Evaluation of In Vitro Digested Mulberry Leaf Tea Kombucha: A Functional Fermented Beverage with Antioxidant, Anti-Inflammatory, Antihyperglycemic, and Antihypertensive Potentials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Analysis Instruments
2.2. Sample Preparation
2.3. Determination of In Vitro Bioactivity
2.3.1. Antihypertensive Activity
2.3.2. Antiglycemic Activity
2.3.3. Antioxidant Activity
2.3.4. Anti-Inflammatory Activity
2.3.5. Color Interference Correction in Spectrophotometric Assays
2.4. Determination of Bioactive Compounds
2.4.1. Determination of 1-Deoxynojirimycin (DNJ) Content
2.4.2. Determination of γ-Aminobutyric Acid (GABA) Content
2.4.3. Total Phenolic and Total Flavonoid Contents
2.4.4. Identification and Quantification of Phenolic Compounds
2.5. Bioaccessibility During In Vitro Digestion
2.6. Statistical Analysis
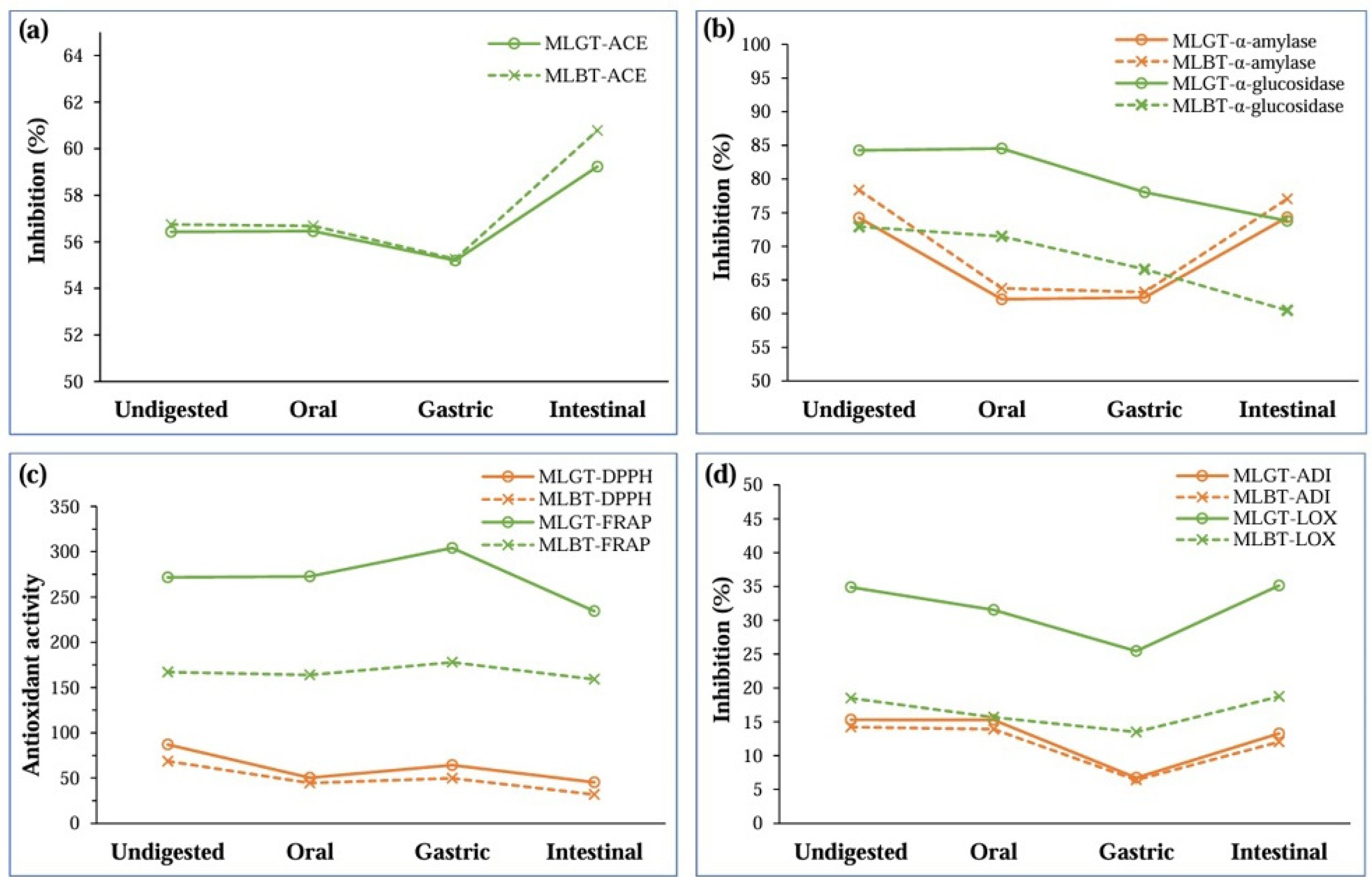
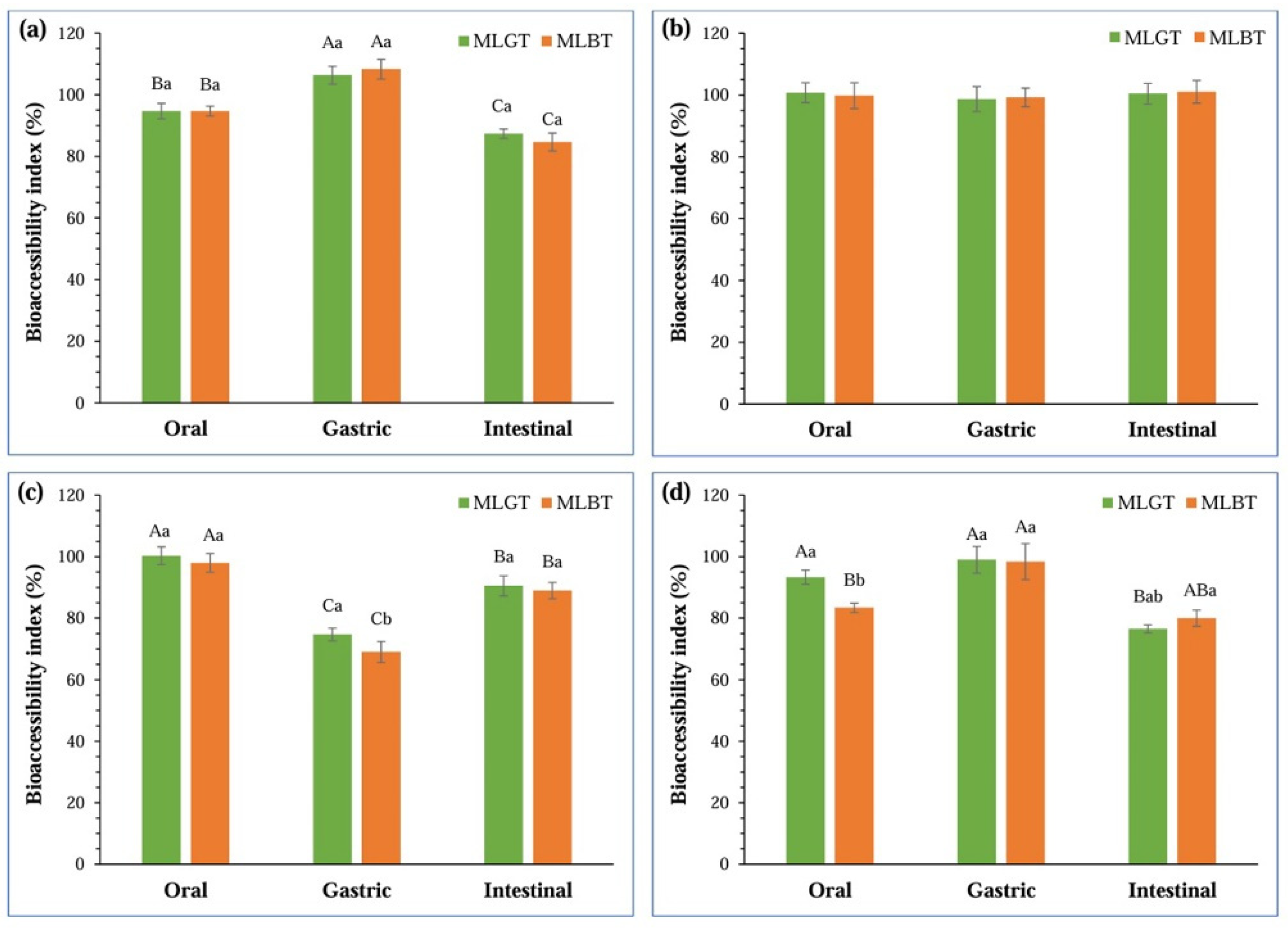
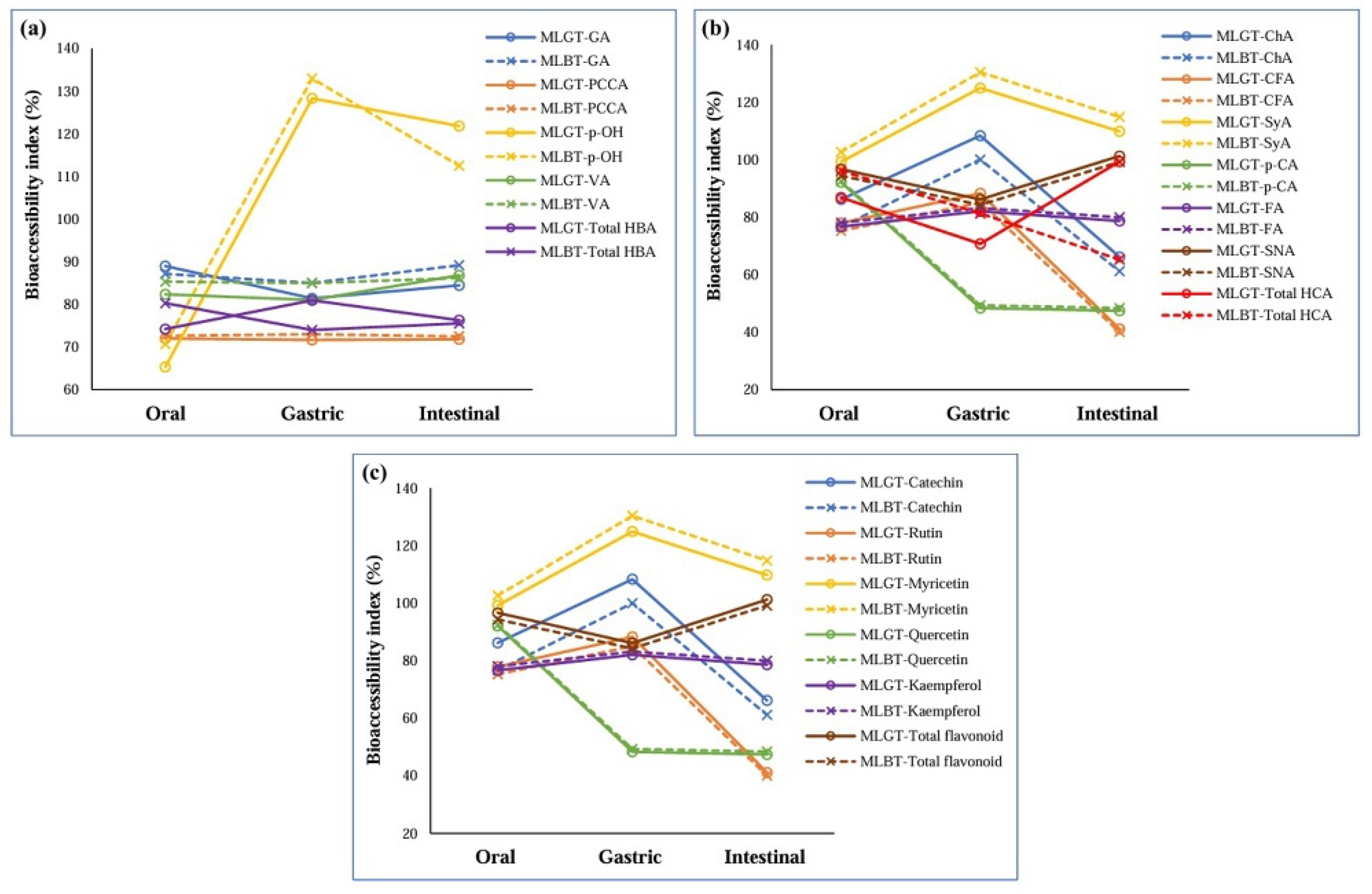
3. Results
3.1. Changes in Bioactivity During Digestion
3.2. Bioaccessibility
3.3. Pearson’s Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ortega-Montiel, J.; Montoya, A.; Soria-Saucedo, R.; Gallegos-Carrillo, K.; Ramirez-Palacios, P.; Salmeron, J.; Salazar-Martinez, E. Trends of antihypertensive, antidiabetic, and nonsteroidal anti-inflammatory drugs use among the health workers cohort study, Mexico 2004 to 2018. Adv. Pharmacol. Pharm. Sci. 2023, 2023, 5555274. [Google Scholar] [CrossRef] [PubMed]
- Mlynarska, E.; Biskup, L.; Mozdzan, M.; Grygorcewicz, O.; Mozdzan, Z.; Semeradt, J.; Uramowski, M.; Rysz, J.; Franczyk, B. The role of oxidative stress in hypertension: The insight into antihypertensive properties of vitamins A, C and E. Antioxidants 2024, 13, 848. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, P.; Lozano, P.; Ros, G.; Solano, F. Hyperglycemia and oxidative stress: An integral, updated and critical overview of their metabolic interconnections. Int. J. Mol. Sci. 2023, 24, 9352. [Google Scholar] [CrossRef] [PubMed]
- Puschel, G.P.; Klauder, J.; Henkel, J. Macrophages, low-grade inflammation, insulin resistance and hyperinsulinemia: A mutual ambiguous relationship in the development of metabolic diseases. J. Clin. Med. 2022, 11, 4358. [Google Scholar] [CrossRef]
- Maranduca, M.A.; Tanase, D.M.; Branisteanu, D.C.; Serban, D.N.; Branisteanu, D.E.; Serban, I.L. Involvement of proinflammatory cytokines in angiotensin II-induced hypertension in rat. Exp. Ther. Med. 2020, 20, 3541–3545. [Google Scholar] [CrossRef]
