Influence of Kefir Grain Concentration on the Nutritional, Microbiological, and Sensory Properties of Camel Milk Kefir and Characterization of Some Technological Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Camel Milk and Kefir Grains (KGs)
2.2. Manufacture of Camel Milk Kefir
2.3. Physicochemical Characteristics (Milk, Kefir, and KGs)
2.4. Microbiological Analysis of Camel Milk and Kefir Grains (KGs)
2.5. Sensory Analysis
2.6. Isolation of Lactic Acid Bacteria (LAB) from Selected Kefir
2.7. Technological Aspects of Selected Kefir
- Acidifying activity
- Proteolytic activity
- Lipolytic activity
- Exopolysaccharide production
2.8. Antimicrobial Activity Tests
2.9. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Camel Milk and KGs
3.2. The Effect of the KG Dose on the Composition of Kefir
3.3. Sensory Evaluation of Camel Kefirs (CKs)
3.4. Physiological and Biochemical Tests
Growth and Activity of Isolated Strains in Sherman’s Milk
3.5. Technological Activities of Lactic Acid Strains Isolated from Selected Kefir
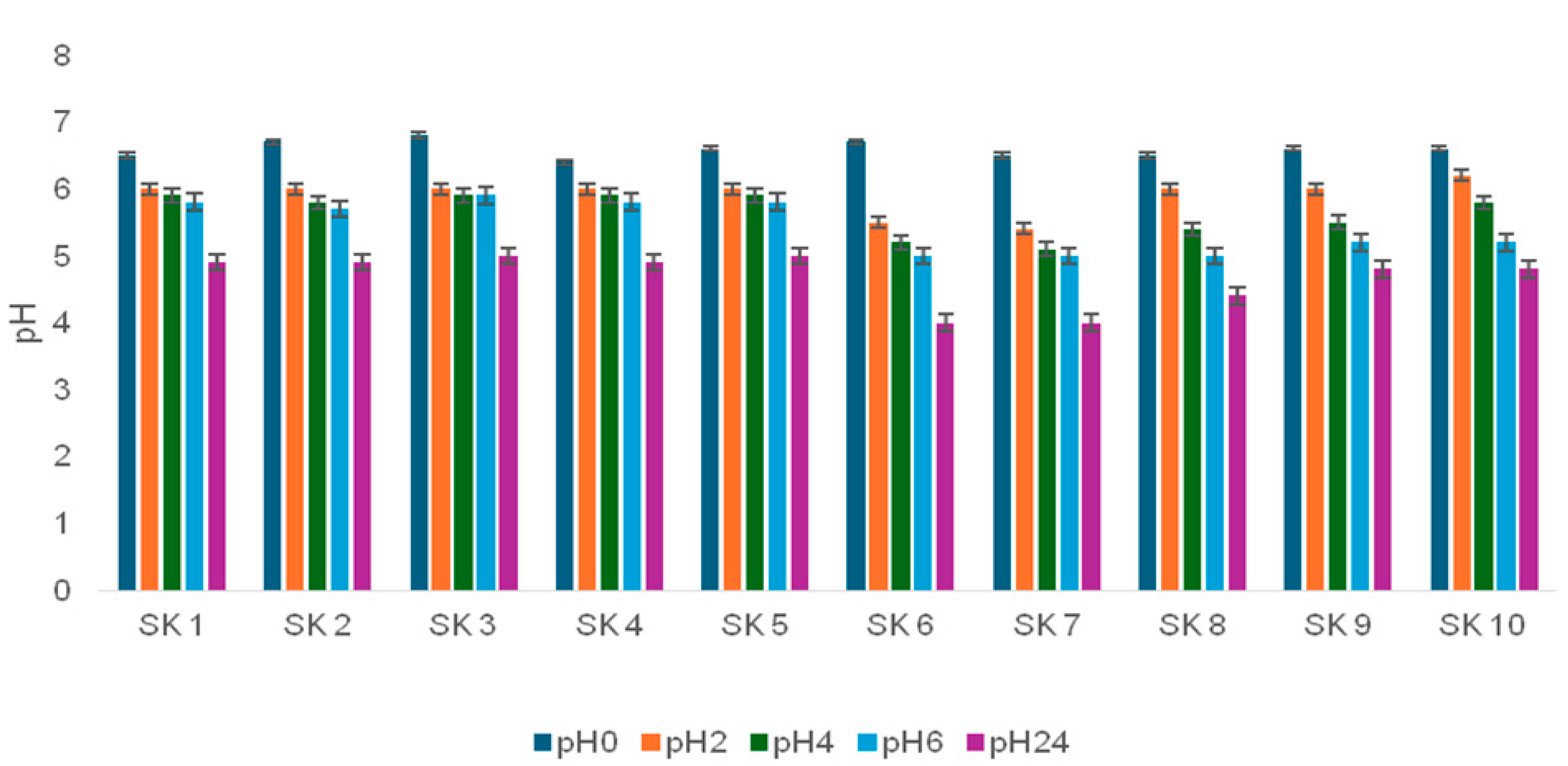
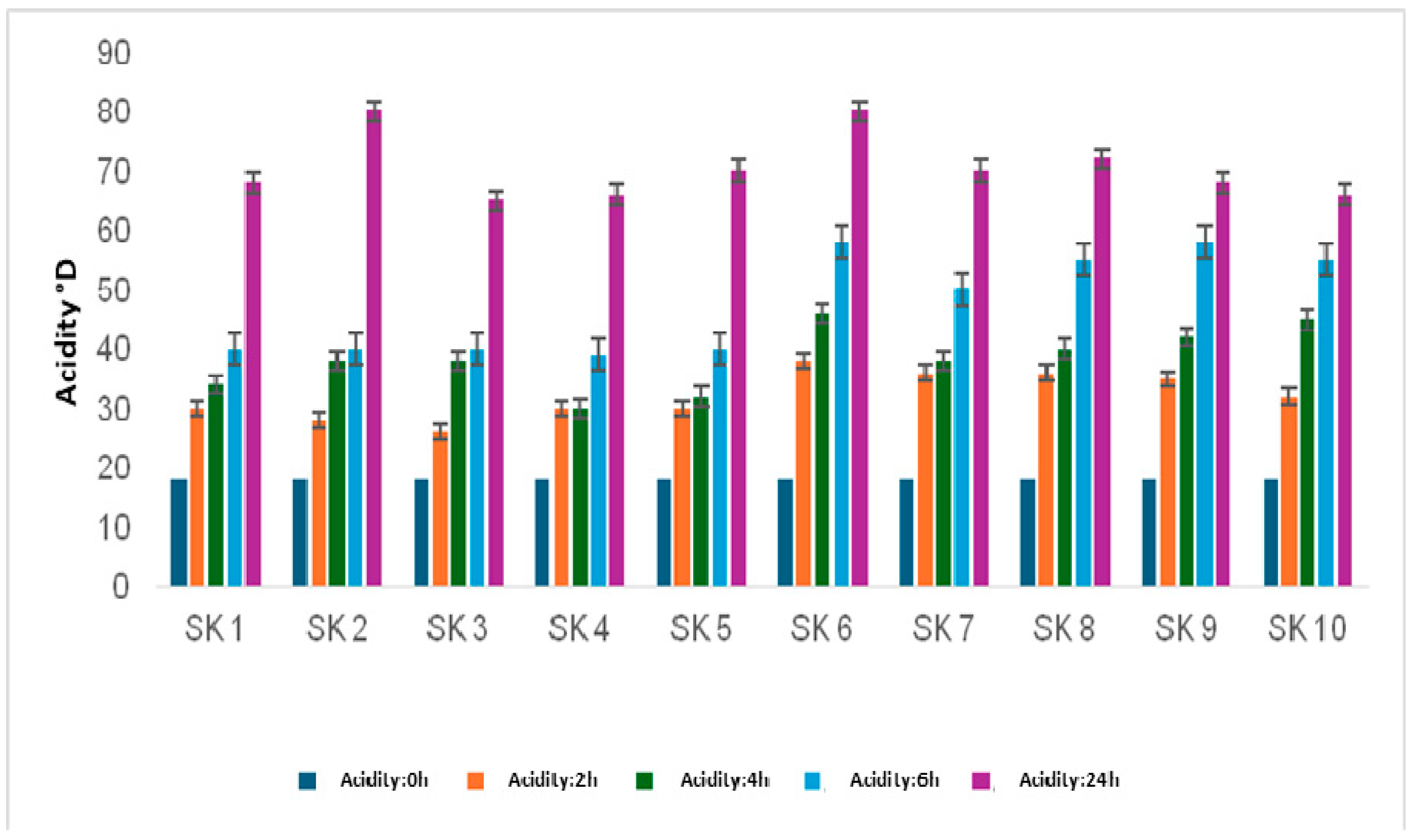
3.5.1. Acidifying Activity
3.5.2. Proteolytic Activity
3.5.3. Lipolytic Activity
3.5.4. Texturizing Activity
3.5.5. Gasogenic Activity
3.6. Antibacterial Properties of Lactic Acid Bacteria Isolated from Camel’s Kefir
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MRS | de Man, Rogosa, and Sharpe |
| LAB | Lactic acid bacteria |
| GK | Kefir grain |
| DM | Dry matter |
| YM | Yeast and molds |
| CK (2%, 18 h) | Camel kefir with 2% kefir grains and 18 h incubation time |
| CK (5%, 18 h) | Camel kefir with 5% kefir grains and 18 h incubation time |
| CK (10%, 18 h) | Camel kefir with 10% kefir grains and 18 h incubation time |
| SK1–SK10 | Lactic strains isolated from selected camel kefir. |
References
- Mohammed, A.A.; Almuyidi, A.; Almarri, H.; Alkhalifah, H.; Alhmad, A.; Alali, H.; AlHuwaish, O.; AlKhawaher, M. Unique Characteristics of Camel Body Systems: Adaptation to Harsh Conditions, Productive and Reproductive Performances: A Review. Indian. J. Anim. Res. 2025, 1–10. [Google Scholar] [CrossRef]
