Optimized Spirulina Fermentation with Lacticaseibacillus rhamnosus: Bioactive Properties and Pilot-Scale Validation
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of Bacterial Strain
2.2. Cultivation of Arthrospira platensis and Biochemical Composition
2.3. Optimization of Bioprocess Conditions 3 L Bioreactor
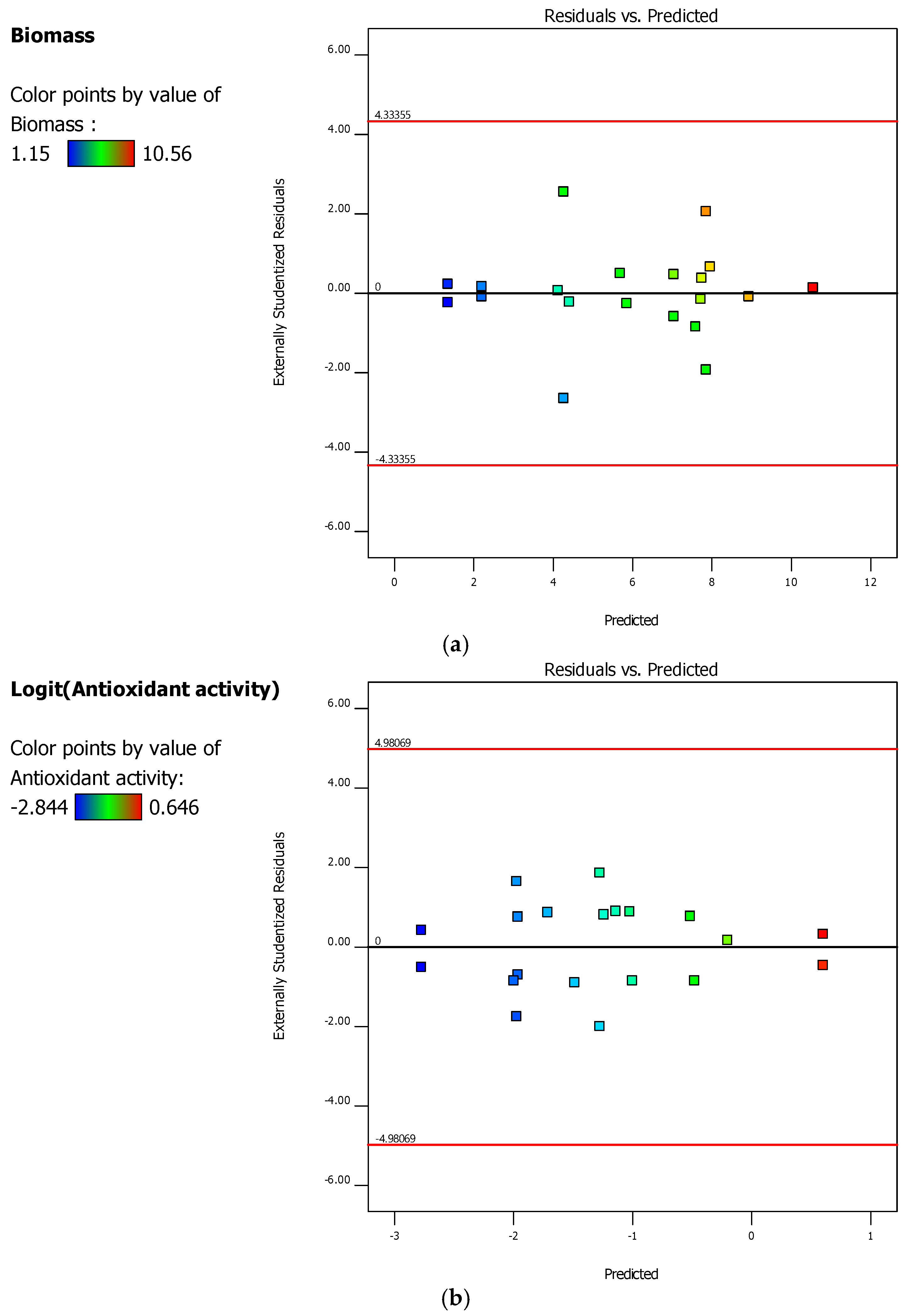
2.4. D-Optimal Experimental Design and the Optimization Phase
2.5. Determination of Biomass
2.6. Analyses of Antioxidant Activity
2.7. Pilot-Scale Production Experiments
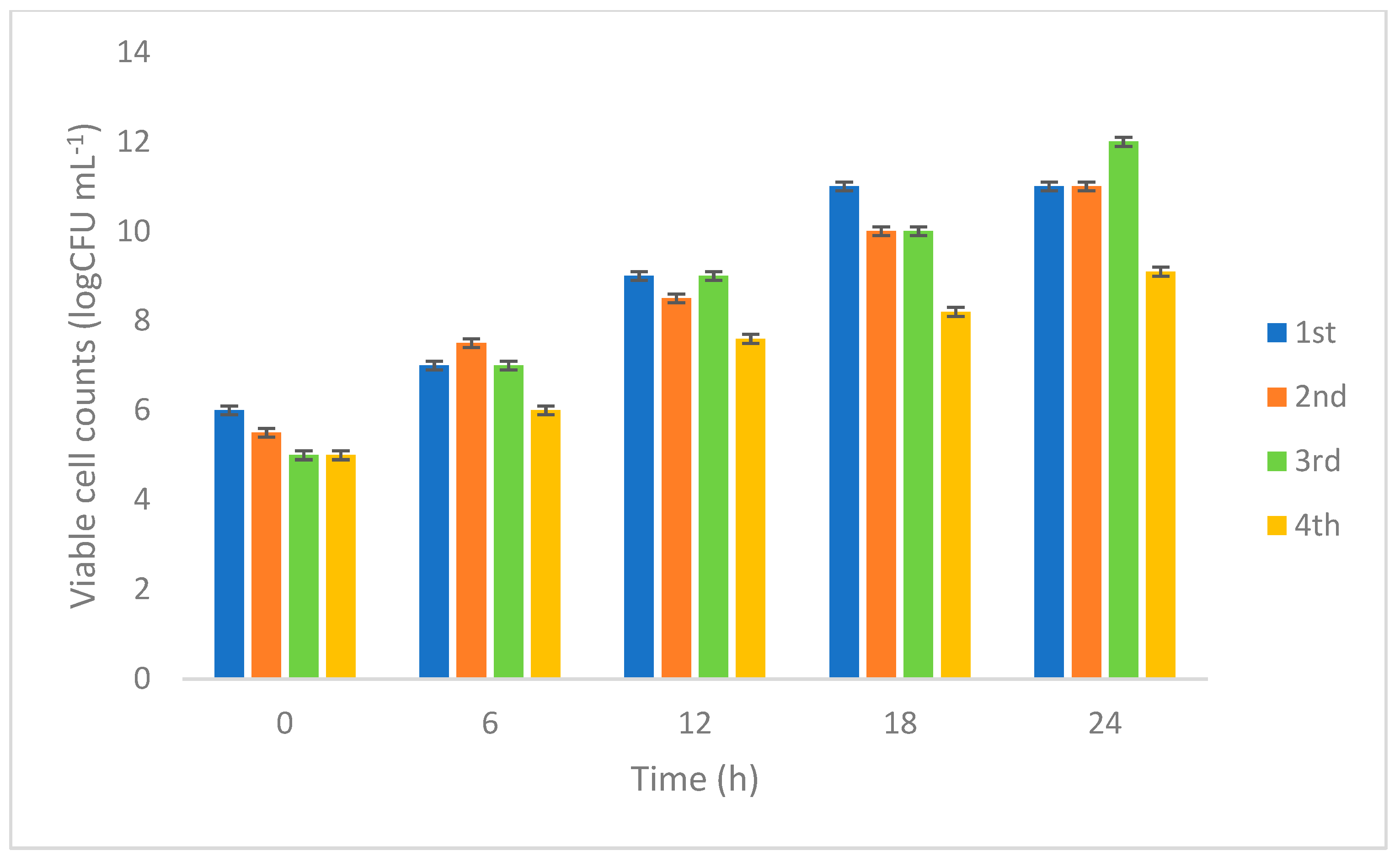
2.7.1. Monitoring Viable Cell Count
2.7.2. Antibacterial Activity
2.7.3. Antioxidant and Total Phenolic Content Determination
2.7.4. Cytotoxic Activity
2.8. Statistical Analysis for Pilot Production
3. Results and Discussion
| Carbohydrate | Protein | Lipid | Ash | Moisture | |
|---|---|---|---|---|---|
| Arthrospira platensis | 21.1 ± 0.7 | 59.6 ± 0.3 | 9.7 ± 0.6 | 5.8 ± 0.1 | 3.8 ± 0.2 |
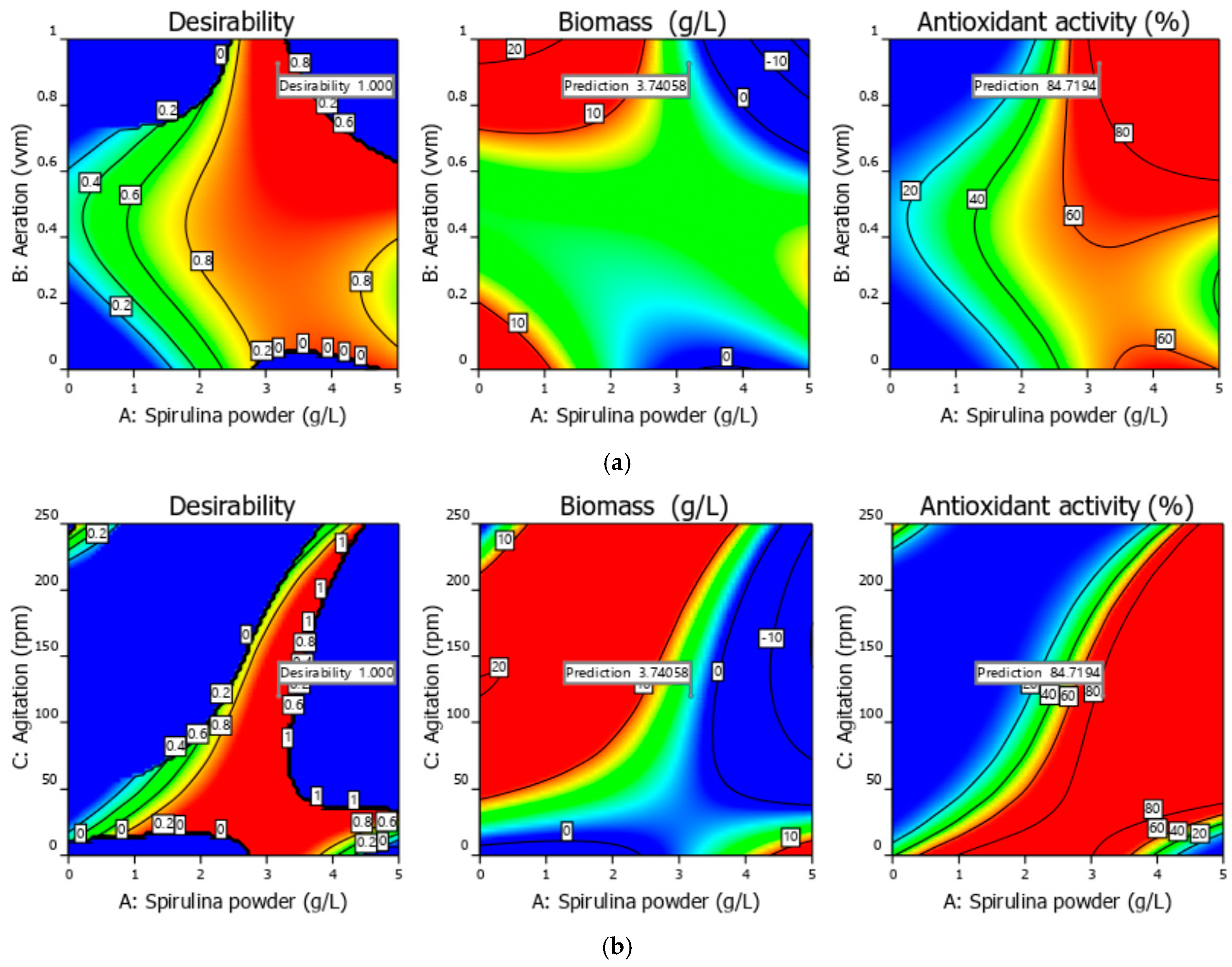
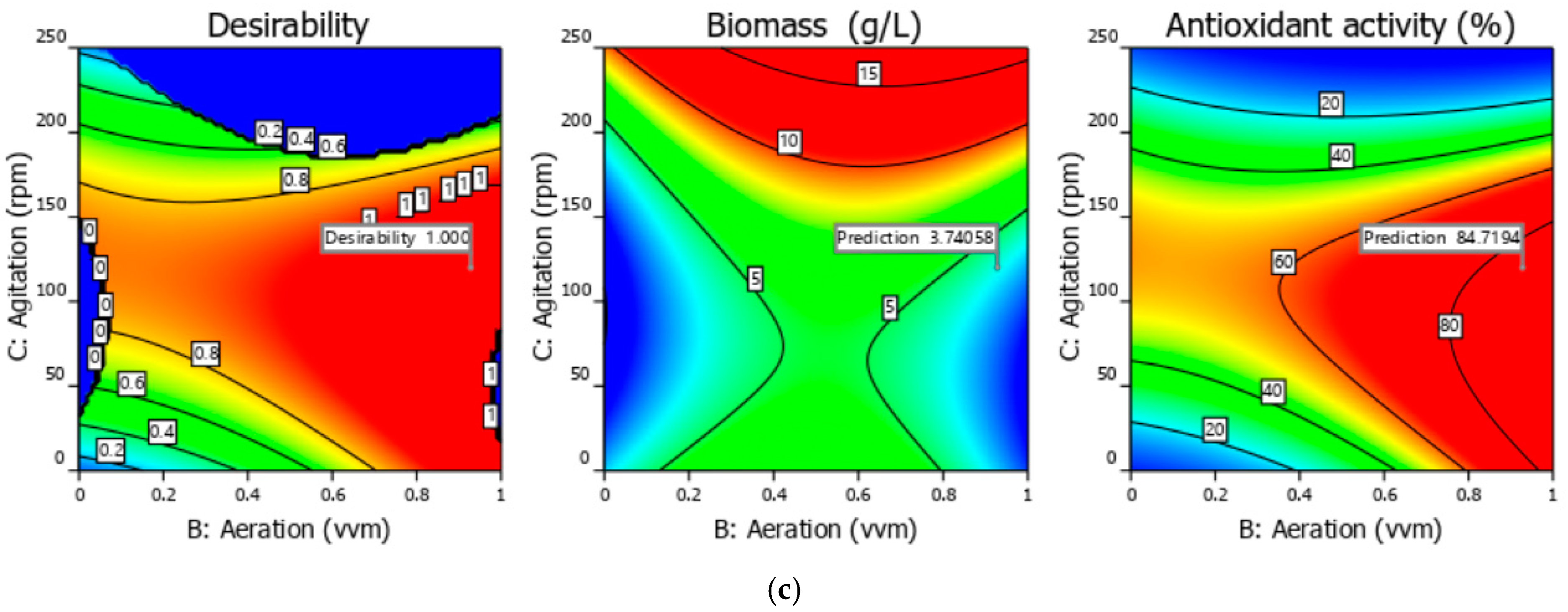
3.1. Experimental and Optimization Results of Bioprocess Conditions 3 L Bioreactor
| Design Factors | |||||
|---|---|---|---|---|---|
| Factor | Name | Units | Coded/Uncoded Low | Coded/Uncoded High | |
| A | Spirulina powder | g/L | −1/0.00 | +1/5.00 | |
| B | Aeration | vvm | −1/0.00 | +1/1.00 | |
| C | Agitation | rpm | −1/0.00 | +1/250.00 | |
| DM of a D-optimal design and collected data for the two responses | |||||
| Factor A | Factor B | Factor C | Response y1 | Response y2 | |
| Spirulina powder | Aeration | Agitation | Biomass | Antioxidant activity | |
| Run | (g/L) | (vvm) | (rpm) | (g/L) | (%) |
| 1 | 5.00 | 1.00 | 0.00 | 6.52 | 10.21 |
| 2 | 0.00 | 0.00 | 0.00 | 1.15 | 5.50 |
| 3 | 0.00 | 1.00 | 250.00 | 2.63 | 25.60 |
| 4 | 5.00 | 0.00 | 88.62 | 7.65 | 16.20 |
| 5 | 1.62 | 0.00 | 108.75 | 6.12 | 11.50 |
| 6 | 1.62 | 0.57 | 250.00 | 8.54 | 36.70 |
| 7 | 0.00 | 1.00 | 0.00 | 2.34 | 13.45 |
| 8 | 2.98 | 1.00 | 149.13 | 7.45 | 63.10 |
| 9 | 5.00 | 0.00 | 250.00 | 7.95 | 17.40 |
| 10 | 5.00 | 0.65 | 250.00 | 8.89 | 23.51 |
| 11 | 0.00 | 0.00 | 0.00 | 1.54 | 6.21 |
| 12 | 3.44 | 0.31 | 171.83 | 6.78 | 45.67 |
| 13 | 0.00 | 1.00 | 0.00 | 2.13 | 11.34 |
| 14 | 2.98 | 0.40 | 0.00 | 4.16 | 37.89 |
| 15 | 2.98 | 1.00 | 149.13 | 6.54 | 65.60 |
| 16 | 0.00 | 1.00 | 250.00 | 5.87 | 18.36 |
| 17 | 5.00 | 1.00 | 0.00 | 9.25 | 14.40 |
| 18 | 1.77 | 0.00 | 250.00 | 5.69 | 27.88 |
| 19 | 5.00 | 0.57 | 81.11 | 10.56 | 25.61 |
| 20 | 0.00 | 0.40 | 149.01 | 4.20 | 24.72 |
3.2. Pilot-Scale Production
3.2.1. Monitoring Viable Cell Count L. rhamnosus 23.2
3.2.2. Antibacterial Activity
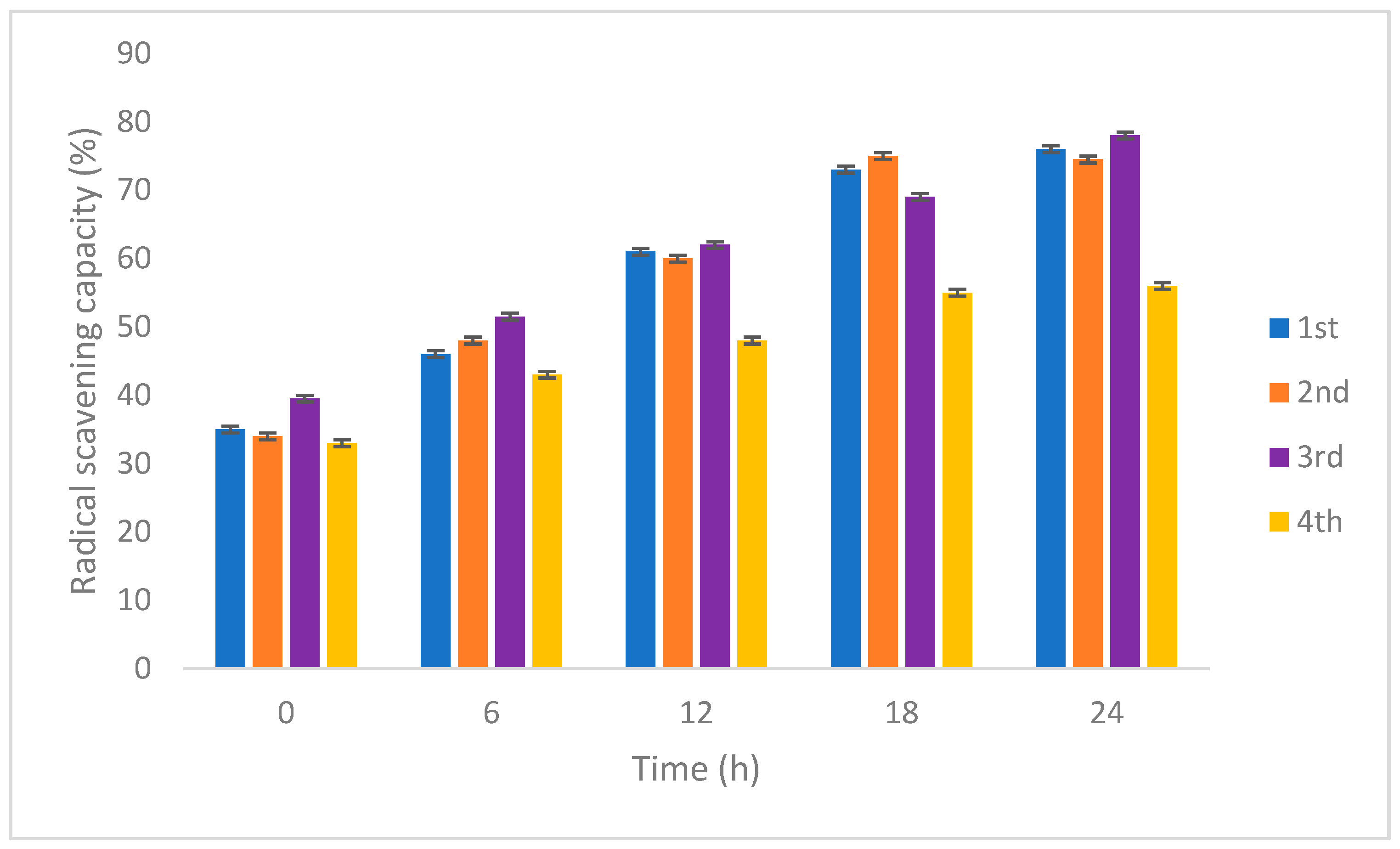
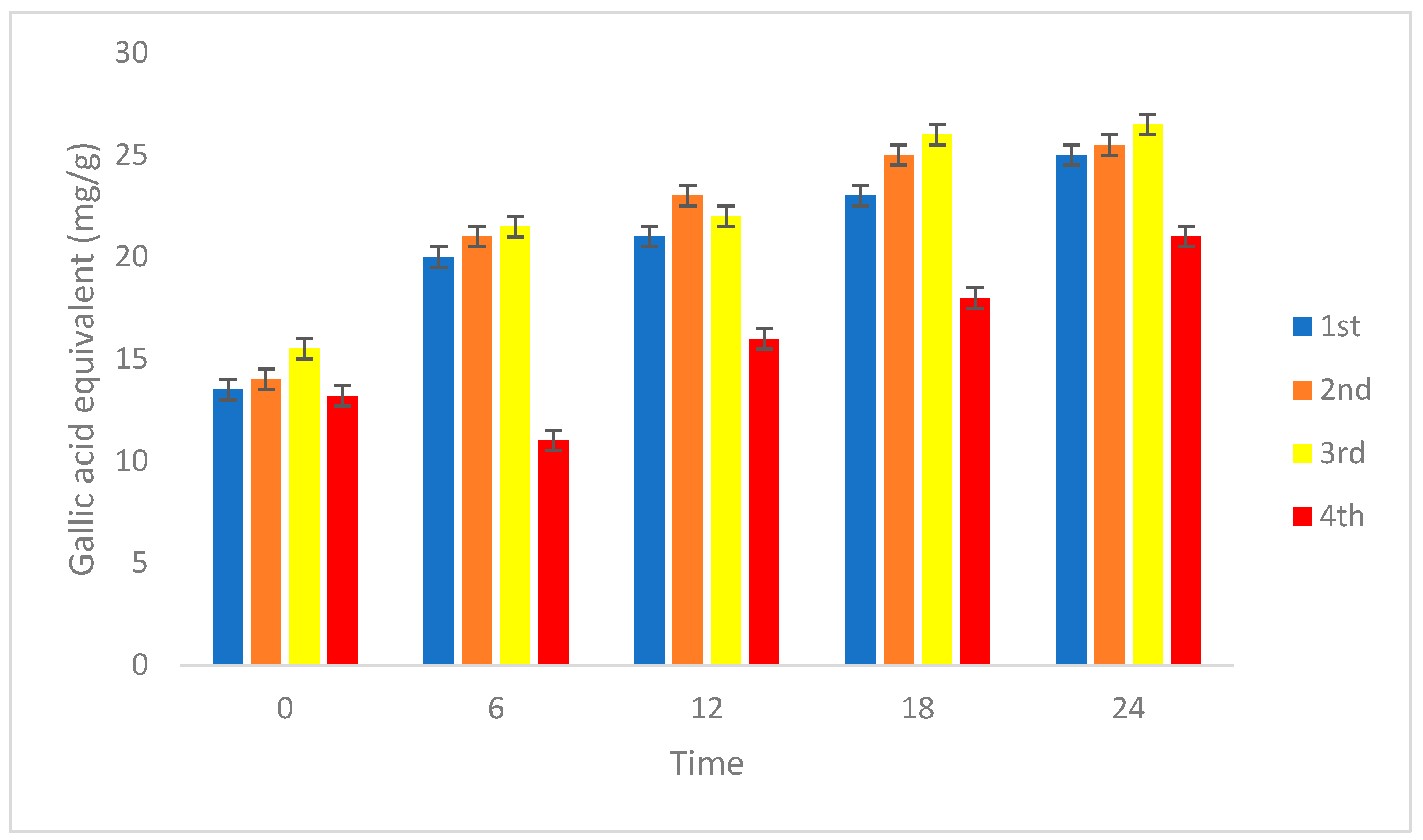
3.2.3. Antioxidant and Total Phenolic Content
3.2.4. Cytotoxic Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chai, K.F.; Voo, A.Y.H.; Chen, W.N. Bioactive peptides from food fermentation: A comprehensive review of their sources, bioactivities, applications, and future development. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3825–3885. [Google Scholar] [CrossRef] [PubMed]
