Different Yeast Strain Effects on ‘King of the North’ Wine Chemical, Chromatic, and Descriptive Sensory Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Grapes
2.2. Winemaking
2.3. Analysis of Chemical and Chromatic Properties of Must and Wine
2.4. Descriptive Sensory Analysis
2.5. Statistical Analysis
3. Results and Discussions
3.1. General Parameters of ‘KON’ Musts
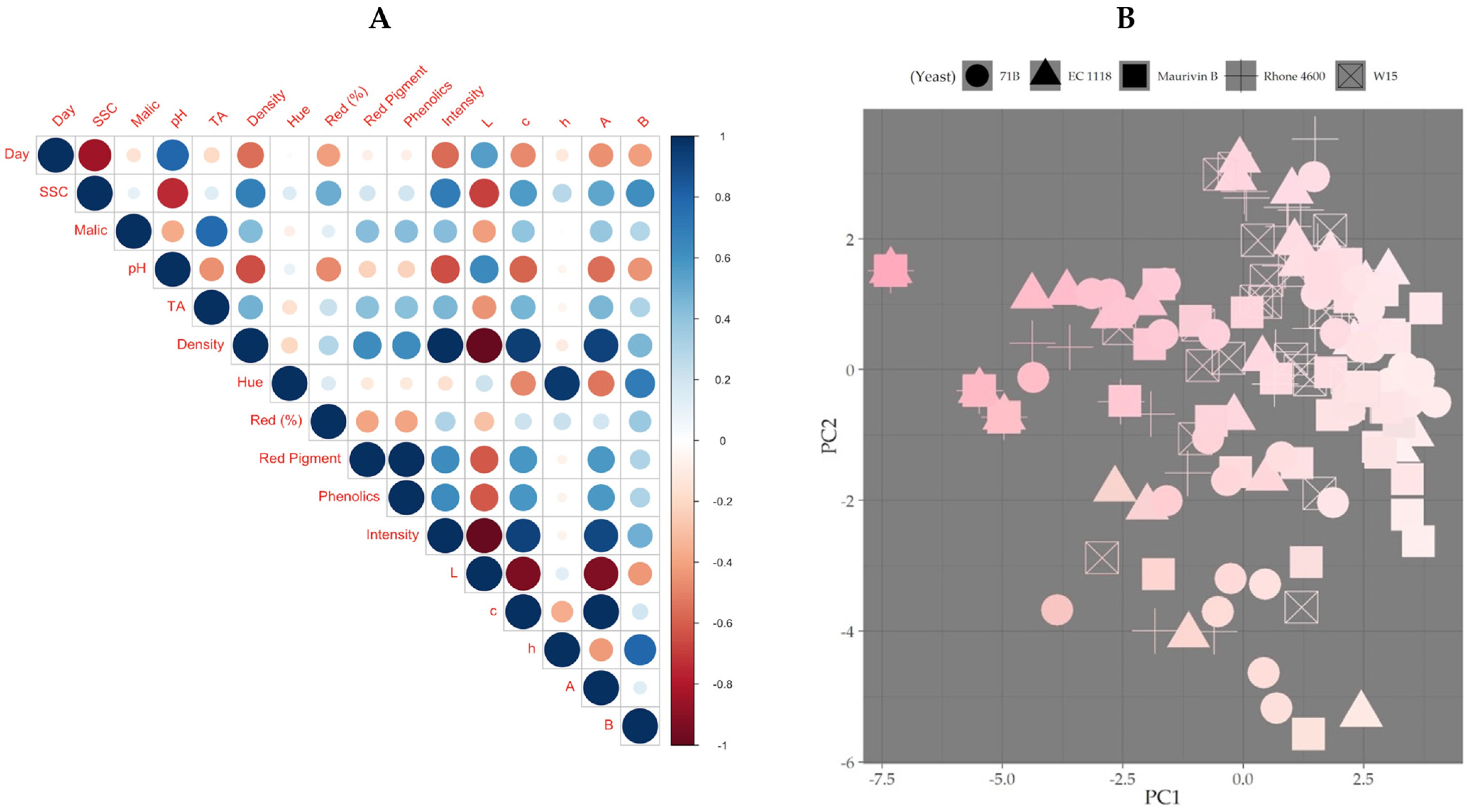
3.2. Effect of Yeast Strains on Color Metrics and Acid Metrics
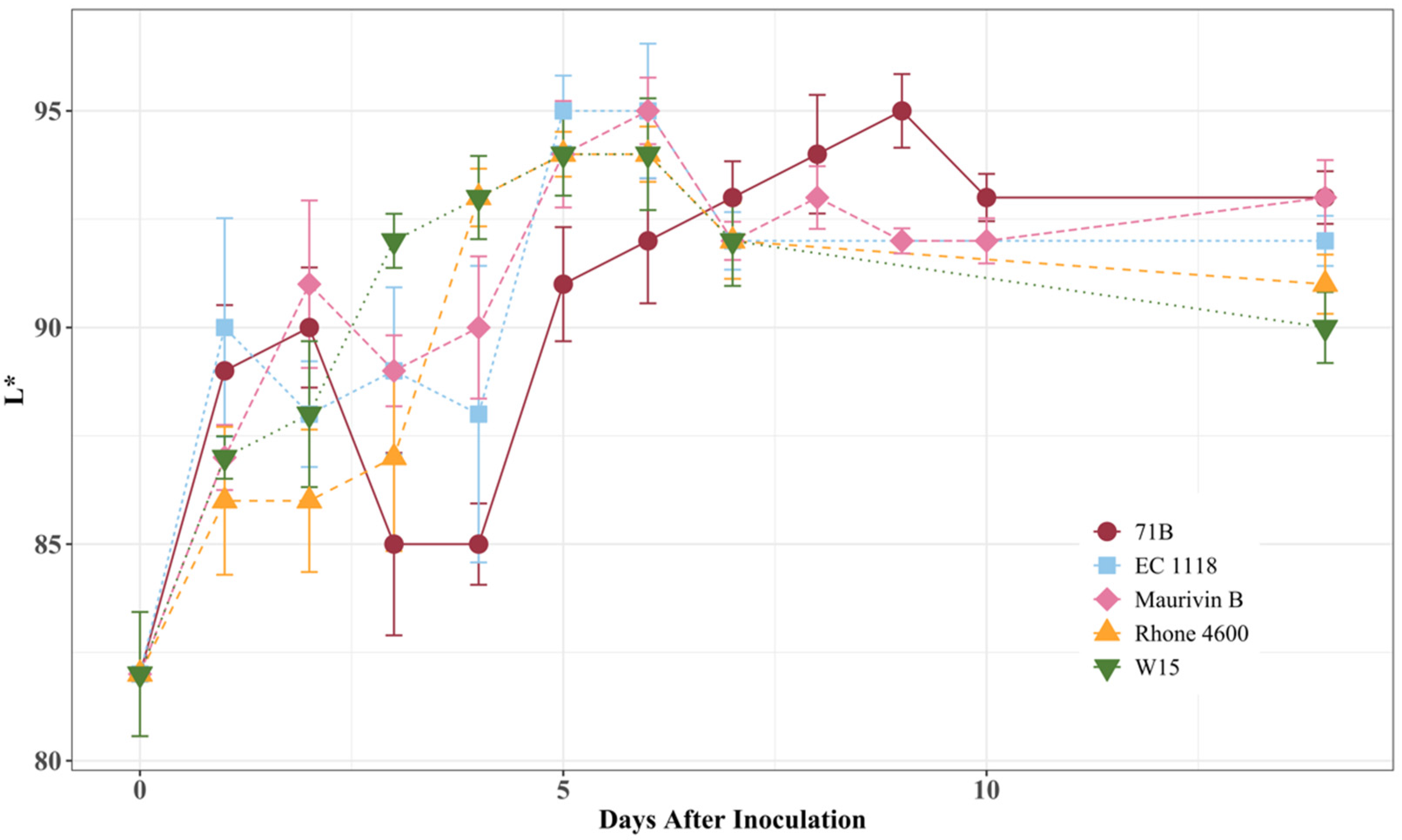
3.3. Fermentation Lightness Changes with Different Yeast Strains
3.4. Sugar Transformation Differences with Different Yeast Strains
3.5. Wine Chemistry
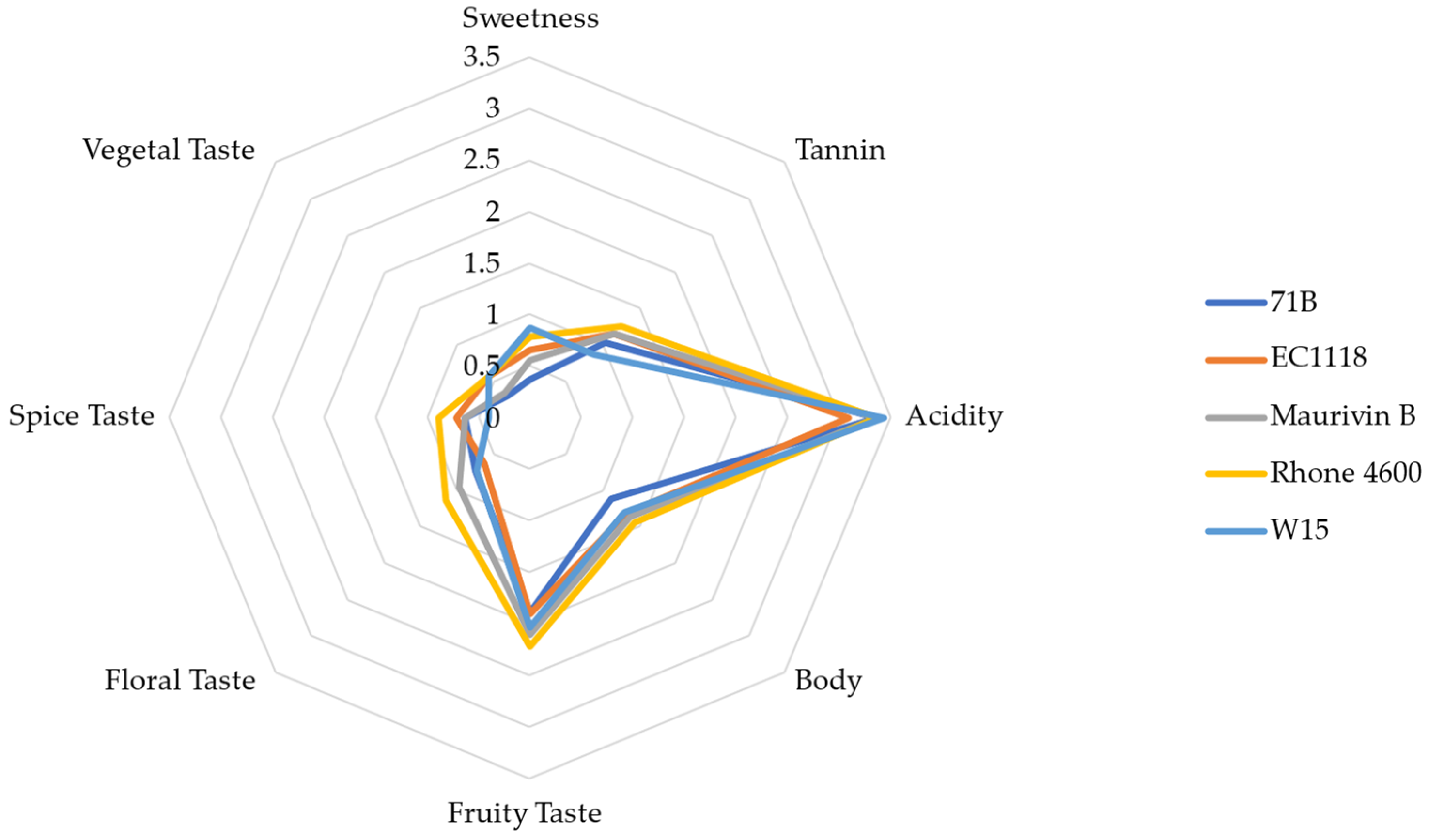
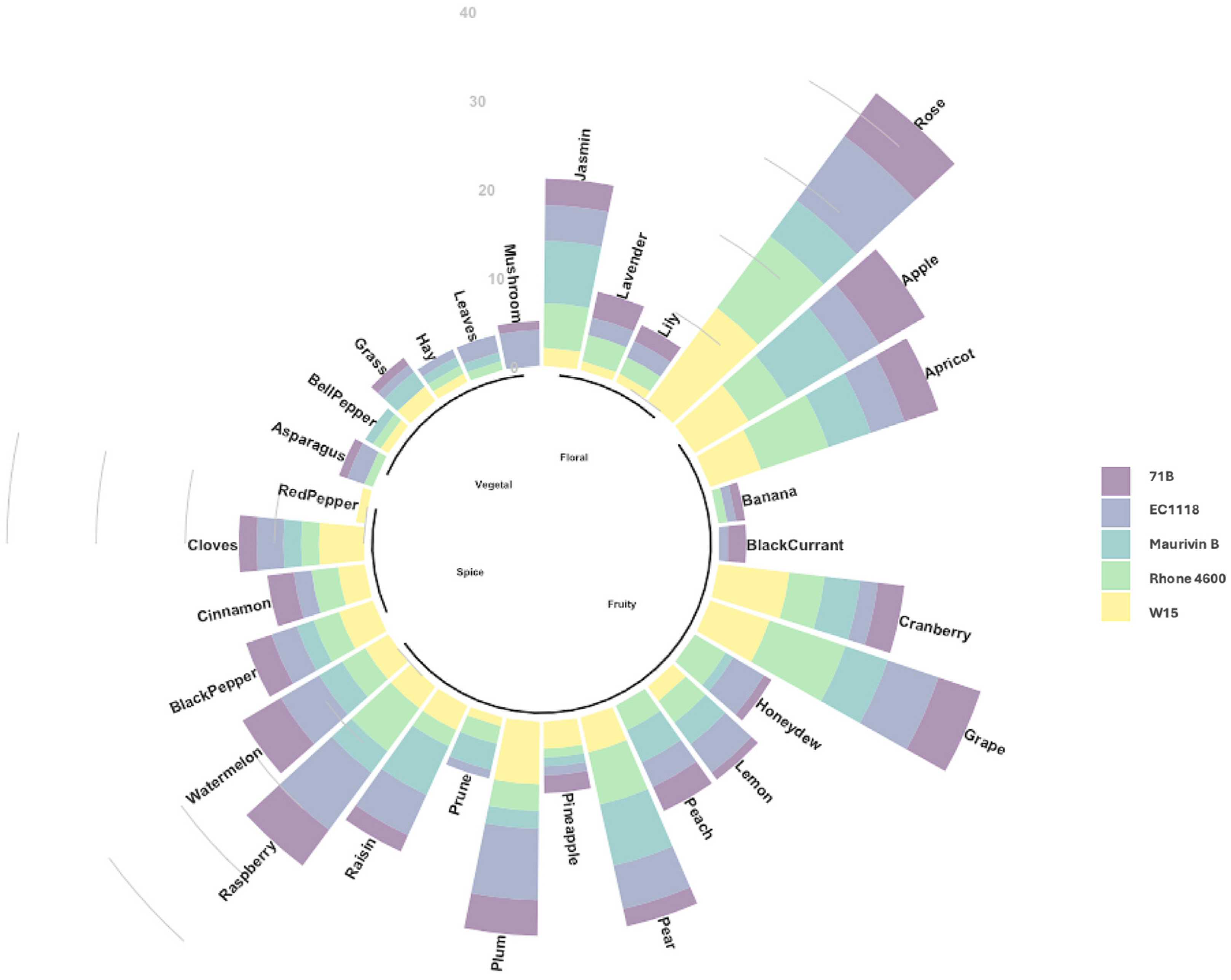
3.6. Descriptive Sensory Analysis of ‘KON’ Wine with Different Yeast Strains
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Samoticha, J.; Wojdyło, A.; Chmielewska, J.; Nofer, J. Effect of Different Yeast Strains and Temperature of Fermentation on Basic Enological Parameters, Polyphenols and Volatile Compounds of Aurore White Wine. Foods 2019, 8, 599. [Google Scholar] [CrossRef] [PubMed]
- Henschke, P.A. Wine Yeast. In Yeast Sugar Metabolism; Taylor: Oxfordshire, UK, 1997; Available online: https://www.taylorfrancis.com/chapters/edit/10.1201/9781003578987-25/wine-yeast-paul-henschke (accessed on 20 February 2025).
