Abstract
In order to secure domestic microbial resources, a fruit-flavored yeast was selected, and a starter manufacturing technology was developed to evaluate its quality characteristics. Among 26 yeast strains analyzed using an electronic nose, Saccharomyces cerevisiae YM45 isolated from Makgeolli was identified as having high levels of methyl formate and propan-2-one, compounds that are associated with fruity and sweet aroma. A powdered starter was then produced with S. cerevisiae YM45, and its quality characteristics were analyzed. When cyclodextrin was used as an excipient, with water content at 4.7%, bacterial contamination was found to be 2.30 log CFU/g, which ensures quality and safety. The rehydration rate, assessed using a PBS buffer, showed a high survival rate of 8.7 log CFU/g, which is a suitable condition for preserving yeast activity. These findings suggest that domestically sourced microbial resources can serve as viable alternatives to imported strains, with potential applications in industrial fermentation.
1. Introduction
With the ‘Nagoya Protocol’ in effect, it is necessary to find ways to manage and nurture microbial resources that are national assets. Although the global microbial market is expanding, South Korea’s market—particularly in agriculture and fermented foods—remains limited and depends heavily on imported starter culture [1]. Furthermore, biological resources, including microorganisms, are subject to high royalty fees when foreign biological resources are used due to regulations restricting movement between countries. As a result, interest in discovering and securing fermented species to minimize the impact on the domestic microbial industry is increasing [2,3].
Among the various microorganisms that require resource utilization, yeast is classified into the genera Saccharomyces and Non-Saccharomyces (Phichia, Candida, Hansenular, etc.). The genus Saccharomyces is the most representative, and most of the yeasts involved in ethanol production for alcohol manufacturing today belong to this genus [4,5,6]. Alcohol is a fermented product made from carbohydrates and decomposed by microorganisms and is consisted of various compounds, including ethanol. Among the different types of alcohol, fermented alcoholic beverages, also known as brewed liquor, can be categorized into single fermentation beverages, such as wine and apple cider, which ferment the sugars naturally present in the raw materials, and multiple fermentation beverages, such as Takju, Yakju, and beer, which ferment starch by saccharifying it with saccharification enzyme [7]. In this process, yeast plays a role as a starter for alcoholic fermentation through sugar decomposition during the fermentation of multiple fermentation beverages [8,9].
During the fermentation process of alcoholic beverages, yeast not only converts sugar into alcohol but also plays an important role in the production of volatile flavor compounds and metabolites. The characteristics of fermented beverages are known to vary depending on the enzymes produced by yeast [10]. Yeasts have been used throughout human history; previous studies have reported that the alcohol quality can be enhanced by yeast strains with strong aroma-producing capabilities [11]. Aromatic compounds are particularly important in brewing as they influence the characteristics of the beverages such as wine, beer, sake, etc. While wine is traditionally made from grapes, varieties such as Chardonnay exhibit fruity aromas reminiscent of apples, pears, and peaches, whereas Riesling wines emphasize floral notes [12]. In beer, yeast plays a key role in fermentation, producing aromas like banana and apple, particularly in Belgian ales [13]. Similarly, sake, made from rice and water, can acquire fruity aromas (such as apple, peach, or pear) or floral notes (like jasmine or rose) depending on the yeast used, and these characteristics are often associated with premium sake [14]. The aromas produced by yeast and microorganisms, particularly through the formation of compounds like esters, alcohols, and aldehydes, highlight the important role yeast plays in the overall flavor profile.
Therefore, in this study, fruit-flavored yeasts were selected to identify and develop suitable strains for brewing, aiming to reduce dependence on imported strains and promote a stable supply of domestically sourced strains. To industrially utilize S. cerevisiae, which is mainly used for brewing, a formulation process is typically applied to enhance its viability and storability. Yeast formulations are produced in both solid and liquid forms, with dry powdered products being the most commonly used in the industry [15]. Based on this, the goal was to select an excipient suitable for maintaining activity during preservation in order to manufacture the selected fruit-flavored yeast in powder form. The suitability of the excipient types as a starter culture was investigated.
2. Materials and Methods
2.1. Strains and Culture Conditions
The yeast strains used in this study consisted of twenty-six strains held by this research team, including twenty-three Saccharomyces strains and three non-Sacccharomyces, as shown in Table 1. These strains were isolated from domestically produced fermentation agents or fermented foods in Korea, with the aim of securing them as resources from indigenous strains. These strains were subcultured on YPD agar medium (BD Difco Co., Detroit, MI, USA) at 30 °C to activate them and then used in the experiments.

Table 1.
List of yeast strains and ethanol production.