- Sarker, A.; Ahmmed, R.; Ahsan, S.M.; Rana, J.; Ghosh, M.K.; Nandi, R. A comprehensive review of food waste valorization for the sustainable management of global food waste. Sustain. Food Technol. 2024, 2, 48–69. [Google Scholar] [CrossRef]
- Liu, F.; Li, M.; Wang, Q.; Yan, J.; Han, S.; Ma, C.; Ma, P.; Liu, X.; McClements, D.J. Future foods: Alternative proteins, food architecture, sustainable packaging, and precision nutrition. Crit. Rev. Food Sci. Nutr. 2022, 63, 6423–6444. [Google Scholar] [CrossRef] [PubMed]
- Niccolai, A.; Bazec, K.; Rodolfi, L.; Biondi, N.; Zlatic, E.; Jamnik, P.; Tredici, M.R. Lactic Acid Fermentation of Arthrospira platensis (Spirulina) in a Vegetal Soybean Drink for Developing New Functional Lactose-Free Beverages. Front. Microbiol. 2020, 11, 560–684. [Google Scholar] [CrossRef]
- Wu, C.; Huang, J.; Zhou, R. Genomics of lactic acid bacteria: Current status and potential applications. Crit. Rev. Microbiol. 2017, 43, 393–404. [Google Scholar] [CrossRef]
- Pfeiler, E.A.; Klaenhammer, T.R. The genomics of lacticacid bacteria. Trends Microbiol. 2007, 15, 546–553. [Google Scholar] [CrossRef]
- Felis, G.E.; Dellaglio, F. Taxonomy of Lactobacilli and Bifidobacteria. Curr. Issues Intest. Microbiol. 2007, 8, 44–61. [Google Scholar] [PubMed]
- Srinivash, M.; Krishnamoorthi, R.; Mahalingam, P.U.; Malaikozhundan, B.; Keerthivasan, M. Probiotic potential of exopolysaccharide producing lactic acid bacteria isolated from homemade fermented food products. J. Agric. Food Res. 2023, 11, 11100517. [Google Scholar] [CrossRef]
- Burgain, J.; Scher, J.; Francius, G.; Borges, F.; Corgneau, M.; Revol-Junelles, A.M.; Cailliez-Grimal, C.; Gaiani, C. Lactic acid bacteria in dairy food: Surface characterization and interactions with food matrix components. Adv. Colloid. Interf. Sci. 2014, 213, 21–35. [Google Scholar] [CrossRef]
- Perez, R.H.; Zendo, T.; Sonomoto, K. Novel bacteriocins from lactic acid bacteria (LAB): Various structures and applications. Microb. Cell Factor. 2014, 13, S3. [Google Scholar] [CrossRef]
- Lafarga, T. Cultured microalgae and compounds derived thereof for food applications: Strain selection and cultivation, drying, and processing strategies. Food Rev. Int. 2020, 36, 559–583. [Google Scholar] [CrossRef]
- Gershwin, M.E.; Belay, A. (Eds.) Spirulina in Human Nutrition and Health; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar] [CrossRef]
- Garrido-Cardenas, J.A.; Manzano-Agugliaro, F.; Acien-Fernandez, F.G.; Molina-Grima, E. Microalgae research worldwide. Algal Res. 2018, 35, 50–60. [Google Scholar] [CrossRef]
- Garofalo, C.; Norici, A.; Mollo, L.; Osimani, A.; Aquilanti, L. Fermentation of Microalgal Biomass for Innovative Food Production. Microorganisms 2022, 10, 2069. [Google Scholar] [CrossRef] [PubMed]
- Barberis, S.E.; Origone, A.L.; Adaro, M.O.; Bersi, G. Bioactive Peptides as Functional Food Ingredients. In Microbial Biotechnology: Principles and Applications, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Sahin, B.; Hosoglu, M.I.; Guneser, O.; Karagul-Yuceer, Y. Fermented Spirulina products with Saccharomyces and non-Saccharomyces yeasts: Special reference to their microbial, physico-chemical and sensory characterizations. Food Biosci. 2022, 47, 101691. [Google Scholar] [CrossRef]