- Maicas, S. The Role of Yeasts in Fermentation Processes. Microorganisms 2020, 8, 1142. [Google Scholar] [CrossRef] [PubMed]
- Pedneault, K.; Dorais, M.; Angers, P. Flavor of Cold-Hardy Grapes: Impact of Berry Maturity and Environmental Conditions. J. Agric. Food Chem. 2013, 61, 10418–10438. [Google Scholar] [CrossRef] [PubMed]
- Hatterman-Valenti, H.M.; Auwarter, C.P.; Stenger, J.E. Evaluation of Cold-Hardy Grape Cultivars for North Dakota and the North Dakota State University Germplasm Enhancement Project. Acta Hortic. 2016, 1115, 13–22. [Google Scholar] [CrossRef]
- Growing Grapes. In Minnesota-Minnesota Grape Growers Association. Available online: https://cdn.ymaws.com/www.mngrapes.org/resource/resmgr/Growing_Grapes_in_MN_Best_Practices/GGIM_Best_Practices-book.pdf (accessed on 18 June 2024).
- Svyantek, A.; Köse, B.; Stenger, J.; Auwarter, C.; Hatterman-Valenti, H. Cold-Hardy Grape Cultivar Winter Injury and Trunk Re-Establishment Following Severe Weather Events in North Dakota. Horticulturae 2020, 6, 75. [Google Scholar] [CrossRef]
- Olson, B.K.; Brooke, M.; Wang, Z.; Svyantek, A.; Stenger, J.; Hatterman-Valenti, H. ‘Frontenac’ Grape Response to Canopy Management in North Dakota. Horticulturae 2021, 7, 288. [Google Scholar] [CrossRef]
- Wang, Z.-L.; Xue, T.-T.; Gao, F.-F.; Zhang, L.; Han, X.; Wang, Y.; Hui, M.; Wu, D.; Li, H.; Wang, H. Intraspecific Recurrent Selection in V. vinifera: An Effective Method for Breeding of High Quality, Disease-, Cold-, and Drought -Resistant Grapes. Euphytica 2021, 217, 111. [Google Scholar] [CrossRef]
