Enhancing Antioxidant Activity and Modulating Gut Microbiota Through Lactiplantibacillus plantarum-Fermented Processing Wastewater of Yuba (FPWY)
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PWY
2.3. Preparation and Fermentation of FPWY
2.4. Comparison of FPWY and NFPWY Flavonoid Components
2.4.1. Determination of Free Flavonoid Content
2.4.2. Determination of Soy Isoflavone Content
2.5. Comparison of Antioxidant Activities of FPWY and NFPWY
2.5.1. Determination of DPPH Radical Scavenging Activity
2.5.2. Determination of ABTS Radical Scavenging Activity
2.5.3. Determination of Ferric Reducing Antioxidant Power (FRAP)
2.6. In Vitro Digestion Fermentation
2.6.1. The Digestion in the Stomach and Intestine
2.6.2. The Fermentation in the Colon
2.7. Analysis of Bioactive Components During Digestion
2.7.1. Changes in Free Flavonoid Content
2.7.2. Changes in Isoflavone Content
2.8. The Effect of In Vitro Digestion on Antioxidant Activity
2.8.1. Determination of DPPH Radical Scavenging Activity
2.8.2. Determination of ABTS Radical Scavenging Activity
2.8.3. Determination of Ferric Reducing Antioxidant Power (FRAP)
2.9. Quantification of the Gut Microbiota by qPCR
2.10. Determination of SCFAs and Lactic Acid
2.11. Statistical Analysis
3. Results and Discussion
3.1. Effect of Fermentation on the Bacterial Count of L. plantarum in PWY
3.2. Effect of Fermentation on Main Components of PWY
3.2.1. Content of Free Flavonoids in NFPWY and FPWY
3.2.2. Content of Soy Isoflavones in NFPWY and FPWY
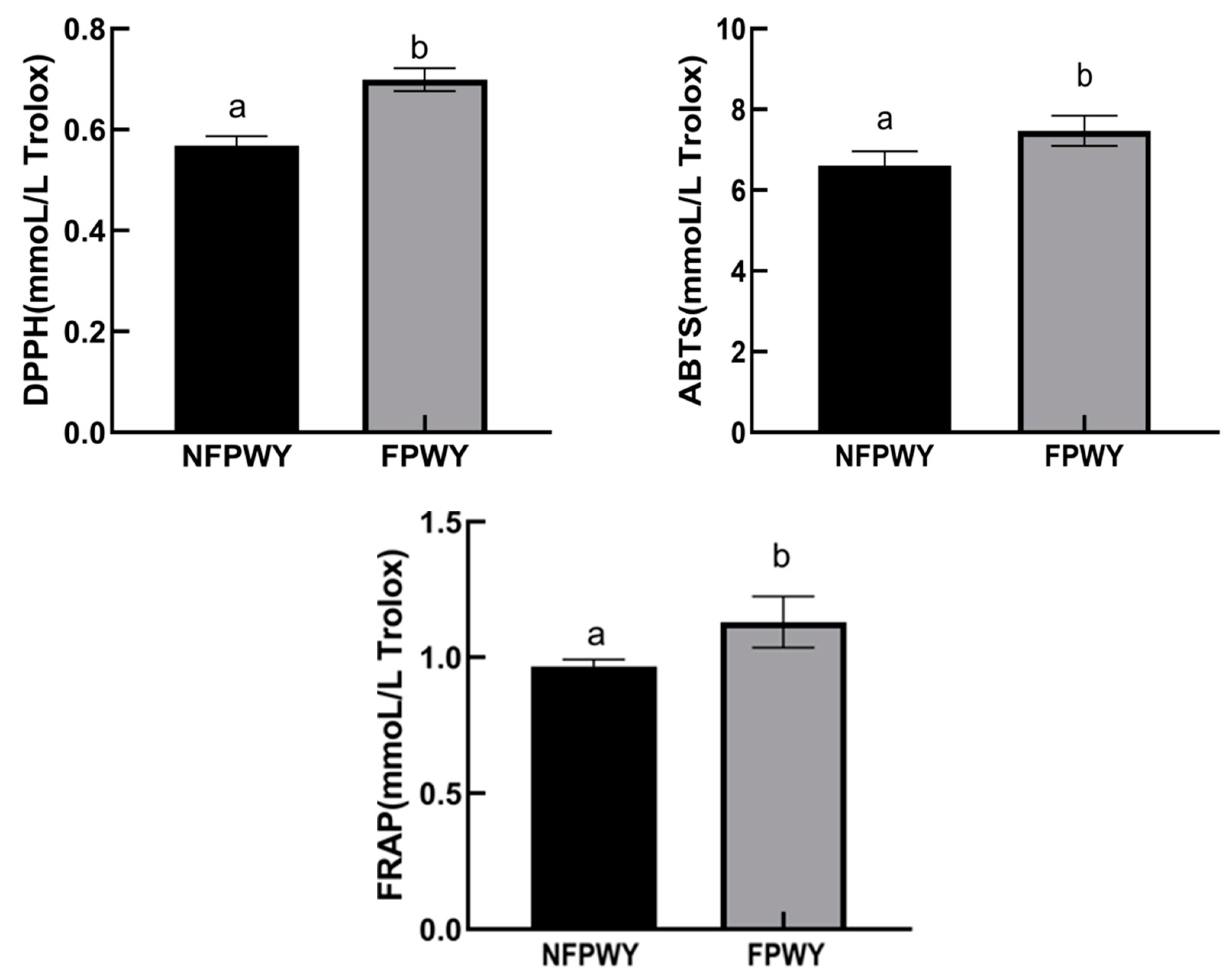
3.3. Comparison of Antioxidant Activity Between NFPWY and FPWY
3.4. Changes in Main Components During In Vitro Simulated Digestion
3.4.1. Changes in Free Flavonoids
3.4.2. Changes in Soy Isoflavones
3.5. Changes in Antioxidant Activity During In Vitro Simulated Digestion
3.6. The Relative Abundances of Fecal Microbiota
3.7. The Contents of SCFAs and Lactic Acid
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kalita, C.; Mehta, U.; Aayush, K.; Sawant, P.; Chavan, P.; Rasane, P.; Sharma, S.; Singh, G.P.; Nawghare, G.K.; Dhruv; et al. Recent trends in antioxidative peptides derived from soybean and other soy-based products: A comprehensive review. Process Biochem. 2024, 136, 311–323. [Google Scholar] [CrossRef]
- Lee, C.H.; Yang, L.; Xu, J.Z.; Yeung, S.Y.V.; Huang, Y.; Chen, Z.-Y. Relative antioxidant activity of soybean isoflavones and their glycosides. Food Chem. 2005, 90, 735–741. [Google Scholar] [CrossRef]
- Mateos-Aparicio, I.; Mateos-Peinado, C.; Jiménez-Escrig, A.; Rupérez, P. Multifunctional antioxidant activity of polysaccharide fractions from the soybean byproduct okara. Carbohydr. Polym. 2010, 82, 245–250. [Google Scholar] [CrossRef]