- Aroua, M. L’élevage Camelin en Tunisie Élevage et Lait de Chamelle; Editions Universitaires Européennes: Saarbrücken, Germany, 2021. [Google Scholar]
- Subbulakshmi, V.; Sheetal, K.R.; Noor Mohamed, M.B.; Renjith, P.S.; Kala, S. Arid Agroforestry for Thar Desert. In Natural Resource Management in the Thar Desert Region of Rajasthan; Springer International Publishing: Cham, Switzerland, 2023; pp. 155–192. [Google Scholar]
- Boudjnah, H.S. Aptitude à la Transformation du Lait de Chamelle en Produit Dérives: Effet des Enzymes Coagulantes Extraites de Caillettes de Dromadaires. Ph.D. Thesis, Universite Mouloud Mameri, Tizi-Ouzou, Algérie, 2012; pp. 40–60. [Google Scholar]
- Sboui, A.; Atig, C.; Khabir, A.; Hammadi, M.; Khorchani, T. Camel milk used as an adjuvant therapy to treat type 2 diabetic patients: Effects on blood glucose, HbA1c, cholesterol, and TG levels. J. Chem. 2022, 2022, 5860162. [Google Scholar] [CrossRef]
- Abdelazez, A.; Abd-elmotaal, H.; Abady, G. Exploring the potential of camel milk as a functional food: Physicochemical characteristics, bioactive components, innovative therapeutic applications, and development opportunities analysis. Food Mater. Res. 2024, 4, e031. [Google Scholar] [CrossRef]
- Soutelino, M.E.M.; da Silva Rocha, R.; Mársico, E.T.; Esmerino, E.A.; de Oliveira Silva, A.C. Innovative approaches to kefir production, challenges, and current remarks. Curr. Opin. Food Sci. 2025, 61, 101252. [Google Scholar] [CrossRef]
- La Torre, C.; Caputo, P.; Fazio, A. Effect of Milk and Water Kefir Grains on the Nutritional Profile and Antioxidant Capacity of Fermented Almond Milk. Molecules 2025, 30, 698. [Google Scholar] [CrossRef]
- Mujahid, M.; Wakeel, M.; Ali, A.M.; Saeed, S.; Nawaz, A.S.; Hafeez, K. Food fermentation: Traditional practices and modern applications in food industry. Int. J. Food Ferment. Technol. 2024, 14, 239–273. [Google Scholar] [CrossRef]
- Codex Alimentarius International Food Standards 2003. Codex Standard for Fermented Milks (Codex Stan CXS 243-2003). Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/es/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B243-2003%252FCXS_243e.pdf (accessed on 22 December 2024).
