Abstract
In this study, wheat bran was used to prepare dietary fiber by Monascus anka in liquid fermentation. The structural and functional characteristics of wheat bran dietary fiber were analyzed. Scanning electron microscopy and X-ray diffraction analysis indicated that the insoluble dietary fiber matrix was disrupted during the liquid fermentation. Infrared spectroscopy and differential scanning calorimetry analysis demonstrated that intramolecular hydrogen bonds were broken and the oligosaccharides increased. The soluble dietary fiber content increased from 10.7 g/100 g to 16.5 g/100 g, which contributed to improvements in the water-holding capacity, oil-holding capacity, and swelling capacity of wheat bran dietary fiber. UV–Vis spectroscopy analysis demonstrated that the M. anka wheat bran fermentation broth (MWFB) mainly contained yellow pigments (236.6 μ mL−1). HPLC-MS spectrometry further showed MWFB contained three known Monascus pigments: monasine (observed. m/z 359.1853 [M+H]+), ankaflavin (observed. m/z 387.2151 [M+H]+), and monascorubrin (observed. m/z 382.2007 [M+H]+). In conclusion, M. anka can make the most use of wheat bran and improve the structure and function of dietary fiber, thereby expanding its application potential in functional food additives, gut microbiota modulation, and low-calorie baked goods.
1. Introduction
Dietary fiber (DF), which comprises non-starch polysaccharides, resistant oligosaccharides, and lignin, is a group of polysaccharides that resist digestion in the human gastrointestinal tract. Depending on the water solubility, those that dissolve in water are called soluble dietary fibers (SDF), and those that do not dissolve in water are called insoluble dietary fibers (IDF) [1]. The structural matrix of plant cellular walls predominantly comprises insoluble fibrous components, including cellulose, hemicellulose, and lignin-like substances. Common water-soluble fibers like pectin and gum, which are found naturally in non-fibrous materials [2]. Although enzymes in the human digestive tract cannot hydrolyze DF, it possesses significant physiological functions, effectively increasing fecal bulk through its ability to form granules and retain water [3]. In recent years, dietary fiber intake worldwide has fallen below nationally recommended levels, and optimizing its consumption could contribute to improving global health and preventing diseases. Since most foods contain low levels of bioavailable dietary fiber, research on fermented dietary fibers holds promise in addressing this challenge [4].
Bran, a by-product of wheat flour, constitutes approximately 15 percent of wheat and is produced globally annually in huge quantities [5]. As a major agricultural byproduct, wheat bran is widely processed into commercially valuable arabinose and sugar alcohols like xylitol and sorbitol. Wheat bran serves as a cheap source of dietary fiber. Not only is it high in dietary fiber, but it also contains minerals and other active ingredients. Dietary fiber of wheat bran accounts for about 50 percent (dry w/w), of which more than 90 percent (w/w) is IDF [6]. IDF is directly related to intestinal regulation, which is beneficial to the growth of intestinal flora and inhibits pancreatic lipase activity, while SDF can reduce blood cholesterol and absorption of glucose. Compared with IDF, SDF exhibits better emulsifying and gelling properties [7]. Moreover, adding IDF to foods can adversely affect the color, flavor, and mouthfeel resulting in a decline in food quality. To improve the flavor characteristics of DF and expand its application range of DF in food, researchers have adopted a series of methods and made significant contributions [8]. The crystallinity and particle size distribution of rice bran DF were changed by using cellulase, xylanase, and ball milling. Meanwhile, the water-holding capacity and oil-holding capacity of rice bran DF were decreased, while the swelling capacity and absorption of cholesterol and sodium taurocholate were enhanced. Niu et al. found that the alkaline method is the best method to increase the extraction rate of SDF in tomato peel, compared with the acid method and enzyme method. And the alkali-produced SDF has the smallest molecular weight and the strongest gel performance [9]. Fermentation has been proven to be an excellent method to change the quality of DF by microbial degradation of phytic acid, macromolecular polysaccharides, proteins, etc. [10]. Marques et al. found that using wheat bran as a substrate could induce endophytic fungi to produce cellulose [11].
Monascus anka (M. anka) is a nonpathogenic filamentous saprophytic fungus commonly used in food fermentation [12]. M. anka fermentation produces Monascus pigment, a widely employed natural pigment that not only possesses antioxidant properties but also can effectively inhibit the growth of bacteria and other microorganisms. The preparation of dietary fiber by microbial fermentation is an efficient method that is easy to adjust and control [13]. In comparison with other microbial fermentation methods for producing dietary fiber, M. anka offers a variety of substrate selections and can be used as the fermentation substrate for grains or agricultural waste residues. Wheat bran is used as a fermentation substrate, and M. anka is inoculated for liquid fermentation. In this process, Monascus pigment can be obtained while wheat bran fiber is modified.
Different sources of dietary fiber have different physiological functions, and the characteristics of dietary fiber prepared by different methods are also quite different. Because the proportion of IDF and SDF in foods is different and has different properties, it is very necessary to analyze and characterize the various fibers in different foods [14]. The purpose of this study was to modify the dietary fiber in wheat bran fermented by M. anka. The effect of M. anka liquid fermentation on the characteristics of soluble and insoluble wheat bran dietary fiber was investigated.
2. Materials and Methods
2.1. Materials
All chemical reagents and enzymes employed in this experiment were purchased from Yili Biotechnology Co., Ltd., (Hefei, Anhui, China). The high-temperature α-amylase, protease, cellulase, and xylanase were purchased by Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China). Glucose, peptone, KH2PO4, and MgSO4·7H2O were purchased by Sinopharm Group Chemical Reagent Co., Ltd. (Shanghai, China). Wheat bran was purchased from local suppliers in Hefei. M. anka was acquired from the General Microbiological Culture Collection of China and stored in the Key Laboratory for Agricultural Products Processing of Anhui Province, Hefei University of Technology.