2.2. Alcohol Production
To confirm suitability for brewing manufacturing, each activated yeast strain was inoculated into 5 mL of YPD broth medium using a single inoculation loop and cultured at 30 °C, 140 rpm for 24 h. Then, it was inoculated at 1% (v/v) into 100 mL of YPD broth adjusted to 24 °Brix and cultured again at 30 °C, 140 rpm for 5 days. The alcohol produced was then measured. Specifically, 100 mL of the sample was distilled with water to obtain 80 mL of distilled solution, and then the alcohol content was analyzed using a density meter (AT/DMA 500 M, Anton Paar, Graz, Austria) (Table 1).
2.3. Determination of Fruit-Flavored Yeast
To select yeast strains capable of producing fruit flavors among the twenty-six tested strains, colonies were recovered from the medium, and 3 mL of the sample solution suspended in distilled water was placed in a 20 mL headspace vial for electronic nose analysis. The volatile compounds were saturated in the vial while stirring at 40 °C for 20 min at 500 rpm, followed by analysis using an electronic nose (Heracles NEO, Alpha Mos, Toulouse, France). The analysis was conducted using a Heracles E-nose (MXT-5 and MXT-1701), which is equipped with two columns of different polarities. The temperature of the oven was maintained at 50 °C for 2 s, then increased to 80 °C at 1.0 °C/s and to 250 °C at a rate of 3 °C/s, where it was maintained for 21 s [16,17]. Compounds were identified based on Kovat’s index library, and the compounds of the separated peaks were identified using AroChemBase 4.7.0, a module within the AlphaSoft 7.2.8 programs provided by Alpha MOS. Data statistical processing was also represented by principal component analysis (PCA) and SIMCA plot using AlphaSoft. A total of six repetitions were analyzed, and four repetitions of data with high reproducibility were used for analysis.
2.4. Optimal Culture Conditions
The culture temperature was set to 20, 25, 30, 35, and 45 °C, respectively, and the optimal temperature was determined by comparing the amount of yeast cells produced after culturing for 3 days at 140 rpm. Yeast was inoculated at 1% (v/v) into YPD broth. To enhance the yeast cell production, five nitrogen sources were tested as substitutes for peptone, and ten carbon sources were evaluated as alternatives to dextrose within the standard YPD medium composition (Yeast extract 1%, Peptone 2%, Dextrose 2%). The optimal cultivation conditions were established using each of these media (Table 2). The cultivation conditions were the same as described above. The cell recovery rate was determined by centrifuging each culture broth at 5 °C, 6000 rpm, for 10 min, followed by removing the supernatant and measuring the cell weight, which was expressed in % (g/g) units.

Table 2.
Addition of nitrogen and carbon sources to set of medium composition.
2.5. Excipients
Freeze-drying during the powdered starter production process is known as the most effective method for preserving bacteria, yeast, and spore-forming fungi. However, the quantitative viability of the dried strains has been reported to be low, around 0.1% [18]. Therefore, based on previous research that suggested using sugars, albumin, milk, honey, and other drying protectants to enhance the drying protection effect during freeze-drying, efforts were made to compensate for the loss [19]. There are previous reports stating that monosaccharides and disaccharides, which are known to effectively protect cells while ensuring uniform treatment of drying protectants, are mainly used [20,21]. Based on these results, skim milk, maltodextrin, cyclodextrin, and lactomeal were used [22,23]. At this time, Lactomeal is made by mixing 89% lactose and 11% maltodextrin [24].
2.6. Manufacturing of Powdered Starter
Based on the selected optimal cultivation conditions, 2000 L of yeast culture was cultivated in a 5000 L fermenter for 3 days with stirring at 60 rpm, an aeration rate of 800 L/min, and an internal pressure of less than 0.2 kgf/cm2. The culture solution was centrifuged at 7400 rpm and 1000 L/h to recover the yeast cells. Four different excipients (skim milk, maltodextrin, cyclodextrin, and lactomeal) were mixed at a ratio of 1:1 (w/w), respectively, and the mixture for each excipient was freeze-dried. Freeze-drying was performed for 3 days at a vacuum level of 500 mTorr or lower and at temperatures of −53 °C or lower. It was ground at 1000 rpm, 80 mesh conditions to produce powdered solid starter (Figure 1). The quality characteristics of the starter for each excipient were then analyzed and compared.
