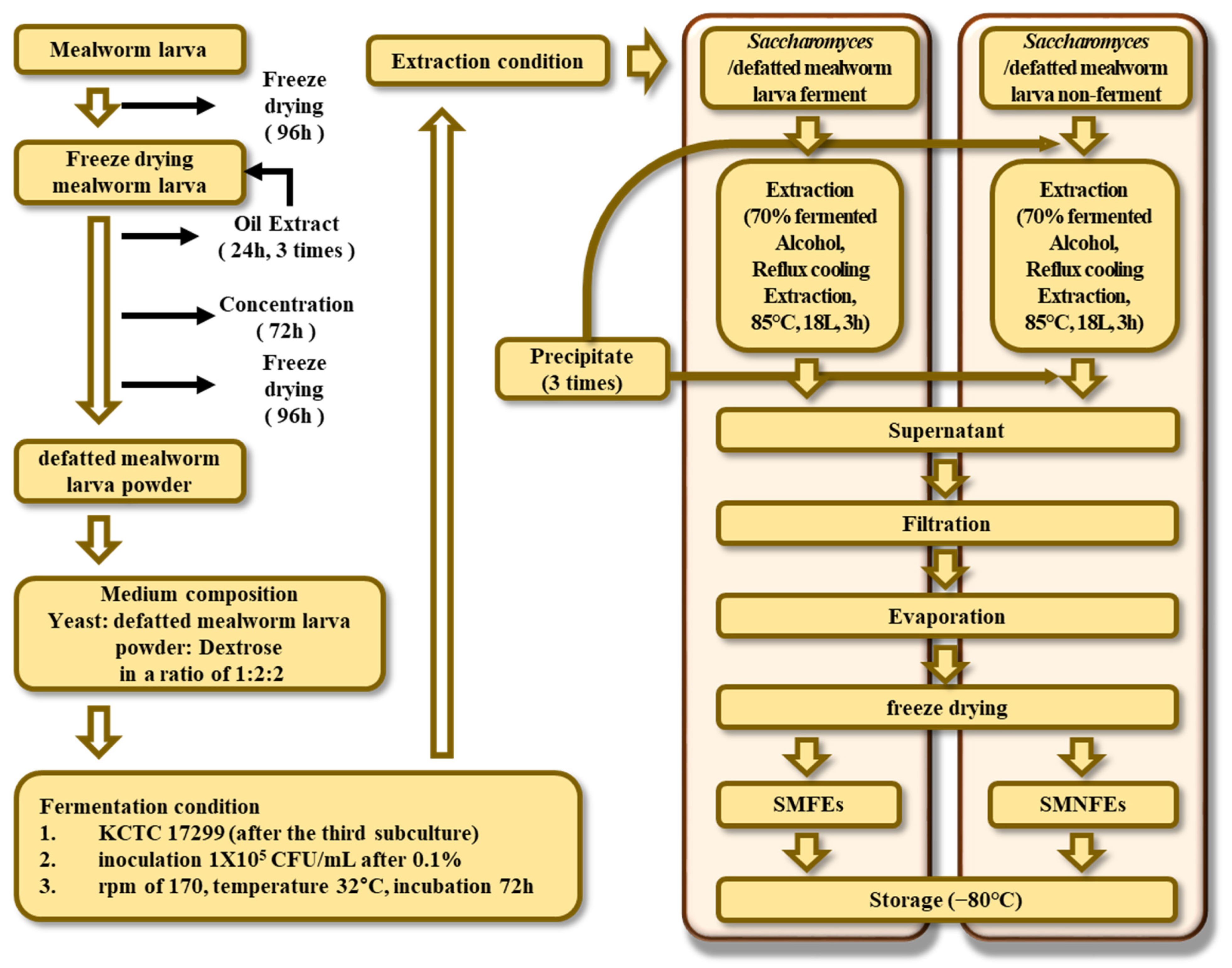
2.2. Pretreatment of Samples
2.3. Fermentation and Extraction of Samples
2.4. Antioxidant Activity
2.4.1. Estimation of Total Phenolics, Tannins, and Flavonoids Content
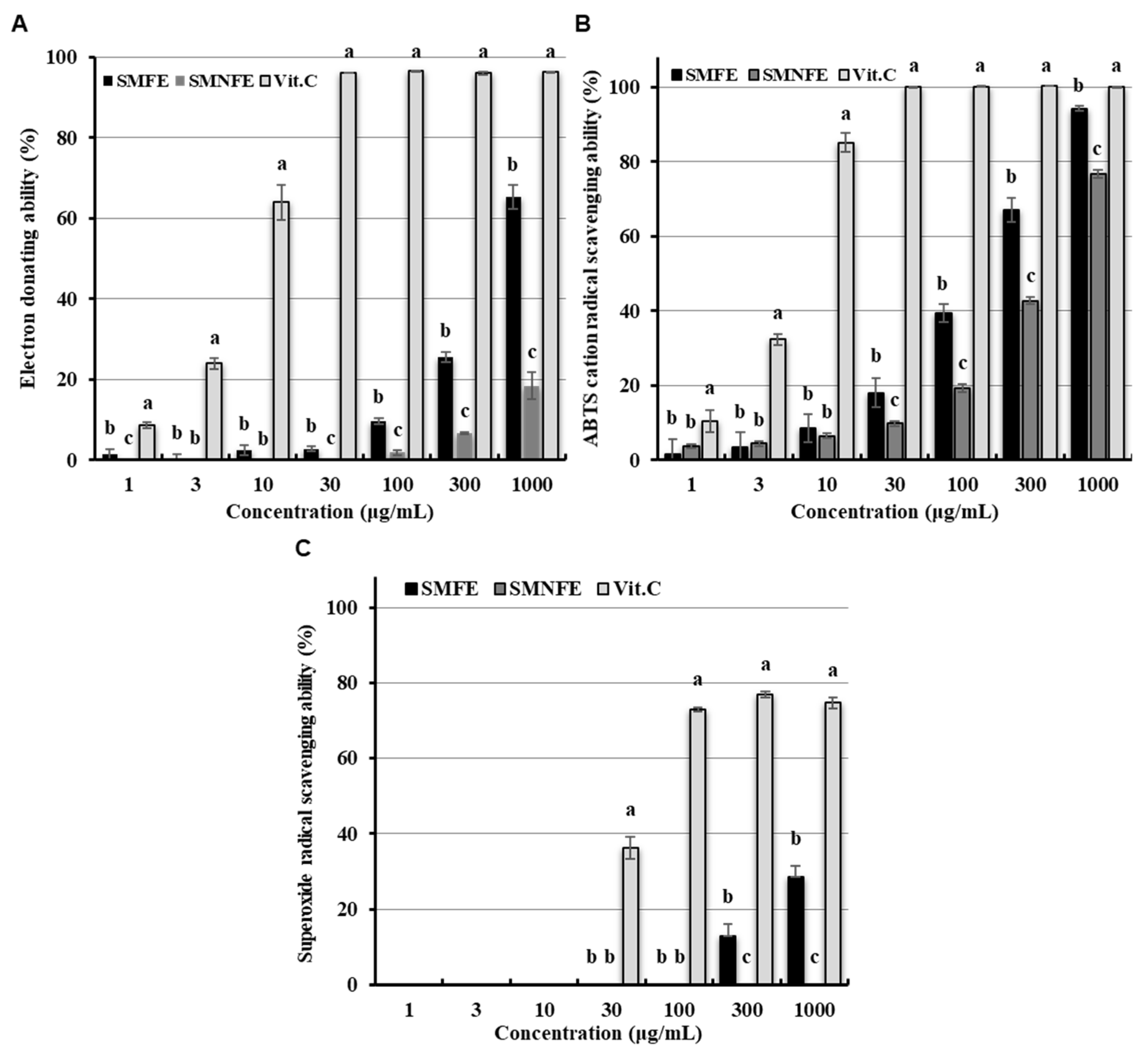
2.4.2. Radical and Anion Scavenging Activity
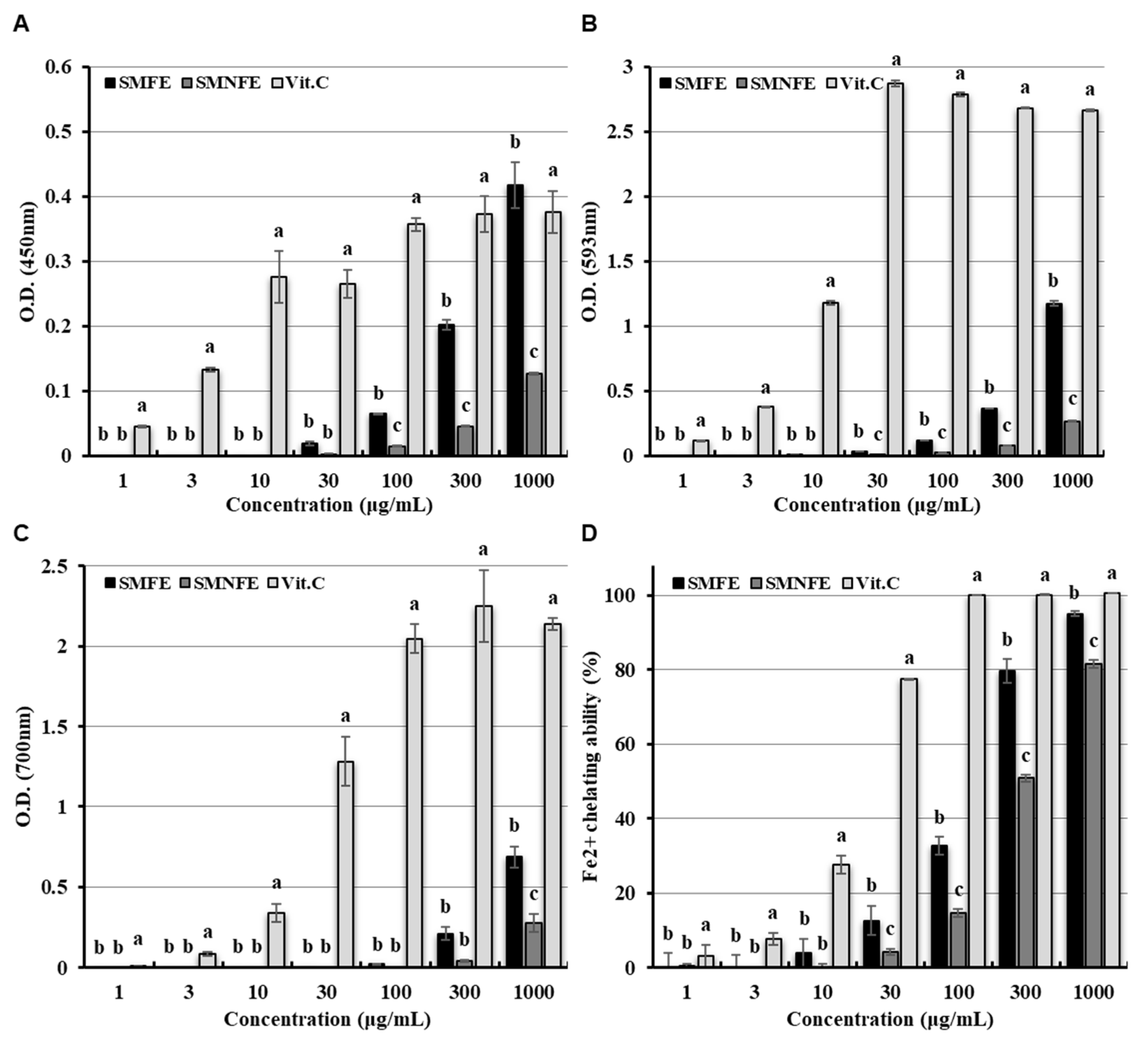
2.4.3. Fenton Reaction and Reducing Power Activity
2.5. Cell and Culture
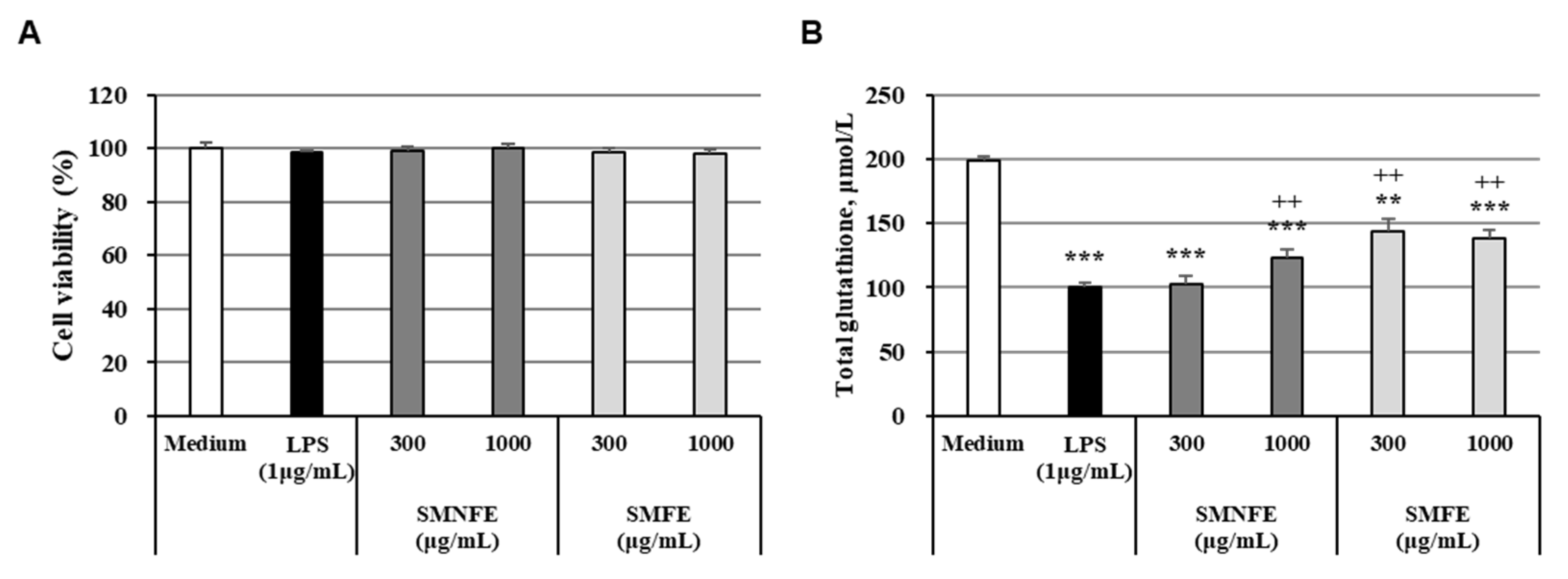
2.6. Cell Viability
2.7. Total Glutathione Quantification Assay
2.8. Analysis of Free Amino Acids
2.9. Statistical Analysis
3. Results and Discussion
3.1. Chemical Composition: Phenolics, Tannins, and Flavonoids
3.2. Determination of Antioxidant Activities: Radical and Anion Scavenging Activities
3.3. Determination of Antioxidant Activities: Fenton Reaction and Reducing Power Activities
3.4. Activity of Antioxidant Enzymes
3.5. Determination of Free Amino Acids
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bors, W.; Saran, M. Radical scavenging by flavonoid antioxidants. Free Radic. Res. Commun. 1987, 2, 289–294. [Google Scholar] [CrossRef]
- Chang, S.S.; Ostric-Matijasevice, B.; Hsieh, O.A.L.; Huang, C. Natural antioxidants from rosemary and sage. J. Food Sci. 1977, 42, 1102–1110. [Google Scholar] [CrossRef]
- Kang, K.Y.; Hwang, Y.H.; Lee, S.J.; Jang, H.Y.; Hong, S.G.; Mun, S.K.; Kim, S.J.; Kim, J.J.; Park, K.W.; Seo, K.S.; et al. Verification of the functional antioxidant activity and antimelanogenic properties of extracts of Poria cocos Mycelium fermented with freeze-dried plum powder. Int. J. Biomater. 2019, 2019, 9283207. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.H.; Cha, H.J.; Lee, H.; Hong, S.H.; Park, C.; Park, S.H.; Kim, G.Y.; Kim, S.; Kim, H.S.; Hwang, H.J.; et al. Protective effect of glutathione against oxidative stress-induced cytotoxicity in RAW 264.7 macrophages through activating the nuclear factor erythroid 2-related factor-2/heme oxygenase-1 pathway. Antioxidants 2019, 8, 82. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Wang, L.; Yi, D.; Wu, G. N-acetylcysteine and intestinal health a focus on its mechanism of action. Front. Biosci. 2015, 20, 872–891. [Google Scholar] [CrossRef]