- Swallah, M.S.; Fan, H.; Wang, S.; Yu, H.; Piao, C. Prebiotic Impacts of Soybean Residue (Okara) on Eubiosis/Dysbiosis Condition of the Gut and the Possible Effects on Liver and Kidney Functions. Molecules 2021, 26, 326. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.T.; Tan, S.S.; Tan, C.X. Soy protein, bioactive peptides, and isoflavones: A review of their safety and health benefits. PharmaNutrition 2023, 25, 100352. [Google Scholar] [CrossRef]
- Chua, J.-Y.; Liu, S.-Q. Soy whey: More than just wastewater from tofu and soy protein isolate industry. Trends Food Sci. Technol. 2019, 91, 24–32. [Google Scholar] [CrossRef]
- Wang, Y.; Serventi, L. Sustainability of dairy and soy processing: A review on wastewater recycling. J. Clean. Prod. 2019, 237, 117821. [Google Scholar] [CrossRef]
- Khubber, S.; Marti-Quijal, F.J.; Tomasevic, I.; Remize, F.; Barba, F.J. Lactic acid fermentation as a useful strategy to recover antimicrobial and antioxidant compounds from food and by-products. Curr. Opin. Food Sci. 2022, 43, 189–198. [Google Scholar] [CrossRef]
- Sabater, C.; Ruiz, L.; Delgado, S.; Ruas-Madiedo, P.; Margolles, A. Valorization of Vegetable Food Waste and By-Products Through Fermentation Processes. Front. Microbiol. 2020, 11, 581997. [Google Scholar] [CrossRef]
- Phomkaivon, N.; Pan-Utai, W.; Surojanametakul, V.; Varichanan, P.; Kaewtathip, T.; Kanyakam, K.; Klinsoda, J. Isoflavone aglycone-rich powder from soybean residue submerged fermentation using Lactobacillus fermentum 44197. NFS J. 2023, 33, 100157. [Google Scholar] [CrossRef]
- Langa, S.; Peirotén, Á.; Curiel, J.A.; de la Bastida, A.R.; Landete, J.M. Isoflavone Metabolism by Lactic Acid Bacteria and Its Application in the Development of Fermented Soy Food with Beneficial Effects on Human Health. Foods 2023, 12, 1293. [Google Scholar] [CrossRef]
- Pyo, Y.-H.; Lee, T.-C.; Lee, Y.C. Effect of Lactic Acid Fermentation on Enrichment of Antioxidant Properties and Bioactive Isoflavones in Soybean. J. Food Sci. 2005, 70, S215–S220. [Google Scholar] [CrossRef]
- Dai, S.; Pan, M.; El-Nezami, H.S.; Wan, J.M.; Wang, M.; Habimana, O.; Louie, J.C.; Shah, N.P. Effects of Lactic Acid Bacteria-Fermented Soymilk on Isoflavone Metabolites and Short-Chain Fatty Acids Excretion and Their Modulating Effects on Gut Microbiota. J. Food Sci. 2019, 84, 1854–1863. [Google Scholar] [CrossRef] [PubMed]
- Madjirebaye, P.; Peng, F.; Mueed, A.; Huang, T.; Mahamat, B.; Pahane, M.M.; Xi, Q.; Chen, X.; Moussa, K.; Kadebe, Z.T.; et al. Exploring Impact of Probiotic-Fermented Soymilk on Dextran-Sulfate-Sodium-Induced Ulcerative Colitis via Modulating Inflammation and Gut Microbiota Profile. Mol. Nutr. Food Res. 2024, 68, 2300586. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Cheng, Y.; Wu, T.; Hu, F.; Pan, S.; Xu, X. Effect of Lactobacillus plantarum-fermented mulberry pomace on antioxidant properties and fecal microbial community. LWT 2021, 147, 111651. [Google Scholar] [CrossRef]