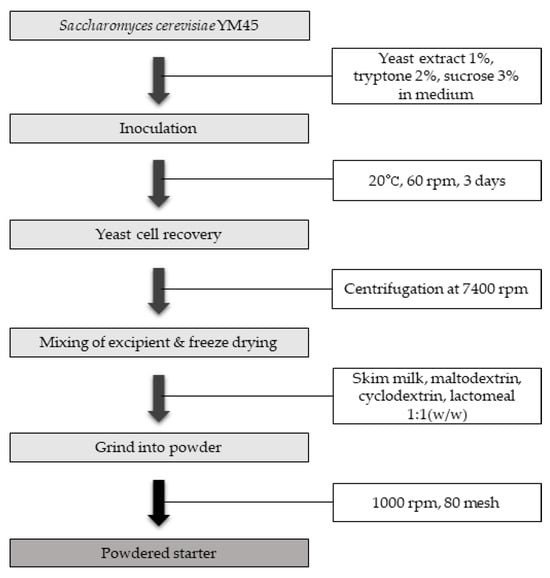
Figure 1.
Manufacturing process of powder starter formulation using fruit-flavored S. cerevisiae YM45.
2.7. Quality Characteristics of Powdered Starter
The quality characteristics of the powdered starters made with different excipients were analyzed by measuring the moisture content using a moisture content meter (MX-50, And Co., Tokyo, Japan) and assessing bacterial contamination using Petrifilm (Petrifilm, 3 M, Maplewood, MN, USA) [25]. To evaluate the activity of the starters under different rehydration conditions, PBS buffer solution, 0.85% NaCl solution, and peptone water (peptone 1%, NaCl 0.05%) were used as rehydration solutions, while sterile distilled water was used as the control group [24]. After rehydrating at 25 °C for 1 h, the survival rate of yeast was determined by measuring the viable cell count using an automatic yeast counter (LUNA-II Automated Cell Counter, Logos Biosystems Inc., Anyang, Republic of Korea).
2.8. Statistical Analysis
The experiments were conducted in triplicate, and the mean values were reported. The results were statistically analyzed by one-way analysis of variance (ANOVA) with Duncan’s test (p < 0.05) using SPSS (ver. 27.0, SPSS Inc., Chicago, IL, USA).
3. Results
3.1. Selection of Fruit-Flavored Yeast
The volatile compounds of twenty-six yeast strains were analyzed, and similar groups were identified through principal component analysis (PCA). The results are shown in Figure 2a. The first principal component accounted for 97.820%, while the second principal component contributed 1.205%, resulting in a total cumulative 99.025%, and classified based on PC1. Therefore, after classifying the twenty-three strains, and excluding the yeast strains YM44, YM47, and YM48, which are positioned in the negative direction as similar groups, a qualitative sniffing test was conducted with reference to the reported research method [26]. Trained panelists directly smelled the colonies on the medium, and based on their collective evaluation of the aromas, the yeast strain NR-09 was selected as the one that most closely resembled a fruity aroma. Based on this, a SIMCA plot analysis was conducted to examine how each yeast clustered and to assess their similarities or differences, as shown in Figure 2b. First, NR-09, described as having a fruit aroma, was modeled to visualize whether it clustered with other yeasts exhibiting similar characteristics. The results showed that 13 yeast strains, including YM4, YM33, YM34, YM38, YM40, YM45, YM50, YM54, YM58, CP-2, D5-P5, NR-06, and NR-09, contained similar aromatic components.
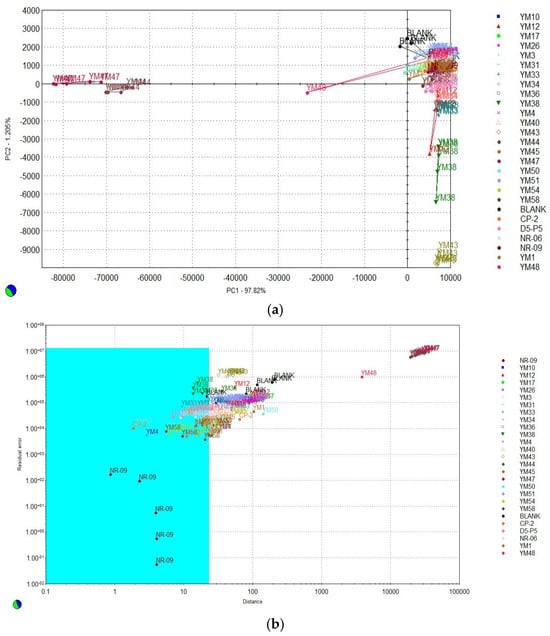
Figure 2.
Selection of fruit-flavored yeast strains using an electronic nose: (a) principal component analysis (PCA) of twenty-six strains; (b) SIMCA plot analysis of the twenty-three classified strains.