- Deponte, M. The incomplete glutathione puzzle: Just guessing at numbers and figures? Antioxid. Redox Signal. 2017, 27, 1130–1161. [Google Scholar] [CrossRef]
- Gomex-Cabrera, M.C.; Salvador-Pascual, A.; Cabo, H.; Ferrando, B.; Viña, J. Redox modulation of mitochondriogenesis in exercise. Does antioxidant supplementation blunt the benefits of exercise training? Free Radic. Biol. Med. 2015, 86, 37–46. [Google Scholar] [CrossRef]
- Purohit, V.; Abdelmalek, M.F.; Barve, S.; Benevenga, N.J.; Halsted, C.H.; Kaplowitz, N.; Kharbanda, K.K.; Liu, Q.Y.; Lu, S.C.; McClain, C.J.; et al. Role of S-adenosylmethionine, folate, and betaine in the treatment of alcoholic liver disease: Summary of a symposium. Am. J. Clin. Nutr. 2007, 86, 14–24. [Google Scholar] [CrossRef]
- Williams, G.M.; Iatropoulos, M.J.; Whysner, J. Safety assessment of butylated hydroxyanisole and butylated hydroxytoluene as antioxidant food additives. Food Chem. Toxicol. 1999, 37, 1027–1038. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Novel antioxidants in food quality preservation and health promotion. Eur. J. Lipid Sci. Technol. 2010, 112, 930–940. [Google Scholar] [CrossRef]
- Zielińska, E.; Baraniak, B.; Karaś, M.; Rybczyńska, K.; Jakubczyk, A. Selected species of edible insects as a source of nutrient composition. Food Res. Int. 2015, 77, 460–466. [Google Scholar] [CrossRef]
- Sogari, G.; Amato, M.; Biasato, I.; Chiesa, S.; Gasco, L. The potential role of insects as feed: A multi-perspective review. Animals 2019, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, T.; Choi, B.D.; Kim, G.E.; Lee, K.H. Effects of containing pigments of dried grasshopper on the lipid deterioration. J. Korean Soc. Food Sci. Nutr. 1987, 16, 300–305. [Google Scholar]