- Zhang, S.; Kim, N.; Yokoyama, W.; Kim, Y. Effects of moisture content on mechanical properties, transparency, and thermal stability of yuba film. Food Chem. 2018, 243, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Shraim, A.M.; Ahmed, T.A.; Rahman, M.M.; Hijji, Y.M. Determination of total flavonoid content by aluminum chloride assay: A critical evaluation. LWT 2021, 150, 111932. [Google Scholar] [CrossRef]
- Tuncil, Y.E.; Thakkar, R.D.; Arioglu-Tuncil, S.; Hamaker, B.R.; Lindemann, S.R. Fecal Microbiota Responses to Bran Particles Are Specific to Cereal Type and In Vitro Digestion Methods That Mimic Upper Gastrointestinal Tract Passage. J. Agric. Food Chem. 2018, 66, 12580–12593. [Google Scholar] [CrossRef]
- Li, E.; Yang, H.; Zou, Y.; Wang, H.; Hu, T.; Li, Q.; Liao, S. In-vitro digestion by simulated gastrointestinal juices of Lactobacillus rhamnosus cultured with mulberry oligosaccharides and subsequent fermentation with human fecal inocula. LWT-Food Sci. Technol. 2019, 101, 61–68. [Google Scholar] [CrossRef]
- Wu, T.; Chu, X.; Cheng, Y.; Tang, S.; Zogona, D.; Pan, S.; Xu, X. Modulation of Gut Microbiota by Lactobacillus casei Fermented Raspberry Juice In Vitro and In Vivo. Foods 2021, 10, 3055. [Google Scholar] [CrossRef]
- Cheng, Y.; Wu, T.; Chu, X.; Tang, S.; Cao, W.; Liang, F.; Fang, Y.; Pan, S.; Xu, X. Fermented blueberry pomace with antioxidant properties improves fecal microbiota community structure and short chain fatty acids production in an in vitro mode. LWT 2020, 125, 109260. [Google Scholar] [CrossRef]
- Vendrame, S.; Guglielmetti, S.; Riso, P.; Arioli, S.; Klimis-Zacas, D.; Porrini, M. Six-Week Consumption of a Wild Blueberry Powder Drink Increases Bifidobacteria in the Human Gut. J. Agric. Food Chem. 2011, 59, 12815–12820. [Google Scholar] [CrossRef] [PubMed]
- Ben Ounis, W.; Champagne, C.; Makhlouf, J.; Bazinet, L. Utilization of tofu whey pre-treated by electromembrane process as a growth medium for Lactobacillus plantarum LB17. Desalination 2008, 229, 192–203. [Google Scholar] [CrossRef]
- Tu, C.; Azi, F.; Huang, J.; Xu, X.; Xing, G.; Dong, M. Quality and metagenomic evaluation of a novel functional beverage produced from soy whey using water kefir grains. LWT 2019, 113, 108258. [Google Scholar] [CrossRef]
- Bhanja, T.; Kumari, A.; Banerjee, R. Enrichment of phenolics and free radical scavenging property of wheat koji prepared with two filamentous fungi. Bioresour. Technol. 2009, 100, 2861–2866. [Google Scholar] [CrossRef]