- Hafeez, Z.; Cakir-Kiefer, C.; Roux, E.; Perrin, C.; Miclo, L.; Dary-Mourot, A. Strategies of producing bioactive peptides from milk proteins to functionalize fermented milk products. Food Res. Int. 2014, 63, 71–80. [Google Scholar] [CrossRef]
- Elam, E.; Feng, J.; Lv, Y.-M.; Ni, Z.-J.; Sun, P.; Thakur, K.; Zhang, J.G.; Ma, Y.-L.; Wei, Z.J. Recent advances on bioactive food derived anti-diabetic hydrolysates and peptides from natural resources. J. Funct. Foods. 2021, 86, 104674. [Google Scholar] [CrossRef]
- Martelli, F.; Cirlini, M.; Lazzi, C.; Neviani, E.; Bernini, V. Solid-state fermentation of Arthrospira platensis to implement new food products: Evaluation of stabilization treatments and bacterial growth on the volatile fraction. Foods 2021, 10, 67. [Google Scholar] [CrossRef]
- Moayedi, A.; Mora, L.; Aristoy, M.-C.; Hashemi, M.; Safari, M.; Toldrá, F. ACE- Inhibitory and Antioxidant Activities of Peptide Fragments Obtained from Tomato Processing By-Products Fermented Using Bacillus subtilis: Effect of Amino Acid Composition and Peptides Molecular Mass Distribution. Appl. Biochem. Biotechnol. 2016, 181, 48–64. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, X.; Zheng, J.; Ren, D.; Lu, J. Mixed fermentation of Spirulina platensis with Lactobacillus plantarum and Bacillus subtilis by random-centroid optimization. Food Chem. 2018, 264, 64–72. [Google Scholar] [CrossRef]
- Verni, M.; Dingeo, C.; Rizzello, C.G.; Pontonio, E. Lactic acid bacteria fermentation and endopeptidase treatment improve the functional and nutritional features of Arthrospira platensis. Front. Microbiol. 2021, 12, 744437. [Google Scholar] [CrossRef]
- Jamnik, P.; Mahnič, N.; Mrak, A.; Pogačnik, L.; Jeršek, B.; Niccolai, A.; Rutar, J.M.; Ogrinc, N.; Dušak, L.; Ferjančič, B.; et al. Fermented Biomass of Arthrospira platensis as a Potential Food Ingredient. Antioxidants 2022, 11, 216. [Google Scholar] [CrossRef] [PubMed]
- Yay, C.; Cinar, Z.O.; Donmez, S.; Tumer, T.B.; Guneser, O.; Hosoglu, M.I. Optimizing bioreactor conditions for Spirulina fermentation by Lactobacillus helveticus and Kluyveromyces marxianus: Impact on chemical & bioactive properties. Bioresour. Technol. 2024, 403, 130832. [Google Scholar] [CrossRef]
- Niccolai, A.; Shannon, E.; Abu-ghannam, N.; Biondi, N.; Rodolfi, L. Lactic acid fermentation of Arthrospira platensis (Spirulina) biomass for probiotic-based products. J. Appl. Phycol. 2019, 31, 1077–1083. [Google Scholar] [CrossRef]
- de Marco Castro, E.; Shannon, E.; Abu-Ghannam, N. Effect of fermentation on enhancing the nutraceutical properties of Arthrospira platensis (Spirulina). Fermentation 2019, 5, 28. [Google Scholar] [CrossRef]
- Liu, J.G.; Hou, C.W.; Lee, S.Y.; Chuang, Y.; Lin, C.C. Antioxidant effects and UVB protective activity of Spirulina (Arthrospira platensis) products fermented with lactic acid bacteria. Process Biochem. 2011, 46, 1405–1410. [Google Scholar] [CrossRef]