- Yu, T.J.; Lee, K.Y.; Lee, S.K. Studies on the development of cocoon pupas for food materials. Korean J. Nutr. 1978, 11, 39–43. [Google Scholar]
- Van Huis, A. Potential of insects as food and feed in assuring food security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef]
- Lensvelt, E.J.S.; Steenbekkers, L.P.A. Exploring consumer acceptance of entomophagy: Determinants in a cross-cultural study among the young Dutch and Chinese. Appetite 2014, 83, 152–161. [Google Scholar]
- Netherlands Enterprise Agency. Insect Sector Development Plan—Towards a Circular Agriculture; Netherlands Enterprise Agency: Den Haag, The Netherlands, 2021. [Google Scholar]
- Chung, M.Y.; Kwon, E.Y.; Hwang, J.S.; Goo, T.W.; Yun, E.Y. Pre-treatment conditions on the powder of Tenebrio molitor for using as a novel food ingredient. J. Seric. Entomol. Sci. 2013, 51, 9–14. [Google Scholar]
- Chung, S.J.; Lee, Y.H.; Chung, J.H.; Lee, B.R.; Han, D.M. Antifungal effect and activity spectrum of crude antifungal proteins from hemolymph of larvae of Tenebrio molitor in Korea. Kor. J. Mycol. 1995, 23, 232–237. [Google Scholar]
- Jang, Y.S.; Park, M.W.; Kim, J.H.; Lee, Y.S. Anti-inflammatory and antioxidant effects of Tenebrio molitor larvae in vitro and in vivo. Food Sci. Biotechnol. 2021, 30, 293–301. [Google Scholar]
- Kim, T.K.; Yong, H.I.; Kim, Y.B.; Kim, H.W.; Choi, Y.S. Functional properties of edible insects and their applications in food: A review. Food Sci. Anim. Resour. 2020, 40, 863–880. [Google Scholar]
- Yi, L.; Lakemond, C.M.M.; Sagis, L.M.C.; Eisner-Schadler, V.; van Huis, A.; van Boekel, M.A.J.S. Extraction and characterisation of protein fractions from five insect species. Food Chem. 2013, 141, 3341–3348. [Google Scholar] [CrossRef] [PubMed]