- Moke, E.G.; Omogbai, E.K.I.; Osagie-Eweka, S.E.; Uchendu, A.P.; Obayuwana, O.M.; Okoro-Akpandu, E.; Ben-Azu, B. Antihypertensive and antihyperglycemic effects of combinations of losartan with metformin and/or glibenclamide in desoxycorticosterone acetate and streptozotocin-induced hypertensive diabetic rats. Lab. Anim. Res. 2023, 39, 7. [Google Scholar] [CrossRef]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, hypertension, and cardiovascular disease: Clinical insights and vascular mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef]
- Nugraha, R.; Kurniawan, F.; Abdullah, A.; Lopata, A.L.; Ruethers, T. Antihypertensive and antidiabetic drug candidates from milkfish (chanos chanos)-identification and characterization through an integrated bioinformatic approach. Foods 2024, 13, 2594. [Google Scholar] [CrossRef]
- Vignesh, A.; Amal, T.C.; Sarvalingam, A.; Vasanth, K. A Review on the influence of nutraceuticals and functional foods on health. Food Chem. Adv. 2024, 2024, 100749. [Google Scholar] [CrossRef]
- Corbo, M.R.; Bevilacqua, A.; Petruzzi, L.; Casanova, F.P.; Sinigaglia, M. Functional beverages: The emerging side of functional foods. Compr. Rev. Food Sci. Food Saf. 2014, 13, 1192–1206. [Google Scholar] [CrossRef]
- Misra, S.; Pandey, P.; Mishra, H.N. Novel approaches for co-encapsulation of probiotic bacteria with bioactive compounds, their health benefits and functional food product development: A review. Trends Food Sci. Technol. 2021, 109, 340–351. [Google Scholar] [CrossRef]
- Gupta, A.; Sanwal, N.; Bareen, M.A.; Barua, S.; Sharma, N.; Joshua Olatunji, O.; Prakash Nirmal, N.; Sahu, J.K. Trends in functional beverages: Functional ingredients, processing technologies, stability, health benefits, and consumer perspective. Food Res. Int. 2023, 170, 113046. [Google Scholar] [CrossRef] [PubMed]
- Antolak, H.; Piechota, D.; Kucharska, A. Kombucha Tea—A double power of bioactive compounds from tea and symbiotic culture of bacteria and yeasts (SCOBY). Antioxidants 2021, 10, 1541. [Google Scholar] [CrossRef]
- Jayabalan, R.; Malbasa, R.V.; Loncar, E.S.; Vitas, J.S.; Sathishkumar, M. A Review on kombucha tea-microbiology, composition, fermentation, beneficial effects, toxicity, and tea fungus. Compr. Rev. Food Sci. Food Saf. 2014, 13, 538–550. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Tan, Q.; Wu, S.; Abbas, B.; Yang, M. Application of kombucha fermentation broth for antibacterial, antioxidant, and anti-inflammatory processes. Int. J. Mol. Sci. 2023, 24, 13948. [Google Scholar] [CrossRef]