- Dahiya, D.; Nigam, P.S. Therapeutic and Dietary Support for Gastrointestinal Tract Using Kefir as a Nutraceutical Beverage: Dairy-Milk-Based or Plant-Sourced Kefir Probiotic Products for Vegan and Lactose-Intolerant Populations. Fermentation 2023, 9, 388. [Google Scholar] [CrossRef]
- Arain, M.A.; Salma, H.M.; Ali, M.; Khaskheli, G.B.; Barham, G.S.; Marghazani, I.B.; Ahmed, S. A review on camel milk composition, techno-functional properties and processing constraints. Food Sci. Anim. Resour. 2023, 44, 739. [Google Scholar] [CrossRef]
- Seifu, E. Camel milk products: Innovations, limitations and opportunities. FPPN 2023, 5, 1–20. [Google Scholar] [CrossRef]
- Farnworth, E.R.; Mainville, I. Kefir—A Fermented Milk Product. In Handbook of Fermented Functional Foods, 2nd ed.; Farnworth, E.R., Ed.; CRC Press Taylor & Francis Group: Boca Raton, FL, USA; London, UK; New York, NY, USA, 2008; pp. 89–127. [Google Scholar]
- Agustina, L.T.; Setyawardani, T.; Astuti, T.Y. Penggunaan starter biji kefir dengan konsentrasi yang berbeda pada susu sapi terhadap pH dan kadar asam laktat (The use of different concentration of kefir grainsion cow’s milk and its effect on pH and lactic acid level). JIPT 2013, 1, 254–259. [Google Scholar]
- Otles, S.; Cagindi, O. Kefir: A Probiotic Dairy-Composition, Nutritional and Therapeutic Aspects. Pak. J. Nutr. 2003, 2, 54–59. [Google Scholar]
- Deak, T. Food safety management: Chapter 17. In Thermal Treatment; Elsevier Inc.: Amsterdam, The Netherlands, 2013. [Google Scholar]
- AFNOR. Contrôle de la Qualité des Produits Alimentaires; Lait et produits laitiers, Afnor: Paris, France, 1993. [Google Scholar]
- Wangoh, J.; Farah, Z.; Puhan, Z. Composition of milk from three camel (Camelus dromedarius) breeds in Kenya during lactation. Milchwissenschaft 1998, 53, 136–139. [Google Scholar]
- International Standard ISO 1443; Meat and Milk Products-Determination of Total Fat Content. International Standard ISO: Geneva, Switzerland, 2010.