- Marco, M.L.; Heeney, D.; Binda, S.; Cifelli, C.J.; Cotter, P.D.; Foligné, B.; Gänzle, M.; Kort, R.; Pasin, G.; Pihlanto, A.; et al. Health benefits of fermented foods: Microbiota and beyond. Curr. Opin. Biotechnol. 2017, 44, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Swieca, M.; Gawlik-Dziki, U.; Dziki, D.; Baraniak, B.; Czyz, J. Antioxidant and nutritional evaluation of fermented wheat sprouts. Food Chem. 2014, 164, 101–107. [Google Scholar]
- van Huis, A.; Tomberlin, J.K.; dos Santos Oliveira, J.V. Edible Insects: Future Prospects for Food and Feed Security, 2nd ed.; FAO: Rome, Italy; Springer: Berlin/Heidelberg, Germany, 2021. [Google Scholar]
- Zielińska, E.; Pankiewicz, U. Fermentation as a tool for improving the quality of edible insect-based foods. Innov. Food Sci. Emerg. Technol. 2020, 67, 102510. [Google Scholar]
- Baek, M.; Kim, S.; Cho, J.; Lee, S.; Yoon, S. Effect of fermentation on antioxidant activity and peptide profile of Tenebrio molitor larvae protein hydrolysate. Korean J. Food Sci. Technol. 2019, 51, 543–550. [Google Scholar]
- Broadhurst, R.B.; Jones, W.T. Analysis of condensed tannins using acidified vanillin. J. Sci. Food Agric. 1978, 29, 788–794. [Google Scholar] [CrossRef]
- Herald, T.J.; Gadgil, P.; Tilley, M. High-throughput micro plate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J. Sci. Food Agric. 2012, 92, 2326–2331. [Google Scholar] [CrossRef]
- Pękal, A.; Pyrzynska, K. Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, J.; Shen, J.; Silva, A.; Dennis, D.A.; Barrow, C.J. A simple 96-well microplate method for estimation of total polyphenol content in seaweeds. J. Appl. Phycol. 2006, 18, 445–450. [Google Scholar] [CrossRef]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Re, E.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–560. [Google Scholar] [CrossRef]
- Dinis, T.C.P.; Madeira, V.M.C.; Almeida, L.M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Gülçin, İ. Comparison of in vitro antioxidant and antiradical activities of L-tyrosine and L-Dopa. Amino Acids 2007, 32, 431–438. [Google Scholar] [CrossRef]
