Abstract
Mead is a fermented alcoholic beverage obtained by diluting honey in water and adding yeast. However, the addition of fruit to this beverage gives rise to melomel. In this study we are proposing an interesting novelty which consists of developing cupuaçu (Theobroma grandiflorum) melomel by fermenting Saccharomyces cerevisiae var. boulardii. The aim of this study was to develop cupuaçu (Theobroma grandiflorum) melomel produced by S. boulardii and to evaluate its physicochemical and microbiological characteristics after refrigerated storage at 4 °C. To do this, a central composite design (CCD) was employed, with two independent variables, i.e., the initial soluble solids content of the honey must (°Brix) and the concentration of the cupuaçu pulp (%). A standardized amount of 1 g/L of S. boulardii yeast was used at a temperature of 25 °C and a fermentation time of 30 days. Using the results of the central composite design (CCD), the best conditions for producing the beverage were defined according to the objectives of the study. Thus, the experimental comparison was carried out under the conditions of 25 °Brix of initial soluble solids in the honey must, 10% cupuaçu pulp, and 10 days of fermentation at 25 °C. The cupuaçu melomel exhibited a cell viability of the probiotic yeast S. boulardii above 107 log CFU/mL, with an alcohol content of 8.22% (v/v), a pH of 3.43, a total acidity of 54.8 of (mEq/L), and soluble solids of 12.42°Brix. In addition, the beverage was subjected to simulated gastric and intestinal juices in vitro to evaluate the survival of the microorganisms under these conditions, and a concentration of 106 log CFU/mL of S. boulardii was obtained. In this way, it was possible to produce a probiotic fermented alcoholic beverage made from honey and cupuaçu.
1. Introduction
Honey is defined as a food product produced by bees from the nectar of flowers or secretions from the living parts of plants [1]. Mead, a fermented alcoholic beverage produced by diluting honey in water and adding yeast [2], has been produced and consumed since ancient times. This beverage has an alcohol content of between 4 and 14% and its sensory and physicochemical characteristics are directly related to the type of honey used, as well as the yeast [2].
Mead has undergone changes over time from its original formulation, mainly related to the addition of spices and fruits, which provides the original beverage with sensory characteristics, antioxidants, and nutrients from the added ingredients [3,4,5]. Melomel, the name given to a fruit-infused honey beverage, has been gaining popularity, given the variety of fruits available [6,7].
One fruit of great prominence is cupuaçu (Theobroma grandiflorum), a fruit produced by T. grandiflorum trees, popularly called “cupuassuzeiro” or “cupuassueiro”; this species is native to the Amazon region and is cultivated and distributed in the northern region of Brazil, as well as in neighboring Latin American countries that are part of the Amazon Biome [8,9]. This fruit has a yellowish, acidic, and very aromatic pulp, and in view of its high popularity and versatility, it has been widely used for the production of different food products such as beverages, ice creams, creams, jams, etc. [10,11]. It is also rich in vitamins K and C, magnesium, phosphorus, and essential amino acids such as leucine, lysine, and valine in concentrations of 68, 62, and 60 mg/g, respectively [9,12]. Cupuaçu also plays a significant role in the economy of the northern region of Brazil, with a production value of BR 54.8 million in 2017, according to the latest IBGE census [13]. From this perspective, cupuaçu (T. grandiflorum), a fruit that is very well consumed and accepted, is a potential alternative for application in the development of melomel.
Currently, the yeast Saccharomyces cerevisiae is the microorganism most commonly used in mead fermentation [14,15]. However, the identification of microorganisms that add improvements to the fermentation process, as well as the discovery new technological and functional properties, continues to be a developing field of research. Probiotics, microorganisms that when consumed in sufficient quantities, promote health benefits for the host, have been used to produce different food products. These microorganisms must also be resistant to the digestive process and be present in minimum concentrations of 109 CFU/mL of viable cells in food [16]. However, studies in the literature indicate that smaller quantities, i.e., 106 CFU/mL of probiotics, are already sufficient and acceptable for products to develop some beneficial effect on the host [14,15,17,18,19,20,21]. As examples of unconventional and probiotic microorganisms used for the production of fermented alcoholic beverages, the use of immobilized kefir culture for the production of wine and cider, both with low alcohol content, is documented in the literature [22,23], as well as the use of Saccharomyces cerevisiae var. boulardii (S. boulardii) for the production of alcoholic orange and blackberry juice [24]. This yeast has also been used to produce beer with probiotic potential [25]. S. boulardii is a probiotic yeast whose use has been well established in the treatment of gastrointestinal diseases; it stands out for its performance in the production of beer, rosé wine, and mead, all of which exhibit probiotic properties, and it also displays adequate viability and excellent ethanol production capacity [18,26,27,28].
In this context, the aim of this study was to develop a fermented honey beverage using cupuaçu, called cupuaçu “melomel”, using the probiotic yeast S. boulardii for fermentation, as well as to evaluate the physicochemical and microbiological characteristics, the survival of the microorganisms in simulated gastric and intestinal juices in vitro, and to determine the shelf life of the product developed.
2. Material and Methods
2.1. Microorganisms
The yeast Saccharomyces cerevisiae var. boulardii CCT 4308 (UFPEDA 1176) was acquired from the culture bank of the André Tosello Foundation (ATF, Research and Technology), Campinas, São Paulo, Brazil. The commercial yeast Saccharomyces cerevisiae (Red star, Premier Rouge, Fermentis Lesaffre for Beverages) was obtained from a local business in the city of Pirassununga, São Paulo, Brazil.