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Sharma, R.; Rajput, Y.S.; Poonam Dogra, G.; Tomar, S.K. Estimation of sugars in milk by HPLC and its application in detection of adulteration of milk with soy milk. Int. J. Dairy Technol. 2009, 62, 514–519. [Google Scholar] [CrossRef]
- De Man, J.C.; Rogosa, M.; Sharpe, M.E. A medium for the cultivation of lactobacilli. J. Appl. Microbiol. 1960, 23, 130–135. [Google Scholar] [CrossRef]
- Jrad, Z.; Oussaief, O.; Bouhemda, T.; Khorchani, T.; EL Hatmi, H. Potential effects of ultrafiltration process and date powder on textural, sensory, bacterial viability, antioxidant properties and phenolic profile of dromedary Greek yogurt. IJFST 2019, 54, 854–861. [Google Scholar] [CrossRef]
- Abdo, E.M.; Mansour, H.M.; Darwish, A.M.G.; El-Sohaimy, S.A.; Gomaa, M.A.; Shaltout, O.E.; Allam, M.G. Beetroot Stalk Extract as a Functional Colorant for Stirred Yogurt Beverages: Effect on Nutritional Value and Stability during Storage. Fermentation 2023, 9, 878. [Google Scholar] [CrossRef]
- Zarour, K.; Benmechernene, Z.; Hadadji, M.; Moussa-Boudjemaa, B.; Henni, D.J.; Kihal, M. Bioprospecting of Leuconostoc mesenteroides strains isolated from Algerian raw camel and goat milk for technological properties useful as adjunct starters. Afr. J. Microbiol. Res. 2012, 6, 3192–3201. [Google Scholar]
- Maghnia, D.A. Etude de Potentiel Technologique des Bactéries Lactiques Isolées des Aliments Fermentés Traditionnels Algériens. Ph.D. Thesis, Université d’Oran, Oran, Algeria, 2011. [Google Scholar]
- Meng, Z.; Zhang, L.; Xin, L.; Lin, K.; Yi, H.; Han, X. Technological characterization of Lactobacillus in semi hard artisanal goat cheeses from different Mediterranean areas for potential use as nonstarter lactic acid bacteria. JDS 2018, 101, 2887–2896. [Google Scholar]
- Vuillemard, J.C. Microbiologie des aliments. Evolution de l’activité protéolytique des bactéries lactiques. Tech. Doc. Lavoisier Paris 1986, 3, 1–65. [Google Scholar]
- Smibert, R.M.; Krieg, N.R. General characterization. In Manual of Methods for General Bacteriology; Gerhardt, P., Ed.; American Society for Microbiology: Washington, DC, USA, 1981; pp. 409–443. [Google Scholar]
- Smith, J.L.; Haas, M.J. Lipolytic microorganisms. In Compendium of Methods for the Microbiological Examination of Foods; APHA Press: Washington, DC, USA, 1992; pp. 183–191. [Google Scholar]
- Van Geel-Schutten, G.H.; Faber, E.J.; Smit, E.; Bonting, K.; Smith, M.R.; TenBrink, B.; Kamerling, J.P.; Vliegenthart, J.F.; Dijkhuizen, G.L. Biochemical and structural characterization of the Glucan and Fructan Exopolysaccharides synthesized by the Lactobacillus reuteriwild-type strain and by mutant strain. Appl. Environ. Microbiol. 1999, 65, 3008–3014. [Google Scholar] [CrossRef] [PubMed]
- Knoshaug, E.P.; Ahlgrent, J.A.; Trempy, J.E. Growth associated exopolysaccharide expression in Lactococcus lactis subspecies cremoris Ropy352. JDS 2000, 83, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Ayad, E.H.E.; Nashat, S.; El-Sadek, N.; El-Soda, M. Selection of wild lactic acid bacteria isolated from traditional Egyptian dairy prod-ucts according to production and technological criteria. Food Microbiol. 2004, 21, 715–725. [Google Scholar] [CrossRef]
- Jrad, Z.; El Hatmi, H.; Fguiri, I.; Arroum, S.; Assadi, M.; Khorchani, T. Antibacterial activity of Lactic acid bacteria isolated from Tunisian camel milk. Afr. J. Microbiol. Res. 2013, 7, 1002–1008. [Google Scholar]
- Fguiri, I.; Manel, Z.; Sboui, A.; Ayeb, N.; Atigui, M.; Arroum, S.; Hammadi, M.; Khorchani, T. Microbiological quality and biochemical characteristics of lactic acid bacteria from camel milk as affected by the production system and stage of lactation. In Milk Protein: New Research Approaches; IntechOpen: London, UK, 2022; Volume 13. [Google Scholar]