- Apak, R.; Gulcu, K.; Ozyurek, M.; Karademir, S.E.; Ercag, E. The cupric ion reducing antioxidant capacity and polyphenolic content of some herbal teas. Int. J. Food Sci. Nutr. 2006, 57, 292–304. [Google Scholar] [CrossRef]
- Gülçin, İ. In vitro prooxidant effect of caffeine. J. Enzyme Inhib. Med. Chem. 2008, 23, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Zou, Y.; Chang, S.K.C.; Gu, Y.; Qian, S.Y. Antioxidant activity and phenolic compositions of lentil (Lens culinaris var. Morton) extract and its fractions. J. Agric. Food Chem. 2011, 59, 2268–2344. [Google Scholar] [CrossRef]
- Göçer, H.; Gülçin, İ. Caffeic acid phenethyl ester (CAPE): Correlation of structure and antioxidant properties. Int. J. Food Sci. Nutr. 2011, 62, 821–825. [Google Scholar] [CrossRef]
- Gülçin, İ.; Elmastaş, M.; Aboul-Enein, H. Determination of antioxidant and radical scavenging activity of Basil (Ocimum basilicum L. Family Lamiaceae) assayed by different methodologies. Phytother. Res. 2007, 21, 354–361. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant properties of resveratrol: A structure–activity insight. Innov. Food Sci. Emerg. Technol. 2010, 11, 210–218. [Google Scholar] [CrossRef]
- Elmastaş, M.; Turkekul, I.; Ozturk, L.; Gülçin, İ.; Isildak, O.; Aboul-Enein, H. Antioxidant activity of two wild edible mushrooms (Morchella vulgaris and Morchella esculanta) from North Turkey. Comb. Chem. High Throughput Screen 2006, 9, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Bursal, E.; Koksal, E.; Gülçin, İ.; Bilsel, G.; Goren, A.C. Antioxidant activity and polyphenol content of cherry stem (Cerasus avium L.) determined by LC–MS/MS. Food Res. Int. 2013, 51, 66–74. [Google Scholar] [CrossRef]
- Marino, R.; Iammarino, M.; Santillo, A.; Muscarella, M.; Caropresse, M.; Albenzio, M. Rapid method for determination of amino acids in milk. J. Dairy Sci. 2010, 93, 2367–2370. [Google Scholar] [CrossRef]