- Greenwalt, C.J.; Steinkraus, K.H.; Ledford, R.A. Kombucha, the fermented tea: Microbiology, composition, and claimed health effects. J. Food Prot. 2000, 63, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Batista, P.; Rodrigues Penas, M.; Vila-Real, C.; Pintado, M.; Oliveira-Silva, P. Kombucha: Challenges for health and mental health. Foods 2023, 12, 3378. [Google Scholar] [CrossRef]
- Chakravorty, S.; Bhattacharya, S.; Chatzinotas, A.; Chakraborty, W.; Bhattacharya, D.; Gachhui, R. Kombucha tea fermentation: Microbial and biochemical dynamics. Int. J. Food Microbiol. 2016, 220, 63–72. [Google Scholar] [CrossRef]
- Tu, J.; Liu, G.; Jin, Y.; Tang, C.; Yao, T.; Zhuo, J.; Li, Q.; Liu, L.; Wang, J. Enrichment of γ-aminobutyric acid in mulberry leaves and the inhibitory effects of the water extract on ACE and α-glucosidase activity. Ind. Crops Prod. 2022, 177, 114485. [Google Scholar] [CrossRef]
- Marchetti, L.; Truzzi, E.; Frosi, I.; Papetti, A.; Cappellozza, S.; Saviane, A.; Pellati, F.; Bertelli, D. In vitro bioactivity evaluation of mulberry leaf extracts as nutraceuticals for the management of diabetes mellitus. Food Funct. 2022, 13, 4344–4359. [Google Scholar] [CrossRef]
- Fongsodsri, K.; Thaipitakwong, T.; Rujimongkon, K.; Kanjanapruthipong, T.; Ampawong, S.; Reamtong, O.; Aramwit, P. Mulberry-derived 1-deoxynojirimycin prevents type 2 diabetes mellitus progression via modulation of retinol-binding protein 4 and haptoglobin. Nutrients 2022, 14, 4538. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Xing, Y.; Ren, X.; Zheng, M.; Yu, S.; Wang, Y.; Xiu, Z.; Dong, Y. Mulberry leaf extract improves metabolic syndrome by alleviating lipid accumulation in vitro and in vivo. Molecules 2022, 27, 5111. [Google Scholar] [CrossRef]
- Li, R.; Zhu, Q.; Wang, X.; Wang, H. Mulberry leaf polyphenols alleviated high-fat diet-induced obesity in mice. Front. Nutr. 2022, 9, 979058. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, C.; Artacho, R.; Giménez, R. Beneficial effects of green tea—A review. J. Am. Coll. Nutr. 2006, 25, 79–99. [Google Scholar] [CrossRef] [PubMed]
- Villarreal-Soto, S.A.; Beaufort, S.; Bouajila, J.; Souchard, J.P.; Taillandier, P. Understanding kombucha tea fermentation: A review. J. Food Sci. 2018, 83, 580–588. [Google Scholar] [CrossRef]
- Wu, Q.; Du, J.; Jia, J.; Kuang, C. Production of ACE inhibitory peptides from sweet sorghum grain protein using alcalase: Hydrolysis kinetic, purification and molecular docking study. Food Chem. 2016, 199, 140–149. [Google Scholar] [CrossRef]
- Kwon, Y.-I.; Apostolidis, E.; Shetty, K. Inhibitory potential of wine and tea against α-amylase and α-glucosidase for management of hyperglycemia linked to type 2 diabetes. J. Food Biochem. 2008, 32, 15–31. [Google Scholar] [CrossRef]