- Adams, D. Genetic Analysis of Cold Hardiness in a Population of Norton (Vitis aestivalis) and Cabernet Sauvignon (Vitis vinifera) Hybrids. Master’s Thesis, Michigan State University, East Lansing, MI, USA, 2017. [Google Scholar]
- Kose, B.; Uray, Y.; Kaya, O.; Turk, F.; Bayram, K.; Svyantek, A. Foliar Applications Improves Grapevine Plant Cold Hardiness. Sci. Hortic. 2024, 330, 113088. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, H.; Fu, X.; Wang, H.; Wang, R. Optimizing Grape Growth, Berry Quality and Phenolic Compounds with Foliar Co-Application of Iron and Calcium. S. Afr. J. Bot. 2024, 169, 146–154. [Google Scholar] [CrossRef]
- Faralli, M.; Mallucci, S.; Bignardi, A.; Varner, M.; Bertamini, M. Four Decades in the Vineyard: The Impact of Climate Change on Grapevine Phenology and Wine Quality in Northern Italy. OENO One 2024, 58. Available online: https://oeno-one.eu/article/view/8083 (accessed on 19 February 2025). [CrossRef]
- van Breda, V.M.; van Jaarsveld, F.P.; van Wyk, J. Pre-Fermentative Cryogenic Treatments: The Effect on Aroma Compounds and Sensory Properties of Sauvignon Blanc and Chenin Blanc Wine—A Review. Appl. Sci. 2024, 14, 1483. [Google Scholar] [CrossRef]