- Cheng, K.-C.; Wu, J.-Y.; Lin, J.-T.; Liu, W.-H. Enhancements of isoflavone aglycones, total phenolic content, and antioxidant activity of black soybean by solid-state fermentation with Rhizopus spp. Eur. Food Res. Technol. 2013, 236, 1107–1113. [Google Scholar] [CrossRef]
- Hur, S.J.; Lee, S.Y.; Kim, Y.-C.; Choi, I.; Kim, G.-B. Effect of fermentation on the antioxidant activity in plant-based foods. Food Chem. 2014, 160, 346–356. [Google Scholar] [CrossRef]
- Lee, J.H.; Hwang, C.E.; Son, K.S.; Cho, K.M. Comparisons of nutritional constituents in soybeans during solid state fermentation times and screening for their glucosidase enzymes and antioxidant properties. Food Chem. 2019, 272, 362–371. [Google Scholar] [CrossRef]
- Otieno, D.O.; Ashton, J.F.; Shah, N.P. Evaluation of enzymic potential for biotransformation of isoflavone phytoestrogen in soymilk by Bifidobacterium animalis, Lactobacillus acidophilus and Lactobacillus casei. Food Res. Int. 2006, 39, 394–407. [Google Scholar] [CrossRef]
- Xiao, Y.; Wang, L.; Rui, X.; Li, W.; Chen, X.; Jiang, M.; Dong, M. Enhancement of the antioxidant capacity of soy whey by fermentation with Lactobacillus plantarum B1–6. J. Funct. Foods 2015, 12, 33–44. [Google Scholar] [CrossRef]
- Sarıtaş, S.; Portocarrero, A.C.M.; López, J.M.M.; Lombardo, M.; Koch, W.; Raposo, A.; El-Seedi, H.R.; Alves, J.L.d.B.; Esatbeyoglu, T.; Karav, S.; et al. The Impact of Fermentation on the Antioxidant Activity of Food Products. Molecules 2024, 29, 3941. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, M.; Huang, H. Changes of heat-treated soymilks in bioactive compounds and their antioxidant activities under in vitro gastrointestinal digestion. Eur. Food Res. Technol. 2014, 239, 637–652. [Google Scholar] [CrossRef]
- Rodríguez-Roque, M.J.; Rojas-Graü, M.A.; Elez-Martínez, P.; Martín-Belloso, O. Soymilk phenolic compounds, isoflavones and antioxidant activity as affected by in vitro gastrointestinal digestion. Food Chem. 2013, 136, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Mao, Q.; Dong, M.; Wang, X.; Rui, X.; Zhang, Q.; Chen, X.; Li, W. Structural Characterization and Antioxidant Activity of Exopolysaccharide from Soybean Whey Fermented by Lacticaseibacillus plantarum 70810. Foods 2021, 10, 2780. [Google Scholar] [CrossRef]
- Huo, C.; Yang, X.; Li, L. Non-beany flavor soymilk fermented by lactic acid bacteria: Characterization, stability, antioxidant capacity and in vitro digestion. Food Chem. X 2023, 17, 100578. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Krishnan, H.B.; Pham, Q.; Yu, L.L.; Wang, T.T.Y. Soy and Gut Microbiota: Interaction and Implication for Human Health. J. Agric. Food Chem. 2016, 64, 8695–8709. [Google Scholar] [CrossRef]