- De Man, J.C.; Rogosam, M.; Sharpe, M.E. A Medium for the Cultivation of Lactobacilli. J. Appl. Bacteriol. 1960, 23, 130–135. [Google Scholar] [CrossRef]
- Richmond, A. Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Blackwell Science: Oxford, UK; Ames, IA, USA, 2004. [Google Scholar]
- Acien, F.G.; Fernandez, J.M.; Magan, J.J.; Molina, E. Production cost of a real microalgae production plant and strategies to reduce it. Biotechnol. Adv. 2012, 30, 1344–1353. [Google Scholar] [CrossRef]
- Niangoran, N.G.U.F.; Buso, D.; Zissis, G.; Prudhomme, T. Influence of light intensity and photoperiod on energy efficiency of biomass and pigment production of Spirulina (Arthrospira platensis). OCL 2021, 28, 37. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.T.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Marsh, J.B.; Weinstein, D.B. Simple charring method for determination of lipids. J. Lipid Res. 1966, 7, 574–576. [Google Scholar] [CrossRef] [PubMed]
- Inegbedion, F. Estimation of the moisture content, volatile matter, ash content, fixed carbon and calorific values of saw dust briquettes. MANAS J. Eng. 2022, 10, 17–20. [Google Scholar] [CrossRef]
- Gökmen, G.G.; Sariyildiz, S.; Cholakov, R.; Nalbantsoy, A.; Baler, B.; Aslan, E.; Düzel, A.; Sargin, S.; Göksungur, Y.; Kişla, D. A novel Lactiplantibacillus plantarum strain: Probiotic properties and optimization of the growth conditions by response surface methodology. World J. Microbiol. Biotechnol. 2024, 40, 66. [Google Scholar] [CrossRef] [PubMed]
- Deniz, I.; Demir, T.; Oncel, S.S.; Hames, E.E.; Vardar-Sukan, F. Effect of Agitation and Aeration on Keratinase Production in Bioreactors Using Bioprocess Engineering Aspects. Protein J. 2021, 40, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Ozdemir, A. D-optimal experimental design for production models in nonstandard experiments. Qual. Reliab. Eng. Int. 2020, 36, 1537–1552. [Google Scholar] [CrossRef]
- Kavak, A.E.; Zent, İ.; Sağir, E.M.; Oncü, G.; Simsek, F.İ. Characterization of nuvita biosearch center (NBC) isolated lactic acid bacteria strains from human origin and determination of growth kinetic profiles of selected cultures under bioreactor. Ann. Microbiol. 2024, 74, 26. [Google Scholar] [CrossRef]
- Rizzello, C.G.; Lorusso, A.; Russo, V.; Pinto, D.; Marzani, B.; Gobbetti, M. Improving the antioxidant properties of quinoa flour through fermentation with selected autochthonous lactic acid bacteria. Int. J. Food Microbiol. 2017, 241, 252–261. [Google Scholar] [CrossRef]
- Sanders, E.R. Aseptic laboratory techniques: Plating methods. J. Vis. Exp. 2012, e3064. [Google Scholar] [CrossRef]
- Tsega, K.T.; Maina, K.J.; Tesema, N.B. Characterization of potential probiotics Lac tobacillus species isolated from the gastrointestinal tract of Rhode Island Red (RIR) chicken in Ethiopia. Heliyon 2023, 9, e17453. [Google Scholar] [CrossRef]