2.2. Expansion Culture of M. anka
M. anka seed broth (MSB) was cultured by activation of the laboratory-preserved M. anka species, inoculating in potato dextrose agar medium (potato 200 g, glucose 20 g, agar 20 g, peptone 10 g, and distilled water 1000 mL). The spores of M. anka were harvested using a platinum loop and subsequently suspended in sterile water to prepare a spore suspension. Dilute the spore suspension with sterile water to a concentration of 5 × l06 spores/mL. This was followed by inoculation of 10 mL (around 10 percent) of the above spore suspension into 100 mL of the sterile seed medium. The medium consists of glucose (30 g/L), peptone (15 g/L), KH2PO4 (3 g/L), and MgSO4 · 7H2O (1 g/L). The inoculated medium was maintained at 28 °C for 3 days while shaking at 160 rpm [15].
2.3. Preparation of Wheat Bran Dietary Fiber and M. anka Wheat Bran Dietary Fiber
Wheat bran dietary fiber (WDF) and M. anka wheat bran dietary fiber (MWDF) were prepared in Figure 1. The enzymatic hydrolysis process was carried out in 250 mL flasks containing 10 g wheat bran powder dissolved in 150 mL distilled water. After heating at 90 °C for 30 min. And then 20,000 U/g of high-temperature α-amylase was added to enzymatically hydrolyze in a thermostat water bath (HWS-26, Shanghai Yiheng Scientific Instrument Co., Ltd., Shanghai, China) at 95 °C, till the supernatant stopped turning blue on the addition of iodine solution. Next, 15,000 U/g proteases was added to the mixture and enzymatic hydrolysis was carried out in a water bath maintained at 60 °C for 30 min. Next, 20,000 U/g cellulases and 20,000 U/g xylanases were sequentially added to the mixture, with enzymatic hydrolysis at 45 °C and 55 °C for 1 h, respectively. After that, the enzymatic hydrolysate was thermally inactivated at 100 °C for 10 min. Finally, the mixture was centrifuged using a high-speed refrigerated centrifuge (BK-TGL-20MB, BIOBASE, Jinan, China) at a speed of 8000 rpm for 10 min to separate the supernatant (A) from the insoluble residue (B). The solution A underwent concentration by heating at 60 °C until reaching one-fourth of its initial volume. Subsequently, a four-fold volume of 95% ethanol was added to the concentrated solution, and the resulting mixture was maintained under static conditions for 24 h to allow complete precipitation. The precipitated mixture was then further concentrated to 25% of its volume at 4 °C. Finally, the processed material was subjected to freeze-drying and mechanical grinding to produce wheat bran soluble dietary fiber (WSDF). The insoluble residue B was similarly freeze-dried and smashed to be the wheat bran insoluble dietary fiber (WIDF). The mixture of WSDF and WIDF was collectively referred to as wheat bran dietary fiber (WDF).
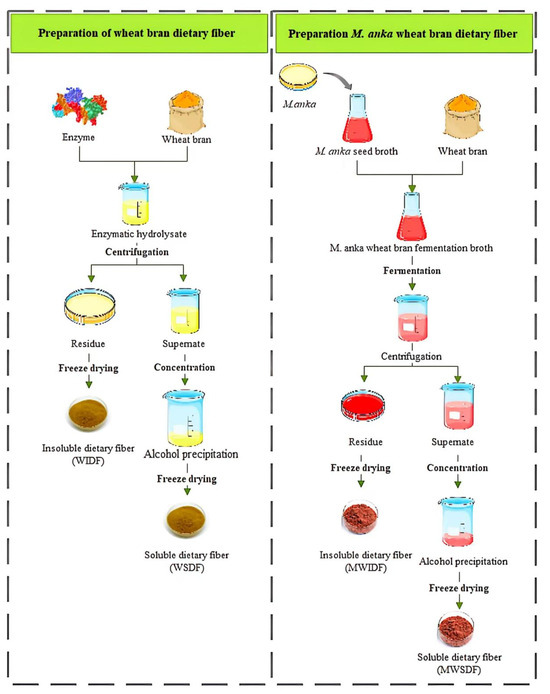
Figure 1.
Flowchart of preparing dietary fiber from wheat bran.
The fermentation substrate was conducted in 250 mL flasks, each with 150 mL of distilled water containing 2.25 g of glucose, 0.3 g of NH4SO4, and 0.2 g of K2HPO4. Then autoclaved (115 °C, 20 min) in a 250 mL flask. Subsequently, 15 mL of MSB suspension (approximately 10%, 5 × 106 spores/mL) was aseptically transferred into the fermentation flask. The culture was maintained under dark conditions at 28 °C and 160 rpm for 6 days. The resulting M. anka wheat bran fermentation broth (MWFB) was sterilized by autoclaving at 1 × 105 Pa for 20 min. M. anka wheat bran soluble (MWSDF) and insoluble dietary fiber (MWIDF) fractions were obtained using the same methods as those described above for WSDF and WIDF. The mixture of MWSDF and MWIDF collectively represents the M. anka wheat bran dietary fiber (MWDF).
2.4. Scanning Electron Microscopy (SEM)
The Jeol JSM-6490LV scanning electron microscope (Japan Electronics Manufacturing, Tokyo, Japan) was used to measure the morphology of WIDF and MWIDF, with the same method in reference to Sun [15]. Using a vacuum evaporator, Using a vacuum evaporator, the sample was placed on a conductive strip and coated with thin gold. For visualization, the SEM was set to a voltage of 20 kV, and magnifications of 500× and 1000× were used.
2.5. X-Ray Crystallography
The structure and crystalline phases of WIDF and MWIDF were determined using the same method described by Sun [15]. X-ray diffractometry (XRD) analysis for WIDF and MWIDF was carried out using a D-MAX 2500 V X-ray (Rigaku, Tokyo, Japan) diffractometer. The measurement conditions were 18 kW benchtop high-frequency X-ray generators with a rotating target, a tube voltage of 40 kV, a scanning range of 5–90°, and a step size of 0.02°; the X-ray source was Cu.