2.2. Preparation of the S. boulardii Inoculum
To maintain the viability of the S. boulardii strains, the yeast was kept in an inclined tube with the yeast extract peptone dextrose (YPD) agar (slant) at 5 °C [29]. To prepare the inoculum, the yeast was then cultivated in a shaker (TECNAL, model TE-424, Niort, France) at 160 rpm at 25 °C for 96 h in 250 mL Erlenmeyer flasks containing yeast extract peptone dextrose (YPD) broth. After this period, the cells were recovered by centrifugation at 5000 rpm for 5 min, washed three times with phosphate buffer at pH 7.2, divided into portions, and set aside to be added to the melomel must.
The freeze-dried commercial S. cerevisiae yeasts were weighed and reactivated in sterile distilled water, according to the manufacturer’s recommendations, and then added to the beverage must.
2.3. Production of Cupuaçu Melomel
The cupuaçu melomel formulations were prepared using an experimental design, combining the independent variables in an experimental matrix (+1; −1), resulting in a central composite design (CCD) 22 with a central point repeated three times (level 0), totaling seven trials. The independent variables evaluated were the concentrations of X1 (°Brix), i.e., the initial soluble solids of the honey must (20, 25, and 30 °Brix), and X2 (%), i.e., the concentration of cupuaçu pulp in the cupuaçu must (5, 10, and 15%). For this purpose, 1 L of cupuaçu melomel was produced for each trial (Table 1).

Table 1.
Experimental design for the production of cupuaçu melomel.
The honey (Organic-LAMBERTUCCI, eucalyptus flowers) was purchased in the city of Pirassununga, São Paulo, Brazil, as was the cupuaçu pulp (Brand: Pura Polpa, Pirassununga, São Paulo, Brazil). The minimum and maximum concentrations (%) were defined based on preliminary tests. Equations (1) and (2) were used to correct the soluble solids of the honey required for each test.
MHo = (MWo × SS (°Brix) desired)/(SS (°Brix) honey)
Thus: MHo: Mass of honey (kg); MWo: Mass of must (kg); SS: Soluble solids (°Brix).
MWa = MWo − MHo
Thus: MWa = Mass of water (kg); MWo = Mass of must (kg); MHo = Mass of honey (kg).
The choice of formulations used in the follow-up experimental comparison was based on the following responses, i.e., Y1: final soluble solids (°Brix); Y2: pH; Y3: total acidity (mEq/L); Y4: alcohol content (%); Y5: viable cell count (log CFU/mL).
The honey and cupuaçu were prepared separately. The honey was diluted in drinking water in the amount calculated (Equations (1) and (2)), while the cupuaçu pulp was liquefied in the proportions of 50 (5%), 100 (10%), and 150 g (15%) of pulp in 1 L of drinking water. The mixtures were then pasteurized at 65 °C for 30 min, mixed in a ratio of 80% honey must and 20% cupuaçu juice, cooled, and the yeast was added (Table 1). The concentration of S. boulardii inoculated at 1 g/L corresponds to 106 CFU/mL. Fermentation was carried out for 30 days under anaerobiosis at 25 °C in polypropylene containers with a capacity of 1 L (Figure 1).
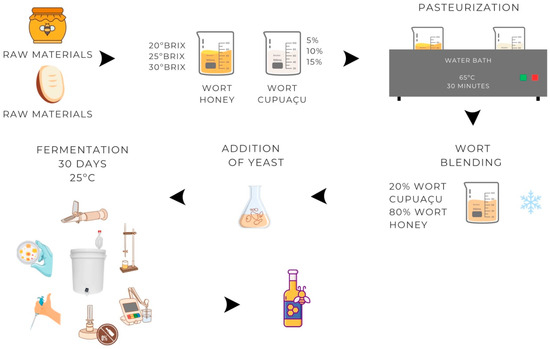
Figure 1.
Flowchart of the stages in the production of cupuaçu melomel.
Throughout fermentation, the following properties were analyzed every 5 days: pH (model PG 1800 FARMA, Miami, FL, USA); soluble solids (°Brix) (portable refractometer—model RSG-100ATC, National Industrial Supply, Temecula, CA, USA); total acidity (mEq/L), assessed by titration with 0.1 M NaOH, using 10 mL of the sample and a phenolphthalein indicator until it turned pink [30]; alcohol content (%) (using an ebulliometer Kit-0700, CIENLAB, Campinas, Brazil); and viable yeast cell count (log CFU/mL). The determination of alcohol content in the ebulliometer is carried out using the boiling temperature of the samples. This equipment is efficient for precise measurement of the boiling temperature of a sample containing ethanol so that, in comparison with the boiling temperature of water (used to calibrate the equipment), alcohol concentrations (%) are determined by the equivalent ratio on a millimeter ruler that relates the boiling temperature to the alcohol content [21]. At the end of fermentation, the melomel was placed in transparent glass bottles (total volume 300 mL) and stored at 4 ± 1 °C.
2.4. Experimental Comparison of Cupuaçu Melomel Fermented by S. cerevisiae and S. boulardii
For the experimental comparison between the beverages produced by S. cerevisiae and S. boulardii, a pilot scale was employed, with a total volume of 2.5 L of melomel for each treatment selected. The best condition obtained in the preliminary tests and defined in Table 1 was selected. This fermentation was carried out in polypropylene containers with a total volumetric capacity of 3 L. During fermentation, pH was analyzed using a bench pH meter (model PG 1800 FARMA), the soluble solids (°Brix) were determined with a portable refractometer (model RSG-100ATC), and total acidity (mEq/L), alcohol content (%) and viable yeast count (log CFU/mL) were evaluated every 5 days. The cupuaçu melomel was poured into glass bottles and stored at 4 ± 1 °C for later analysis.