- Kin, Y.H.; Yook, H.S. Antioxidant activites of Yam (Dioscorea japonica Thunb.) fermented with various microorganisms. J. Korean Soc. Food Sci. Nutr. 2024, 53, 965–972. [Google Scholar]
- Kim, G.S.; Yang, K.H.; Kim, H.K.; Kim, J.E.; Yun, H.N.; Yu, J.W.; Kim, B.S. Antioxidant and immunomodulatory effect of lactic acid bacteria fermented barley sprout hot water extract. J. Korean Soc. Food Sci. Nutr. 2022, 51, 1027–1035. [Google Scholar] [CrossRef]
- Anagnostopoulou, M.A.; Kefalas, P.; Papageorgiou, V.P.; Assimopoulou, A.N.; Boskou, D. Radical scavenging activity of various extracts and fractions of sweet orange peel (Citrus sinensis). Food Chem. 2006, 94, 19–25. [Google Scholar] [CrossRef]
- Seong, H.Y.; Kim, M.S. Enhanced protein quality and antioxidant activity of fermented brown rice with Gryllus bimaculatus. LWT 2021, 150, 111948. [Google Scholar] [CrossRef]
- Jang, S.H.; Sim, S.Y.; Ahn, H.Y.; Seo, K.W.; Cho, Y.S. Physicochemical properties and biological activities of Tenebrio molitor fermented by several kinds of micro-organisms. J. Life Sci. 2018, 28, 923–930. [Google Scholar]
- Foyer, C.H.; Lopez-Delgado, H.; Dat, J.F.; Scott, I.M. Hydrogen peroxide- and glutathione-associated mechanisms of acclimatory stress tolerance and signalling. Physiol. Plant. 1997, 100, 241–254. [Google Scholar] [CrossRef]
- Lu, S.C. Regulation of glutathione synthesis. Mol. Aspects Med. 2009, 30, 42–59. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.T.; Oh, C.H.; Oh, N.S. Production of glutathione by the yeast mutant Saccharomyces cerevisiae Sa59. Korean J. Food Sci. Technol. 2013, 45, 801–804. [Google Scholar] [CrossRef]
- Lee, H.L.; Kim, J.M.; Go, M.J.; Lee, H.S.; Kim, J.H.; Kim, I.Y.; Seong, G.S.; Heo, H.J. Fermented Protaetia brevitarsis larvae alleviates high-fat diet-induced non-alcoholic fatty liver disease in C57BL/6 mice via regulation of lipid accumulation and inflammation. J. Microbiol. Biotechnol. 2025, 35, e2409025. [Google Scholar] [CrossRef]