- Braga, A.R.C.; Mesquita, L.M.D.S.; Martins, P.L.G.; Habu, S.; de Rosso, V.V. Lactobacillus fermentation of jussara pulp leads to the enzymatic conversion of anthocyanins increasing antioxidant activity. J. Food Compos. Anal. 2017, 69, 162–170. [Google Scholar] [CrossRef]
- Louis, P.; Young, P.; Holtrop, G.; Flint, H.J. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ. Microbiol. 2010, 12, 304–314. [Google Scholar] [CrossRef]
- Rist, V.T.S.; Weiss, E.; Sauer, N.; Mosenthin, R.; Eklund, M. Effect of dietary protein supply originating from soybean meal or casein on the intestinal microbiota of piglets. Anaerobe 2014, 25, 72–79. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Fagan, A.; White, M.B.; Wade, J.B.; Hylemon, P.B.; Heuman, D.M.; Fuchs, M.; John, B.; Acharya, C.; Sikaroodi, M.; et al. Specific Gut and Salivary Microbiota Patterns Are Linked with Different Cognitive Testing Strategies in Minimal Hepatic Encephalopathy. Am. J. Gastroenterol. 2019, 114, 1080–1090. [Google Scholar] [CrossRef]
- Huang, L.; Zheng, T.; Hui, H.; Xie, G. Soybean isoflavones modulate gut microbiota to benefit the health weight and metabolism. Front. Cell. Infect. Microbiol. 2022, 12, 1004765. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, P.; Zhou, Y.; Zhuang, S. Quercetin Supplementation Improves Intestinal Digestive and Absorptive Functions and Microbiota in Rats Fed Protein-Oxidized Soybean Meal: Transcriptomics and Microbiomics Insights. Animals 2024, 14, 2326. [Google Scholar] [CrossRef]
- Han, F.; Wang, Y.; Han, Y.; Zhao, J.; Han, F.; Song, G.; Jiang, P.; Miao, H. Effects of Whole-Grain Rice and Wheat on Composition of Gut Microbiota and Short-Chain Fatty Acids in Rats. J. Agric. Food Chem. 2018, 66, 6326–6335. [Google Scholar] [CrossRef] [PubMed]
- Han, W.; Zhuang, X.; Liu, Q.; Sun, B.; Miao, H.; Zhang, X. Fermented soy whey induced changes on intestinal microbiota and metabolic influence in mice. Food Sci. Hum. Wellness 2022, 11, 41–48. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Y.; Ma, F.; Sun, M.; Mu, G.; Tuo, Y. The ameliorative effect of Lactobacillus plantarum Y44 oral administration on inflammation and lipid metabolism in obese mice fed with a high fat diet. Food Funct. 2020, 11, 5024–5039. [Google Scholar] [CrossRef]
- Xiao, X.; Cui, T.; Qin, S.; Wang, T.; Liu, J.; Sa, L.; Wu, Y.; Zhong, Y.; Yang, C. Beneficial effects of Lactobacillus plantarum on growth performance, immune status, antioxidant function and intestinal microbiota in broilers. Poult. Sci. 2024, 103, 104280. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Lin, W.; Wen, L.; Wen, J.; Xiang, G. Effects of Sleeve Gastrectomy on Fecal Gut Microbiota and Short-Chain Fatty Acid Content in a Rat Model of Polycystic Ovary Syndrome. Front. Endocrinol. 2021, 12. [Google Scholar] [CrossRef]