- Fouzia, R.; Noureddine, S.; Mebrouk, K. Evaluation of the factors affecting the variation of the physicochemical composition of Algerian camel’s raw milk during different seasons. Adv. Environ. Biol. 2013, 7, 4879–4884. [Google Scholar]
- Ismaili, M.A.; Saidi, B.; Zahar, M.; Hamama, A.; Ezzaier, R. Composition and microbial quality of raw camel milk produced in Morocco. J. Saudi Soc. Agric. Sci. 2019, 18, 17–21. [Google Scholar] [CrossRef]
- Arroum, S.; Zamouli, K.; Gaddour, A.; Fguiri, I.; Ayeb, N.; Khorhani, T. Etude comparative des caractéristiques physicochimiques et microbiologiques du lait camelin en fonction du mode d’élevage. J. New Sci. 2015, 4, 4648. [Google Scholar]
- Hazebrouck, S. Laits de chèvre, d’ânesse et de chamelle: Une alternative en cas d’allergie au lait de vache ? Innov. Agron. 2016, 52, 73–84. [Google Scholar]
- Chethouna, F.; Boudjenah, S.H.; Beldi, N.; Siboukeur, O. Comparative Study of the Physico-Chemical and Microbiological Characteristics of Raw and Pasteurized Camel Milk. Emir. J. Food Agric. 2022, 34, 10. [Google Scholar] [CrossRef]
- Chamekh, L.; Calvo, M.; Khorchani, T.; Castro-Gómez, P.; Hammadi, M.; Fontecha, J.; Yahyaoui, M.H. Impact of management system and lactation stage on fatty acid composition of camel milk. J. Food Compos. Anal. 2020, 87, 103–418. [Google Scholar] [CrossRef]
- Fguiri, I.; Ziadi, M.; Sboui, A.; Ayeb, N.; Atigui, M.; Arroum, S.; Khorchani, T. Effect of the production system and stage of lactation on the microbiological and biochemical characteristics of camel milk. J. Camelid Sci. 2018, 11, 57–63. [Google Scholar]
- Sraïri, M.T.; Alaoui, H.I.; Hamama, A. Relations entre pratiques d’élevage et qualité globale du lait de vache en étables suburbaines au Maroc. Rev. Med. Vet. 2005, 156, 155–162. [Google Scholar]
- Garrote, G.L.; Abraham, A.G.; De Antoni, G.L. Chemical and microbiological characterisation of kefir grains. Res. J. Dairy Sci. 2001, 68, 639–652. [Google Scholar] [CrossRef]
- Jelen, P.; Fuquay, J.; Fox, P.; McSweeney, P. Encyclopedia of Dairy Sciences; Academic Press: Oxford, UK, 2003. [Google Scholar]
- Chen, Z.; Shi, J.; Yang, X.; Nan, B.; Liu, Y.; Wang, Z. Chemical and physical characteristics and antioxidant activities of the exopolysaccharide produced by Tibetan kefir grains during milk fermentation. Int. Dairy J. 2015, 43, 15–21. [Google Scholar] [CrossRef]
- Suriasih, K.; Sucipta, I.N.; Putri, W.C.W.; Wirawan, I.P.S. Chemical Characteristics And Microbiological Kefir Beverages from Bali Cattle During Storage. IJSTR 2020, 9, 135–138. [Google Scholar]
- Irigoyen, A.; Arana, I.; Castiella, M.; Torre, P.; Ibanez, F.C. Microbiological, physicochemical, and sensory characteristics of kefir during storage. Food Chem. 2005, 90, 613–620. [Google Scholar] [CrossRef]
- Dedhia, N.; Marathe, S.J.; Singhal, R.S. Food polysaccharides: A review on emerging microbial sources, bioactivities, nanoformulations and safety considerations. Carbohydr. Polym. 2022, 287, 119–355. [Google Scholar] [CrossRef]
- Sarkar, S. Biotechnological innovations in kefir production: A review. Br. Food J. 2008, 110, 283–295. [Google Scholar] [CrossRef]
- Ferreira, C. Quefir como alimento funcional. In Alimentos Funcionais–Componentes Bioativos e Efeitos Fisiológicos [NMB Costa and CO Rosa, Editors]; Editora Rubio LTDA: Rio de Janeiro, Brazil, 2010; Volume 1, pp. 111–122. [Google Scholar]
- Cheng, T.; Zhang, T.; Zhang, P.; He, X.; Sadiq, F.A.; Li, J.; Gao, J. The complex world of kefir: Structural insights and symbiotic relationships. CRFSFS 2024, 23, e13364. [Google Scholar] [CrossRef]