- Deed, R.C.; Fedrizzi, B.; Gardner, R.C. Influence of Fermentation Temperature, Yeast Strain, and Grape Juice on the Aroma Chemistry and Sensory Profile of Sauvignon Blanc Wines. J. Agric. Food Chem. 2017, 65, 8902–8912. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, A.; Cliff, M.; Girard, B.; Kopp, T.G. Influence of Fermentation Temperature on Composition and Sensory Properties of Semillon and Shiraz Wines. Am. J. Enol. Vitic. 2001, 52, 235–240. [Google Scholar] [CrossRef]
- Svyantek, A.; Wang, Z.; Hatterman-Valenti, H. Impact of Steam Extraction and Maceration Duration on Wines from Frozen ‘Frontenac’ Must. Fermentation 2023, 9, 317. [Google Scholar] [CrossRef]
- Rice, S.; Koziel, J.A.; Dharmadhikari, M.; Fennell, A. Evaluation of Tannins and Anthocyanins in Marquette, Frontenac, and St. Croix Cold-Hardy Grape Cultivars. Fermentation 2017, 3, 47. [Google Scholar] [CrossRef]
- Gapinski, A.D.; Horton, A.C.; Watrelot, A.A. Effect of Whole Cluster Fermentation on Phenolics in Cold-Hardy Hybrid Wines. Food Bioprocess Technol. 2023, 16, 1595–1608. [Google Scholar] [CrossRef]
- Kunkee, R.E. Selection and Modification of Yeasts and Lactic Acid Bacteria for Wine Fermentation. Food Microbiol. 1984, 1, 315–332. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, W.; Yi, H.; Wang, B.; Xiao, J.; Zhou, X.; Jiankun, X.; Jiang, L.; Shi, X. Microbial Community Composition and Its Role in Volatile Compound Formation during the Spontaneous Fermentation of Ice Wine Made from Vidal Grapes. Process Biochem. 2020, 92, 365–377. [Google Scholar] [CrossRef]
