Beyond Saccharomyces: Exploring the Bioethanol Potential of Wickerhamomyces anomalus and Diutina rugosa in Xylose and Glucose Co-Fermentation
Abstract
1. Introduction
2. Materials and Methods
2.1. Yeast Strains and Maintenance
2.2. Culture Medium and Inoculum Preparation
2.3. Analysis of Glucose Repression Using 2-Deoxyglucose
2.4. Fermentation Setup
2.5. Analytical Methods
2.5.1. Growth Measurement
2.5.2. Sugar and Ethanol Quantification
2.6. Kinetic and Yield Parameters
2.7. Statistical Analysis
3. Results
3.1. Effect of 2-DG on Xylose Utilization
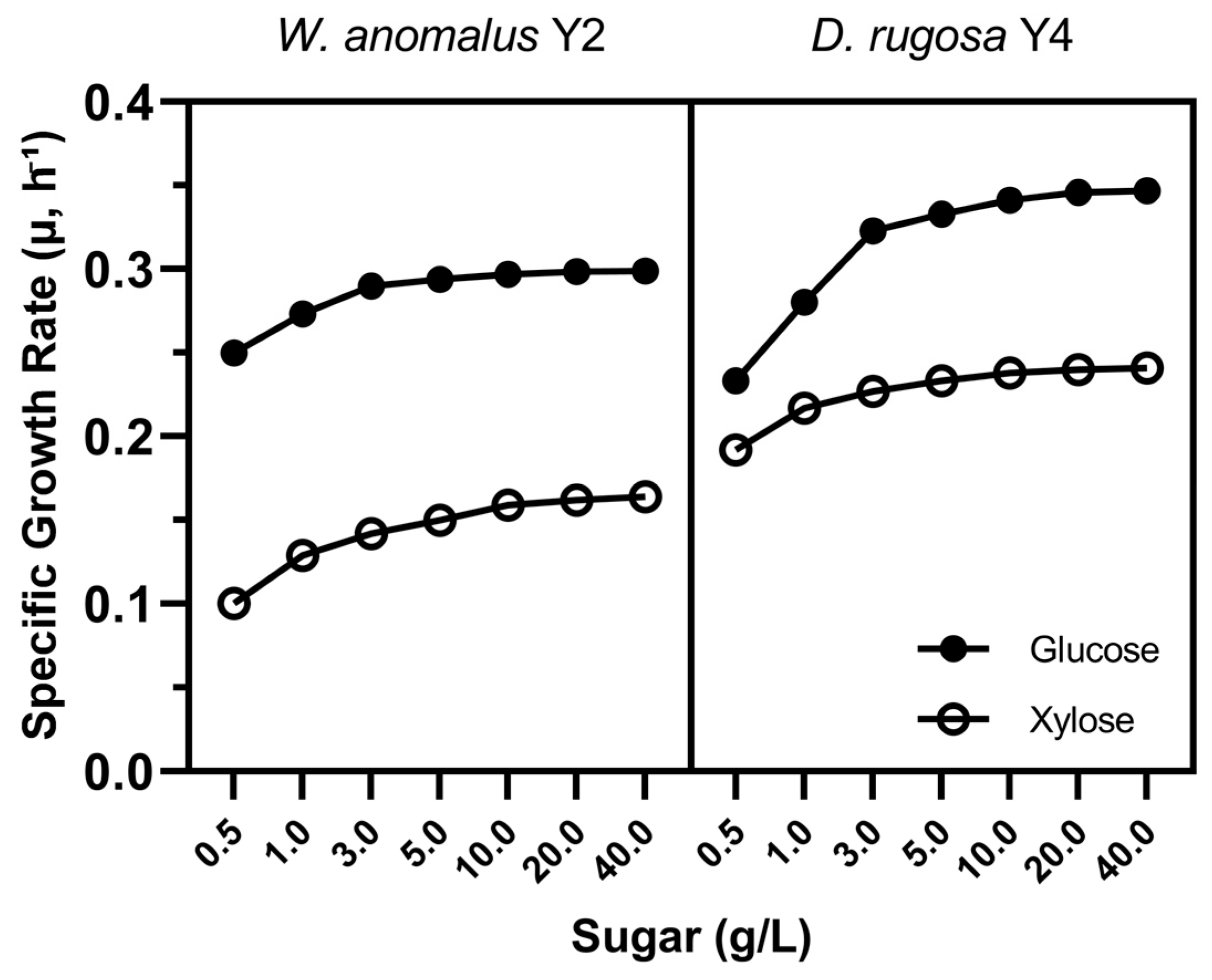
3.2. Growth Kinetics in Different Glucose/Xylose Ratios
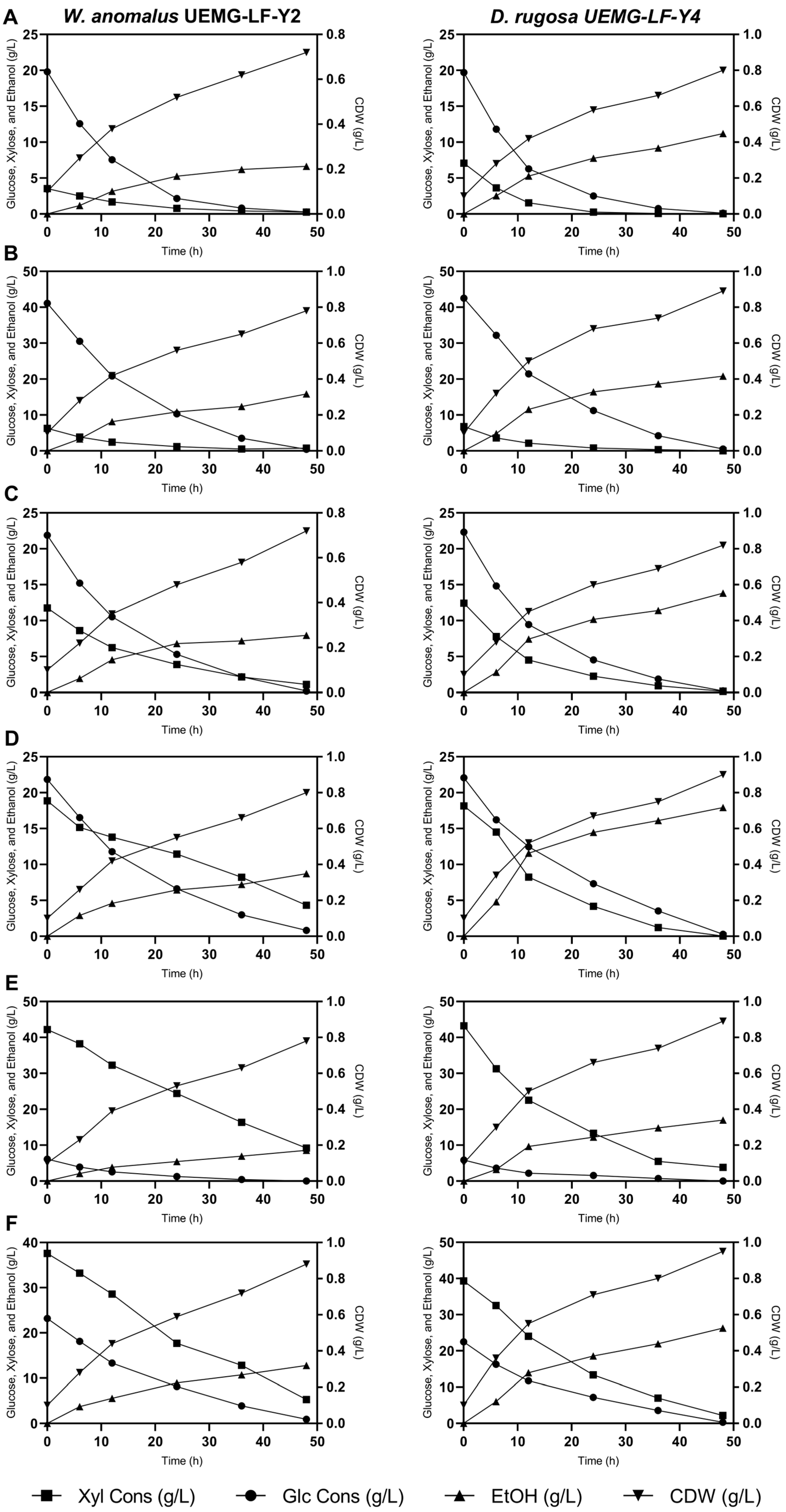
3.3. Substrate Consumption and Ethanol Production
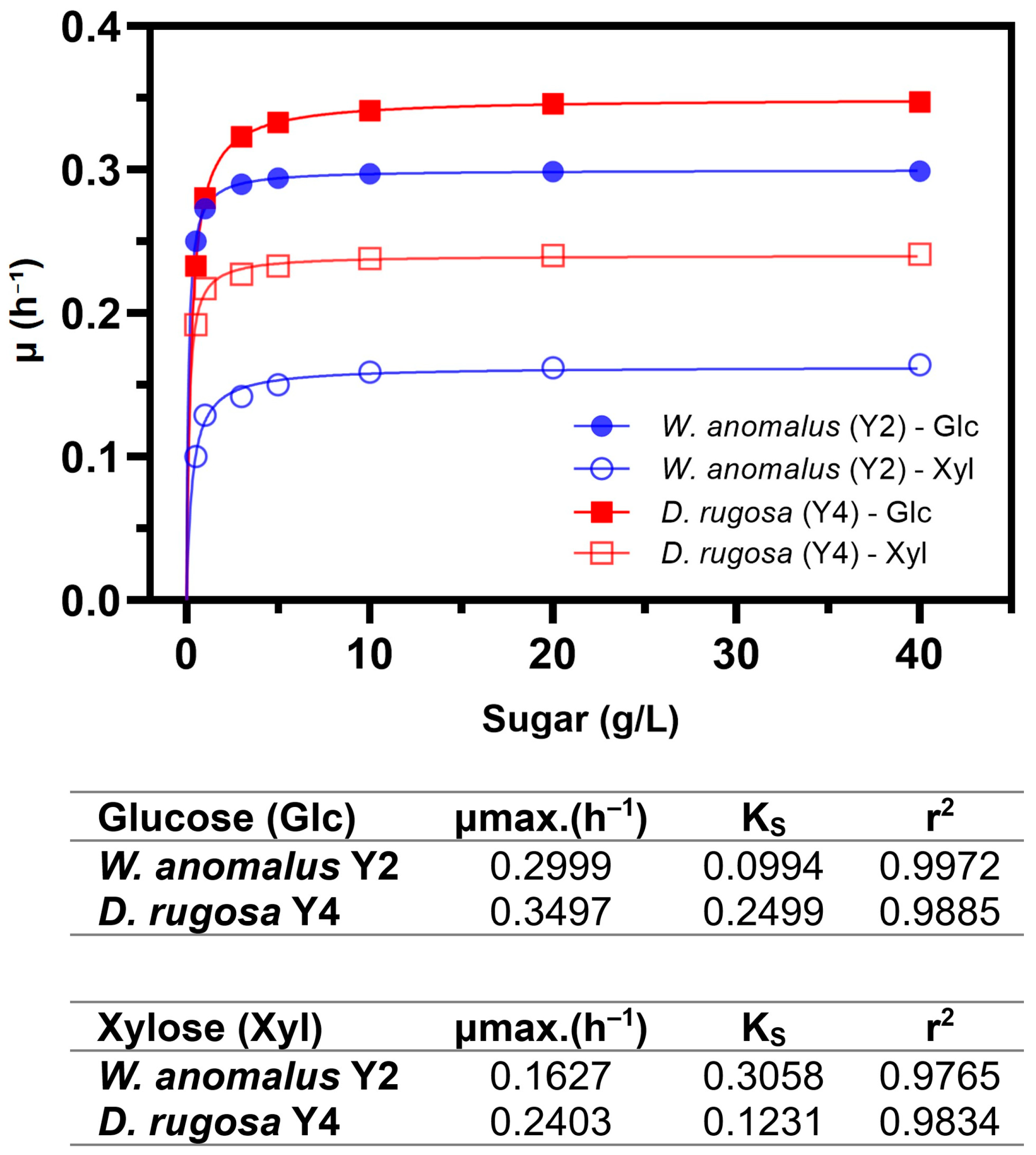
3.4. Monod Model Fitting and Kinetic Parameters
3.5. Yield and Productivity Parameters
3.6. Inhibition of Xylose Consumption by Ethanol
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mehejabin, F.; Musharrat, A.; Ahmed, S.F.; Kabir, Z.; Khan, T.M.Y.; Saleel, C.A. Sustainable Biofuel Production Utilizing Nanotechnology: Challenges and Potential Solutions. GCB Bioenergy 2024, 16, e70001. [Google Scholar] [CrossRef]
- Blay-Roger, R.; Saif, M.; Bobadilla, L.F.; Ramirez-Reina, T.; Nawaz, M.A.; Odriozola, J.A. Embracing the Sustainable Horizons through Bioenergy Innovations: A Path to a Sustainable Energy Future. Front. Chem. 2024, 12, 1416102. [Google Scholar] [CrossRef] [PubMed]
- Anekwe, I.M.S.; Isa, Y.M.; Oboirien, B. Bioethanol as a Potential Eco-Friendlier Feedstock for Catalytic Production of Fuels and Petrochemicals. J. Chem. Technol. Biotechnol. 2023, 98, 2077–2094. [Google Scholar] [CrossRef]
- Russo, C.; Cirillo, V.; Pollaro, N.; Terribile, F.; Chiodini, A.; Maggio, A. The Global Energy Challenge: Second-Generation Feedstocks on Marginal Lands for a Sustainable Biofuel Production. Chem. Biol. Technol. Agric. 2025, 12, 10. [Google Scholar] [CrossRef]
- Woźniak, A.; Kuligowski, K.; Świerczek, L.; Cenian, A. Review of Lignocellulosic Biomass Pretreatment Using Physical, Thermal and Chemical Methods for Higher Yields in Bioethanol Production. Sustainability 2025, 17, 287. [Google Scholar] [CrossRef]
- Pereira, L.M.S.; Taveira, I.C.; Maués, D.B.; de Paula, R.G.; Silva, R.N. Advances in Fungal Sugar Transporters: Unlocking the Potential of Second-Generation Bioethanol Production. Appl. Microbiol. Biotechnol. 2025, 109, 19. [Google Scholar] [CrossRef]
- Afedzi, A.E.K.; Afrakomah, G.S.; Gyan, K.; Khan, J.; Seidu, R.; Baidoo, T.; Sultan, I.N.; Tareen, A.K.; Parakulsuksatid, P. Enhancing Economic and Environmental Sustainability in Lignocellulosic Bioethanol Production: Key Factors, Innovative Technologies, Policy Frameworks, and Social Considerations. Sustainability 2025, 17, 499. [Google Scholar] [CrossRef]