2.6. Fourier Transform Infrared Spectrum (FT-IR)
Structural modifications in SDF and IDF following fermentation were analyzed by Fourier-transform infrared spectroscopy using a Nicolet 6700 spectrometer (Thermo Fisher Scientific, Waltham, MA, USA). Mixed the sample thoroughly with potassium bromide (KBr) powder and compressed it into transparent flakes. Spectral acquisition was conducted across the mid-infrared range (4000–400 cm−1) at 1.0 cm−1 resolution, enabling identification of characteristic functional groups through spectral interpretation.
2.7. Thermal Properties
The thermal properties of SDF and IDF of wheat bran were analyzed by a differential scanning calorimeter DSC Q2000 (The NETZSCH, Selb, Germany). For DSC analysis, precisely weighed samples (3 mg) were loaded into aluminum crucibles and sealed with perforated lids. An empty aluminum pan served as the reference during calibration with the indium standard. Thermal scanning was performed from 0 to 300 °C at a constant heating rate of 10 °C/min under controlled atmosphere.
2.8. Composition and Functional Properties of WDF and MWDF
The water-holding capacity (WHC) of MWDF and WDF was determined by the method described by Guan et al. [16]. Different preparations of wheat bran dietary fiber were hydrated in 30 mL ultrapure water with continuous agitation for 24 h at ambient temperature. Following saturation, the samples were centrifuged at 4000 rpm for 15 min to separate the hydrated fraction. The WHC was determined gravimetrically by calculating the mass difference between the original dry weight of the sample (W1) and the weight of the residue (W2) using the standard formula.
The oil-holding capacity (OHC) of MWDF and WDF was measured by the methods of Moczkowska et al. [17]. The methods described in the previous work [18] were used to determine the swelling capacity (SC) of MWDF and WDF. For oil-binding capacity determination, wheat bran dietary fiber was mixed with olive oil at room temperature for 1 h. The initial dry mass (m1) and post-centrifugation residue (4000 rpm, 15 min; m2) were recorded for quantitative analysis. Wheat bran dietary fiber (0.2 g) prepared in different methods was put into a 10 mL sealed graduated cylinder with 5 mL of ultrapure water. Following 24 h incubation at 4 °C, the final hydrated volume (V) was measured for subsequent calculations. OHC and SC were calculated by the following equations:
The Coomassie brilliant blue method was used to determine the protein content of wheat bran dietary fiber, and the SZF-06C crude fat analyzer (Zhejiang Top Instrument Co., Ltd., Hangzhou, China) was used to determine its fat content. And the DNS method was used to determine the starch content in wheat bran dietary fiber.
2.9. Quantification of Pigments in MSB and MWFB
A total of 10 mL of MSB and MWFB samples were added to 90 mL of 75% ethanol, respectively. The mixture was kept in a 60 °C constant-temperature water bath for 1 h. After the reaction was complete, 1 mL of the extract mixed with 9 mL of 75% ethanol was transferred to a 10 mL centrifuge tube, and the mixture was shaken well and stood for 10 min. The absorption spectra of the two fermentation broths were recorded on a TU-1901 dual beam UV–Vis spectrophotometer (Zhongke Zhongjian Scientific Instrument Co., Ltd., Guangzhou, China) in the wavelength range from 300 to 600 nm using a 1 cm cuvette (75% ethanol was as control). The full-wavelength scanning spectrum was used to determine the optical density of Monascus pigments [19], and the color values corresponding to the Monascus pigments present in MSB and MWFB were calculated through OD value and dilution factor multiplication [20].
2.10. HPLC-MS of Monascus Pigments in MWFB
The MWFB described in 2.3 was filtered to obtain the filter residue. Then 200 mL of ethanol was added to the filter residue (20 g). The mixture was sonicated for 30 min and then filtered to discard the filter residue. Afterward, the filtrate was then concentrated into a paste under reduced pressure and dissolved in 10 mL methanol. Finally, the sample solution for further analysis was filtered by a 0.22-micron organic membrane.
LC-CAD-ESI/MS was performed with a Thermo Scientific Vanquish Q Exactive Plus (Thermo Fisher Scientific, Dreieich, Germany), fitted with a CAD detector and Orbitrap triple quadrupole mass spectrometer. The sample solution was separated on an ACCUCORE C30 column (2.1 mm × 100 mm, 2.6 µm) with a flow rate of 0.25 mL/min. The temperature of the sample and column was 45 °C, and the total run time was 15 min. The mobile phase consists of methanol (A) and water (B), and the elution mode of the mobile phase was as follows: 0–14 min 70% A; 14–14.1 min 95% A; 14.1–15 min 70% A. The pigment was detected by a triple quadrupole detector equipped with an electrospray ionization (ESI) source (mass number scanning range of 100–1500 m/z). All mass spectrometry measurements were performed in positive ion mode [21].
2.11. Statistical Analysis
Each trial was run in parallel three times, and statistical analysis of the acquired data was performed using the SPSS software V19.0 (IBM, Armonk, NY, USA). Prior to ANOVA, the normality of data distribution was confirmed by Shapiro–Wilk test (p > 0.05), and homogeneity of variances was verified by Levene’s test (p > 0.05). One-way ANOVA followed by Duncan’s new multiple range test was adopted for multiple comparisons, as this method is particularly suitable for exploratory studies requiring sensitive detection of differences between group means with equal sample sizes. Statistical significance was defined at p < 0.05.