- Kim, Y.M.; Jeong, Y.K.; Wang, M.H.; Lee, W.Y.; Rhee, H.I. Inhibitory effect of pine extract on alpha-glucosidase activity and postprandial hyperglycemia. Nutrition 2005, 21, 756–761. [Google Scholar] [CrossRef]
- Wanyo, P.; Kaewseejan, N.; Meeso, N.; Siriamornpun, S. Bioactive compounds and antioxidant properties of different solvent extracts derived from Thai rice by-products. Appl. Biol. Chem. 2016, 59, 373–384. [Google Scholar] [CrossRef]
- Williams, L.A.; O’Connar, A.; Latore, L.; Dennis, O.; Ringer, S.; Whittaker, J.A.; Conrad, J.; Vogler, B.; Rosner, H.; Kraus, W. The in vitro anti-denaturation effects induced by natural products and non-steroidal compounds in heat treated (immunogenic) bovine serum albumin is proposed as a screening assay for the detection of anti-inflammatory compounds, without the use of animals, in the early stages of the drug discovery process. West Indian Med. J. 2008, 57, 327–331. [Google Scholar]
- Yawer, M.A.; Ahmed, E.; Malik, A.; Ashraf, M.; Rasool, M.A.; Afza, N. New Lipoxygenase-inhibiting constituents from calligonum polygonoides. Chem. Biodivers. 2007, 4, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Kim, S.U.; Lee, H.S.; Kim, I.; Ahn, M.Y.; Ryu, K.S. Determination of 1-deoxynojirimycin in Morus alba L. leaves by derivatization with 9-fluorenylmethyl chloroformate followed by reversed-phase high-performance liquid chromatography. J. Chromatogr. A 2003, 1002, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Tu, J.; Han, X.; Zhuo, J.; Liu, G.; Han, Y.; Du, H.; Wang, J.; Xiao, H. Characteristics of mulberry leaf powder enriched with gamma-aminobutyric acid and its antioxidant capacity as a potential functional food ingredient. Front. Nutr. 2022, 9, 900718. [Google Scholar] [CrossRef] [PubMed]
- Santana Andrade, J.K.; Chagas Barros, R.G.; Pereira, U.C.; Nogueira, J.P.; Gualberto, N.C.; Santos de Oliveira, C.; Shanmugam, S.; Narain, N. Bioaccessibility of bioactive compounds after in vitro gastrointestinal digestion and probiotics fermentation of Brazilian fruits residues with antioxidant and antidiabetic potential. LWT 2022, 153, 112469. [Google Scholar] [CrossRef]
- Prado, N.J.; Ramirez, D.; Mazzei, L.; Parra, M.; Casarotto, M.; Calvo, J.P.; Cuello Carrion, D.; Ponce Zumino, A.Z.; Diez, E.R.; Camargo, A.; et al. Anti-inflammatory, antioxidant, antihypertensive, and antiarrhythmic effect of indole-3-carbinol, a phytochemical derived from cruciferous vegetables. Heliyon 2022, 8, e08989. [Google Scholar] [CrossRef]