- Zielińska, M.; Bułkowska, K. Agricultural Wastes and Their By-Products for the Energy Market. Energies 2024, 17, 2099. [Google Scholar] [CrossRef]
- Subudhi, S.; Mudgil, D.; Saha, K.; Sarangi, P.K.; Pal, P. Yeast as a Cell Factory for Fermentative Production of Ethanol from Xylose. J. Taiwan Inst. Chem. Eng. 2024, 105616. [Google Scholar] [CrossRef]
- Taveira, I.C.; Carraro, C.B.; Nogueira, K.M.V.; Pereira, L.M.S.; Bueno, J.G.R.; Fiamenghi, M.B.; dos Santos, L.V.; Silva, R.N. Structural and Biochemical Insights of Xylose MFS and SWEET Transporters in Microbial Cell Factories: Challenges to Lignocellulosic Hydrolysates Fermentation. Front. Microbiol. 2024, 15, 1452240. [Google Scholar] [CrossRef]
- Tsegaye, K.N.; Alemnew, M.; Berhane, N. Saccharomyces Cerevisiae for Lignocellulosic Ethanol Production: A Look at Key Attributes and Genome Shuffling. Front. Bioeng. Biotechnol. 2024, 12, 1466644. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, L.V.; Neitzel, T.; Lima, C.S.; de Carvalho, L.M.; de Lima, T.B.; Ienczak, J.L.; Corrêa, T.L.R.; Pereira, G.A.G. Engineering Cellular Redox Homeostasis to Optimize Ethanol Production in Xylose-Fermenting Saccharomyces Cerevisiae Strains. Microbiol. Res. 2025, 290, 127955. [Google Scholar] [CrossRef] [PubMed]
- Procópio, D.P.; Lee, J.W.; Shin, J.; Tramontina, R.; Ávila, P.F.; Brenelli, L.B.; Squina, F.M.; Damasio, A.; Rabelo, S.C.; Goldbeck, R.; et al. Metabolic Engineering of Saccharomyces Cerevisiae for Second-Generation Ethanol Production from Xylo-Oligosaccharides and Acetate. Sci. Rep. 2023, 13, 19182. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.; Tafur Rangel, A.; Kerkhoven, E.J.; Nygård, Y. Engineering of Saccharomyces Cerevisiae for Enhanced Metabolic Robustness and L-Lactic Acid Production from Lignocellulosic Biomass. Metab. Eng. 2024, 84, 23–33. [Google Scholar] [CrossRef]
- Wagner, E.R.; Gasch, A.P. Advances in S. Cerevisiae Engineering for Xylose Fermentation and Biofuel Production: Balancing Growth, Metabolism, and Defense. J. Fungi 2023, 9, 786. [Google Scholar] [CrossRef]
- Duperray, M.; Delvenne, M.; François, J.M.; Delvigne, F.; Capp, J.P. Genomic and Metabolic Instability during Long-Term Fermentation of an Industrial Saccharomyces Cerevisiae Strain Engineered for C5 Sugar Utilization. Front. Bioeng. Biotechnol. 2024, 12, 1357671. [Google Scholar] [CrossRef]
- Fan, L.; Doucette, C.; McSweeney, M.B.; English, M.; Song, J.; Vinqvist-Tymchuk, M.; Kernaghan, G. Non-Traditional Yeasts from Cool-Climate Vineyards for Novel Low-Alcohol Wines. Plants People Planet 2024. [Google Scholar] [CrossRef]
- da Costa, M.V.A.; Bragança, C.R.S.; Sousa, A.C.R.; Batista, A.G.; Paula, F.E.G.M.; Cardozo, M.V.; Vargas, S.R.; Philippini, R.R. Innovative Pathways for Ethanol Production: Harnessing Xylose’s Bioenergy Potential Using Brazilian Wild Isolated Yeasts. Bioresour. Technol. 2024, 404, 130930. [Google Scholar] [CrossRef]