- Efsa Panel On Additives And Products Or Substances Used In Animal Feed (Feedap); Rychen, G.; Aquilina, G.; Azimonti, G.; Bampidis, V.; Bastos, M.d.L.; Bories, G.; Chesson, A.; Cocconcelli, P.S.; Flachowsky, G.; et al. Guidance on the characterisation of microorganisms used as feed additives or as production organisms. EFSA J. 2018, 16, 5206. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Acad. Press. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Cakir-Koc, R.; Budama-Kilinc, Y.; Kaya, Z.; Berber Orcen, B.; Ucarkus, E. Coconut oil-loaded chitosan nanoparticles for the treatment of acne vulgaris: Cytotoxicity, antibacterial activity, and antibiofilm properties. Fresenius Environ. Bull. 2018, 27, 2642–2648. [Google Scholar]
- Wu, W.J.; Ahn, B.Y. Statistical Optimization of Medium Components by Response Surface Methodology to Enhance Menaquinone-7 (Vitamin K-2) Production by Bacillus subtilis. J. Microbiol. Biotechn. 2018, 28, 902–908. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, W.; Mahsa, G.C.; Zhang, C.; Ma, K.; Rui, X.; Li, W. Co-cultivation effects of Lactobacillus helveticus SNA12 and Kluveromyces marxiensis GY1 on the probiotic properties, flavor, and digestion in fermented milk. Food Res. Int. 2023, 169, 112843. [Google Scholar] [CrossRef] [PubMed]
- Hamme, V.; Sannier, F.; Piot, J.M.; Didelot, S.; Bordenave-Juchereau, S. Crude goat whey fermentation by Kluyveromyces marxianus and Lactobacillus rhamnosus: Contribution to proteolysis and ACE inhibitory activity. J. Dairy Res. 2009, 76, 152–157. [Google Scholar] [CrossRef]
- Jain, S.; Anal, A.K. Production and characterization of functional properties of protein hydrolysates from egg shell membranes by lactic acid bacteria fermentation. J. Food Sci. Technol. 2017, 54, 1062–1072. [Google Scholar] [CrossRef]
- Ovando, C.A.; de Carvalho, J.C.; Vinícius de Melo Pereira, G.; Jacques, P.; Soccol, V.T.; Soccol, C.R. Functional Properties and Health Benefits of Bioactive Peptides Derived from Spirulina: A Review. Food Rev. Int. 2018, 34, 34–51. [Google Scholar] [CrossRef]
- Lafarga, T.; Sánchez-Zurano, A.; Villaró, S.; Morillas-España, A.; Acién, G. Industrial production of spirulina as a protein source for bioactive peptide generation. Trends Food Sci. Technol. 2021, 116, 176–185. [Google Scholar] [CrossRef]
- Sun, Y.; Chang, R.; Li, Q. Isolation and characterization of an antibacterial peptide from protein hydrolysates of Spirulina platensis. Eur. Food Res. Technol. 2015, 242, 685–692. [Google Scholar] [CrossRef]
- Hwanhlem, N.; Watthanasakphuban, N.; Riebroy, S.; Benjakul, S.; H-Kittikun, A.; Maneerat, S. Probiotic lactic acid bacteria from Kung-Som: Isolation, screening, inhibition of pathogenic bacteria. Int. J. Food Sci. Technol. 2010, 45, 594–601. [Google Scholar] [CrossRef]
- Georgieva, R.; Yocheva, L.; Tserovska, L.; Zhelezova, G.; Stefanova, N.; Atanasova, A.; Danguleva, A.; Ivanova, G.; Karapetkov, N.; Rumyan, N.; et al. Antimicrobial activity and antibiotic susceptibility of Lactobacillus and Bifidobacterium spp. intended for use as starter and probiotic cultures. Biotechnol. Biotechnol. Equip. 2015, 29, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Poimenidou, S.V.; Skarveli, A.; Saxami, G.; Mitsou, E.K.; Kotsou, M.; Kyriacou, A. Inhibition of Listeria monocytogenes Growth, Adherence and Invasion in Caco-2 Cells by Potential Probiotic Lactic Acid Bacteria Isolated from Fecal Samples of Healthy Neonates. Microorganisms 2023, 11, 363. [Google Scholar] [CrossRef]