- Arabshomali, A.; Bazzazzadehgan, S.; Mahdi, F.; Shariat-Madar, Z. Potential benefits of antioxidant phytochemicals in type 2 diabetes. Molecules 2023, 28, 7209. [Google Scholar] [CrossRef]
- Acharya, K.R.; Gregory, K.S.; Sturrock, E.D. Advances in the structural basis for angiotensin-1 converting enzyme (ACE) inhibitors. Biosci. Rep. 2024, 44, BSR20240130. [Google Scholar] [CrossRef]
- Anantachoke, N.; Duangrat, R.; Sutthiphatkul, T.; Ochaikul, D.; Mangmool, S. Kombucha beverages produced from fruits, vegetables, and plants: A review on their pharmacological activities and health benefits. Foods 2023, 12, 1818. [Google Scholar] [CrossRef]
- Yu, M.; Kim, H.J.; Heo, H.; Kim, M.; Jeon, Y.; Lee, H.; Lee, J. Comparison of the antihypertensive activity of phenolic acids. Molecules 2022, 27, 6185. [Google Scholar] [CrossRef]
- Abdullah, R.; Arshad, H.; Kaleem, A.; Iqtedar, M.; Aftab, M.; Saleem, F. Assessment of angiotensin converting enzyme inhibitory activity and quality attributes of yoghurt enriched with Cinnamomum verum, Elettaria cardamomum, Beta vulgaris and Brassica oleracea. Saudi J. Biol. Sci. 2023, 30, 103556. [Google Scholar] [CrossRef]
- Yang, G.; Zhu, Y.; Shi, J.; Peng, Q.; Lin, Z.; Lv, H. Effects of anaerobic treatment on the non-volatile components and angiotensin-converting enzyme (ACE) inhibitory activity of purple-colored leaf tea. Food Chem. X 2024, 23, 101649. [Google Scholar] [CrossRef] [PubMed]
- Kapp, J.M.; Sumner, W. Kombucha: A systematic review of the empirical evidence of human health benefit. Ann. Epidemiol. 2019, 30, 66–70. [Google Scholar] [CrossRef]
- Cardoso, R.R.; Neto, R.O.; dos Santos D’Almeida, C.T.; do Nascimento, T.P.; Pressete, C.G.; Azevedo, L.; Martino, H.S.D.; Cameron, L.C.; Ferreira, M.S.L.; Barros, F.A.R.d. Kombuchas from green and black teas have different phenolic profile, which impacts their antioxidant capacities, antibacterial and antiproliferative activities. Food Res. Int. 2020, 128, 108782. [Google Scholar] [CrossRef] [PubMed]
- Gaggìa, F.; Baffoni, L.; Galiano, M.; Nielsen, D.S.; Jakobsen, R.R.; Castro-Mejía, J.L.; Bosi, S.; Truzzi, F.; Musumeci, F.; Dinelli, G.; et al. Kombucha beverage from green, black and rooibos teas: A comparative study looking at microbiology, chemistry and antioxidant activity. Nutrients 2019, 11, 1. [Google Scholar] [CrossRef] [PubMed]
- Padhi, S.; Nayak, A.K.; Behera, A. Type II diabetes mellitus: A review on recent drug based therapeutics. Biomed. Pharmacother. 2020, 131, 110708. [Google Scholar] [CrossRef]