- Chen, L.; Li, K.; Chen, H.; Li, Z. Reviewing the Source, Physiological Characteristics, and Aroma Production Mechanisms of Aroma-Producing Yeasts. Foods 2023, 12, 3501. [Google Scholar] [CrossRef]
- Li, Q.; Du, B.; Chen, X.; Zhao, Y.; Zhu, L.; Ma, H.; Sun, B.; Hao, J.; Li, X. Microbial Community Dynamics and Spatial Distribution of Flavor Compound Metabolism during Solid-State Fermentation of Baijiu Enhanced by Wickerhamomyces Anomalus. Food Biosci. 2024, 59, 103909. [Google Scholar] [CrossRef]
- Wang, J.; Yan, J.; Gao, H.; Li, X.; Dong, Z.; Yan, S.; Shi, F. Comparative Metabolic Profiling of Cabernet Sauvignon Wines Reveals the Potential of Different Wickerhamomyces Anomalus Co-Fermented with Commercial Saccharomyces Cerevisiae. LWT 2023, 186, 115229. [Google Scholar] [CrossRef]
- Rahmayetty; Whulanza, Y.; Sukirno; Rahman, S.F.; Suyono, E.A.; Yohda, M.; Gozan, M. Use of Candida Rugosa Lipase as a Biocatalyst for L-Lactide Ring-Opening Polymerization and Polylactic Acid Production. Biocatal. Agric. Biotechnol. 2018, 16, 683–691. [Google Scholar] [CrossRef]
- Vanleeuw, E.; Winderickx, S.; Thevissen, K.; Lagrain, B.; Dusselier, M.; Cammue, B.P.A.; Sels, B.F. Substrate-Specificity of Candida Rugosa Lipase and Its Industrial Application. ACS Sustain. Chem. Eng. 2019, 7, 15828–15844. [Google Scholar] [CrossRef]
- Almulaiky, Y.Q.; Altalhi, T.; El-Shishtawy, R.M. Enhanced Catalytic Performance of Candida Rugosa Lipase through Immobilization on Zirconium-2-Methylimidazole: A Novel Biocatalyst Approach. Int. J. Biol. Macromol. 2024, 279, 135211. [Google Scholar] [CrossRef]
- Sierra-Ibarra, E.; Vargas-Tah, A.; Moss-Acosta, C.L.; Trujillo-Martínez, B.; Molina-Vázquez, E.R.; Rosas-Aburto, A.; Valdivia-López, Á.; Hernández-Luna, M.G.; Vivaldo-Lima, E.; Martínez, A. Co-Fermentation of Glucose–Xylose Mixtures from Agroindustrial Residues by Ethanologenic Escherichia Coli: A Study on the Lack of Carbon Catabolite Repression in Strain MS04. Molecules 2022, 27, 8941. [Google Scholar] [CrossRef]
- An, N.; Chen, X.; Sheng, H.; Wang, J.; Sun, X.; Yan, Y.; Shen, X.; Yuan, Q. Rewiring the Microbial Metabolic Network for Efficient Utilization of Mixed Carbon Sources. J. Ind. Microbiol. Biotechnol. 2021, 48, 40. [Google Scholar] [CrossRef]
- Kaplan, N.A.; Islam, K.N.; Kanis, F.C.; Verderber, J.R.; Wang, X.; Jones, J.A.; Koffas, M.A.G. Simultaneous Glucose and Xylose Utilization by an Escherichia Coli Catabolite Repression Mutant. Appl. Environ. Microbiol. 2024, 90, e0216923. [Google Scholar] [CrossRef]
- Dos Santos, V.C.; Bragança, C.R.S.; Passos, F.J.V.; Passos, F.M.L. Kinetics of Growth and Ethanol Formation from a Mix of Glucose/Xylose Substrate by Kluyveromyces Marxianus UFV-3. Antonie Van Leeuwenhoek Int. J. General. Mol. Microbiol. 2013, 103, 153–161. [Google Scholar] [CrossRef]