- Louis, P.; Flint, H.J. Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 2009, 294, 1–8. [Google Scholar] [CrossRef]
- Tan, J.K.; Macia, L.; Mackay, C.R. Dietary fiber and SCFAs in the regulation of mucosal immunity. J. Allergy Clin. Immunol. 2023, 151, 361–370. [Google Scholar] [CrossRef]
- Aldunate, M.; Srbinovski, D.; Hearps, A.C.; Latham, C.F.; Ramsland, P.A.; Gugasyan, R.; Cone, R.A.; Tachedjian, G. Antimicrobial and immune modulatory effects of lactic acid and short chain fatty acids produced by vaginal microbiota associated with eubiosis and bacterial vaginosis. Front. Physiol. 2015, 6, 164. [Google Scholar] [CrossRef] [PubMed]





| Name | Primers |
|---|---|
| Bifidobacterium | F: GGGTGGTAATGCCGGATG |
| R: TAAGCGATGGACTTTCACACC | |
| Ruminococcus | F: TTAACACAATAAGTWATCCACCTGG |
| R: ACCTTCCTCCGTTTTGTCAAC | |
| Butyrate bacteria | F: GCIGAICATTTCACITGGAAYWSITGGCAYATG |
| R: CCTGCCTTTGCAATRTCIACRAANGC | |
| Lactobacillus | F: AGCAGTAGGGAATCTTCCA |
| R: CACCGCTACACATGGAG | |
| Akkermansia | F: CAGCACGTGAAGGTGGGGAC |
| R: CCTTGCGGTTGGCTTCAGAT | |
| Escherichia coli | F: GTTAATACCTTTGCTCATTA |
| R: ACCAGGGTATCTTAATCCTGTT | |
| Bacteroides | F: ATAGCCTTTCGAAAGRAAGAT |
| R: CCAGTATCAACTGCAATTTTA |
| NFPWY | FPWY | |
|---|---|---|
| Daidzein | 116.85 ± 8.52 a | 312.77 ± 15.24 b |
| Daidzin | 371.06 ± 6.14 a | 77.51 ± 19.68 b |
| Glycitein | 3.67 ± 0.65 a | 46.82 ± 4.12 b |
| Glycitin | 30.03 ± 2.97 a | n.d. |
| Genistein | 22.66 ± 1.76 a | 93.52 ± 2.77 b |
| Genistin | 399.64 ± 29.86 a | 115.37 ± 20.43 b |
| Soy Isoflavone | Undigested | Stomach Digestion at 60 min | Stomach Digestion at 120 min | Intestine Digestion at 60 min | Intestine Digestion at 120 min | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| NFPWY | FPWY | NFPWY | FPWY | NFPWY | FPWY | NFPWY | FPWY | NFPWY | FPWY | |
| Daidzein | 116.85 ± 8.52 c | 312.77 ± 15.24 B | 79.34 ± 4.51 b | 303.96 ± 5.32 B | 80.24 ± 9.37 b | 298.33 ± 10.33 B | 59.70 ± 0.38 a | 215.57 ± 18.50 A | 59.39 ± 1.27 a | 218.85 ± 10.82 C |
| Daidzin | 371.06 ± 6.14 c | 77.51 ± 19.68 B | 222.09 ± 10.87 b | 55.23 ± 5.06 A | 212.84 ± 19.78 b | 56.36 ± 9.75 A | 162.00 ± 3.13 a | 38.97 ± 3.47 A | 171.78 ± 1.36 a | 42.80 ± 1.64 A |
| Glycitein | 3.67 ± 0.65 a | 46.82 ± 4.12 C | 2.94 ± 0.34 a | 36.48 ± 0.42 B | 3.37 ± 1.17 a | 43.25 ± 2.64 C | 6.22 ± 0.32 b | 30.49 ± 2.64 A | 5.70 ± 0.25 b | 30.87 ± 1.13 A |
| Glycitin | 30.03 ± 2.97 d | n.d. | 20.10 ± 0.93 c | n.d. | 17.80 ± 1.59 bc | n.d. | 13.70 ± 0.09 a | n.d. | 14.71 ± 0.19 ab | n.d. |
| Genistein | 22.66 ± 1.76 c | 93.52 ± 2.77 B | 15.44 ± 0.76 b | 96.00 ± 1.49 B | 14.91 ± 1.50 b | 92.26 ± 3.06 B | 10.31 ± 0.54 a | 64.92 ± 6.39 A | 11.59 ± 0.44 a | 67.70 ± 3.36 A |