- Son, H.-S.; Hwang, G.-S.; Park, W.-M.; Hong, Y.-S.; Lee, C.-H. Metabolomic Characterization of Malolactic Fermentation and Fermentative Behaviors of Wine Yeasts in Grape Wine. J. Agric. Food Chem. 2009, 57, 4801–4809. [Google Scholar] [CrossRef]
- Feng, C.T.; Du, X.; Wee, J. Microbial and Chemical Analysis of Non-Saccharomyces Yeasts from Chambourcin Hybrid Grapes for Potential Use in Winemaking. Fermentation 2021, 7, 15. [Google Scholar] [CrossRef]
- Beigbeder, J.-B.; de Medeiros Dantas, J.M.; Lavoie, J.-M. Optimization of Yeast, Sugar and Nutrient Concentrations for High Ethanol Production Rate Using Industrial Sugar Beet Molasses and Response Surface Methodology. Fermentation 2021, 7, 86. [Google Scholar] [CrossRef]
- Vasyagin, E.A.; Urakov, V.N.; Shalamitskiy, M.Y.; Cherviak, S.N.; Ivanova, E.V.; Zagoruyko, V.I.; Beletsky, A.V.; Rakitin, A.L.; Mardanova, E.S.; Kushnirov, V.V.; et al. Development of a Wine Yeast Strain Capable of Malolactic Fermentation and Reducing the Ethyl Carbamate Content in Wine. Foods 2024, 14, 54. [Google Scholar] [CrossRef]
- Malolactic Fermentation—An Overview|ScienceDirect Topics. Available online: https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/malolactic-fermentation (accessed on 18 February 2025).
- Malolactic Fermentation: The ABC’s of MLF. Available online: https://www.sasev.org/journal-entry/malolactic-fermentation-the-abcs-of-mlf/ (accessed on 7 April 2025).