- Cristina Oliveira, A.; Fernanda Rosa, M.; Cabral, J.M.S.; Raquel Aires-Barros, M. Effect of Extraction and Enzymatic Esterification of Ethanol on Glucose Consumption by Two Saccharomyces Cerevisiae Strains: A Comparative Study. J. Chem. Technol. Biotechnol. 2001, 76, 285–290. [Google Scholar] [CrossRef]
- Slininger, P.J.; Thompson, S.R.; Weber, S.; Liu, Z.L.; Moon, J. Repression of Xylose-Specific Enzymes by Ethanol in Scheffersomyces (Pichia) Stipitis and Utility of Repitching Xylose-Grown Populations to Eliminate Diauxic Lag. Biotechnol. Bioeng. 2011, 108, 1801–1815. [Google Scholar] [CrossRef]
- Koh, H.G.; Yook, S.; Oh, H.; Rao, C.V.; Jin, Y.S. Toward Rapid and Efficient Utilization of Nonconventional Substrates by Nonconventional Yeast Strains. Curr. Opin. Biotechnol. 2024, 85, 103059. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Long, H.; Jiang, G.; Gong, X.; Yu, Z.; Huang, M.; Guan, T.; Guan, Y.; Liu, X. Analysis of the Ethanol Stress Response Mechanism in Wickerhamomyces Anomalus Based on Transcriptomics and Metabolomics Approaches. BMC Microbiol. 2022, 22, 275. [Google Scholar] [CrossRef] [PubMed]
- Padilla, B.; Gil, J.V.; Manzanares, P. Challenges of the Non-Conventional Yeast Wickerhamomyces Anomalus in Winemaking. Fermentation 2018, 4, 68. [Google Scholar] [CrossRef]
- Cappelli, A.; Favia, G.; Ricci, I. Wickerhamomyces Anomalus in Mosquitoes: A Promising Yeast-Based Tool for the “Symbiotic Control” of Mosquito-Borne Diseases. Front. Microbiol. 2021, 11, 621605. [Google Scholar] [CrossRef]
- de Freitas, M.d.F.M.; Cavalcante, L.S.; Gudiña, E.J.; Silvério, S.C.; Rodrigues, S.; Rodrigues, L.R.; Gonçalves, L.R.B. Sustainable Lipase Production by Diutina Rugosa NRRL Y-95 Through a Combined Use of Agro-Industrial Residues as Feedstock. Appl. Biochem. Biotechnol. 2021, 193, 589–605. [Google Scholar] [CrossRef]
- Perera, I.A.; Abinandan, S.; Panneerselvan, L.; Subashchandrabose, S.R.; Venkateswarlu, K.; Naidu, R.; Megharaj, M. Co-Culturing of Microalgae and Bacteria in Real Wastewaters Alters Indigenous Bacterial Communities Enhancing Effluent Bioremediation. Algal Res. 2022, 64, 102705. [Google Scholar] [CrossRef]
- Farias, D.; Maugeri Filho, F. Co-Culture Strategy for Improved 2G Bioethanol Production Using a Mixture of Sugarcane Molasses and Bagasse Hydrolysate as Substrate. Biochem. Eng. J. 2019, 147, 29–38. [Google Scholar] [CrossRef]
- Gharechahi, J.; Vahidi, M.F.; Sharifi, G.; Ariaeenejad, S.; Ding, X.Z.; Han, J.L.; Salekdeh, G.H. Lignocellulose Degradation by Rumen Bacterial Communities: New Insights from Metagenome Analyses. Environ. Res. 2023, 229, 115925. [Google Scholar] [CrossRef]
- Jia, M.; Zhu, S.; Xue, M.Y.; Chen, H.; Xu, J.; Song, M.; Tang, Y.; Liu, X.; Tao, Y.; Zhang, T.; et al. Single-Cell Transcriptomics across 2,534 Microbial Species Reveals Functional Heterogeneity in the Rumen Microbiome. Nat. Microbiol. 2024, 9, 1884–1898. [Google Scholar] [CrossRef]