- Lee, B.H.; Eskandari, R.; Jones, K.; Reddy, K.R.; Quezada-Calvillo, R.; Nichols, B.L.; Rose, D.R.; Hamaker, B.R.; Pinto, B.M. Modulation of starch digestion for slow glucose release through “toggling” of activities of mucosal alpha-glucosidases. J. Biol. Chem. 2012, 287, 31929–31938. [Google Scholar] [CrossRef]
- Lin, A.H.; Lee, B.H.; Nichols, B.L.; Quezada-Calvillo, R.; Rose, D.R.; Naim, H.Y.; Hamaker, B.R. Starch source influences dietary glucose generation at the mucosal alpha-glucosidase level. J. Biol. Chem. 2012, 287, 36917–36921. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Y.; Huang, G.; Xin, X.; Tang, L.; Li, H.; Lee, K.S.; Jin, B.R.; Gui, Z. Chemical synthesis, inhibitory activity and molecular mechanism of 1-deoxynojirimycin-chrysin as a potent alpha-glucosidase inhibitor. RSC Adv. 2021, 11, 38703–38711. [Google Scholar] [CrossRef]
- An, H.; Thanh, L.N.; Khanh, L.Q.; Ryu, S.H.; Lee, S.; Yeon, S.W.; Lee, H.H.; Turk, A.; Lee, K.Y.; Hwang, B.Y.; et al. Characterization of antioxidant and alpha-glucosidase inhibitory compounds of Cratoxylum formosum ssp. pruniflorum and optimization of extraction condition. Antioxidants 2023, 12, 511. [Google Scholar] [CrossRef]
- Barber, E.; Houghton, M.J.; Williamson, G. Flavonoids as human intestinal alpha-glucosidase inhibitors. Foods 2021, 10, 1939. [Google Scholar] [CrossRef]
- Saarniit, K.; Lang, H.; Kuldjarv, R.; Laaksonen, O.; Rosenvald, S. The stability of phenolic compounds in fruit, berry, and vegetable purees based on accelerated shelf-life testing methodology. Foods 2023, 12, 1777. [Google Scholar] [CrossRef]
- Chaves, J.O.; de Souza, M.C.; da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.; Forster-Carneiro, T.; Vazquez-Espinosa, M.; Gonzalez-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of flavonoids from natural sources using modern techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef] [PubMed]
- Kluz, M.I.; Pietrzyk, K.; Pastuszczak, M.; Kacaniova, M.; Kita, A.; Kapusta, I.; Zaguła, G.; Zagrobelna, E.; Struś, K.; Marciniak-Lukasiak, K.; et al. Microbiological and physicochemical composition of various types of homemade kombucha beverages using alternative kinds of sugars. Foods 2022, 11, 1523. [Google Scholar] [CrossRef]
- Wu, P.; Deng, R.; Wu, X.; Wang, Y.; Dong, Z.; Dhital, S.; Chen, X.D. In vitro gastric digestion of cooked white and brown rice using a dynamic rat stomach model. Food Chem. 2017, 237, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Pouvreau, C.; Dayre, A.; Butkowski, E.G.; de Jong, B.; Jelinek, H.F. Inflammation and oxidative stress markers in diabetes and hypertension. J. Inflamm. Res. 2018, 11, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Odriozola-Serrano, I.; Nogueira, D.P.; Esparza, I.; Vaz, A.A.; Jimenez-Moreno, N.; Martin-Belloso, O.; Ancin-Azpilicueta, C. Stability and bioaccessibility of phenolic compounds in rosehip extracts during in vitro digestion. Antioxidants 2023, 12, 1035. [Google Scholar] [CrossRef]