- Fernandes, F.H.A.; Salgado, H.R.N. Gallic Acid: Review of the Methods of Determination and Quantification. Crit. Rev. Anal. Chem. 2016, 46, 257–265. [Google Scholar] [CrossRef]
- Gobbi, M.; Comitini, F.; Domizio, P.; Romani, C.; Lencioni, L.; Mannazzu, I.; Ciani, M. Lachancea thermotolerans and Saccharomyces cerevisiae in Simultaneous and Sequential Co-Fermentation: A Strategy to Enhance Acidity and Improve the Overall Quality of Wine. Food Microbiol. 2013, 33, 271–281. [Google Scholar] [CrossRef]
- Kapsopoulou, K.; Kapaklis, A.; Spyropoulos, H. Growth and Fermentation Characteristics of a Strain of the Wine Yeast Kluyveromyces Thermotolerans Isolated in Greece. World J. Microbiol. Biotechnol. 2005, 21, 1599–1602. [Google Scholar] [CrossRef]
- Bonciani, T.; Solieri, L.; De Vero, L.; Giudici, P. Improved Wine Yeasts by Direct Mating and Selection under Stressful Fermentative Conditions. Eur. Food Res. Technol. 2016, 242, 899–910. [Google Scholar] [CrossRef]
- Minnaar, P.; Nyobo, L.; Jolly, N.; Ntushelo, N.; Meiring, S. Anthocyanins and Polyphenols in Cabernet Franc Wines Produced with Saccharomyces cerevisiae and Torulaspora delbrueckii Yeast Strains: Spectrophotometric Analysis and Effect on Selected Sensory Attributes. Food Chem. 2018, 268, 287–291. [Google Scholar] [CrossRef]
- Fan, L.; Doucette, C.; McSweeney, M.B.; English, M.; Song, J.; Vinqvist-Tymchuk, M.; Kernaghan, G. Non-Traditional Yeasts from Cool-Climate Vineyards for Novel Low-Alcohol Wines. Plants People Planet 2025, 7, 776–788. [Google Scholar] [CrossRef]
- Haggerty, L. Understanding the Ripening Chemistry of Cold-Hardy Wine Grapes to Predict Optimal Harvest Time; University of Minnesota: Minneapolis, MN, USA, 2014. [Google Scholar]
- Tahim, C.M.; Mansfield, A.K. Yeast Assimilable Nitrogen Optimization for Cool-Climate Riesling. Am. J. Enol. Vitic. 2019, 70, 127–138. [Google Scholar] [CrossRef]
- Sims, C.A.; Eastridge, J.S.; Bates, R.P. Changes in Phenols, Color, and Sensory Characteristics of Muscadine Wines by Pre- and Post-Fermentation Additions of PVPP, Casein, and Gelatin. Am. J. Enol. Vitic. 1995, 46, 155–158. [Google Scholar] [CrossRef]
- Departamento de Química. Universidad de La Rioja. Available online: https://www.unirioja.es/color/descargas.shtml (accessed on 7 April 2025).
- Wang, Z.; Svyantek, A.; Bogenrief, S.; Kadium, V.R.; Hatterman-Valenti, H. The Influence of Yeast Strain on the Chemical, Chromatic, and Sensory Characteristics of ‘Wodarz’ Apple Cider. Appl. Sci. 2024, 14, 4851. [Google Scholar] [CrossRef]
- Iland, P.; Bruer, N.; Edwards, G.; Caloghiris, S.; Wilkes, E. Patrick Iland Wine Promotions. In Chemical Analysis of Grapes and Wine: Techniques and Concepts, 2nd ed.; Patrick Iland Wine Promotions Pty Ltd.: Campbelltown, SA, Australia, 2013; ISBN 978-0-9581605-7-5. [Google Scholar]
- The Wine Cellar Insider. Wine Aroma Wheel from UC Davis. Available online: https://www.thewinecellarinsider.com/wine-topics/wine-educational-questions/davis-aroma-wheel/ (accessed on 18 June 2024).
- Marquette Grape Cultivar|Minnesota Hardy. Available online: https://mnhardy.umn.edu/marquette (accessed on 19 June 2024).
- Svyantek, A.; Stenger, J.E.; Theisen, N.; Brooke, M.; Auwarter, C.; Hatterman-Valenti, H. Assessment of ‘Frontenac’ and ‘King of the North’ as Potential Genotypes for Late Harvest and Ice Wine Production in North Dakota. Acta Hortic. 2025, 1418, 229–236. [Google Scholar] [CrossRef]
- Lodi Growers. WINE GRAPE ACIDITY, pH, & POTASSIUM. Available online: https://lodigrowers.com/wine-grape-acidity-ph-potassium/ (accessed on 18 February 2025).
- Boulton, R. The Relationships between Total Acidity, Titratable Acidity and pH in Wine. Am. J. Enol. Vitic. 1980, 31, 76–80. [Google Scholar] [CrossRef]
- Testing for Titratable Acidity. Available online: https://winemakermag.com/technique/testing-for-titratable-acidity (accessed on 20 February 2025).