- Novak, S.; D’Amore, T.; Russell, I.; Stewart, G.G. Sugar Uptake in a 2-Deoxy-d-Glucose Resistant Mutant of Saccharomyces Cerevisiae. J. Ind. Microbiol. 1991, 7, 35–39. [Google Scholar] [CrossRef]
- Lane, S.; Xu, H.; Oh, E.J.; Kim, H.; Lesmana, A.; Jeong, D.; Zhang, G.; Tsai, C.S.; Jin, Y.S.; Kim, S.R. Glucose Repression Can Be Alleviated by Reducing Glucose Phosphorylation Rate in Saccharomyces Cerevisiae. Sci. Rep. 2018, 8, 2613. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.; won Kim, J.; Ye, S.; Kim, S.; Jeong, D.; Lee, D.Y.; Kim, J.N.; Jin, Y.S.; Kim, K.H.; Kim, S.R. Comparative Global Metabolite Profiling of Xylose-Fermenting Saccharomyces Cerevisiae SR8 and Scheffersomyces Stipitis. Appl. Microbiol. Biotechnol. 2019, 103, 5435–5446. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, S.C.; Soares, L.B.; Biazi, L.E.; Nascimento, V.M.; Costa, A.C.; Rocha, G.J.M.; Ienczak, J.L. Fermentation Strategy for Second Generation Ethanol Production from Sugarcane Bagasse Hydrolyzate by Spathaspora Passalidarum and Scheffersomyces Stipitis. Biotechnol. Bioeng. 2017, 114, 2211–2221. [Google Scholar] [CrossRef]
- Acevedo, A.; Conejeros, R.; Aroca, G. Ethanol Production Improvement Driven by Genome-Scale Metabolic Modeling and Sensitivity Analysis in Scheffersomyces Stipitis. PLoS ONE 2017, 12, e0180074. [Google Scholar] [CrossRef]
- Phommachan, K.; Keo-Oudone, C.; Nurcholis, M.; Vongvilaisak, N.; Chanhming, M.; Savanhnaly, V.; Bounphanmy, S.; Matsutani, M.; Kosaka, T.; Limtong, S.; et al. Adaptive Laboratory Evolution for Multistress Tolerance, Including Fermentability at High Glucose Concentrations in Thermotolerant Candida Tropicalis. Energies 2022, 15, 561. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Z.; Wang, J.; Shen, W.; Li, Q.; Chen, X. Stepwise Metabolic Engineering of Candida Tropicalis for Efficient Xylitol Production from Xylose Mother Liquor. Microb. Cell Fact. 2021, 20, 105. [Google Scholar] [CrossRef]
- Singh, A.K.; Deeba, F.; Kumar, M.; Kumari, S.; Wani, S.A.; Paul, T.; Gaur, N.A. Development of Engineered Candida Tropicalis Strain for Efficient Corncob-Based Xylitol-Ethanol Biorefinery. Microb. Cell Fact. 2023, 22, 201. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Sung, B.H. Isolation and Characterization of the Stress-Tolerant Candida Tropicalis YHJ1 and Evaluation of Its Xylose Reductase for Xylitol Production from Acid Pretreatment Wastewater. Front. Bioeng. Biotechnol. 2019, 7, 464705. [Google Scholar] [CrossRef]
- Unrean, P.; Khajeeram, S. Model-Based Optimization of Scheffersomyces Stipitis and Saccharomyces Cerevisiae Co-Culture for Efficient Lignocellulosic Ethanol Production. Bioresour. Bioprocess. 2015, 2, 41. [Google Scholar] [CrossRef]