The top fifteen volatile components with the highest intensity, among the main volatile compounds detected in the thirteen yeast strains isolated for a similar flavor, are listed in Table 3. The sensory descriptors identified components representing fruity-flavored, including methyl formate, propan-2-one, methyl acetate, and dimethyl sulfide. The methyl formate component, which describes a fruity aroma, was measured at 66.6 in the reference yeast NR-09 but showed higher levels of 75.1 and 73.5 in YM45 and YM40, respectively. In addition, propan-2-one, which is associated with fruit aromas such as apple, fruity, pear, and sweet, was detected at levels exceeding 80 in most samples, indicating a strong presence. Among them, YM4 and YM50 were measured at 92.6 and 92.9, respectively, which are the highest levels, suggesting that these yeasts strongly exhibit a fruit-flavored. The propan-2-one component, which showed particularly high levels of detection, was identified to be fruit-flavored yeast based on previous research analyzing the volatile components of coffee beans using an electronic nose. In that study, the detection of propan-2-one led to the classification of the beans as having fruity and sweet aromas [27]. However, methanol, which should be minimized in yeast-based alcoholic beverage production, was detected at relatively low levels, 31.3% in YM45 and 34.7 in YM38 [28]. These results suggested the potential for use in alcoholic beverage production. On the other hand, although yeasts such as YM50, CP-2, D5-P5, and NR-06 were measured at relatively high levels of methyl formate and propan-2-one, which describe fruit-flavored, methanol was also detected at levels of 60.0 or higher, making them unsuitable for alcoholic beverage production.

Table 3.
Volatile compounds detected in thirteen identified yeast strains using an electronic nose.
Based on these results, this study aimed to select yeasts that had a high fruit-flavored index and were considered suitable for use in alcoholic beverages. Therefore, based on the alcohol production ability results of each yeast presented in Table 1, yeasts with high levels of fruit-flavored indicators and reduced methanol levels were selected, focusing on those that produced an alcohol content of 10% or higher. As a result, Saccharomyces cerevisiae YM34, YM38, and YM45 were selected.
3.2. Determination of Optimal Culture Conditions
3.2.1. Culture Temperature
To determine the optimal culture conditions for S. cerevisiae YM34, YM38 and YM45, which was selected as a strong fruit-flavored property, the cell production rate was assessed at various incubation temperatures to evaluate its suitability for starter production (Figure 3a, Table S1). Both YM34 and YM38 showed higher cell production rates at 35 °C, with cell recovery rates of 1.7% and 1.6%, respectively, compared to 1.5% at 30 °C. Both yeast strains were measured at 0.8% at 20 °C and 0.5% at 45 °C, indicating that their growth abilities are somewhat inhibited at low and high temperatures. In contrast, yeast strain YM45 showed the highest cell recovery rate of 1.7% at the cultivation temperature of 20 °C within the range of 20–45 °C, suggesting that it maintains activity at low temperatures. Based on previous research findings, these results confirmed that the YM45 has excellent growth ability at low temperatures [29]. Given the reported benefits of low-temperature fermentation, including increased volatile compound production and stable fermentation, selecting yeasts with both strong fruit-flavor production and low-temperature adaptability is expected to enhance the fruitness of alcoholic beverages.
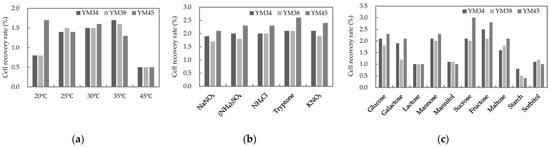
Figure 3.
Optimal culture conditions for fruit-flavored S. cerevisiae YM34, YM38 and YM45: (a) culture temperature; (b) nitrogen source availability in medium; (c) carbon source availability in medium.
3.2.2. Nitrogen and Carbon Source Availability in Medium
To increase the cell production ability based on the utilization of nitrogen and carbon sources, the culture was carried out with different medium compositions. Based on the selected culture temperatures for each strain, S. cerevisiae YM34 and YM38 were cultured at 35 °C, while YM45 was cultured at 20 °C to confirm the cell production ability with different medium compositions. In the media prepared with five different nitrogen sources, the YM34 strain showed the highest cell recovery rate of 2.1% with tryptone and potassium nitrate (KNO3). The yeast strain YM38 showed a cell recovery rate with potassium nitrate (KNO3) (2.1%) and ammonium chloride (NH4Cl) (2.0%), respectively. The yeast strain YM45 showed a cell recovery rate of tryptone (2.6%), potassium nitrate (KNO3) (2.4%), ammonium chloride (NH4Cl) (2.3%), ammonium sulfate ((NH4)2SO4) (2.3%), and sodium nitrate (NaNO3) (2.1%), in that order. These results showed that the yeast strain YM45 exhibited higher cell recovery rates under all nitrogen source conditions compared to the YM34 and YM38 (Figure 3b, Table S2). According to previous reports, when measuring the yeast cell growth with various nitrogen sources, both tryptone and casitone measured equally good growth. However, due to its economic advantages, tryptone was ultimately selected as the final nitrogen source [30]. These previous research findings align with the results of this study, where the highest cell production ability (2.6%) was observed with tryptone among the nitrogen sources.