- Yang, X.; Peng, Z.; He, M.; Li, Z.; Fu, G.; Li, S.; Zhang, J. Screening, probiotic properties, and inhibition mechanism of a Lactobacillus antagonistic to Listeria monocytogenes. Sci. Total Environ. 2024, 906, 167587. [Google Scholar] [CrossRef] [PubMed]
- Tolpeznikaite, E.; Bartkevics, V.; Skrastina, A.; Pavlenko, R.; Ruzauskas, M.; Starkute, V.; Zokaityte, E.; Klupsaite, D.; Ruibys, R.; Rocha, J.M.; et al. Submerged and Solid-State Fermentation of Spirulina with Lactic Acid Bacteria Strains: Antimicrobial Properties and the Formation of Bioactive Compounds of Protein Origin. Biology 2023, 12, 248. [Google Scholar] [CrossRef]
- Chaiklahan, R.; Chirasuwan, N.; Triratana, P.; Loha, V.; Tia, S.; Bunnag, B. Polysaccharide Extraction from Spirulina sp. and Its Antioxidant Capacity. Int. J. Biol. Macromol. 2013, 58, 73–78. [Google Scholar] [CrossRef]
- Perez-Alva, A.; MacIntosh, A.J.; Baigts-Allende, D.K.; García-Torres, R.; Ramírez-Rodrigues, M.M. Fermentation of algae to enhance their bioactive activity: A review. Algal Res. 2022, 64, 102684. [Google Scholar] [CrossRef]
- Li, S.; Zhao, Y.; Zhang, L.; Zhang, X.; Huang, L.; Li, D.; Niu, C.; Yang, Z.; Wang, Q. Antioxidant activity of Lactobacillus plantarum strains iso lated from traditional Chinese fermented foods. Food Chem. 2012, 135, 1914–1919. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.M.; Sujay, S.; Tejaswini, M.; Sushma, P.P.; Prithvi, S.; Ramu, R. Bioactive peptides: Its production and potential role on health. Innov. Food Sci. Emerg. Technol. 2020, 7, 167–182. [Google Scholar]
- Hayes, M.; García-Vaquero, M. Bioactive compounds from fermented food products. In Novel Food Fermentation Technologies, 1st ed.; Shikha Ojha, K., Tiwari, B.K., Eds.; Springer: Cham, Switzerland, 2016; pp. 293–310. [Google Scholar] [CrossRef]
- Das, D.; Goyal, A. Antioxidant activity and γ-aminobutyric acid (GABA) producing ability of probiotic Lactobacillus plantarum DM5 isolated from Marcha of Sikkim. LWT-Food Sci. Technol. 2015, 61, 263–268. [Google Scholar] [CrossRef]
- Ayyash, M.; Al-Nuaimi, A.K.; Al-Mahadin, S.; Liu, S.Q. In vitro investigation of anticancer and ACE-inhibiting activity, α-amylase and α-glucosidase inhibition, and antioxidant activity of camel milk fermented with camel milk probiotic: A comparative study with fermented bovine milk. Food Chem. 2018, 239, 588–597. [Google Scholar] [CrossRef]
- AbdEl-Baki, H.H.; El Baz, F.K.; El-Baroty, G.S. Production of phenolic compounds from Spirulina maxima microalgae and its protective effects. Afr. J. Biotechnol. 2009, 8, 7059–7067. [Google Scholar]
- Hartmann, R.; Meisel, H. Food-derived peptides with biological activity: From research to food applications. Curr. Opin. Biotechnol. 2007, 18, 163–169. [Google Scholar] [CrossRef]