- Mastella, L.; Senatore, V.; Beltrani, T.; Branduardi, P. Scheffersomyces Stipitis Ability to Valorize Different Residual Biomasses for Vitamin B9 Production. Microb. Biotechnol. 2023, 16, 392–403. [Google Scholar] [CrossRef]
- Lohmeier-Vogel, E.M.; Bä, B.; Hahn-Hägerdal, B.; Hägerdal, H.; Vogel, H.J. Phosphorus-31 and Carbon-13 Nuclear Magnetic Resonance Study of Glucose and Xylose Metabolism in Agarose-Immobilized Candida Tropicalis. Appl. Environ. Microbiol. 1995, 61, 1420–1425. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Jin, M.; Huang, M.; Zhu, Y.; Yuan, W.; Kang, Y.; Kong, M.; Ali, S.; Jia, Z.; Xu, Z.; et al. Engineered Polyploid Yeast Strains Enable Efficient Xylose Utilization and Ethanol Production in Corn Hydrolysates. Front. Bioeng. Biotechnol. 2021, 9, 655272. [Google Scholar] [CrossRef] [PubMed]
- Wei, F.; Li, M.; Wang, M.; Li, H.; Li, Z.; Qin, W.; Wei, T.; Zhao, J.; Bao, X. A C6/C5 Co-Fermenting Saccharomyces Cerevisiae Strain with the Alleviation of Antagonism between Xylose Utilization and Robustness. GCB Bioenergy 2021, 13, 83–97. [Google Scholar] [CrossRef]
- Kastner, J.R.; Jones, W.J.; Roberts, R.S. Ethanol Fermentation of Mixed-Sugars Using a Two-Phase, Fed-Batch Process: Method to Minimize D-Glucose Repression of Candida Shehatae D-Xylose Fermentations. J. Ind. Microbiol. Biotechnol. 1999, 22, 65–70. [Google Scholar] [CrossRef]
- Ruchala, J.; Sibirny, A.A. Pentose Metabolism and Conversion to Biofuels and High-Value Chemicals in Yeasts. FEMS Microbiol. Rev. 2021, 45, fuaa069. [Google Scholar] [CrossRef]
- Nijland, J.G.; Driessen, A.J.M. Engineering of Pentose Transport in Saccharomyces Cerevisiae for Biotechnological Applications. Front. Bioeng. Biotechnol. 2020, 7, 512089. [Google Scholar] [CrossRef]
- Chroumpi, T.; Peng, M.; Aguilar-Pontes, M.V.; Müller, A.; Wang, M.; Yan, J.; Lipzen, A.; Ng, V.; Grigoriev, I.V.; Mäkelä, M.R.; et al. Revisiting a ‘Simple’ Fungal Metabolic Pathway Reveals Redundancy, Complexity and Diversity. Microb. Biotechnol. 2021, 14, 2525–2537. [Google Scholar] [CrossRef]
- Nijland, J.G.; Shin, H.Y.; De Jong, R.M.; De Waal, P.P.; Klaassen, P.; Driessen, A.J.M. Engineering of an Endogenous Hexose Transporter into a Specific D-Xylose Transporter Facilitates Glucose-Xylose Co-Consumption in Saccharomyces Cerevisiae. Biotechnol. Biofuels 2014, 7, 168. [Google Scholar] [CrossRef]
- Young, E.M.; Tong, A.; Bui, H.; Spofford, C.; Alper, H.S. Rewiring Yeast Sugar Transporter Preference through Modifying a Conserved Protein Motif. Proc. Natl. Acad. Sci. USA 2014, 111, 131–136. [Google Scholar] [CrossRef]
- Martínez, A.A.; Conboy, A.; Buskirk, S.W.; Marad, D.A.; Lang, G.I. Long-Term Adaptation to Galactose as a Sole Carbon Source Selects for Mutations Outside the Canonical GAL Pathway. J. Mol. Evol. 2023, 91, 46–59. [Google Scholar] [CrossRef]