| Genistin | 399.64 ± 29.86 c | 115.37 ± 20.43 C | 263.48 ± 12.17 b | 90.53 ± 7.85 B | 266.32 ± 15.35 b | 95.43 ± 26.12 BC | 189.90 ± 6.23 a | 63.37 ± 5.80 A | 184.39 ± 6.06 a | 67.79 ± 2.00 A |
| Blank | NFPWY | FPWY | L. plantarum | |
|---|---|---|---|---|
| Bifidobacterium | 1.00 ± 0.00 a | 2.17 ± 0.66 b | 4.24 ± 0.19 c | 1.22 ± 0.3 ab |
| Ruminococcus | 1.00 ± 0.00 b | 0.55 ± 0.04 a | 1.32 ± 0.10 c | 1.13 ± 0.18 bc |
| Butyrate bacteria | 1.00 ± 0.00 a | 6.52 ± 0.16 c | 2.50 ± 0.10 b | 1.06 ± 0.03 a |
| Lactobacillus | 1.00 ± 0.00 a | 2.70 ± 0.16 c | 1.26 ± 0.04 ab | 1.03 ± 0.01 ab |
| Akkermansia | 1.00 ± 0.00 ab | 1.13 ± 0.22 bc | 1.50 ± 0.21 c | 0.68 ± 0.00 a |
| E. coli | 1.00 ± 0.00 d | 0.46 ± 0.03 c | 0.27 ± 0.00 b | 0.01 ± 0.00 a |
| Bacteroides | 1.00 ± 0.00 a | 6.78 ± 0.83 c | 3.94 ± 0.75 b | 1.44 ± 0.04 a |
| Blank | NFPWY | FPWY | L. plantarum | |
|---|---|---|---|---|
| Acetic acid | 52.672 ± 3.337 a | 57.541 ± 4.341 a | 59.420 ± 1.688 a | 70.010 ± 4.628 b |
| Propionic acid | 5.006 ± 0.080 a | 8.151 ± 0.270 b | 9.299 ± 0.219 c | 0.305 ± 0.015 a |
| Isobutyric acid | 0.173 ± 0.074 b | 0.032 ± 0.001 a | 0.098 ± 0.001 ab | 0.119 ± 0.003 ab |
| Butyric acid | 0.107 ± 0.017 a | 2.428 ± 0.695 b | 2.536 ± 0.232 b | 0.038 ± 0.001 a |
| Isovaleric acid | 0.322 ± 0.031 c | 0.177 ± 0.010 b | 0.425 ± 0.002 d | 0.104 ± 0.013 a |
| Valeric acid | 0.029 ± 0.006 b | 0.022 ± 0.001 ab | 0.032 ± 0.006 b | 0.016 ± 0.004 a |
| Lactic acid | 0.344 ± 0.018 a | 3.193 ± 0.251 c | 1.122 ± 0.239 b | 0.533 ± 0.070 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, T.; Hu, F.; Tang, S.; Xu, X.; Li, D. Enhancing Antioxidant Activity and Modulating Gut Microbiota Through Lactiplantibacillus plantarum-Fermented Processing Wastewater of Yuba (FPWY). Fermentation 2025, 11, 212. https://doi.org/10.3390/fermentation11040212
Wu T, Hu F, Tang S, Xu X, Li D. Enhancing Antioxidant Activity and Modulating Gut Microbiota Through Lactiplantibacillus plantarum-Fermented Processing Wastewater of Yuba (FPWY). Fermentation. 2025; 11(4):212. https://doi.org/10.3390/fermentation11040212
Chicago/Turabian StyleWu, Ting, Feiting Hu, Shuxin Tang, Xiaoyun Xu, and Duo Li. 2025. "Enhancing Antioxidant Activity and Modulating Gut Microbiota Through Lactiplantibacillus plantarum-Fermented Processing Wastewater of Yuba (FPWY)" Fermentation 11, no. 4: 212. https://doi.org/10.3390/fermentation11040212
APA StyleWu, T., Hu, F., Tang, S., Xu, X., & Li, D. (2025). Enhancing Antioxidant Activity and Modulating Gut Microbiota Through Lactiplantibacillus plantarum-Fermented Processing Wastewater of Yuba (FPWY). Fermentation, 11(4), 212. https://doi.org/10.3390/fermentation11040212