3. Results and Discussion
3.1. Microstructure
To further evaluate the performance of the different methods for extracting wheat bran IDFs, the microstructure of WIDF and MWIDF was observed under a scanning electron microscope (SEM) as illustrated in Figure 2. The figure indicated that WIDF had a regular shape and size, with an intact and dense fiber surface. However, the MWIDF has a loose structure, irregular protruding surface, rough edges, and a large number of micropores, which destroy the layered structure of MWIDF. M. anka liquid fermentation promoted the cracking and detachment of the IDF surface, resulting in a discontinuous and loose fiber matrix, exposing a large number of its internal structures. Looser structure with larger specific surface area and porosity in the internal structure, which would lead to improving the hydration properties of MWIDF [22]. This phenomenon may result from the action of cellulase, hemicellulase, and protease produced during the fermentation process on insoluble dietary fiber, which causes the destruction of the plant cell wall structure.
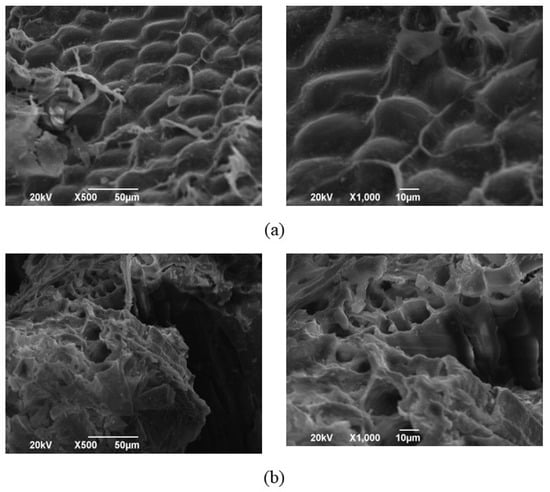
Figure 2.
The scanning electron microscopy pictures of WIDF (a) and MWIDF (b). Samples were systematically observed at 500× (left) and 1000× (right) magnification levels.
3.2. X-Ray Diffraction Analysis
Figure 3 showed the XRD patterns of WIDF and MWIDF. In XRD analysis, sharper diffraction peaks indicate higher crystallinity, which is positively associated with enhanced mechanical properties such as tensile strength and material hardness [23]. Both WIDF and MWIDF exhibited strong diffraction peaks at 21.8° in the diffraction direction, indicating the characteristic cellulose I crystalline configuration in WIDF. The sharper peak observed in WIDF compared to MWIDF suggests that the fermentation using M. anka led to a reduction in the crystallinity of IDF [15]. The broadening of the MWIDF diffraction peak may originate from enzymatic hydrolysis-induced cleavage of partial β-1, 4-glycosidic bonds and disruption of hydrogen bonds, which leads to crystal defects, increased intermolecular chain spacing, or disordered arrangement of microfibrils. During M. anka fermentation of wheat bran, enzymes like cellulases, proteases, and lipases were crucial as they degrade phytic acid, polysaccharides, proteins, and fats in wheat bran. This led to the structural modification of WIDF. Exposure of the internal structure promoted enzymatic hydrolysis in the crystalline region, which was the primary factor for the decrease in the crystallinity of MWIDF. This was consistent with the observations of the scanning electron microscopy pictures of WIDF and MWIDF.
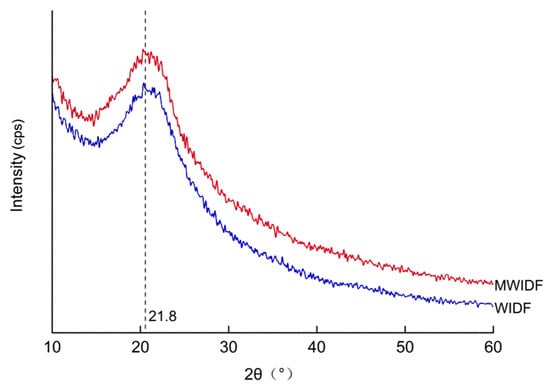
Figure 3.
X-ray diffraction diagrams of WIDF and MWIDF. 2θ: diffraction angle.
3.3. FT-IR Analysis
Figure 4 showed the FT-IR spectrum of wheat bran SDF and IDF, which could explain the functional groups and structure information of wheat bran dietary fiber prepared by M. anka fermentation and without fermentation. Both SDF and IDF preparations exhibited characteristic O-H stretching vibrations at 3266 cm−1, which is attributed to polysaccharide hydroxyl groups. Compared with SDF and IDF prepared by non-fermented, the peak intensity of SDF and IDF produced by M. anka was lower, and the peak shifted to a lower wave number, which indicated the degradation of polysaccharides and the breakage of hydrogen bonds in cellulose and hemicellulose. And this led to the bond length being longer and the characteristic frequency decreasing. The 2924 cm−1 band was attributed to the C-H stretch band from the polysaccharide methylene. The characteristic absorption at 1730 cm−1 represents C=O stretching vibrations from ester linkages in lignin and hemicellulose structures. The peak at 1638 cm−1 was due to the bending or stretching of the aromatics of lignin. The peak intensity of the IDF produced by M. anka liquid fermentation at this point became weaker, indicating the degradation of lignin. The 1420 cm−1 band represents crystalline region absorption. After fermentation treatment by M. anka, the absorption peaks of SDF and IDF in wheat bran were obviously enhanced. Characteristic oligosaccharide absorptions at 1050 cm−1 and 890 cm−1 were observed in both wheat bran SDF and IDF spectra [24]. The peak intensities of SDF and IDF obtained after fermentation by M. anka were increased, indicating that the polysaccharides in the dietary fiber were enzymatically hydrolyzed. The long-chain molecules were broken and degraded into small-molecule polysaccharides or oligosaccharides. Proteases could hydrolyze the proteins bound to the dietary fiber, disrupt the interactions between proteins and polysaccharides, and further promote the alteration of the dietary fiber structure.