2.5. Antioxidant Activity and Phenolic Compounds
The antioxidant capacity was assessed using the 2,2′-azinobis-(3-ethyl-benzothiazoline-6-sulfonic acid) (ABTS) method [31]. The antioxidant potential of cupuaçu melomel was assessed using the ferric reducing antioxidant power (FRAP) method [32]. The Folin–Ciocalteu method was used to assess the total phenolic compounds [33].
2.6. Viable Yeast Cell Counts
To count the viable yeast cells, the melomel samples were serially diluted in peptone water (0.1%). Subsequently, 100 μL were plated on YPD agar (yeast extract, peptone, and D-glucose) using the surface method and incubated at 35 °C for 48 h [34].
2.7. Survival of Microorganisms in Simulated In Vitro Gastric and Intestinal Juices
According to the methodology adopted by Souza et al. [15], 1 mL of cupuaçu melomel was mixed in 9 mL of sterile saline solution (0.85% w/v NaCl) with pH adjusted to 2.0 using 1 M hydrochloric acid (HCl) containing 0.5% pepsin (w/v). It was then incubated for 90 min at 37 °C, and cell viability was determined by surface plating on YPD agar. To prepare the simulated intestinal juice, solutions with final concentrations of 0.3% oxgall and 0.1% pancreatin (w/v) were used. The pH was adjusted to 7.0 by adding 1 M sodium hydroxide (NaOH). After homogenization, the samples were incubated at 37 °C for 150 min, and then the viable cell counts were determined by plating on YPD agar.
2.8. Cupuaçu Melomel Shelf Life
The melomel was stored at 4 ± 1 °C for shelf-life analysis. Samples were taken every 10 days for analysis of soluble solids (°Brix), pH, total acidity (mEq/L), alcohol content (%, Ebulliometer Kit-0700, CIENLAB), viable cell count (log CFU/mL), and antioxidant potential using the ferric reductant and ABTS radical assay methodologies and phenolic compounds.
2.9. Statistical Analysis
The central composite design (CCD) was analyzed using Statistica ® software, version 10.0 (New York, NY, USA), and its effects were evaluated using the variance test (ANOVA). For the experimental comparison, the results were also evaluated by an ANOVA analysis of variance and subjected to Tukey’s test, with a 95% confidence limit (p ≤ 0.05). The results were tabulated and expressed as mean ± standard deviation.
3. Results and Discussion
3.1. Central Composite Design (CCD) for the Production of Cupuaçu Melomel
Over the 30 days of fermentation, the concentrations of soluble solids (°Brix) were gradually reduced (Table 2), indicating the consumption of the substrate by the yeasts and the consequent production of ethanol. The pH of the melomel during the fermentation process showed variations (Table 2), as did the total acidity content (Table 2). These observations are in line with the results of Starowicza and Granvogl [6], who also found no significant difference in the pH of mead produced with different yeast strains. The cell viability (log CFU/mL) of S. boulardii was reduced as the alcohol content of the beverage increased (Table 2).

Table 2.
Analyses of the central composite design (CCD) for the response variables during the fermentation process for the production of cupuaçu melomel.
According to Table 2, it can be seen that with the increase in fermentation time to 30 days, there is a reduction in viable yeast cell counts, which can be correlated with the increase in alcohol content in the melomel. According to the literature, high levels of alcohol can contribute to decreasing the vitality and increasing the death of microorganisms [15,26,29]. Therefore, a fermentation time of 10 days is more suitable for the production of melomel. In addition, the conditions in the central points at 10 days of fermentation resulted in an average alcohol content of 7.3%, which, when compared to the other treatments, is an intermediate amount of alcohol, neither low nor high for alcoholic beverages. Taking these aspects into account and according to Table 2, the best conditions for the production of melomel were designated as the central point conditions.
The Pareto chart (Figure 2) demonstrates the standardized estimated effects of the central composite design. The horizontal bars demonstrate the main effects divided by the standard error. Thus, bars that exceed the red vertical line indicate statistically significant effects at the 95% confidence limit (Figure 2). Therefore, a statistically significant effect can be observed only for the final variable of the soluble solids.
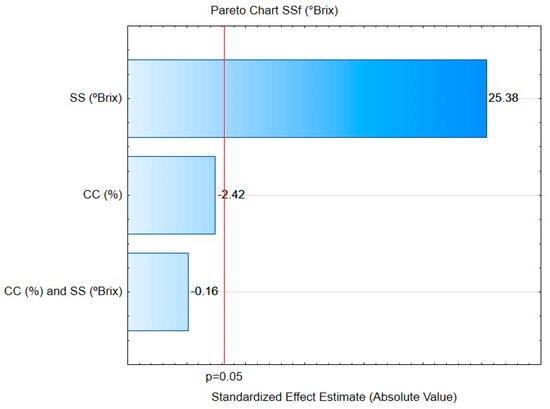
Figure 2.
Pareto diagram for the standardized estimated effect of the central composite design (CCD) for two independent variables for cupuaçu melomel production during 10 days of fermentation at p ≤ 0.05. SSi = soluble solids initial (°Brix); SSf = soluble solids final (°Brix); CC = cupuaçu concentration (%).
Thus, from the first-order general equation (Equation (3)), Equation (4) can be obtained to predict the behavior of the final soluble solids in melomel.
Y = β0 + β1·X1 + β2·X2 + β1.2·X1·X2
Substituting the values of the regression coefficients:
SSf = *13.329 + *3.925·SSi + 0.375·CC + 0.025·SSi·CC
* statistically significant at the 95% confidence limit (p ≤ 0.05). SSi = soluble solids initial (°Brix); SSf = soluble solids final (°Brix); CC = cupuaçu concentration (%).
Table 3 presents the analysis of variance (ANOVA) of the central composite design (CCD). It can be observed from the variance explained by the R2 and F test that it is possible to obtain the response surface for the final response of the soluble solids (Figure 3).