- Ma, X.Y.; Coleman, B.; Prabhu, P.; Yang, M.; Wen, F. Engineering Compositionally Uniform Yeast Whole-Cell Biocatalysts with Maximized Surface Enzyme Density for Cellulosic Biofuel Production. ACS Synth. Biol. 2024, 13, 1225–1236. [Google Scholar] [CrossRef] [PubMed]
- Fentahun, M.; Andualem, B. Optimization of Bioethanol Production Using Stress-Tolerant Yeast Strains Isolated from Household Alcoholic Beverages (Tella, Tej, and Areke) and Molasses (as Substrate). F1000Research 2024, 13, 286. [Google Scholar] [CrossRef]
- Patané, A.; Jansen, G.; Conca, P.; Carapezza, G.; Costanza, J.; Nicosia, G. Multi-Objective Optimization of Genome-Scale Metabolic Models: The Case of Ethanol Production. Ann. Oper. Res. 2019, 276, 211–227. [Google Scholar] [CrossRef]
- AbdElhafez, S.E.; Taha, T.; Mansy, A.E.; El-Desouky, E.; Abu-Saied, M.A.; Eltaher, K.; Hamdy, A.; El Fawal, G.; Gamal, A.; Hashim, A.M.; et al. Experimental Optimization with the Emphasis on Techno-Economic Analysis of Production and Purification of High Value-Added Bioethanol from Sustainable Corn Stover. Energies 2022, 15, 6131. [Google Scholar] [CrossRef]
- Milessi, T.S.; Silva, C.R.; Moraes, G.S.; Aquino, P.M.; Giordano, R.C.; Giordano, R.L.C.; Zangirolami, T.C. Continuous 2G Ethanol Production from Xylose in a Fixed-Bed Reactor by Native Saccharomyces Cerevisiae Strain Through Simultaneous Isomerization and Fermentation. Cellulose 2020, 27, 4429–4442. [Google Scholar] [CrossRef]
- Moimenta, A.R.; Troitiño-Jordedo, D.; Henriques, D.; Contreras-Ruíz, A.; Minebois, R.; Morard, M.; Barrio, E.; Querol, A.; Balsa-Canto, E.; Zhang, Y. An Integrated Multiphase Dynamic Genome-Scale Model Explains Batch Fermentations Led by Species of the Saccharomyces Genus. mSystems 2025, 10, e0161524. [Google Scholar] [CrossRef]
- Yang, P.; Jiang, S.; Lu, S.; Jiang, S.; Jiang, S.; Deng, Y.; Lu, J.; Wang, H.; Zhou, Y. Ethanol Yield Improvement in Saccharomyces Cerevisiae GPD2 Delta FPS1 Delta ADH2 Delta DLD3 Delta Mutant and Molecular Mechanism Exploration Based on the Metabolic Flux and Transcriptomics Approaches. Microb. Cell Fact. 2022, 21, 160. [Google Scholar] [CrossRef]
- Du, Q.; Liu, Y.; Song, Y.; Qin, Y. Creation of a Low-Alcohol-Production Yeast by a Mutated SPT15 Transcription Regulator Triggers Transcriptional and Metabolic Changes During Wine Fermentation. Front. Microbiol. 2020, 11, 597828. [Google Scholar] [CrossRef]
- Naghshbandi, M.P.; Tabatabaei, M.; Aghbashlo, M.; Gupta, V.K.; Sulaiman, A.; Karimi, K.; Moghimi, H.; Maleki, M. Progress toward Improving Ethanol Production through Decreased Glycerol Generation in Saccharomyces Cerevisiae by Metabolic and Genetic Engineering Approaches. Renew. Sustain. Energy Rev. 2019, 115, 109353. [Google Scholar] [CrossRef]
- Mesquita, T.J.B.; Sargo, C.R.; Fuzer, J.R.; Paredes, S.A.H.; Giordano, R.D.C.; Horta, A.C.L.; Zangirolami, T.C. Metabolic Fluxes-Oriented Control of Bioreactors: A Novel Approach to Tune Micro-Aeration and Substrate Feeding in Fermentations. Microb. Cell Fact. 2019, 18, 150. [Google Scholar] [CrossRef]