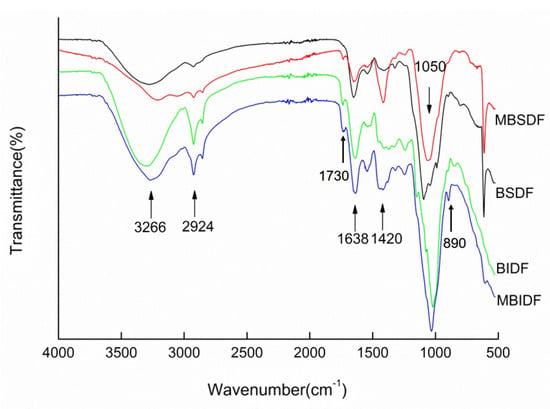
Figure 4.
FT-IR spectrum of wheat bran SDF and IDF.
3.4. Thermal Properties
Differential scanning calorimetry (DSC) can be used to determine the thermodynamic properties of substances and reflect the various phase transition processes of samples. It is widely used in the analysis of inorganic substances, organic substances, and drugs [25]. Figure 5 showed the thermal properties of WSDF, MWSDF, WIDF, and MWIDF. WSDF and MWSDF thermograms displayed endothermic peaks near 100 °C, attributed to water molecule liberation from the SDF matrix. In Figure 5, the endothermic peak of MWSDF was significantly enhanced and moved to a high-temperature direction, which indicated that MWSDF had better hydration performance, thermal stability, and phase transition characteristics. DSC thermograms of WIDF and MWIDF exhibited characteristic endothermic peaks near 70 °C, corresponding to oligosaccharide melting peaks. The melting peak of MWIDF shifted to a low temperature, indicating that MWIDF was degraded by M. anka to produce more oligosaccharides than that in WIDF.
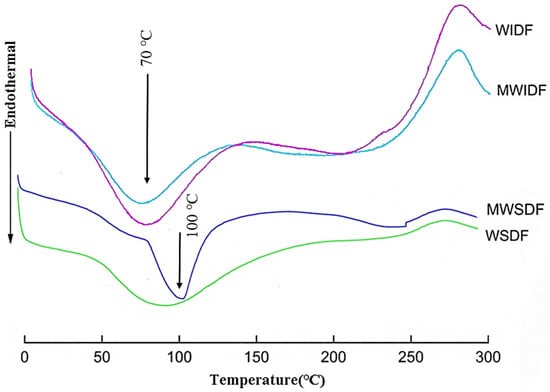
Figure 5.
Differential scanning calorimetry curves of wheat bran SDF and IDF.
3.5. Changes in Composition and Functional Properties of Wheat Bran Dietary Fiber
Table 1 showed the composition and functional properties of WDF and MWDF. High WHC can change the viscosity and texture of some foods, which helps maintain the shape of the food. The high oil-holding power of dietary fiber is also important in food applications. In food processing, it can prevent the loss of fat and affect the regulation of human intestinal absorption [26]. Fermentation enhanced the WHC and OHC of wheat bran dietary fiber, which might be attributed to the disruption of glycosidic bonds. These structural modifications, coupled with the increased SDF content, created additional binding sites for water and oil molecules [27]. WDF was obtained by enzymatic treatment. Enzymatic hydrolysis disrupted cellulose-hemicellulose hydrogen bonding, altering absorption of hydrated hydroxides, carboxyl group absorption, and capillary dynamics. Therefore, WDF also had good WHC and OHC. However, the layered structure of insoluble dietary fiber was destroyed, and the specific surface area and porosity were increased by M. anka fermentation, which was beneficial to the contact between dietary fiber and water and oil. At the same time, the reduction in the content of protein and fat indicates that fermentation hydrolyzed these components, thereby decreasing the steric hindrance around WDF. This likely increased the accessibility to hydrophilic groups, enhancing WHC and SC [28]. The decrease in starch content may reduce competition for water absorption, thereby improving WHC. M. anka fermentation metabolized wheat bran nutrients, generating lots of short-chain fatty acids that enhanced lipid absorption capacity, thereby improving MWDF oil retention properties [29]. Dietary fiber expansion properties directly correlate with its physical configuration. Compared with WDF, MWDF had more micropores and stronger water absorption. Amino acids or small peptides released by protease may also be involved in these functional changes. Moreover, the increase of SDF content in MWDF contributes to the improvement of water solubility, resulting in a larger swelling volume [30].

Table 1.
Composition and functional properties of WDF and MWDF.
3.6. Pigment Content in MSB and MWFB
The full-wavelength scan and color value measurement results of MSB and MWFB were shown in Table 2. The full-wavelength scan manifested three characteristic absorption peaks of red (532 nm) Monascus pigments (RMPs), orange (410 nm) Monascus pigments (OMPs), and yellow (370 nm) Monascus pigments (YMPs). After wheat bran was added, no OMPs absorption peak was detected in the fermentation broth because part of the OMPs was converted into RMPs and YMPs. It is generally believed that the color value of a pigment is positively related to its content [31]. Therefore, the color value of the pigment can be used to analyze the pigment content produced by M. anka from wheat bran to preliminarily explore the effect of wheat bran on the characteristics of Monascus pigments produced by M. anka. As illustrated in Table 2, the color values of RMPs, OMPs, and YMPs in MSB were similar, which are 143.8 μ mL−1, 142.7 μ mL−1, and 128.4 μ mL−1, indicating that MSB contained the same amount of three Monascus pigments. There were absorption peaks of RMPs and YMPs in MWFB, so the two color values in MWFB were investigated. The color values of RMPs and YMPs in MWFB are 154.4 μ mL−1 and 236.6 μ mL−1, respectively. The contents of RMPs and YMPs in MWFB were increased. Therefore, the type and content of pigments in the fermentation broth changed after inoculating M. anka in wheat bran. And YMPs were the main pigment product in MWFB after fermentation of wheat bran by M. anka. The increased content of YMPs aligns with the findings of Li et al., which may be attributed to the nitrogen source in the wheat bran fermentation process being more conducive to the production of yellow pigments [32]. The study by Moradi et al. also demonstrated that abundant nitrogen sources positively influence the production of Monascus pigments [33].