Table 3.
Analysis of variance (ANOVA) of the central composite design (CCD).
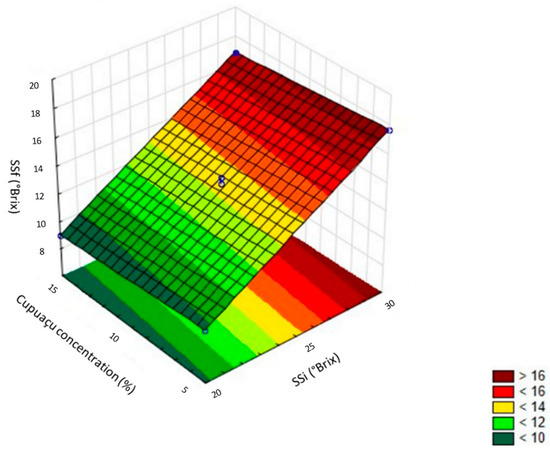
Figure 3.
Response surface for final soluble solids in the production of cupuaçu melomel from the central composite design (CCD). SSi = soluble solids initial (°Brix); SSf = soluble solids final (°Brix).
3.2. Experimental Comparison of Melomel with S. cerevisiae and S. boulardii Yeasts
Considering the results obtained in the central composite design (CCD), the best condition for melomel production was defined as the initial soluble solids in the must of 25 °Brix and 10% cupuaçu pulp. Table 4 shows the physicochemical and microbiological parameters of cupuaçu melomel produced with the probiotic yeast S. boulardii compared to that produced with S. cerevisiae. Cupuaçu melomel produced with probiotic yeast showed a statistically significant difference at p ≤ 0.05 when compared to melomel produced with commercial yeast, as determined via the responses of soluble solids (°Brix) and the viable cell count (log CFU/mL). The concentration of S. boulardii was 7.99 log CFU/mL, and the control had a concentration of 8.36 log CFU/mL. This significant difference can be justified by the high levels of ethanol that render fermented alcoholic beverages a stressful environment for the maintenance of probiotic microorganisms [26]. In the study by Souza et al. [14], the viable cell count of S. boulardii remained above 106 CFU/mL in probiotic mead fermented for 20 days.

Table 4.
Physicochemical and microbiological analyses of cupuaçu melomel after 10 days of fermentation with S. boulardii compared with cupuaçu melomel produced using commercial yeast S. cerevisiae.
3.3. Antioxidant Activity and Phenolic Compounds
Regarding bioactive compounds, in general, it is noted that the fermentation process allowed for an increase in the concentration of antioxidants, determined by the ABTS and FRAP methods (Table 5). The melomel produced by probiotic yeast obtained an antioxidant concentration of 47.81 µmol TE/100 mL, while the beverage produced by commercial yeast presented 51.81 µmol TE/100 mL, using the ABTS methodology, and these values that were statistically different at p ≤ 0.05. These results are in agreement with those of Fu et al. [35], who evaluated the production of mead using S. cerevisiae yeast and mixed culture with Lactobacillus paracasei M-8R. In this study, there was also an increase in the ABTS scavenging activity after the fermentation process.

Table 5.
Bioactive compounds before and after 10 days of fermentation of cupuaçu melomel.
For antioxidants assessed using the FRAP method, melomel with S. boulardii presented a higher value of 3.23 µmol TE/100 mL when compared to that of the S. cerevisiae beverage (2.74 µmol TE/100 mL) (Table 5). In the production of mead with fruits and herbs, the study by Rygielska et al. [7] showed higher concentrations of antioxidants after the fermentation process using the FRAP method. In this study, using mead produced with red grape seed flour, approximate values of 0.75 and 1.17 µmol TE/mL were collected in the initial must and after 16 days of fermentation, respectively.
Regarding total phenolic compounds, the two treatments evaluated in our study did not show significant differences (p ≤ 0.05) after 10 days of fermentation (Table 5). In mead, also produced by probiotic yeast, the total phenolic concentration was 10.71 ± 0.54 mg GAE/100, which allows us to infer that the beverages are sources of bioactive compounds [14]. Similar to our findings, in a study using S. boulardii in the production of probiotic mead, the presence of bioactive compounds such as phenolics and antioxidants in the mead was observed [14].
3.4. Survival of Microorganisms in Simulated In Vitro Digestion
To achieve the greatest beneficial effects on host health, probiotics must survive during processing, storage, and throughout the digestive process, maintaining their viability in the site of greatest interest, the intestine [36]. Figure 4 shows the count of viable yeast cells and the survival of microorganisms in simulated gastric and intestinal juices in vitro. It is observed that the yeasts S. boulardii and S. cerevisiae contain concentrations greater than 6 log CFU/mL in the initial product and at the end of the intestinal phase (Figure 4). In the study by Fu et al. [37], five strains of the yeast S. boulardii were tested for their ability to survive simulated gastric juice. As in our study, in which S. boulardii proved to be resistant to intestinal simulation, the results of Fu et al. [37] showed that all the strains displayed a survival rate of over 88%.
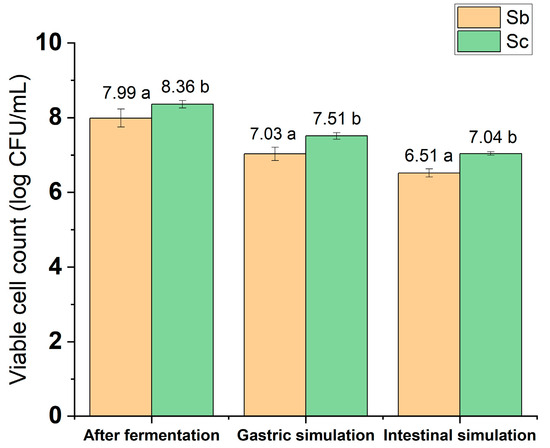
Figure 4.