- Martinson, T.E.; Mansfield, A.K.; Luby, J.J.; Gartner, W.C.; Dharmadhikari, M.; Domoto, P.; Fennell, A. The Northern Grapes Project: Integrating Viticulture, Enology, and Marketing of New Cold-Hardy Wine Grape Cultivars in the Midwest and Northeast United States. Acta Hortic. 2016, 1115, 3–12. [Google Scholar] [CrossRef]
- He, F.; Mu, L.; Yan, G.-L.; Liang, N.-N.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Biosynthesis of Anthocyanins and Their Regulation in Colored Grapes. Molecules 2010, 15, 9057–9091. [Google Scholar] [CrossRef]
- Gonzalez-Ramirez, M.; Kazakova, J.; Garcia-Serrano, P.; Ubeda, C.; Valero, E.; Cerezo, A.B.; Troncoso, A.M.; Garcia-Parrilla, M.C. Commercial Wine Yeast Nitrogen Requirement Influences the Production of Secondary Metabolites (Aroma, Hydroxytyrosol, Melatonin and Other Bioactives) during Alcoholic Fermentation. Int. J. Food Microbiol. 2024, 421, 110788. [Google Scholar] [CrossRef]
- He, F.; Liang, N.-N.; Mu, L.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Anthocyanins and Their Variation in Red Wines I. Monomeric Anthocyanins and Their Color Expression. Molecules 2012, 17, 1571–1601. [Google Scholar] [CrossRef]
- Novo, M.; Gonzalez, R.; Bertran, E.; Martínez, M.; Yuste, M.; Morales, P. Improved Fermentation Kinetics by Wine Yeast Strains Evolved under Ethanol Stress. LWT-Food Sci. Technol. 2014, 58, 166–172. [Google Scholar] [CrossRef]
- Bordet, F.; Romanet, R.; Bahut, F.; Ferreira, V.; Peña, C.; Julien-Ortiz, A.; Roullier-Gall, C.; Alexandre, H. Impact of Saccharomyces cerevisiae Yeast Inoculation Mode on Wine Composition. Food Chem. 2024, 441, 138391. [Google Scholar] [CrossRef]
- Bianchi, F.; Avesani, M.; Lorenzini, M.; Zapparoli, G.; Simonato, B. Fermentation Performances and Aroma Contributions of Selected Non-Saccharomyces Yeasts for Cherry Wine Production. Foods 2024, 13, 2455. [Google Scholar] [CrossRef]
- Hao, Y.; Li, J.; Zhao, Z.; Xu, W.; Wang, L.; Lin, X.; Hu, X.; Li, C. Flavor Characteristics of Shanlan Rice Wines Fermented for Different Time Based on HS-SPME-GC-MS-O, HS-GC-IMS, and Electronic Sensory Analyses. Food Chem. 2024, 432, 137150. [Google Scholar] [CrossRef] [PubMed]
- Matei, F.; Kosseva, M.R. Chapter 2—Microbiology of Fruit Wine Production. In Science and Technology of Fruit Wine Production; Kosseva, M.R., Joshi, V.K., Panesar, P.S., Eds.; Academic Press: San Diego, CA, USA, 2017; pp. 73–103. ISBN 978-0-12-800850-8. [Google Scholar]



| Yeast | Yeast Species | Company | Location of Origin |
|---|---|---|---|
| 71B | Saccharomyces cerevisiae | Scott Lab (Petaluma, CA, USA) | Narbonne, FRA |
| EC1118 | Saccharomyces cerevisiae (ex-bayanus) | Scott Lab (Petaluma, CA, USA) | Champagne, FRA |
| Maurivin B | Saccharomyces cerevisiae | Lallemand Inc. (Montreal, QC, Canada) | Burgundy region of France |
| Rhône 4600 | Saccharomyces cerevisiae | Scott Lab (Petaluma, CA, USA) | Côtes du Rhône, FRA |
| W15 | Saccharomyces cerevisiae | Lallemand Inc., (Montreal, QC, Canada) | Wädenswil, CHE |
| Sugar content | |||||
|---|---|---|---|---|---|
| SSC (°Brix) | Glucose (g/L) | Fructose (g/L) | |||
| 17.53 ± 0.47 | 88.28 ± 3.10 | 90.78 ± 1.87 | |||
| Acid attributes | |||||
| pH | Titratable acidity (g/L) | Malic acid (g/L) | |||
| 2.58 ± 0.01 | 22.7 ± 0.05 | 13.44 ± 0.57 | |||
| Total phenolics and total red pigments | |||||