Table 2.
Effect of wheat bran on the Monascus pigments properties of M. anka fermentation.
3.7. HPLC-MS Analysis
The composition of Monascus pigments is quite complex, and the colors of Monascus pigments are divided into three categories: red, orange, and yellow. Several Monascus pigments were recognized and determined by numerous advanced instruments and methods. Six pigments identified so far are listed in Table 3 [34].

Table 3.
Information on six Monascus pigments.
The samples of MWFB were analyzed by HPLC-MS equipped with a CAD detector. Several main peaks were observed after separation by chromatographic column (Figure 6). According to the comparative analysis of the spectra in the control by HPLC-CAD and HPLC-MS, molecular formulae of C23H27NO4 (calculated m/z at 382.2012 [M+H]+), C21H26O5 (calculated m/z at 359.1853 [M+H]+), and C23H30O5 (calculated m/z at 387.2166 [M+H]+) were detected. They were monascorubramine with a retention time of 2.62 (observed. m/z 382.2007 [M+H]+, m/z 420.1568 [M+K]+) (Figure 7a), monascine with a retention time of 3.64 (observed. m/z 359.1853 [M+H]+, m/z 397.1394 [M+K]+) (Figure 7b), and ankaflavine with a retention time of 5.92 (observed. m/z 387.2151 [M+H]+, m/z 409.1974 [M+Na]+, m/z 425.1708 [M+K]+) (Figure 7c), respectively. It indicates that MWFB contains two kinds of YMPs: monasine and ankaflavin, and one RMP: monascorubrin. This was in accordance with the results in Table 2.
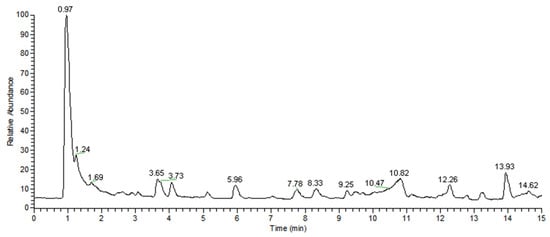
Figure 6.
Total ion chromatographic of MWFB pigments.
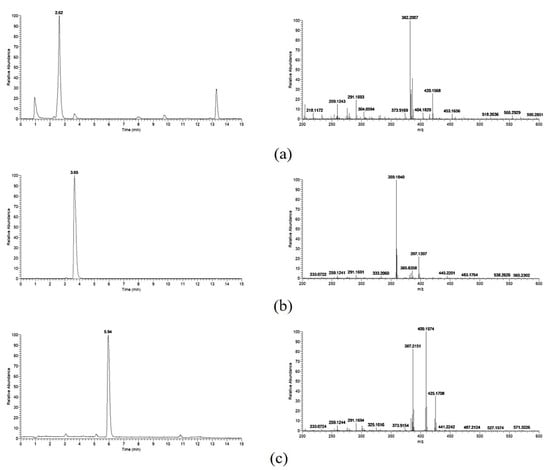
Figure 7.
Retention time and MS spectrum of monascorubramine (a), monascine (b), and ankaflavine (c).
Six Monascus pigments and several other metabolites are known to be present in Monascus rice. Zheng et al. detected six known pigments and two unknown yellow pigments in rice samples fermented by Monascus [35]. However, other pigments in MWFB have not been detected except for the above three pigments in this study. Environmental conditions significantly influence Monascus metabolic activity. When wheat bran was added to the Monascus fermentation broth, the Monascus pigment may have undergone some chemical reaction. For example, the pyran ring of the orange pigment could react with the amine to complete the O-to-N substitution [36], thus converting the OMPs to the RMPs. L. Martinkova et al. [37] studied the bioactivity of Monascus pigments. Their results showed that YMPs were less toxic than OMPs. The teratogenic effect of the YMPs on the embryo was less, and ankaflavin can selectively show toxicity to cancer cells [38]. Therefore, YMPs can be used as a functional natural colorant. The results obtained from UV–Vis (Figure A1) spectroscopy and HPLC-MS analysis demonstrated a decrease in the content of OMPs and a concomitant increase in YMPs in MWFB. Therefore, wheat bran is a good substrate for the fermentation to produce YMPs.
4. Conclusions
The fermentation of wheat bran with M. anka results in structural and physicochemical changes in its dietary fiber and yields a pro-health MWDF with high SDF content. The M. anka fermentation treatment changed the structure of IDF and SDF in wheat bran and increased the content of oligosaccharides in MWDF. MWDF exhibits many remarkable physicochemical characteristics, such as excellent WHC, OHC, and SC. In addition, MWFB also contains Monascus pigments; two yellow pigments, monasine and ankaflavin, and one red pigment, monascorubin, were found in it. Therefore, MWDF is an excellent value-added dietary fiber product transformed by biotechnology. This study primarily focused on the physicochemical properties of MWDF, while its health benefits remain to be explored to facilitate the development and utilization of related fortified foods. Future research will further investigate mixed fermentation strategies to enhance the yield of target products while minimizing the formation of by-products.
Author Contributions
Conceptualization, X.W. and S.H.; methodology, S.H., H.X. and L.L.; software, H.X. and L.L.; validation, X.W., S.H. and Z.L.; formal analysis, S.H.; investigation, S.H., H.X. and L.L.; resources, X.W., H.X., H.Z. and X.L.; data curation, S.H.; writing—original draft preparation, S.H.; writing—review and editing, X.W., D.M., M.Z., H.Z. and X.L.; visualization, J.C.; supervision, X.W., Z.L., D.M. and X.L.; project administration, X.W.; funding administration, X.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Project of Anhui Province [grant numbers 2023n06020049, 202423110050044, and 2308085MC112] and the Project of Suzhou City in Anhui Province [grant number SZSZDZX2023078].