- Farag, M.A.; Jomaa, S.A.; Abd El-Wahed, A.; El-Seedi, H.R. The many faces of kefir fermented dairy products: Quality characteristics, flavour chemistry, nutritional value, health benefits, and safety. Nutrients 2020, 12, 346. [Google Scholar] [CrossRef]
- Schmidt, K.A. Dairy: Ice cream. In Food Processing: Principles and Applications; Wiley-Blackwell: Hoboken, NJ, USA, 2004; pp. 287–296. [Google Scholar]
- Hu, Y.; Zhang, L.; Wen, R.; Chen, Q.; Kong, B. Role of lactic acid bacteria in flavor development in traditional Chinese fermented foods: A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2741–2755. [Google Scholar] [CrossRef] [PubMed]
- Arroum, S.; Sboui, A.; Fguiri, I.; Ayeb, N.; Hammadi, M.; Khorchani, T. Qualité du kéfir camelin issu du système d’élevage extensif en Tunisie. Rev. Marocaine Des Sci. Agron. Et Vétérinaires 2023, 11, 269–277. [Google Scholar]
- Arroum, S.; Sboui, A.; Atigui, M.; Fguiri, I.; Dbara, M.; Ayeb, N.; Khorchani, T. Camel’s Kefir Milk: Optimisation of Processing Conditions. JCPR 2023, 30, 221–227. [Google Scholar] [CrossRef]
- Larpen, J.P. Mémento Technique de Microbiologie, 3rd ed.; Technique et Documentation: Lavoisier, Paris, France, 1997; p. 910. [Google Scholar]
- Axelsson, L. Lactic acid bacteria: Classification and physiology. In Lactic Acid Bacteria: Microbiology and Functional Aspects, 2nd ed.; Revised and Expanded; Salminen, S., von Wright, A., Eds.; Marcel Dekker: New York, NY, USA, 1998; pp. 1–72. [Google Scholar]
- Luquet, F.M.; Corrieu, G. Bactéries Lactiques et Probiotiques; Technique et Documentation: Lavoisier, Paris, France, 2005. [Google Scholar]
- Guan, Y.; Cui, Y.; Qu, X.; Li, B.; Zhang, L. Post-acidification of fermented milk and its molecular regulatory mechanism. Int. J. Food Microbiol. 2024, 426, 110920. [Google Scholar] [CrossRef]
- Shirai, K.; Guerrero, I.; Huerta, S.; Saucedo, G.; Castillo, A.; Gonzalez, R.O.; Hall, G.M. Effect of initial glucose concentration and inoculation level of lactic acid bacteria in shrimp waste ensilation. Enzyme Microb. Technol. 2001, 28, 446–452. [Google Scholar] [CrossRef]
- Karam, N.E.; Dellali, A.; Zadi-Karam, H. Activité lipolytique chez les bactéries lactiques. Rencontres Autour Des Rech. Sur Les Rumin. 2012, 19, 415. [Google Scholar]
- Akın, N.; Aydemir, S.; Koçak, C.; Yıldız, M.A. Changes of free fatty acid contents and sensory properties of white pickled cheese during ripening. Food Chem. 2003, 80, 77–83. [Google Scholar] [CrossRef]
- Walling, É.; Gindreau, E.; Lonvaud-Funel, A. La biosynthèse d’exopolysaccharide par des souches de Pediococcus damnosus isolées du vin: Mise au point d’outils moléculaires de détection. Le Lait 2001, 81, 289–300. [Google Scholar] [CrossRef][Green Version]
- Cerning, J. Exocellular polysaccharides produced by lactic acid bacteria. FEMS Microbiol. Rev. 1990, 7, 113–130. [Google Scholar] [CrossRef]
- Latreche, B.; Kharroub, K. Caractérisation des Bactéries Lactiques Isolées du Beurre cru, Evaluation de Leurs Aptitudes Technologiques et Leur Utilisation Dans la Fabrication de la Crème Sure. 2016. Available online: https://theses-algerie.com/2437191150150627/memoire-de-magister/universite-freres-mentouri---constantine-1/caract%C3%A9risation-des-bact%C3%A9ries-lactiques-isol%C3%A9es-du-beurre-cru-%C3%A9valuation-de-leurs-aptitudes-technologiques-et-leur-utilisation-dans-la-fabrication-de-la-cr%C3%A8me-sure (accessed on 24 December 2024).
- Dupont, I.; Roy, D.; Lapointe, G. Comparison of exopolysaccharide production by strains of Lactobacillus rhamnosus and Lactobacillus paracasei grown in chemically defined medium and milk. J. Ind. Microbiol. Biotechnol. 2000, 24, 251–255. [Google Scholar]