- Sim, S.Y.; Jang, S.H.; Cho, Y.S.; Ahn, H.Y. Effect of Tenebrio molitor larvae fermented with Saccharomyces cerevisiae M1 (KACC 93023) on non-alcoholic fatty liver-induced rats. J. Life Sci. 2020, 30, 434–442. [Google Scholar]
- Cao, Y.; Lu, J.; Cai, G. Quality improvement of soybean meal by yeast fermentation based on the degradation of anti-nutritional factors and accumulation of beneficial metabolites. J. Sci. Food Agric. 2024, 104, 1441–1449. [Google Scholar] [CrossRef]
- Cooper, T.G. Nitrogen metabolism in Saccharomyces cerevisiae. Annu. Rev. Microbiol. 1982, 36, 1–32. [Google Scholar]
- Zaman, S.; Lippman, S.I.; Zhao, X.; Broach, J.R. How Saccharomyces responds to nutrients. Annu. Rev. Genet. 2008, 42, 27–81. [Google Scholar] [CrossRef]
- Jung, S.H. Effect of amino acids on anoxia-induced cell injury. J. Biomed. Lab. Sci. 2001, 7, 127–131. [Google Scholar]
- Bellia, F.; Amorini, A.M.; Mendola, D.L.; Vecchio, G.; Tavazzi, B.; Giardina, B.; Pietro, V.D.; Lazzarino, G.; Rizzarelli, E. New glycosidic derivatives of histidine-containing dipeptides with antioxidant properties and resistant to carnosinase activity. Eur. J. Med. Chem. 2008, 43, 373–380. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Aluko, R.E. Chemometric analysis of the amino acid requirements of antioxidant food protein hydrolysates. Int. J. Mol. Sci. 2011, 12, 3148–3161. [Google Scholar] [CrossRef]
- Xu, N.; Chen, G.; Liu, H. Antioxidative categorization of twenty amino acids based on experimental evaluation. Molecules 2017, 22, 2066. [Google Scholar] [CrossRef] [PubMed]
| Type of Sample | Polyphenols (GAE) a | Flavonoids (QE) b | Tannins (CE) c |
|---|---|---|---|
| SMFE | 36.04 ± 1.04 a | 12.69 ± 0.76 a | 0.55 ± 0.11 a |
| SMNFE | 11.11 ± 0.13 b | 3.61 ± 0.00 b | N.D. b |
| Contents (mg/g) | SMFE | SMNFE |