The cell production ability was tested using media composed of ten different carbon sources. The results showed that both yeast strains, YM34 and YM38, exhibited the highest recovery rates with fructose, measuring 2.5% and 2.1%, respectively. Similarly to the nitrogen source utilization results, yeast strain YM45 showed high cell recovery rates under a variety of carbon source conditions. It was measured in the order of sucrose (3.0%), fructose (2.8%), glucose (2.3%), mannose (2.3%), galactose (2.1%), and maltose (2.1%), confirming relatively high cell recovery rates (Figure 3c, Table S3). In particular, the yeast strain YM45 showed the highest cell recovery rate of 3.0% when utilizing sucrose among the 10 carbon sources, suggesting that it could potentially increase cell production during powdered starter production, thereby expanding its usability. These results are consistent with previous research, where the growth ability of yeast was compared with twenty-six different carbon sources added to the medium. Although the highest activity was observed with sucrose and glucose, sucrose, which is commercially more widely used, was ultimately selected, confirming the significance of the findings [31].
Therefore, among the yeasts with high fruit-flavored indicators, S. cerevisiae YM45, which demonstrated excellent utilization abilities based on nitrogen and carbon source compositions, was selected as the strain for producing powdered starter in this study. This strain was registered at the National Institute of Agricultural Sciences (KACC) as S. cerevisiae YM45 (KACC 93408P) and subsequently used. Based on these findings, the optimal culture conditions for the fruit-flavored yeast S. cerevisiae YM45 were established using a medium containing Yeast extract (1%), tryptone (2%), and sucrose (2%) (YTS medium) at 20 °C. To further enhance cell recovery and improve starter culture productivity, additional nutrients were incorporated based on previous studies: 0.16% MgSO4, 0.90% (NH4)2SO4, 0.45% KH2PO4, and 0.04% CaCl2.These modifications were implemented to enhance cell production for large-scale industrial cultivation [32,33].
3.3. Quality Characteristics of Powdered Starter by Excipient
During the production of the powdered starter, the freeze-drying process was carried out with the addition of four types of excipients as drying protectants. The excipients connect water molecules around protein molecules to maintain hydrogen bonds during the drying process while preventing protein degradation [34]. In this way, to effectively protect the cells and maintain the yeast activity, skim milk, maltodextrin, cyclodextrin, and lactomeal were used as excipients.
The moisture content, microbial contamination, and rehydration rate of four types of powdered starters were analyzed to assess their quality characteristics (Table 4). The moisture content of S. cerevisiae YM45 powdered starters was measured at 4.15% and 4.16% when skim milk and lactomeal were used as excipients, respectively. When maltodextrin and cyclodextrin were used, the moisture content was 4.4% and 4.7%, respectively, with all values falling within a similar range. These results were consistent with the previous studies, where moisture content varied between 2% and 6%, depending on the drying method used in powdered starter production, confirming their suitability of quality as a powdered starter [35]. To ensure the safety of the manufactured powdered starters, bacterial contamination levels were assessed. All four types of excipients showed similar contamination levels, ranging from 2.30 to 2.57 log CFU/g. In particular, cyclodextrin resulted in the lowest bacterial contamination at 2.30 log CFU/g. According to microbiological safety standards, which require a general bacterial count of 6 log CFU/g or lower, all four types of powdered starters were deemed safe for use as food ingredients [36,37].

Table 4.
Quality characteristics of powdered starter by excipients.
Since powdered starters undergo a drying process, rehydration is essential to activate dried microorganisms. Selecting an appropriate rehydration solution is crucial to enhance the survival rate of the dried cells [38,39]. A previous study reported that rehydration for 1 h results in a higher survival rate compared to immediate use (0 h) [40]. Following this finding, rehydration was carried out for 1 h using each rehydration solution, after which the viable cell count was measured to assess the survival rate. As a result, when PBS buffer and 0.85% NaCl were used as rehydration solutions, both powdered starters showed viable cell counts exceeding 8 log CFU/g. Among them, the cyclodextrin-based starter rehydrated with PBS buffer exhibited the highest survival rate at 8.74 log CFU/g. Previous studies investigated the optimal growth conditions of S. cerevisiae and reported approximately 6 log CFU/g under optimal cultivation. In comparison to this result, a relatively high survival rate was observed even when treated with excipients [41]. These results confirm that PBS buffer and 0.85% NaCl solutions are effective rehydration conditions for dried yeasts treated with skim milk and various sugars as excipients, supporting previous studies that reported higher survival rates with PBS buffer [24]. In addition, the excipient cyclodextrin, which exhibited the highest survival rate, is non-hygroscopic and, further, is a uniform, pure substance that enhances moisture retention. It has been reported that cyclodextrin could be used as a carrier for encapsulating flavors and other sensitive components, with potential applications in pharmaceuticals, food, and other fields [42,43].