Viable cell count and survival of microorganisms in simulated in vitro digestion. Sc = Saccharomyces cerevisiae; Sb = Saccharomyces boulardii. Different lowercase letters on the bars indicate statistically significant differences using the Tukey test (p ≤ 0.05).
At the end of the gastric simulation, there was a reduction of approximately 1 log CFU/mL among the evaluated treatments (Figure 4). In the initial phase of the simulation, the cupuaçu melomel produced by S. boulardii presented a viable cell count of 7.03 log CFU/mL, while the beverage produced with commercial yeast presented 7.51 log CFU/mL, reaching statistical significance (p ≤ 0.05) (Figure 4). Statistically significant results were also found in the final phase of the intestinal simulation, with a viable yeast count of 6.51 log CFU/mL in the beverage with probiotic yeast, while the beverage with S. cerevisiae presented a result of 7.04 log CFU/mL (Figure 4).
The viable cell count of 106 CFU/mL in melomel produced with S. boulardii, after intestinal simulation, is highly relevant, since this is the minimum concentration required for the products to be classified as probiotics [14,15,18,21,26] In the development of probiotic mead using the mixed fermentation of S. boulardii and water kefir, Souza et al. [15] also found quantities of yeasts in excess of 6 log (CFU/mL) after simulated in vitro digestion, results that are similar to those found in the present study.
3.5. Cupuaçu Melomel Shelf Life
Figure 5 shows the shelf life of the physicochemical and microbiological parameters of cupuaçu melomel during refrigerated storage at 4 ± 1 °C.
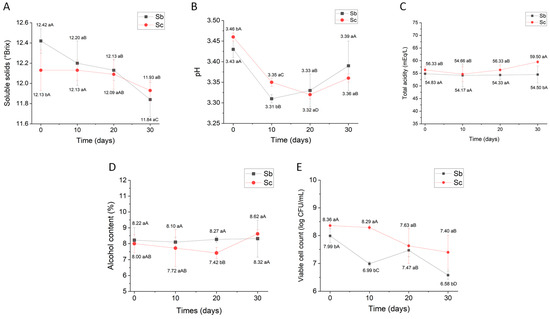
Figure 5.
Shelf life of cupuaçu melomel during 30 days of refrigerated storage at 4 ± 1 °C. (A) Soluble solids (°Brix), (B) pH, (C) total acidity (mEq/L), (D) alcohol content (%), and (E) viable cell count (log CFU/mL). Sc = Saccharomyces cerevisiae; Sb = Saccharomyces boulardii. Different lowercase letters indicate statistically significant differences between the treatments for each storage time using the Tukey test (p ≤ 0.05). Different capital letters indicate statistically significant differences for each specific treatment during the 30-day storage period using the Tukey test (p ≤ 0.05).
Figure 5A shows that during storage, there was a reduction in soluble solids for both treatments, indicating the continued metabolic activity of the yeasts; even though reduced under refrigeration, the microorganisms continue to develop their functions through the synthesis of substrates. On the other hand, there was a sharp drop in pH (Figure 5B) up to 20 days of storage, with a slight increase at 30 days of storage. However, even though there is a drop and variation in pH during the storage of melomel, the total acidity levels (Figure 5C) show a constant behavior, with only a slight increase for melomel with S. cerevisiae at 30 days of storage. With regard to alcohol content (Figure 5D), there were no variations in the concentrations for melomel with S. boulardii, while there was a sharp drop in ethanol in melomel with S. cerevisiae at 20 days of storage and a slight increase at 30 days of storage. These results show that the consumption of soluble solids (Figure 5A) by S. cerevisiae up to 20 days of storage was not enough to increase the alcohol content in the melomel, which remained statistically the same (p ≤ 0.05) during this period (Figure 5D). On the other hand, there was a significant increase (p ≤ 0.05) in the alcohol content of melomel by S. cerevisiae at 30 days of storage (Figure 5D) due to the significant consumption of soluble solids, as shown in Figure 5A. This result may occur mainly due to the fact that the S. cerevisiae yeast specific for mead production is a strain with characteristics suitable for the production of selective fermented beverages, exhibiting high fermentative activity and ethanol production and tolerance [5].
Figure 5E shows the results of viable yeast cell counts during storage at 4 ± 1 °C. Figure 5E shows a drop in yeast counts, corroborating the literature which mentions that high alcohol content in beverages can contribute to reducing the vitality and increasing the death of microorganisms such as yeasts and bacteria [15,26,29]. However, it can be seen that at 30 days of storage, the viable yeast cell counts in melomel with S. boulardii are still higher than 6 log CFU/mL, and is widely accepted that a minimum count of 6 log CFU/mL is the therapeutic dose for probiotic products to have a positive effect on the host [15,17,18,20,21].
With regard to the bioactive compounds in melomel after storage for 30 days (Table 6), there was a reduction in antioxidant concentrations when compared to those in the product obtained after fermentation for 10 days (Table 5). However, there was an increase in phenolic compounds for cupuaçu melomel with S. boulardii and a reduction for melomel with S. cerevisiae at the end of the storage time (Table 6) when compared to the results for the beverage obtained after fermentation for 10 days (Table 5). The presence of antioxidant compounds in beverages such as mead and melomel depends on different factors, but mainly on the type of honey used, along with the processing and aging of the beverage [7,14,15,38]. However, the presence of phenolic compounds and specifically, antioxidants, in beverages such as mead and melomel is important due to the reported functional properties of these compounds, with added health benefits [5,6]. Therefore, as also corroborated by Pereira [39], cupuaçu melomel with S. boulardii is an alternative product for probiotic consumption, as well as being a source of bioactive compounds such as phenolic compounds and antioxidants.