- Filannino, P.; Bai, Y.; Di Cagno, R.; Gobbetti, M.; Ganzle, M.G. Metabolism of phenolic compounds by Lactobacillus spp. during fermentation of cherry juice and broccoli puree. Food Microbiol. 2015, 46, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Lahrichi, S.L.; Affolter, M.; Zolezzi, I.S.; Panchaud, A. Food peptidomics: Large scale analysis of small bioactive peptides—A pilot study. J. Proteom. 2013, 88, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Lamuela-Raventós, R.M. Folin–Ciocalteu method for the measurement of total phenolic content and antioxidant capacity. In Measurement of Antioxidant Activity & Capacity; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 107–115. [Google Scholar] [CrossRef]
- Wu, Q.; Liu, L.; Miron, A.; Klímová, B.; Wan, D.; Kuča, K. The antioxidant, immunomodulatory, and anti-inflammatory activities of Spirulina: An overview. Arch. Toxicol. 2016, 90, 1817–1840. [Google Scholar] [CrossRef]
- Ozturk, N.; Yay, C.; Cinar, Z.O.; Atalay, H.N.; Guneser, O.; Hosoglu, M.I.; Tumer, T.B. Optimizations related to the use of Lactobacillus helveticus, Kluyveromyces marxianus in monoculture, and co-culture for production of Spirulina-based fermented products. Algal Res. 2025, 86, 103948. [Google Scholar] [CrossRef]
- Rosa, L.S.; Santos, M.L.; Abreu, J.P.; Balthazar, C.F.; Rocha, R.S.; Silva, H.L.; Esmerino, E.A.; Duarte, M.C.K.H.; Pimentel, T.C.; Freitas, M.Q.; et al. Antiproliferative and apoptotic effects of probiotic whey dairy beverages in human prostate cell lines. Food Res. Int. 2020, 137, 109450. [Google Scholar] [CrossRef]
- Kayacan Çakmakoğlu, S.; Dere, S.; Bekiroğlu, H.; Bozkurt, F.; Karasu, S.; Dertli, E.; Türker, M. Production of bioactive peptides during yogurt fermentation, their extraction and functional characterization. Food Biosci. 2024, 61, 104805. [Google Scholar] [CrossRef]
- Grover, P.; Bhatnagar, A.; Kumari, N.; Narayan, B.A.; Kumar, N.D.; Purkayastha, J. C-Phycocyanin-a novel protein from Spirulina platensis-In vivo toxicity, antioxidant and immunomodulatory studies. Saudi J. Biol. Sci. 2021, 28, 1853–1859. [Google Scholar] [CrossRef]

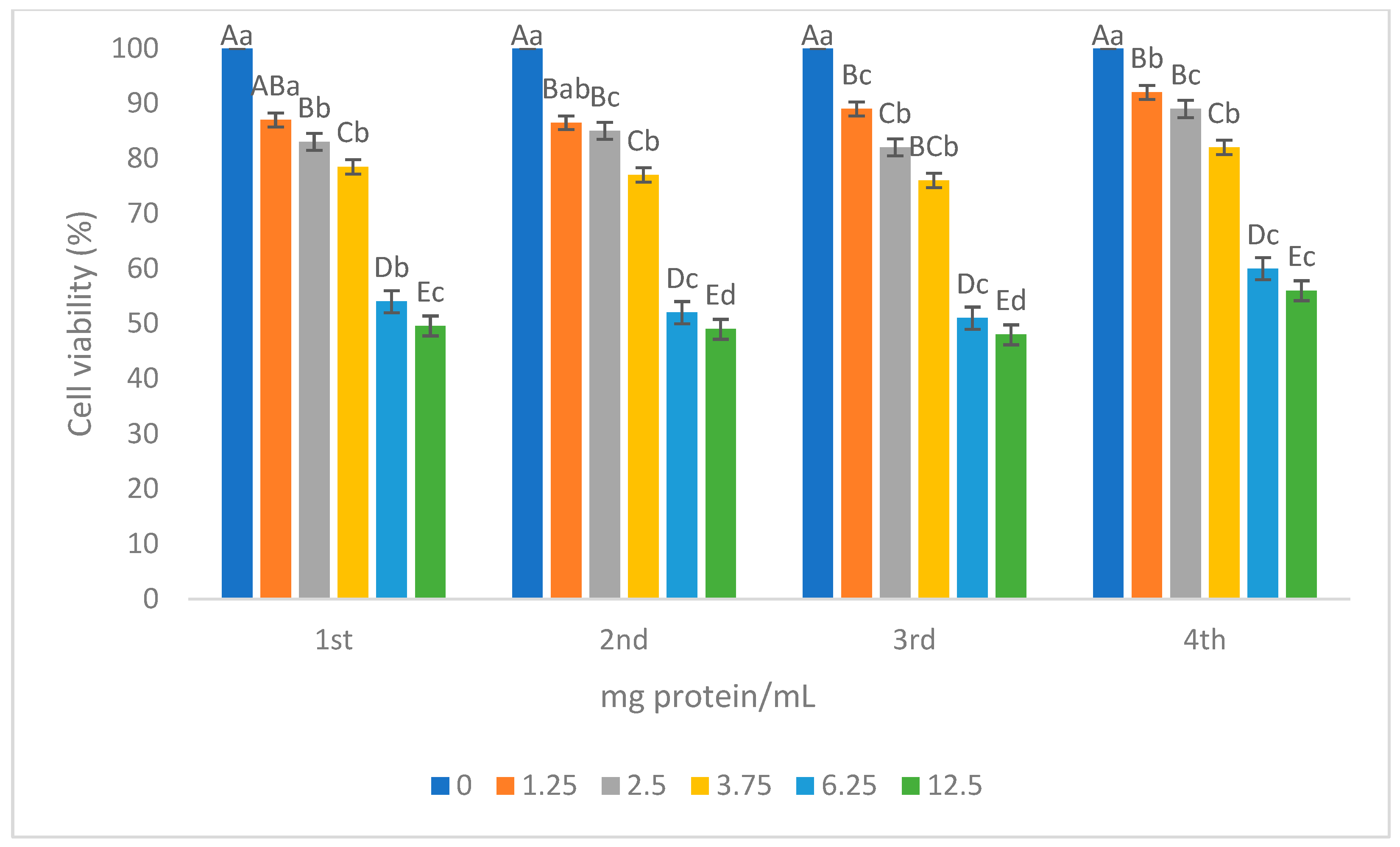
| Solution A | 500 mL | Solution B | 500 mL |
|---|---|---|---|
| NaHCO3 | 13.61 g | NaNO3 | 2.50 g |
| Na2CO3 | 4.03 g | K2SO4 | 1.00 g |
| K2HPO4 | 0.50 g | NaCl | 1.00 g |
| MgSO4·7H2O | 0.20 g | ||
| CaCl2·2H2O | 0.04 g | ||
| P-IV Metal Solution | 6 mL | ||
| Chu Micronutrient Solution | 1 mL |
| Component | Amount |
|---|---|
| Na2EDTA | 0.75 g |
| Na2MoO4·2H2O | 4 mg |
| CoCl2·6H2O | 2 mg |
| MnCl2·4H2O | 41 mg |
| ZnCl2 | 5 mg |
| FeCl3·6H2O | 97 mg |
| Component | Amount |
|---|---|
| Na2EDTA | 50 mg |
| CoCl2·6H2O | 20 mg |
| MnCl2·4H2O | 12.6 mg |
| ZnSO4·7H2O | 44 mg |
| H3BO3 | 618 mg |
| Na2MoO4·2H2O | 12.6 mg |
| CuSO4·5H2O | 19.6 mg |
| Summaries of Fits | y1/y2 | |||||
|---|---|---|---|---|---|---|
| R2 | 0.9255/0.9863 | |||||
| 0.8428/0.9629 | ||||||
| 0.6781/0.7730 | ||||||
| Adequate precision | 11.3290/22.9862 | |||||
| ANOVA results biomass (y1) and antioxidant activity (y2) responses | ||||||