Therefore, in this study, cyclodextrin was used as an excipient to enhance the safety of the S. cerevisiae YM45 powdered starter, which produces fruit-like flavors. Additionally, the effects of rehydration with PBS buffer on yeast activity preservation were investigated. Based on these findings, the development of quality maintenance technology is expected to support the stable supply of domestically produced starters, reducing reliance on imported ones and contributing to commercialization and industrial applications.
4. Conclusions
In this study, twenty-three yeast strains from the genus Saccharomyes and three non-Sacccharomyes strains, isolated from traditional Korean fermented foods by our research team, were analyzed using an electronic nose. Among them, Saccharomyces cerevisiae YM45 was selected due to its high levels of methyl formate and propan-2-one, which contribute to fruity and sweet aromas. To evaluate its potential as a powdered starter, S. cerevisiae YM45 was processed using different excipients. When cyclodextrin was used as an excipient, with moisture content at 4.7%, bacterial contamination was found to be 2.30 log CFU/g, which confirmed its microbiological safety. Additionally, rehydration using PBS buffer resulted in a high survival rate of 8.74 log CFU/g, demonstrating effective preservation of yeast viability. These findings highlight the high-quality powdered yeast starters, and the results contribute to optimizing starter culture formulation, ensuring safety, and improving yeast viability for commercial applications.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/fermentation11040203/s1, Tables S1–S3: Optimal culture conditions for fruit-flavored S. cerevisiae YM45.
Author Contributions
This work was carried out in collaboration with all authors. C.-W.K. and S.-H.Y. conceived the study idea, verified the analytical methods, and analyzed the data; S.J.L. conducted the experiments and wrote the initial and final drafts; J.-Y.K. and S.-Y.K. performed data curation, review, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research Program for Agricultural Science and Technology Development, grant number RS-2022-RD010225, National Institute of Crop and Food Science, Rural Development Administration, Republic of Korea.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The financial support of the Rural Development Administration is gratefully acknowledged.
Conflicts of Interest
Author S.-H.Y. was employed by the company Sulseam, an agricultural corporation. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Kim, S.Y.; Gil, N.Y.; Mun, J.Y.; Song, G.C.; Yeo, S.H. Research for utilization and industrialization of Korean starter. Food Ind. Nutr. 2018, 23, 14–19. [Google Scholar]
- Baek, C.H.; Bae, S.Y.; Mun, J.Y.; Choi, H.S.; Kang, J.E.; Jung, S.T.; Yeo, S.H. Quality characteristics and preparing of solid starter using fungal strains for Takju. Korean J. Food Preserv. 2016, 23, 797–803. [Google Scholar] [CrossRef]
- So, M.H. Characteristics of Koji molds isolated from Koji-starters for brewing in Korea and Japan. Korean J. Food Nutr. 1993, 6, 1–7. [Google Scholar]
- Casey, G.P.; Ingledew, W.M. Ethanol tolerance in yeasts. Crit. Rev. Microbiol. 1986, 13, 219–280. [Google Scholar] [CrossRef]
- Jolly, N.P.; Augustyn, O.P.H.; Pretorius, I.S. The role and use of non-Saccharomyces yeast in wine production. S. Afr. J. Enol. Vitic. 2006, 27, 15–39. [Google Scholar] [CrossRef]
- Lee, H.S.; Lee, T.S.; Noh, B.S. Volatile flavor components in the mashes of Takju prepared using different yeasts. Korean J. Food Sci. Technol. 2007, 39, 593–599. [Google Scholar]
- Kim, Z.U. Food Processing; Moonwoondang: Seoul, Republic of Korea, 1985; p. 5. [Google Scholar]
- Kim, C.J.; Kim, K.C.; Kim, D.Y.; Oh, M.J.; Lee, S.K.; Lee, S.O.; Chung, S.T.; Jung, J.H. Fermentation Technology; Sunjinmunwhasa: Seoul, Republic of Korea, 1990; pp. 79–103. [Google Scholar]