Table 6.
Bioactive compounds of cupuaçu melomel after refrigerated storage at a temperature of 4 ± 1 °C for 30 days.
This study presented the potential of S. boulardii as a probiotic yeast in the development of cupuaçu melomel. In fact, the survival of S. boulardii in simulated gastric and intestinal juices in vitro (Figure 4) and the shelf life of the viable cell counts (Figure 5E) show that cupuaçu melomel is a potential source of probiotics, since the counts were higher than 6 log CFU/mL. Although the product had a high alcohol content during its shelf life, with values above 8% (Figure 5D), a positive relationship between alcohol and probiotic strains can be justified, as the yeast is resistant to high concentrations of ethanol. In the same respect, we can point out that the intake of alcoholic beverages in adequate doses, without harm to health, has been recommended by various organizations and countries. As an example, several European countries stipulate consumption recommendations in their legislation that can be found in the document “National Low-Risk Drinking Recommendations (or Drinking Guidelines) and Standard Units” [40]. In countries such as Brazil, where there is no specific legislation for the adequate and moderate consumption of alcohol, the standards set by the World Health Organization (WHO) are adopted, which defines a recommended daily dose of 10 g of pure ethanol as the standard dose, equivalent to 250 mL of beer, 230 mL of cider, 100 mL of wine, or 31 mL of distilled spirits [41]. Based on WHO recommendations and the alcohol content of the beverages, the consumption of cupuaçu melomel would be equivalent to drinking 230 mL, which is within the recommended limits of alcohol content and daily dosage for cider. In addition, the consumption of cupuaçu melomel will provide the ingestion of probiotic quantities above 6 log CFU/mL, presenting the beverage as a new alternative for the consumption of probiotics.
The benefits of consuming probiotic microorganisms are directly related to intestinal health. S. boulardii is the best documented yeast in terms of significant clinical effects and proven probiotic efficacy. The application of S. boulardii yeast in probiotic fermented beverages can be considered recent. This yeast has been used to produce beer, rosé wine, fermented juices (cashew, orange, blackberry), and mead [15,21,24,25]. Its performance throughout the fermentation process demonstrates its resistance to stressful conditions, especially with regard to ethanol content and pH. In addition, its presence is linked to a higher concentration of aromatic and bioactive compounds, which are considered beneficial to melomel production and consumer health [6,42].
4. Conclusions
This study investigated the use of the yeast S. boulardii in the production of a fermented beverage based on honey and cupuaçu. This research also demonstrated the ability to use S. boulardii to obtain a beverage with probiotic characteristics, taking into account the minimum quantities of viable cells above the minimum therapeutic dose of 6 log CFU/mL for probiotic products to provide beneficial effects to the host. Cupuaçu melomel is a source of bioactive compounds such as phenolics and antioxidants, which are important for human health. The results obtained show the potential of S. boulardii yeast for the development of a fermented honey beverage made with cupuaçu. The development of cupuaçu melomel is therefore an alternative for the probiotic fermented beverage market as another probiotic product option for consumers and the food industry. For future research, it is important to investigate possible ways of optimizing the fermentation process, with a view to improve the concentration of viable S. boulardii cells and bioactive compounds, which could promote greater effects on consumer health.
Author Contributions
Conceptualization, methodology, investigation, project administration, and writing—original draft preparation, K.N.P., H.F.d.S. and A.C.D.d.O.; conceptualization, writing—original draft preparation, and writing—review and editing, K.N.P., H.F.d.S., M.A.D., V.D.D.P.G., M.V.d.C. and E.S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brasil (CAPES), Finance Code 001. The publication of this research was funded by the Fundação de Estudos Agrários Luiz de Queiroz (Fealq) and the Universidade de São Paulo (USP), Brazil.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brasil (CAPES), Finance Code 001. The authors wish to thank the São Paulo Research Foundation (FAPESP), Brazil, for grant #2022/12187–7. The publication of this research was funded by the Fundação de Estudos Agrários Luiz de Queiroz (Fealq) and the Universidade de São Paulo (USP), Brazil.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ministério da Agricultura e Abastecimento. Instrução Normativa n.11, de 20 de Outubro de 2000. Aprova o Regulamento Técnico de Identidade e Qualidade do Mel; Diário Oficial da República Federativa do Brasil: Brasília, DF, Brasil, 2000.
- Ministério de Agricultura, Pecuária e Abastecimento. Instrução Normativa n. 34, de 29 de Novembro de 2012. Aprova o Regulamento Técnico de Identidade e Qualidade de Bebidas Fermentadas: Fermentado de Fruta; Fermentado de Fruta Licoroso; Fermentado de Fruta Composto; Sidra; Hidromel; Fermentado de Cana; Saquê ou Sake; Diário Oficial da República Federativa do Brasil: Brasília, DF, Brasil, 2012.