- Badis, A.; Laouabdia-Sellami, N.; Guetarni, D.; Kihal, M.; Ouzrout, R. Caractérisation phénotypique des bactéries lactiques isolées à partir de lait cru de chèvre de deux populations caprines locales «Arabia et Kabyle». Sci. Technol. C Biotechnol. 2005, 23, 30–37. [Google Scholar]
- Aslam, S.; Qazi, J.I. Isolation of acidophilic lactic acid bacteria antagonistic to microbial contaminants. Pak. J. Zool. 2010, 42, 567–573. [Google Scholar]
- Liévin-Le Moal, V.; Servin, A.L. The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: Mucins, antimicrobial peptides, and microbiota. Clin. Microbiol. Rev. 2006, 19, 315–337. [Google Scholar] [CrossRef] [PubMed]
- González-Orozco, B.D.; García-Cano, I.; Jiménez-Flores, R.; Alvárez, V.B. Invited review: Milk kefir microbiota—Direct and indirect antimicrobial effects. J. Dairy Sci. 2022, 105, 3703–3715. [Google Scholar] [CrossRef]


| Parameters | Camel Milk n = 9 | Kefir Grains n = 7 |
|---|---|---|
| pH | 6.77 ± 0.11 | 3.9 ± 0.70 |
| Acidity °D | 17.36 ± 0.34 | 135.00 ± 1.20 |
| Viscosity (cP) | 2.56 ± 0.22 | - |
| Chemical composition | (g/L) | (g/kg) |
| Fat | 41.70 ± 3.18 | 0.02 ± 0.01 |
| (DM) | 114.21 ± 0.11 | 122.79 ± 0.02 |
| Ash | 8.92 ± 0.61 | 7.90 ± 0.81 |
| Protein | 37.82 ± 0.66 | 44.50 ± 0.10 |
| Lactose | 41.3 ± 0.21 | - |
| LAB (log10 CFU/mL) | 3.55 ± 0.22 | 8.48 ± 0.15 |
| YM (log10 CFU/mL) | 3.75 ± 0. 36 | 6.63 ± 0.19 |
| Parameters | 2% | 5% | 10% |
|---|---|---|---|
| pH | 5.1 ± 0.4 a | 4.8 ± 0.38 b | 4.5 ± 0.35 c |
| Acidity °D | 89.95 ± 5.4 c | 99.4 ± 16.5 b | 112.03 ± 2 a |
| Viscosity | 6.24 ± 0.57 c | 7.88 ± 0.84 b | 10.83 ± 1.8 a |
| Chemical composition (g/L) | |||
| Fat | 41.0 ± 7.1 a | 40.27 ± 7.1 a | 40 ± 7.05 a |
| Protein | 22.04 ± 1.3 b | 25.34 ± 3.6 b | 31.1 ± 5.4 a |
| DM | 114.1 ± 7.7 a | 113.8 ± 7.8 a | 113.7 ± 7.8 a |
| Ash | 8.99 ± 0.49 a | 8.97 ± 0.53 a | 8.97 ± 0.48 a |
| Lactose | 12.66 ± 1 a | 12.2 ± 0.3 a | 11.3 ± 1.3 a |
| LAB (log10 CFU/mL) | 5.32 ± 0.24 a | 5.33 ± 0.25 a | 5.10 ± 0.6 a |
| YM (log10 CFU/mL) | 3.98 ± 0.25 a | 4.53 ± 0.64 a | 4.24 ± 0.83 a |
| Strains | Catalase | Gram | Forms | Citrate |
|---|---|---|---|---|
| SK1 | - | + | Cocci | - |
| SK2 | - | + | Cocci | - |
| SK3 | - | + | Coccobacilli | - |
| SK4 | - | + | Cocci | - |
| SK5 | - | + | Spherical | - |
| SK6 | - | + | Cocci | - |
| SK7 | - | + | Cocci | - |
| SK8 | - | + | Spherical | - |
| SK9 | - | + | Cocci | - |
| SK10 | - | + | Cocci | - |
| SK11 | - | + | Spherical | - |
| SK12 | - | + | Cocci | - |
| SK13 | - | + | Cocci | - |
| SK14 | - | + | Cocci | - |
| SK15 | - | + | Cocci | - |
| SK16 | - | + | Cocci | - |
| SK17 | - | + | Spherical | - |
| SK18 | - | + | Rounded | - |
| Isolated Strains | Growth at Different Temperatures | Growth at Different pH | Growth at Different [NaCl] | |||||
|---|---|---|---|---|---|---|---|---|
| 10 °C | 39 °C | 45 °C | 4.2 | 9.6 | 4% | 6% | 9% | |
| SK1 | + | + | + | + | + | + | + | + |
| SK2 | + | + | + | + | + | + | + | + |
| SK3 | + | + | + | + | + | + | + | + |
| SK4 | + | + | + | + | + | + | + | + |
| SK5 | + | + | + | + | + | + | + | + |
| SK6 | + | + | + | + | + | + | + | + |
| SK7 | + | + | + | + | + | + | + | + |
| SK8 | + | + | + | + | + | + | + | + |
| SK9 | + | + | + | + | + | + | + | + |
| SK10 | + | + | + | + | + | + | + | + |
| SK11 | + | + | + | + | + | + | + | + |
| SK12 | + | + | + | + | + | + | + | + |
| SK13 | + | + | + | + | + | + | + | + |
| SK14 | + | + | + | + | + | + | + | + |
| SK15 | + | + | + | + | + | + | + | + |
| SK16 | + | + | + | + | + | + | + | + |
| SK17 | + | + | + | + | + | + | + | + |
| SK18 | + | + | + | + | + | + | + | + |
| Lactic Strains | Methylene Blue | |