| Total phenolics (AU) | Total red pigments (AU) | ||||
| 12.56 ± 1.05 | 5.15 ± 1.11 | ||||
| Nitrogen content | |||||
| YAN (mg/L) | Ammonia (mg/L) | PAN (mg/L) | |||
| 196.05 ± 15.99 | 7.71 ± 0.69 | 188.34 ± 15.44 | |||
| Yeast | Malic Acid (g/L) | Tartaric Acid (g/L) | Lactic Acid (g/L) | Acetic Acid (g/L) | pH | Titratable Acidity (g/L) 1 |
|---|---|---|---|---|---|---|
| 71B | 9.83 ± 0.39 b2 | 1.12 ± 0.20 b | 0.05 ± 0.01 ns | 0.15 ± 0.01 b | 3.15 ± 0.02 a | 11.2 ± 0.2 c |
| EC1118 | 13.27 ± 0.48 a | 1.21 ± 0.06 ab | 0.05 ± 0.01 | 0.05 ± 0.01 c | 3.11 ± 0.05 ab | 13.0 ± 0.2 ab |
| Maurivin B | 8.93 ± 0.39 b | 1.33 ± 0.19 ab | 0.09 ± 0.04 | 0.33 ± 0.02 a | 3.08 ± 0.03 b | 11.7 ± 1.0 bc |
| Rhône 4600 | 13.03 ± 0.39 a | 1.50 ± 0.13 a | 0.06 ± 0.01 | 0.07 ± 0.01 c | 3.05 ± 0.01 b | 12.9 ± 0.3 ab |
| W15 | 12.81 ± 0.39 a | 1.51 ± 0.17 a | 0.05 ± 0.01 | 0.14 ± 0.01 b | 3.06 ± 0.02 b | 13.5 ± 0.1 a |
| Yeast | Glycerol (g/L) | Ethanol (% v/v) |
|---|---|---|
| 71B | 6.31 ± 0.12 a1 | 8.01 ± 0.11 ns |
| EC1118 | 5.39 ± 0.14 b | 8.20 ± 0.10 |
| Maurivin B | 5.81 ± 0.15 ab | 8.20 ± 0.12 |
| Rhône 4600 | 5.94 ± 0.15 ab | 8.15 ± 0.11 |
| W15 | 6.15 ± 0.29 a | 8.12 ± 0.11 |
| Yeast | Color Density | Color Intensity | Color Hue | Total Phenolics (AU) |
|---|---|---|---|---|
| 71B | 0.56 ± 0.03 ns1 | 0.57 ± 0.03 ns | 1.87 ± 0.43 a | 11.62 ± 1.37 ns |
| EC1118 | 0.73 ± 0.06 | 0.77 ± 0.08 | 1.41 ± 0.20 b | 10.65 ± 1.57 |
| Maurivin B | 0.73 ± 0.04 | 0.77 ± 0.04 | 1.49 ± 0.20 b | 10.68 ± 1.06 |
| Rhône 4600 | 0.68 ± 0.03 | 0.70 ± 0.04 | 1.32 ± 0.13 b | 11.52 ± 1.43 |
| W15 | 0.66 ± 0.12 | 0.67 ± 0.13 | 1.46 ± 0.17 b | 11.02 ± 1.11 |
| Yeast | Lightness (L*) | Red (a*) | Yellow (b*) | Chroma (C*) | Hue (h°) |
|---|---|---|---|---|---|
| 71B | 97.77 ± 0.29 ns | 2.13 ± 0.57 ns | 4.26 ± 0.15 ns | 4.83 ± 0.16 ns | 63.74 ± 6.69 ns |
| EC1118 | 96.43 ± 0.49 | 3.50 ± 0.56 | 4.07 ± 0.21 | 5.41 ± 0.42 | 49.76 ± 4.44 |
| Maurivin B | 95.57 ± 0.17 | 3.19 ± 0.75 | 4.42 ± 0.17 | 5.52 ± 0.46 | 55.17 ± 6.25 |
| Rhône 4600 | 96.73 ± 0.18 | 3.50 ± 0.42 | 3.73 ± 0.30 | 5.14 ± 0.33 | 46.92 ± 4.21 |
| W15 | 97.03 ± 0.67 | 3.44 ± 0.77 | 4.07 ± 0.51 | 5.37 ± 0.82 | 50.42 ± 4.56 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Svyantek, A.; Kadium, V.R.; Bogenrief, S.; Hatterman-Valenti, H. Different Yeast Strain Effects on ‘King of the North’ Wine Chemical, Chromatic, and Descriptive Sensory Characteristics. Fermentation 2025, 11, 262. https://doi.org/10.3390/fermentation11050262
Wang Z, Svyantek A, Kadium VR, Bogenrief S, Hatterman-Valenti H. Different Yeast Strain Effects on ‘King of the North’ Wine Chemical, Chromatic, and Descriptive Sensory Characteristics. Fermentation. 2025; 11(5):262. https://doi.org/10.3390/fermentation11050262
Chicago/Turabian StyleWang, Zhuoyu, Andrej Svyantek, Venkateswara Rao Kadium, Sarah Bogenrief, and Harlene Hatterman-Valenti. 2025. "Different Yeast Strain Effects on ‘King of the North’ Wine Chemical, Chromatic, and Descriptive Sensory Characteristics" Fermentation 11, no. 5: 262. https://doi.org/10.3390/fermentation11050262
APA StyleWang, Z., Svyantek, A., Kadium, V. R., Bogenrief, S., & Hatterman-Valenti, H. (2025). Different Yeast Strain Effects on ‘King of the North’ Wine Chemical, Chromatic, and Descriptive Sensory Characteristics. Fermentation, 11(5), 262. https://doi.org/10.3390/fermentation11050262