- Amorim, T.S.; Lopes, S.B.; Bispo, J.A.C.; Bonafé, C.F.S.; Carvalho, G.B.M.; Martínez, E.A. Influence of acerola pulp concentration on mead production by Saccharomyces cerevisiae AWRI 796. LWT 2018, 97, 561–569. [Google Scholar] [CrossRef]
- Adamenko, K.; Rygielska, K.J.; Kucharska, A.Z.; Piórecki, N. Characteristics of Biologically Active Compounds in Cornelian Cherry Meads. Molecules 2018, 23, 2024. [Google Scholar] [CrossRef]
- Pereira, A.P.; Oliveira, J.M.; Mendes, A.F.; Estevinho, L.M.; Mendes, F.A. 14-Mead and Other Fermented Beverages. In Current Developments in Biotechnology and Bioengineering: Food and Beverages Industry; Elsevier: Amsterdam, The Netherlands, 2017; pp. 407–434. [Google Scholar] [CrossRef]
- Starowicza, M.; Granvogl, M. Trends in food science & technology an overview of mead production and the physicochemical, toxicological, and sensory characteristics of mead with a special emphasis on flavor. Trends Food Sci. Technol. 2020, 106, 402–416. [Google Scholar] [CrossRef]
- Rygielska, J.K.; Adamenko, K.; Kucharska, A.Z.; Szatkowska, K. Fruit and herbal meads–Chemical composition and antioxidant properties. Food Chem. 2019, 283, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Benlloch-Tinoco, M.; Ramírez, J.M.N.; García, P.; Gentile, P.; Girón-Hernández, J. Theobroma genus: Exploring the therapeutic potential of T. grandiflorum and T. bicolor in biomedicine. Food Biosci. 2024, 61, 104755. [Google Scholar] [CrossRef]
- Silva, C.V.A.; Salimo, Z.M.; Souza, T.A.; Reyes, D.E.; Bassicheto, M.C.; Medeiros, L.S.; Sartim, M.A.; Carvalho, J.C.; Gonçalves, J.F.C.; Monteiro, W.M.; et al. Cupuaçu (Theobroma grandiflorum): A Multifunctional Amazonian Fruit with Extensive Benefits. Food Res. Int. 2024, 192, 114729. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.V.V.A.; Silva, J.P.L.; Mathias, S.P.; Rosethal, A.; Calado, V.M.A. Caracterização físico-química e reológicas da polpa de cupuaçu congelada (Theobroma grandiflorumSchum). Perspect. Online Exatas Eng. 2013, 3, 46–53. [Google Scholar]
- Bezerra, J.A.; Corrêa, R.F.; Sanches, E.A.; Lamarão, C.V.; Stringheta, P.C.; Martins, E.; Campelo, P.H. “Cupuaçu” (Theobroma grandiflorum): A brief review on chemical and technological potential of this Amazonian fruit. Food Chem. Adv. 2024, 5, 100747. [Google Scholar] [CrossRef]
- Rogez, H.; Buxant, R.; Mignolet, E.; Souza, J.; Silva, E.; Larondelle, Y. Química e composição da polpa de três frutos típicos da Amazônia: Araça-boi (Eugenia stipitata), bacuri (Platonia insignis) e cupuaçu (Theobroma grandiflorum). Eur. Food Res. Technol. 2004, 218, 380–384. [Google Scholar] [CrossRef]
- Instituto Brasileiro de Geografia e Estatística (IBGE). Cupuaçu Production in Brazil. 2017. Available online: https://www.ibge.gov.br/explica/producao-agropecuaria/cupuacu/br (accessed on 5 October 2024).
- Souza, H.F.; Bessa, M.S.; Gonçalves, V.D.D.P.; Santos, J.V.; Pinheiro, C.; Chagas, E.G.L.; Carvalho, M.V.; Brandi, I.V.; Kamimura, E.S. Growing Conditions of Saccharomyces boulardii for the Development of Potentially Probiotic Mead: Fermentation Kinetics, Viable Cell Counts and Bioactive Compounds. Food Sci. Technol. Int. 2023, 30, 603–613. [Google Scholar] [CrossRef]
- Souza, H.F.; Bogáz, L.T.; Monteiro, G.F.; Freire, E.N.S.; Pereira, K.N.; Carvalho, M.V.; Silva Rocha, R.; Cruz, A.G.; Brandi, I.V.; Kamimura, E.S. Water Kefir in Co-Fermentation with Saccharomyces boulardii for the Development of a New Probiotic Mead. Food Sci. Biotechnol. 2024, 33, 3299–3311. [Google Scholar] [CrossRef] [PubMed]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health Benefits of Probiotics: A Review. ISRN Nutr. 2013, 2013, 481651. [Google Scholar] [CrossRef]
- Capece, A.; Romaniello, R.; Pietrafesa, A.; Siesto, G.; Pietrafesa, R.; Zambuto, M.; Romano, P. Use of Saccharomyces cerevisiae var. boulardii in co-fermentations with S. cerevisiae for the production of craft beers with potential healthy value-added. Int. J. Food Microbiol. 2018, 284, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Sertović, E.; Sarić, Z.; Barać, M.; Barukčić, I.; Kostić, A.; Božanić, R. Physical, chemical, microbiological and sensory characteristics of a probiotic beverage produced from different mixtures of cow’s milk and soy beverage by Lactobacillus acidophilus La5 and yoghurt culture. Food Technol. Biotechnol. 2019, 57, 461–471. [Google Scholar] [CrossRef]
- Zendeboodi, F.; Khorshidian, N.; Mortazavian, A.M.; Da Cruz, A.G. Probiotic: Conceptualization from a New Approach. Curr. Opin. Food Sci. 2020, 32, 103–123. [Google Scholar] [CrossRef]
- Souza, H.F.D.; Freire, E.N.S.; Monteiro, G.F.; Bogáz, L.T.; Teixeira, R.D.; Junior, F.V.S.; Teixeira, F.D.; Santos, J.V.D.; Carvalho, M.V.D.; Rocha, R.D.S.; et al. Development of Potentially Probiotic Mead from Co-Fermentation by Saccharomyces cerevisiae var. boulardii and Kombucha Microorganisms. Fermentation 2024, 10, 482. [Google Scholar] [CrossRef]