- Phukoetphim, N.; Chan-U-Tit, P.; Laopaiboon, P.; Laopaiboon, L. Improvement of Bioethanol Production from Sweet Sorghum Juice under Very High Gravity Fermentation: Effect of Nitrogen, Osmoprotectant, and Aeration. Energies 2019, 12, 3620. [Google Scholar] [CrossRef]
- Samakkarn, W.; Ratanakhanokchai, K.; Soontorngun, N. Reprogramming of the Ethanol Stress Response in Saccharomyces Cerevisiae by the Transcription Factor Znf1 and Its Effect on the Biosynthesis of Glycerol and Ethanol. Appl. Environ. Microbiol. 2021, 87, AEM0058821. [Google Scholar] [CrossRef] [PubMed]
- Udom, N.; Chansongkrow, P.; Charoensawan, V.; Auesukaree, C. Coordination of the Cell Wall Integrity and Highosmolarity Glycerol Pathways in Response to Ethanol Stress in Saccharomyces Cerevisiae. Appl. Environ. Microbiol. 2019, 85, e00551-19. [Google Scholar] [CrossRef] [PubMed]



| Condition 1 | Ethanol Yield (YP/S) (g/g) | Volumetric Productivity (QP) (g/L·h) | Sugar Consumption Rate (QS) (g/L·h) | Biomass Yield (YX/S) (g/g) | Specific Ethanol Production Rate (QP/CDW) (g/g·h) |
|---|---|---|---|---|---|
| Best | 0.45 | 0.55 | 1.18 | 0.02 | 0.48 |
| Worst | 0.22 | 0.14 | 0.48 | 0.01 | 0.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batista, A.G.; da Costa, M.V.A.; Cardozo, M.V.; Vargas, S.R.; Pereira, M.G.; D’Ávila, V.d.A.; Coelho, J.J.; Bragança, C.R.S. Beyond Saccharomyces: Exploring the Bioethanol Potential of Wickerhamomyces anomalus and Diutina rugosa in Xylose and Glucose Co-Fermentation. Fermentation 2025, 11, 204. https://doi.org/10.3390/fermentation11040204
Batista AG, da Costa MVA, Cardozo MV, Vargas SR, Pereira MG, D’Ávila VdA, Coelho JJ, Bragança CRS. Beyond Saccharomyces: Exploring the Bioethanol Potential of Wickerhamomyces anomalus and Diutina rugosa in Xylose and Glucose Co-Fermentation. Fermentation. 2025; 11(4):204. https://doi.org/10.3390/fermentation11040204
Chicago/Turabian StyleBatista, Arthur Gasetta, Marcus Vinicius Astolfo da Costa, Marita Vedovelli Cardozo, Sarah Regina Vargas, Marita Gimenez Pereira, Vinícius de Abreu D’Ávila, Janerson José Coelho, and Caio Roberto Soares Bragança. 2025. "Beyond Saccharomyces: Exploring the Bioethanol Potential of Wickerhamomyces anomalus and Diutina rugosa in Xylose and Glucose Co-Fermentation" Fermentation 11, no. 4: 204. https://doi.org/10.3390/fermentation11040204
APA StyleBatista, A. G., da Costa, M. V. A., Cardozo, M. V., Vargas, S. R., Pereira, M. G., D’Ávila, V. d. A., Coelho, J. J., & Bragança, C. R. S. (2025). Beyond Saccharomyces: Exploring the Bioethanol Potential of Wickerhamomyces anomalus and Diutina rugosa in Xylose and Glucose Co-Fermentation. Fermentation, 11(4), 204. https://doi.org/10.3390/fermentation11040204