- Lee, H.S.; Park, C.S.; Choi, J.Y. Quality characteristics of the mashes of Takju prepared using different yeasts. Korean J. Food Sci. Technol. 2010, 42, 56–62. [Google Scholar]
- Seo, D.J.; Yeo, S.H.; Mun, J.Y.; Baek, S.Y. Effects of low temperature-adapted Saccharomyces cerevisiae Y297 strain and fermentation temperature on the quality characteristics of Yakju. Korean J. Food Preserv. 2016, 23, 666–672. [Google Scholar] [CrossRef][Green Version]
- Shin, K.R.; Kim, B.C.; Yang, J.Y.; Kim, Y.D. Characterization of Yakju prepared with yeasts from fruits. J. Korean Soc. Food Sci. Nutr. 1999, 28, 794–800. [Google Scholar]
- Armin, S.; Matthias, F.; Rainer, J.; Doris, R.; Philippe, D. Characterizing aromatic typicality of Riesling wines: Merging volatile compositional and sensory aspects. Food Res. Int. 2015, 69, 26–37. [Google Scholar]
- Marcos, E.H.; Oliver, B.; Marcus, F. Aroma component analysis by HS-SPME/GC-MS to characterize Lager, Ale, and sour beer styles. Food Res. Int. 2024, 194, 114763. [Google Scholar]
- Atsuko, I.; Hrroshi, U.; Ryoko, K.; Hiroshi, I. Changes in the aroma compounds of sake during aging. J. Agric. Food Chem. 2005, 53, 4118–4123. [Google Scholar]
- Pires, E.J.; Teixeira, J.A.; Branyik, T.; Vicente, A.A. Yeast: The soul of beer’s aroma-a review of flavor-active esters and higher alcohols produced by brewing yeast. Appl. Microbiol. Biotechnol. 2014, 98, 1937–1949. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.R.; Kim, D.H.; Kang, J.E.; Han, G.J.; Jeong, S.T.; Kim, C.W. Effects of enzyme treatment and skin contact time on the characteristics of Dae-hong peach wine. J. Korean Soc. Food Cult. 2023, 38, 442–455. [Google Scholar]
- Kang, J.E.; Kim, Y.M.; Lee, J.E.; Im, B.R.; Choi, J.H.; Han, G.J.; Jeong, H.N. Quality characteristics of distilled soju using Dae-hong peaches. Korea J. Food Preserv. 2023, 30, 683–690. [Google Scholar] [CrossRef]
- Berny, J.F.; Hennebert, G.L. Viability and stability of yeast cells and filamentous fungus spores during freeze drying: Effects of protectants and cooling rates. Mycologia 1991, 83, 805–815. [Google Scholar] [CrossRef]
- Abadias, M.; Benabarre, A.; Teixido, N.; Usall, J.; Vinas, I. Effect of freeze drying and protectants on viability of the biocontrol yeast Candida sake. Int. J. Food Microbiol. 2001, 65, 173–182. [Google Scholar] [CrossRef]
- Susanna, R.; Pirjo, R. Protecting probiotic bacteria by microencapsulation: Challenges for industrial applications. Eur. Food Res. Technol. 2010, 231, 1–12. [Google Scholar]
- Sofia, C.A.; Silva, J.; Ho, P.; Teixeira, P.; Xavier, M.F.; Gibbs, P. Survival of freeze-dried Lactobacillus plantarum and Lactobacillus rhamnosus during storage in the presence of protectants. Biotechnol. Lett. 2002, 24, 1587–1591. [Google Scholar]
- Park, S.K.; Jin, H.; Lee, C.M.; Baik, S.H. Effect of protectants and fermentative properties of Saccharomyces cerevisiae JBCC-95 isolated from Makgeolli after freeze drying. J. Korean Soc. Food Sci. Nutr. 2023, 52, 516–521. [Google Scholar] [CrossRef]
- Oh, H.W.; Lee, S.H. A study on the manufacture and quality characteristics of freeze-dried honey powders by forming agents. J. Apic. 2022, 37, 217–227. [Google Scholar]
- Chae, M.K.; Choi, J.S.; Moon, H.B.; Park, J.B.; Choi, K.T.; Yeo, S.H.; Park, H.D. Development of air-blast dried yeast starter for ‘Yakju’ and monitoring on its fermentation characteristics. Korean J. Food Preserv. 2021, 28, 810–819. [Google Scholar] [CrossRef]
- Lee, S.J.; Kang, H.B.; Kim, S.H.; Jeong, W.S.; Kim, S.Y.; Yeo, S.H. Solid fungi starters using Aspergillus spp. under different manufacturing conditions. Fermentation 2023, 9, 487. [Google Scholar] [CrossRef]
- Lee, M.J.; Lee, Y.B.; Yang, J.Y.; Kwon, H.S.; Yoon, J.R. Isolation and identification of volatile compounds extracted from twigs of Pinus densiflora with Likens-Nickerson apparatus. J. Korean Soc. Food Sci. Nutr. 1998, 27, 568–573. [Google Scholar]