- Nikolaou, A.; Kourkoutas, Y. High-Temperature Semi-Dry and Sweet Low Alcohol Wine-Making Using Immobilized Kefir Culture. Fermentation 2021, 7, 45. [Google Scholar] [CrossRef]
- Nikolaou, A.; Nelios, G.; Kanellaki, M.; Kourkoutas, Y. Freeze-dried immobilized kefir culture in cider-making. J. Sci. Food Agric. 2020, 100, 3319–3327. [Google Scholar] [CrossRef] [PubMed]
- Patelski, A.M.; Dziekońska-Kubczak, U.; Ditrych, M. The Fermentation of Orange and Black Currant Juices by the Probiotic Yeast Saccharomyces cerevisiae var. boulardii. Appl. Sci. 2024, 14, 3009. [Google Scholar] [CrossRef]
- Diaz, A.B.; Guerrero, E.D.; Valiente, S.; Castro, R.; Lasanta, C. Development and characterization of probiotic beers with Saccharomyces boulardii as an alternative to conventional brewer’s yeast. Foods 2023, 12, 2912. [Google Scholar] [CrossRef]
- De Paula, B.P.; de Souza Lago, H.; Firmino, L.; Júnior, W.J.F.L.; Corrêa, M.F.D.; Guerra, A.F.; Coelho, M.A.Z. Technological features of Saccharomyces cerevisiae var. boulardii for potential probiotic wheat beer development. LWT 2021, 135, 110233. [Google Scholar] [CrossRef]
- Ramírez-Cota, G.Y.; López-Villegas, E.O.; Jiménez-Aparicio, A.R.; Hernández-Sánchez, H. Modeling the Ethanol Tolerance of the Probiotic Yeast Saccharomyces cerevisiae var. boulardii CNCM I-745 for its Possible Use in a Functional Beer. Probiotics Antimicrob. Proteins 2021, 13, 187–194. [Google Scholar] [CrossRef]
- Cerezo, J.M.; Molina, A.T.; Vicent, A.C.; Colomer, L.P.; Martí, M.; Aroca, Á.S. Alcoholic and Non-Alcoholic Rosé Wines Made with Saccharomyces cerevisiae var. boulardii Probiotic Yeast. Arch. Microbiol. 2023, 205, 201. [Google Scholar] [CrossRef]
- Paula, B.P.; Chavéz, D.W.; Lemos Junior, W.J.F.; Guerra, A.F.; Corrêa, M.F.D.; Pereira, K.S.; Coelho, M.A. Growth parameters and survivability of Saccharomyces boulardii for probiotic alcoholic beverages development. Front. Microbiol. 2019, 10, 2092. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis; Association of Official Analytical Chemists: Gaithersburg, MD, USA, 2000. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Everette, J.D.; Bryant, Q.M.; Green, A.M.; Abbey, Y.A.; Wangila, G.W.; Walker, R.B. Thorough study of reactivity of various compound classes toward the Folin-Ciocalteou reagent. J. Agric. Food Chem. 2010, 58, 8.139–8.144. [Google Scholar] [CrossRef]
- Vega, R.Z.; Soto, J.L.M.; Flores, H.E.M.; Magallón, R.F.; Ruiz, C.V.M.; González, J.V.; Ortega, T.J.A. Effect of incorporating prebiotics in coating materials for the microencapsulation of Sacharomyces boulardii. Int. J. Food Sci. Nutr. 2012, 63, 930–935. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Shi, X.; Li, F.; Yan, X.; Li, B.; Luo, Y.; Jiang, G.; Liu, X.; Wang, L. Fermentation of mead using Saccharomyces cerevisiae and Lactobacillus paracasei: Strain growth, aroma components and antioxidant capacity. Food Biosci. 2023, 52, 102402. [Google Scholar] [CrossRef]
- Silva, T.F.; Glória, R.A.; Americo, M.F.; Freitas, A.S.; Jesus, L.C.L.; Barroso, F.A.L.; Laguna, J.G.; Rocha, N.D.C.; Loir, Y.L.; Jan, G.; et al. Unlocking the Potential of Probiotics: A comprehensive review on research, production and regulation of probiotics. Probiotics Antimicrob. Proteins 2024. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.J.; Liu, J.; Wen, X.P.; Zhang, G.; Cai, J.; Qiao, Z.; An, Z.; Zheng, J.; Li, L. Unique Probiotic Properties and Bioactive Metabolites of Saccharomyces boulardii. Probiotics Antimicrob. Proteins 2022, 15, 967–982. [Google Scholar] [CrossRef] [PubMed]
- Akalin, H.; Bayram, M.; Anli, R.E. Determination of some individual phenolic compounds and antioxidant capacity of mead produced from different types of honey. J. Inst. Brew. 2017, 123, 167–174. [Google Scholar] [CrossRef]
- Pereira, K.N. Production of Cupuassu (Theobroma grandiflorum) Melomel with Probiotic Yeast. Master’s Thesis, University of São Paulo, Faculty of Animal Science and Food Engineering, Pirassununga, São Paulo, SP, Brazil, 2024; p. 70. Available online: https://www.teses.usp.br/teses/disponiveis/74/74132/tde-26072024-085931/en.php (accessed on 5 October 2024).
- European Commission. National Low-Risk Drinking Recommendations (or Drinking Guidelines) and Standard Units Knowledge for Policy. 2024. Available online: https://knowledge4policy.ec.europa.eu/health-promotion-knowledge-gateway/national-low-risk-drinking-recommendations-drinking-guidelines_en (accessed on 27 October 2024).
- Alcohol—PAHO/WHO Pan American Health Organization. 2024. Available online: https://www.paho.org/en/topics/alcohol (accessed on 27 October 2024).
- Mulero-Cerezo, J.; Briz-Redón, Á.; Serrano-Aroca, Á. Saccharomyces cerevisiae var. boulardii: Valuable probiotic starter for craft beer production. Appl. Sci. 2019, 9, 3250. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).