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed at the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
Appendix A
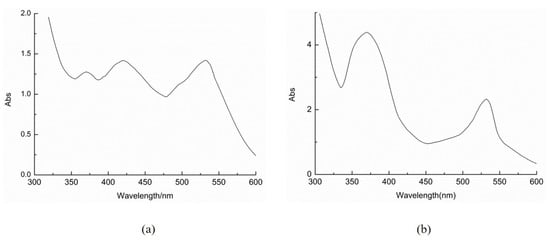
Figure A1.
UV–visible spectrum of Monascus pigments in MSB (a) and MWFB (b).
References
- Mocanu, V.; Madsen, K.L. Dietary fibre and metabolic health: A clinical primer. Clin. Transl. Med. 2024, 14, e70018. [Google Scholar] [CrossRef] [PubMed]
- Pathania, S.; Kaur, N. Utilization of fruits and vegetable by-products for isolation of dietary fibres and its potential application as functional ingredients. Bioact. Carbohydr. Diet. Fibre 2022, 27, 100295. [Google Scholar] [CrossRef]
- Wu, L.; Tang, C.; Chen, L.; Zhao, J. Modified dietary fiber from soybean dregs by fermentation alleviated constipation in mice. Food Chem. X 2023, 19, 100810. [Google Scholar] [CrossRef]
- Delcour, J.A.; Aman, P.; Courtin, C.M.; Hamaker, B.R.; Verbeke, K. Prebiotics, Fermentable Dietary Fiber, and Health Claims. Adv. Nutr. 2016, 7, 1–4. [Google Scholar] [CrossRef]
- Cingöz, A.; Akpinar, Ö.; Sayaslan, A. Effect of addition of wheat bran hydrolysate on bread properties. J. Food Sci. 2024, 89, 2567–2580. [Google Scholar] [CrossRef]
- Ma, S.; Wang, Z.; Liu, H.; Li, L.; Zheng, X.; Tian, X.; Sun, B.; Wang, X. Supplementation of wheat flour products with wheat bran dietary fiber: Purpose, mechanisms, and challenges. Trends Food Sci. Technol. 2022, 123, 281–289. [Google Scholar] [CrossRef]
- Li, X.; Wang, B.; Hu, W.; Chen, H.; Sheng, Z.; Yang, B.; Yu, L. Effect of γ-irradiation on structure, physicochemical property and bioactivity of soluble dietary fiber in navel orange peel. Food Chem. X 2022, 14, 100274. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Lei, H.; Zhen, X.; Liu, J.; Xie, W.; Tang, Q.; Gou, D.; Zhao, J. Advancements in modifying insoluble dietary fiber: Exploring the microstructure, physicochemical properties, biological activity, and applications in food industry—A review. Food Chem. 2024, 458, 140154. [Google Scholar] [CrossRef]
- Niu, Y.; Li, N.; Xia, Q.; Hou, Y.; Xu, G. Comparisons of three modifications on structural, rheological and functional properties of soluble dietary fibers from tomato peels. LWT 2018, 88, 56–63. [Google Scholar] [CrossRef]
- Chen, Q.; Wang, R.; Wang, Y.; An, X.; Liu, N.; Song, M.; Yang, Y.; Yin, N.; Qi, J. Characterization and antioxidant activity of wheat bran polysaccharides modified by Saccharomyces cerevisiae and Bacillus subtilis fermentation. J. Cereal Sci. 2021, 97, 103157. [Google Scholar] [CrossRef]
- Marques, N.P.; de Cassia Pereira, J.; Gomes, E.; da Silva, R.; Araújo, A.R.; Ferreira, H.; Rodrigues, A.; Dussán, K.J.; Bocchini, D.A. Cellulases and xylanases production by endophytic fungi by solid state fermentation using lignocellulosic substrates and enzymatic saccharification of pretreated sugarcane bagasse. Ind. Crops Prod. 2018, 122, 66–75. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Yan, W.; Wang, P.; Li, J.; Lu, Y. Light stability and mechanism of monascus pigment under different lights. LWT 2024, 191, 115666. [Google Scholar] [CrossRef]
- Zhang, S.; Shu, M.; Gong, Z.; Liu, X.; Zhang, C.; Liang, Y.; Lin, Q.; Zhou, B.; Guo, T.; Liu, J. Enhancing extracellular monascus pigment production in submerged fermentation with engineered microbial consortia. Food Microbiol. 2024, 121, 104499. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xiong, M.; Bai, T.; Chen, D.; Zhang, Q.; Lin, D.; Liu, Y.; Liu, A.; Huang, Z.; Qin, W. Comparative study on the structure, physicochemical, and functional properties of dietary fiber extracts from quinoa and wheat. LWT 2021, 149, 111816. [Google Scholar] [CrossRef]
- Sun, C.; Wu, X.; Chen, X.; Li, X.; Zheng, Z.; Jiang, S. Production and characterization of okara dietary fiber produced by fermentation with Monascus anka. Food Chem. 2020, 316, 126243. [Google Scholar] [CrossRef]
- Guan, Y.; Xie, C.; Zhang, R.; Zhang, Z.; Tian, Z.; Feng, J.; Shen, X.; Li, H.; Chang, S.; Zhao, C.; et al. Characterization and the cholesterol-lowering effect of dietary fiber from fermented black rice (Oryza sativa L.). Food Funct. 2023, 14, 6128–6141. [Google Scholar] [CrossRef]
- Moczkowska, M.; Karp, S.; Niu, Y.; Kurek, M.A. Enzymatic, enzymatic-ultrasonic and alkaline extraction of soluble dietary fibre from flaxseed—A physicochemical approach. Food Hydrocoll. 2019, 90, 105–112. [Google Scholar] [CrossRef]
- Bai, X.; He, Y.; Quan, B.; Xia, T.; Zhang, X.; Wang, Y.; Zheng, Y.; Wang, M. Physicochemical properties, structure, and ameliorative effects of insoluble dietary fiber from tea on slow transit constipation. Food Chem. X 2022, 14, 100340. [Google Scholar] [CrossRef]