|---|---|---|
| 0.1% | 0.3% | |
| SK1 | Coagulation of milk | Coagulation of milk |
| SK2 | Coagulation of milk | Coagulation of milk |
| SK3 | Coagulation of milk | Coagulation of milk |
| SK4 | Coagulation of milk | Coagulation of milk |
| SK5 | Coagulation of milk | Coagulation of milk |
| SK6 | Coagulation of milk | Coagulation of milk |
| SK7 | Coagulation of milk | Coagulation of milk |
| SK8 | Coagulation of milk | Coagulation of milk |
| SK9 | Coagulation of milk | Coagulation of milk |
| SK10 | Coagulation of milk | Coagulation of milk |
| SK11 | - | - |
| SK12 | - | - |
| SK13 | - | - |
| SK14 | - | - |
| SK15 | - | - |
| SK16 | - | - |
| SK17 | - | - |
| SK18 | - | - |
| Camel Lactic Strains | Diameter of Proteolysis Zone (mm) | Diameter of Opaque Area (mm) | Exopolysaccharide Production | Gas Release | ||
|---|---|---|---|---|---|---|
| 1% Tween 80 | 3% Tween 80 | 5% Tween 80 | ||||
| KS1 | 8 | 12 | 16 | 5 | ± | ± |
| KS2 | 9 | 7 | 10 | 8 | ± | ± |
| KS3 | 10 | 9 | 12 | 6 | + | + |
| KS4 | 5 | 6 | 8 | 7 | + | + |
| KS5 | 11 | 5 | 9 | 6 | + | + |
| KS6 | 7 | 6 | 7 | 5 | + | + |
| KS7 | 12 | 7 | 10 | 6 | + | + |
| KS8 | 15 | 8 | 10 | 5 | + | + |
| KS9 | 14 | 9 | 9 | 7 | + | + |
| KS10 | 13 | 10.2 | 9 | 8 | + | + |
| Pseudomonas | E. coli | Micrococcus | Klepsiella | Staphylocoque | Streptocoque | |
|---|---|---|---|---|---|---|
| KS1 | 9 ± 0.4 ab | 6.4 ± 0.5 b | 4.1 ± 1.6 ab | 6.1 ± 1 ab | 3.9 ± 0.7 c | 4.3 ± 1.4 b |
| KS2 | 4.2 ± 1.1 c | 3.8 ± 0.8 c | 4.1 ± 1.1 ab | 3.6 ± 2 b | 3.7 ± 1.4 bc | 4 ± 1.2 b |
| KS3 | 7.2 ± 0.3 b | 3.9 ± 1.1 c | 5.9 ± 0.8 a | 6 ± 1.2 ab | 4.4 ± 1.5 b | 4.5 ± 1 ab |
| KS4 | 9.9 ± 0.2 a | 10 ± 0.5 a | 3 ± 0.5 b | 4.4 ± 1.3 b | 4.1 ± 2 b | 6.4 ± 1 a |
| KS5 | 5.9 ± 1 b | 4.9 ± 0.4 bc | 5 ± 1 a | 3.9 ± 0.5 b | 4 ± 1.6 b | 4 ± 2.2 ab |
| KS6 | 6 ± 0.5 b | 5.1 ± 1.5 b | 5 ± 0.9 a | 4.1 ± 0.7 b | 4 ± 2.1 b | 4.1 ± 1.3 b |
| KS7 | 9.8 ± 0.34 a | 5 ± 2 b | 4.2 ± 1.2 ab | 4.1 ± 1.4 b | 6 ± 0.6 ab | 6 ± 1 a |
| KS8 | 6.5 ± 1.3 b | 5.3 ± 1 b | 3 ± 1.5 b | 6.3 ± 0.8 ab | 4.9 ± 0.8 ab | 5 ± 1.5 ab |
| KS9 | 9 ± 0.7 ab | 4 ± 0.5 bc | 4.8 ± 0.8 a | 6 ± 0.7 ab | 8.2 ± 0.6 a | 4.6 ± 1.7 ab |
| KS10 | 7 ± 1.6 b | 5 ± 0.43 bc | 6.3 ± 1 a | 8 ± 1.3 a | 6.7 ± 0.9 a | 4 ± 2 ab |
| P | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arroum, S.; Sboui, A.; Fguiri, I.; Dbara, M.; Ayeb, N.; Hammadi, M.; Khorchani, T. Influence of Kefir Grain Concentration on the Nutritional, Microbiological, and Sensory Properties of Camel Milk Kefir and Characterization of Some Technological Properties. Fermentation 2025, 11, 170. https://doi.org/10.3390/fermentation11040170
Arroum S, Sboui A, Fguiri I, Dbara M, Ayeb N, Hammadi M, Khorchani T. Influence of Kefir Grain Concentration on the Nutritional, Microbiological, and Sensory Properties of Camel Milk Kefir and Characterization of Some Technological Properties. Fermentation. 2025; 11(4):170. https://doi.org/10.3390/fermentation11040170
Chicago/Turabian StyleArroum, Samira, Amel Sboui, Imen Fguiri, Mohamed Dbara, Naziha Ayeb, Mohamed Hammadi, and Touhami Khorchani. 2025. "Influence of Kefir Grain Concentration on the Nutritional, Microbiological, and Sensory Properties of Camel Milk Kefir and Characterization of Some Technological Properties" Fermentation 11, no. 4: 170. https://doi.org/10.3390/fermentation11040170
APA StyleArroum, S., Sboui, A., Fguiri, I., Dbara, M., Ayeb, N., Hammadi, M., & Khorchani, T. (2025). Influence of Kefir Grain Concentration on the Nutritional, Microbiological, and Sensory Properties of Camel Milk Kefir and Characterization of Some Technological Properties. Fermentation, 11(4), 170. https://doi.org/10.3390/fermentation11040170