|---|---|---|
| Aspartic Acid | 5.22 ± 0.09 a | 3.72 ± 0.09 b |
| Glutamic Acid | 11.25 ± 0.25 a | 8.02 ± 0.11 b |
| Serine | 5.60 ± 0.17 a | 5.27 ± 0.05 b |
| Histidine | 4.99 ± 0.05 a | 4.19 ± 0.04 b |
| Glycine | 4.04 ± 0.16 a | 3.05 ± 0.09 b |
| Threonine | 4.84 ± 0.06 a | 4.17 ± 0.10 b |
| Arginine | 4.41 ± 0.09 b | 7.62 ± 0.08 a |
| Alanine | 14.12 ± 0.38 a | 10.96 ± 0.09 b |
| Tyrosine | 7.72 ± 0.19 a | 5.24 ± 0.07 b |
| Valine | 10.50 ± 0.14 a | 7.68 ± 0.11 b |
| Methionine | 2.52 ± 0.08 a | 1.46 ± 0.02 b |
| Phenyalanine | 7.8 ± 0.16 a | 6.02 ± 0.06 b |
| Isoleucine | 7.66 ± 0.15 a | 6.16 ± 0.10 b |
| Leucine | 12.20 ± 0.37 a | 10.45 ± 0.11 b |
| Lysine | 9.69 ± 0.20 a | 7.91 ± 0.05 b |
| Total | 112.60 ± 2.36 a | 91.93 ± 0.75 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, K.-Y.; Jeong, B.-G.; Kim, J.-H.; Park, K.-W. Enhanced Antioxidant Properties of Saccharomyces-Fermented Defatted Tenebrio molitor Larvae Extract: A Sustainable Alternative Protein Source. Fermentation 2025, 11, 272. https://doi.org/10.3390/fermentation11050272
Kang K-Y, Jeong B-G, Kim J-H, Park K-W. Enhanced Antioxidant Properties of Saccharomyces-Fermented Defatted Tenebrio molitor Larvae Extract: A Sustainable Alternative Protein Source. Fermentation. 2025; 11(5):272. https://doi.org/10.3390/fermentation11050272
Chicago/Turabian StyleKang, Kyung-Yun, Beom-Gyun Jeong, Jeong-Ho Kim, and Kyung-Wuk Park. 2025. "Enhanced Antioxidant Properties of Saccharomyces-Fermented Defatted Tenebrio molitor Larvae Extract: A Sustainable Alternative Protein Source" Fermentation 11, no. 5: 272. https://doi.org/10.3390/fermentation11050272
APA StyleKang, K.-Y., Jeong, B.-G., Kim, J.-H., & Park, K.-W. (2025). Enhanced Antioxidant Properties of Saccharomyces-Fermented Defatted Tenebrio molitor Larvae Extract: A Sustainable Alternative Protein Source. Fermentation, 11(5), 272. https://doi.org/10.3390/fermentation11050272