| Source (y1/y2) | SoS (y1/y2) | DoF (y1/y2) | MS (y1/y2) | F-values (y1/y2) | p-values (y1/y2) | Comment (y1/y2) |
| Model | 134.24/16.72 | 10/12 | 13.42/1.39 | 11.19/42.05 | 0.0006/<0.0001 | S/S |
| A | -/1.62 | -/1 | -/1.62 | -/48.82 | -/0.0002 | -/S |
| B | 12.15/- | 1/- | 12.15/- | 10.13/- | 0.0111/- | S/- |
| C | 38.27/1.67 | 1/1 | 38.27/1.67 | 31.89/50.24 | 0.0003/0.0002 | S/S |
| AB | 26.61/2.42 | 1/1 | 26.61/2.42 | 22.17/73.07 | 0.0011/<0.0001 | S/S |
| AC | 18.42/3.05 | 1/1 | 18.42/3.05 | 15.35/92.02 | 0.0035/<0.0001 | S/S |
| BC | 7.13/1.23 | 1/1 | 7.13/1.23 | 5.94/37.06 | 0.0375/0.0005 | S/S |
| A2 | 3.63/0.89 | 1/1 | 3.63/0.89 | 3.02/26.96 | 0.1160/0.0013 | NS/S |
| B2 | -/1.62 | -/1 | -/1.62 | -/48.89 | -/0.0002 | -/S |
| C2 | -/1.20 | -/1 | -/1.20 | -/36.33 | -/0.0005 | -/S |
| A2B | 13.69/4.25 | 1/1 | 13.69/4.25 | 11.40/128.22 | 0.0082/<0.0001 | S/S |
| A2C | 31.55/2.63 | 1/1 | 31.55/2.63 | 26.29/79.23 | 0.0006/<0.0001 | S/S |
| AB2 | 18.20/3.53 | 1/1 | 18.20/3.53 | 15.16/106.44 | 0.0037/<0.0001 | S/S |
| AC2 | 28.71/3.13 | 1/1 | 28.71/3.13 | 23.92/94.35 | 0.0009/<0.0001 | S/S |
| Residual | 10.80/0.23 | 9/7 | 1.20/0.03 | |||
| Lack of Fit | 1.31/0.03 | 4/2 | 0.3283/0.02 | 0.1730/0.3952 | 0.9430/0.6929 | NS/NS |
| Pure Error | 9.49/0.20 | 5/5 | 1.90/0.04 | |||
| Cor Total | 145.04/16.96 | 19/19 | ||||
| Solution from | A = Spirulina Powder (g/L) | B = Aeration (vvm) | C = Agitation (rpm) | y1 = Biomass (g/L) | y2 = Antioxidant Activity (%) |
|---|---|---|---|---|---|
| The bi-objective optimization | 3.18 | 0.93 | 119.97 | 3.74 | 84.72 |
| Validation run | 3.18 | 0.93 | 119.97 | 4.23 | 81.59 |
| Gram-Negative | Gram-Positive | |||
|---|---|---|---|---|
| Study No. | Escherichia coli | Salmonella enterica | Listeria monocytogenes | Staphylococcus aureus |
| 1st | 7.3 | 4.5 | 15.4 | 5.4 |
| 2nd | 7.5 | 4.7 | 9.8 | 4.1 |
| 3rd | 10.4 | 5.2 | 16.1 | 7.6 |
| 4th | 6.2 | 4.4 | 8.0 | 5.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kavak, A.E.; Balkanlı, D.; Sagdıc, O.; Özdemir, A.; Dertli, E. Optimized Spirulina Fermentation with Lacticaseibacillus rhamnosus: Bioactive Properties and Pilot-Scale Validation. Fermentation 2025, 11, 248. https://doi.org/10.3390/fermentation11050248
Kavak AE, Balkanlı D, Sagdıc O, Özdemir A, Dertli E. Optimized Spirulina Fermentation with Lacticaseibacillus rhamnosus: Bioactive Properties and Pilot-Scale Validation. Fermentation. 2025; 11(5):248. https://doi.org/10.3390/fermentation11050248
Chicago/Turabian StyleKavak, Akif Emre, Didem Balkanlı, Osman Sagdıc, Akın Özdemir, and Enes Dertli. 2025. "Optimized Spirulina Fermentation with Lacticaseibacillus rhamnosus: Bioactive Properties and Pilot-Scale Validation" Fermentation 11, no. 5: 248. https://doi.org/10.3390/fermentation11050248
APA StyleKavak, A. E., Balkanlı, D., Sagdıc, O., Özdemir, A., & Dertli, E. (2025). Optimized Spirulina Fermentation with Lacticaseibacillus rhamnosus: Bioactive Properties and Pilot-Scale Validation. Fermentation, 11(5), 248. https://doi.org/10.3390/fermentation11050248