- Boo, C.G.; Hong, S.J.; Shin, E.C. Comparative evaluation of the volatile profiles and taste properties of commercial coffee products using electronic nose, electronic tongue, and GC/MSD. J. Korean Soc. Food Sci. Nutr. 2021, 50, 810–822. [Google Scholar] [CrossRef]
- Dai, Z.; Gu, H.; Zhang, S.; Xin, F.; Zhang, W.; Dong, W.; Ma, J.; Kia, H.; Jiang, M. Metabolic construction strategies for direct methanol uilization in Saccharomyces cerevisiae. Bioresour. Technol. 2017, 245, 1407–1412. [Google Scholar] [CrossRef]
- Seo, D.J.; Yeo, S.H.; Mun, J.Y.; Jung, W.J.; Cho, Y.S.; Baek, S.Y. Characteristics of yeasts with low temperature adaptation for yakju brewed. Korean J. Food Preserv. 2015, 22, 908–914. [Google Scholar] [CrossRef]
- Shin, H.J.; Byun, O.H.; Kim, Y.J.; Bang, B.Y.; Park, J.M.; Jeong, Y.S.; Bai, D.H. Study of tannin reducing effect of Aronia by yeast isolated from Jeotgal. Korean J. Mycol. 2015, 43, 247–252. [Google Scholar]
- Kim, G.J.; Chung, H.C.; Kwon, O.H. Characteristics of culture and isolating lactic acid bacteria and yeast from Sourdough. J. Korean Soc. Food Sci. Nutr. 2004, 33, 1180–1185. [Google Scholar]
- Kim, Y.H.; Kang, S.W.; Lee, J.H.; Chang, H.I.; Yun, C.W.; Paik, H.D.; Kang, C.W.; Kim, S.W. Optimization of medium components for cell mass production of Saccharomyces cerevisiae JUL3 using response surface methodology. Korean J. Biotechnol. Bioeng. 2006, 21, 479–483. [Google Scholar]
- Kang, H.R.; Lee, A.R.; Kwon, Y.H.; Kim, J.H.; Kim, H.R.; Ahn, B.H. Optimization of culture conditions for the yeast and analysis of qualities of Makgeolli brewed with the yeast isolated from Korean traditional Nuruk. Korean J. Mycol. 2012, 40, 204–209. [Google Scholar] [CrossRef]
- Morgan, C.A.; Herman, N.; White, P.A.; Vesey, G. Preservation of micro-organisms by drying; A review. J. Microbiol. Methods 2006, 66, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Chalat, S.; Ulrich, K.; Petra, F. Alternative drying processes for the industrial preservation of lactic acid starter cultures. Biotechnol. Prog. 2007, 23, 302–315. [Google Scholar]
- Solberg, M.; Buckalew, J.J.; Chen, C.C.; Schaffner, D.W.; O’Neil, K.; Mcdowell, J.J.; Post, L.S.; Boderck, M. Microbiological safety assurance system of foodservice facilities. Food Technol. 1990, 44, 68–73. [Google Scholar]
- Jeon, E.B.; Kim, J.Y.; Choi, M.S.; Choi, S.H.; Bang, H.J.; Park, S.Y. Microbial contamination levels in the raw materials of home meal replacement Shabu-Shabu meal kit distributed in markets. J. Food Hyg. Saf. 2020, 35, 375–381. [Google Scholar] [CrossRef]
- Fry, R.M.; Greaves, R.I.N. The survival of bacteria during and after drying. Epidemiol. Infect. 1951, 49, 220–246. [Google Scholar] [CrossRef]
- Lee, S.B.; Kim, D.H.; Park, H.D. Effects of protectant and rehydration conditions on the survival rate and malolactic fermentation efficiency of freeze-dried Lactobacillus plantarum JH287. Appl. Microbiol. Biotechnol. 2016, 100, 7853–7862. [Google Scholar] [CrossRef]
- Lee, S.B.; Choi, W.S.; Jo, H.J.; Yeo, S.H.; Park, H.D. Optimization of air-blast drying process for manufacturing Saccharomyces cerevisiae and non-Saccharomyces cerevisiae yeast as industrial wine starters. AMB Express 2016, 6, 105. [Google Scholar] [CrossRef]
- Roshanak, S.; Rosita, S. Investigation of the best Saccharomyces cerevisiae growth condition. Electron. Physician 2017, 9, 3592–3597. [Google Scholar]
- Lajos, S.; Jozsef, S. Cyclodextrins as food ingredients. Trends Food Sci. Technol. 2004, 15, 137–142. [Google Scholar]
- Zhang, Z.; Niu, J.; Wang, J.; Zheng, Q.; Maio, W.; Lin, Q.; Li, X.; Jin, Z.; Qiu, C.; Sang, S.; et al. Advances in the preparation and application of cyclodextrin derivatives in food and the related fileds. Food Res. Int. 2024, 195, 114952. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).