- Guan, H.; Zhang, W.; Sun-Waterhouse, D.; Jiang, Y.; Li, F.; Waterhouse, G.I.; Li, D. Phenolic-protein interactions in foods and post ingestion: Switches empowering health outcomes. Trends Food Sci. Technol. 2021, 118, 71–86. [Google Scholar] [CrossRef]
- Minekus, M.; Alminger, M.; Alvito, P.; Ballance, S.; Bohn, T.; Bourlieu, C.; Carriere, F.; Boutrou, R.; Corredig, M.; Dupont, D.; et al. A standardised static in vitro digestion method suitable for food—An international consensus. Food Funct. 2014, 5, 1113–1124. [Google Scholar] [CrossRef]
- Bouayed, J.; Bohn, T. Exogenous antioxidants—Double-edged swords in cellular redox state: Health beneficial effects at physiologic doses versus deleterious effects at high doses. Oxid. Med. Cell Longev. 2010, 3, 228–237. [Google Scholar] [CrossRef]
- Macagnan, F.T.; da Silva, L.P.; Hecktheuer, L.H. Dietary fibre: The scientific search for an ideal definition and methodology of analysis, and its physiological importance as a carrier of bioactive compounds. Food Res. Int. 2016, 85, 144–154. [Google Scholar] [CrossRef]
- De Filippis, F.; Troise, A.D.; Vitaglione, P.; Ercolini, D. Different temperatures select distinctive acetic acid bacteria species and promotes organic acids production during kombucha tea fermentation. Food Microbiol. 2018, 73, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Morales, D. Biological activities of kombucha beverages: The need of clinical evidence. Trends Food Sci. Technol. 2020, 105, 323–333. [Google Scholar] [CrossRef]
| Variables | Pearson’s Correlation Coefficient (R2) | ||||||
|---|---|---|---|---|---|---|---|
| DPPH | FRAP | α-Amylase Inhibition | α-Glucosidase Inhibition | ADI | LOX Inhibition | ACE Inhibition | |
| DNJ | 0.583 ** | 0.949 ** | −0.227 | 0.771 ** | −0.030 | 0.772 ** | −0.280 |
| GABA | −0.092 | −0.014 | 0.080 | 0.029 | 0.166 | 0.037 | 0.294 |
| TPC | −0.336 | −0.892 ** | 0.300 | −0.584 ** | 0.220 | −0.751 ** | 0.183 |
| TFC | −0.502 * | −0.096 | −0.106 | 0.042 | −0.271 | −0.449 * | −0.464 * |
| GA | −0.380 | −0.925 ** | 0.232 | −0.736 ** | −0.084 | −0.887 ** | 0.125 |
| PCCA | −0.302 | −0.904 ** | 0.270 | −0.684 ** | −0.068 | −0.869 ** | 0.064 |
| ρ-OH | −0.399 | −0.776 ** | 0.185 | −0.837 ** | −0.477 * | −0.818 ** | 0.092 |