- Yang, X.; Dong, Y.; Liu, G.; Zhang, C.; Cao, Y.; Wang, C. Effects of nonionic surfactants on pigment excretion and cell morphology in extractive fermentation of Monascus sp. NJ1. J. Sci. Food Agric. 2020, 100, 1832. [Google Scholar] [CrossRef]
- Huang, Z.; Hu, T.; Liu, H.; Xie, H.; Tian, X.; Wu, Z. Biosynthesis and polyketide oxidation of Monascus red pigments in an integrated fermentation system with microparticles and surfactants. Food Chem. 2022, 394, 133545. [Google Scholar] [CrossRef]
- Gong, P.; Shi, R.; Liu, Y.; Luo, Q.; Wang, C.; Chen, W. Recent advances in monascus pigments produced by Monascus purpureus: Biosynthesis, fermentation, function, and application. LWT 2023, 185, 115162. [Google Scholar] [CrossRef]
- Yang, B.; Li, K.; Niu, M.; Wei, J.; Zhao, S.; Jia, C.; Xu, Y. Structural characteristics of wheat bran insoluble dietary fiber with various particle size distributions and their influences on the kinetics of gastrointestinal emptying in mice. Int. J. Biol. Macromol. 2024, 272, 132905. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Yang, L.; Guo, J.; Zhang, X.; Huang, Y.; Fu, Q. Preparation, Structural Characterization, and Hypoglycemic Activity of Dietary Fiber from Sea Buckthorn Pomace. Foods 2024, 13, 3665. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Zheng, S.; Bai, T.; Chen, D.; Qin, W.; Zhang, Q.; Lin, D.; Liu, Y.; Liu, A.; Huang, Z.; et al. The difference among structure, physicochemical and functional properties of dietary fiber extracted from triticale and hull-less barley. LWT 2022, 154, 112771. [Google Scholar] [CrossRef]
- Li, S.; Hu, N.; Zhu, J.; Zheng, M.; Liu, H.; Liu, J. Influence of modification methods on physicochemical and structural properties of soluble dietary fiber from corn bran. Food Chem. X 2022, 14, 100298. [Google Scholar] [CrossRef]
- Tang, C.; Wu, L.; Zhang, F.; Kan, J.; Zheng, J. Comparison of different extraction methods on the physicochemical, structural properties, and in vitro hypoglycemic activity of bamboo shoot dietary fibers. Food Chem. 2022, 386, 132642. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, X.; Gao, Z.; Zhang, M.; Mu, S.; Cheng, Y.; Qu, K. Effects of modification methods on the structural characteristics and functional properties of dietary fiber from cucumber. Food Chem. X 2024, 24, 101808. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Li, Y.; Yu, H.; Wang, Y.; Piao, C. Insoluble dietary fibre from okara (soybean residue) modified by yeast Kluyveromyces marxianus. LWT 2020, 134, 110252. [Google Scholar] [CrossRef]
- Kang, J.; Yin, S.; Liu, J.; Li, C.; Wang, N.; Sun, J.; Li, W.; He, J.; Guo, Q.; Cui, S.W. Fermentation models of dietary fibre in vitro and in vivo—A review. Food Hydrocoll. 2022, 131, 107685. [Google Scholar] [CrossRef]
- Jiang, G.; Bai, X.; Wu, Z.; Li, S.; Zhao, C.; Ramachandraiah, K. Modification of ginseng insoluble dietary fiber through alkaline hydrogen peroxide treatment and its impact on structure, physicochemical and functional properties. LWT 2021, 150, 111956. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, W.; Guo, R.; Yu, J.; Wang, Y. Enhancement of yellow pigments production via high CaCl2 stress fermentation of Monascus purpureus. FEMS Microbiol. Lett. 2024, 371, fnae012. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Yu, W.; Li, A.; Wang, Y. Study on production of yellow pigment from potato fermented by Monascus. Food Biosci. 2022, 50, 102088. [Google Scholar] [CrossRef]
- Moradi, S.; Mortazavi, S.A. Evaluation of Monascus purpureus fermentation in dairy sludge-based medium for enhanced production of vibrant red pigment with minimal citrinin content. PLoS ONE 2024, 19, e0315006. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.-R.; He, M.-H.; Sang, Z.-P.; Dong, J.-H.; Ma, Q.-G. Structurally diverse Monascus pigments with hypolipidemic and hepatoprotective activities from highland barley Monascus. Fitoterapia 2022, 156, 105090. [Google Scholar] [CrossRef]
- Zheng, Y.; Xin, Y.; Guo, Y. Study on the fingerprint profile of Monascus products with HPLC–FD, PAD and MS. Food Chem. 2009, 113, 705–711. [Google Scholar] [CrossRef]
- Li, L.; Chen, S.; Gao, M.; Ding, B.; Zhang, J.; Zhou, Y.; Liu, Y.; Yang, H.; Wu, Q.; Chen, F. Acidic conditions induce the accumulation of orange Monascus pigments during liquid-state fermentation of Monascus ruber M7. Appl. Microbiol. Biotechnol. 2019, 103, 8393–8402. [Google Scholar] [CrossRef]
- Martinkova, L. Biological activities of oligoketide pigments of Monascus purpureus. Food Addit. Contam. 1999, 16, 15–24. [Google Scholar] [CrossRef]
- Tan, H.; Xing, Z.; Chen, G.; Tian, X.; Wu, Z. Evaluating Antitumor and Antioxidant Activities of Yellow Monascus Pigments from Monascus ruber Fermentation. Molecules 2018, 23, 3242. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).