| VA | −0.193 | −0.855 ** | 0.345 | −0.639 ** | 0.038 | −0.760 ** | 0.087 |
| HBAs | −0.314 | −0.908 ** | 0.266 | −0.698 ** | −0.083 | −0.874 ** | 0.071 |
| ChA | −0.073 | −0.731 ** | 0.018 | −0.505 * | −0.288 | −0.880 ** | −0.211 |
| CFA | 0.872 ** | 0.806 ** | −0.266 | 0.842 ** | 0.066 | 0.523 ** | −0.478 * |
| SyA | 0.231 | 0.838 ** | −0.321 | 0.448 * | −0.384 | 0.604 ** | −0.137 |
| ρ-CA | 0.663 ** | 0.738 ** | −0.093 | 0.895 ** | 0.540 ** | 0.802 ** | −0.189 |
| FA | 0.555 ** | 0.932 ** | −0.94 | 0.790 ** | 0.181 | 0.923 ** | −0.132 |
| SNA | 0.325 | 0.513 * | 0.144 | 0.587 ** | 0.452 * | 0.752 ** | 0.149 |
| HCAs | 0.382 | −0.341 | −0.103 | −0.080 | −0.276 | −0.612 ** | −0.469 * |
| Catechin | 0.829 ** | 0.590 ** | 0.251 | 0.623 ** | 0.223 | 0.626 ** | −0.307 |
| Rutin | 0.702 | −0.106 | 0.149 | 0.319 | 0.291 | −0.176 | −0.357 |
| Myricetin | 0.122 | −0.660 ** | 0.211 | −0.385 | −0.087 | −0.722 ** | −0.144 |
| Quercetin | −0.275 | −0.748 ** | 0.302 | −0.689 ** | −0.124 | −0.727 ** | 0.120 |
| Kaempferol | −0.354 | −0.450 * | −0.097 | −0.371 | −0.236 | −0.543 ** | −0.003 |
| TFs | −0.725 ** | −0.107 | 0.198 | 0.296 | 0.266 | −0.169 | −0.365 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wanyo, P.; Chamsai, T.; Toontom, N.; Nghiep, L.K.; Tudpor, K. Evaluation of In Vitro Digested Mulberry Leaf Tea Kombucha: A Functional Fermented Beverage with Antioxidant, Anti-Inflammatory, Antihyperglycemic, and Antihypertensive Potentials. Fermentation 2025, 11, 258. https://doi.org/10.3390/fermentation11050258
Wanyo P, Chamsai T, Toontom N, Nghiep LK, Tudpor K. Evaluation of In Vitro Digested Mulberry Leaf Tea Kombucha: A Functional Fermented Beverage with Antioxidant, Anti-Inflammatory, Antihyperglycemic, and Antihypertensive Potentials. Fermentation. 2025; 11(5):258. https://doi.org/10.3390/fermentation11050258
Chicago/Turabian StyleWanyo, Pitchaporn, Tossaporn Chamsai, Nitchara Toontom, Le Ke Nghiep, and Kukiat Tudpor. 2025. "Evaluation of In Vitro Digested Mulberry Leaf Tea Kombucha: A Functional Fermented Beverage with Antioxidant, Anti-Inflammatory, Antihyperglycemic, and Antihypertensive Potentials" Fermentation 11, no. 5: 258. https://doi.org/10.3390/fermentation11050258
APA StyleWanyo, P., Chamsai, T., Toontom, N., Nghiep, L. K., & Tudpor, K. (2025). Evaluation of In Vitro Digested Mulberry Leaf Tea Kombucha: A Functional Fermented Beverage with Antioxidant, Anti-Inflammatory, Antihyperglycemic, and Antihypertensive Potentials. Fermentation, 11(5), 258. https://doi.org/10.3390/fermentation11050258








