Improving Organic Acid Secretion of Aspergillus niger by Overexpression C4-Dicarboxylic Acid Transporters
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Culture Condition
2.2. Phylogenetic Tree and Multiple Sequence Alignments of DCTs
2.3. Plasmid Construction
2.4. Transformation and Screening of Positive Strains
2.5. Shake Flask Cultivation
2.6. Transformants Cultured on Plate with Different Carbon Sources
2.7. RNA Extraction and cDNA Preparation for qRT-PCR
2.8. Scale-Up Experiments and Parameterisation of a 30 L Bioreactor
2.9. Analytical Methods
3. Results and Discussion
3.1. Amino Acid Multiple Sequence Alignment and Phylogenetic Tree Analysis
3.2. Construction of Overexpression Plasmid and Screening of Positive Transformants
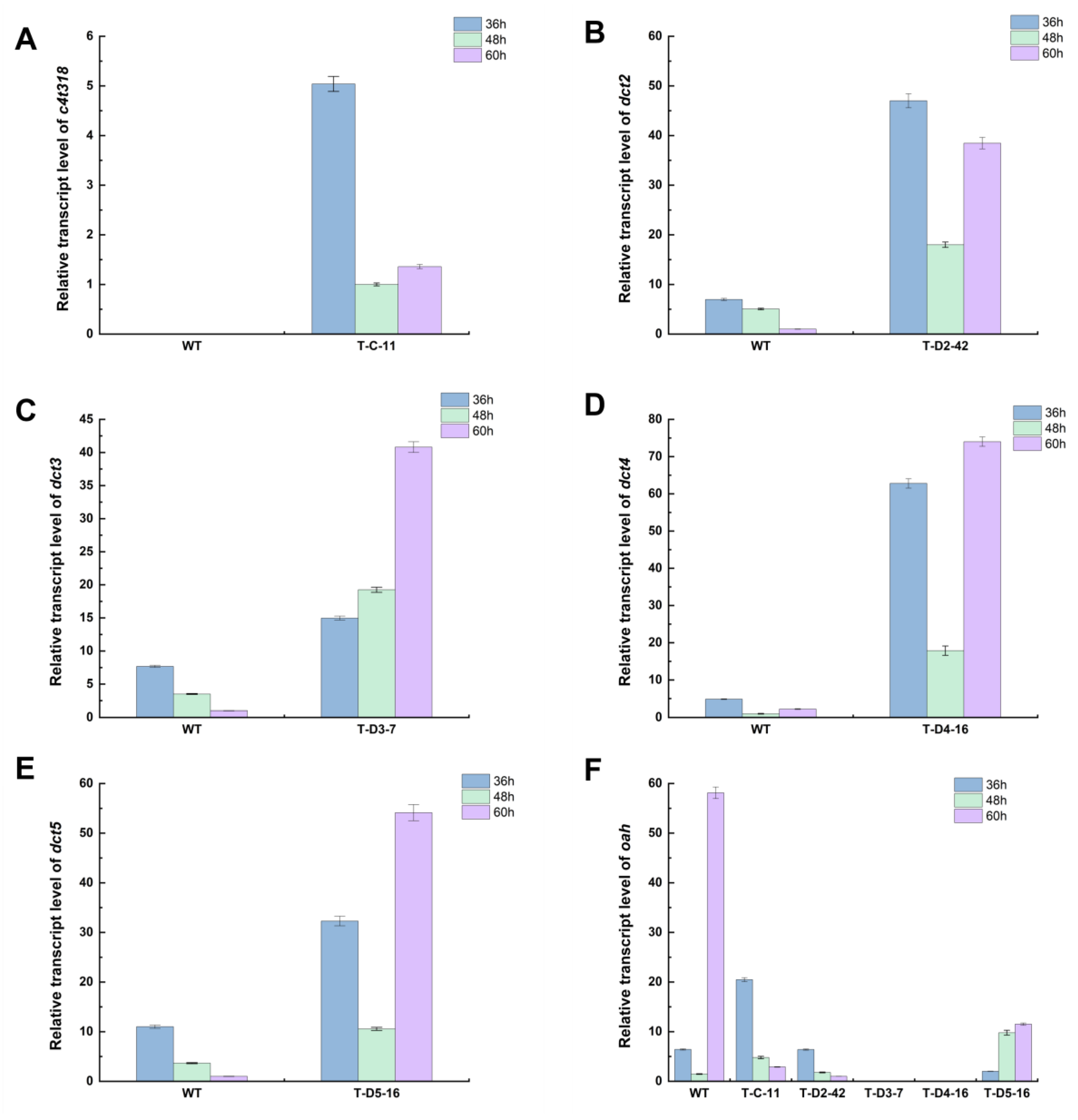
3.3. Analysis of Transcript Levels of Overexpressed Genes in Positive Transformants
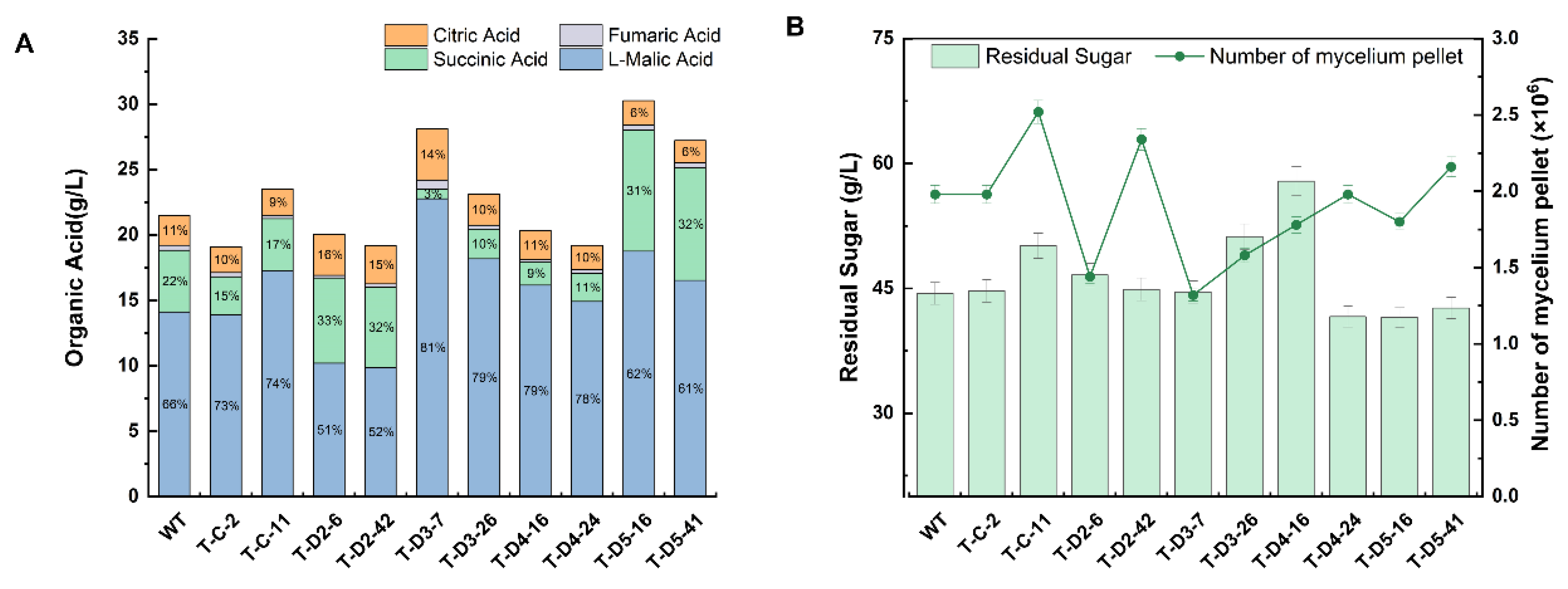
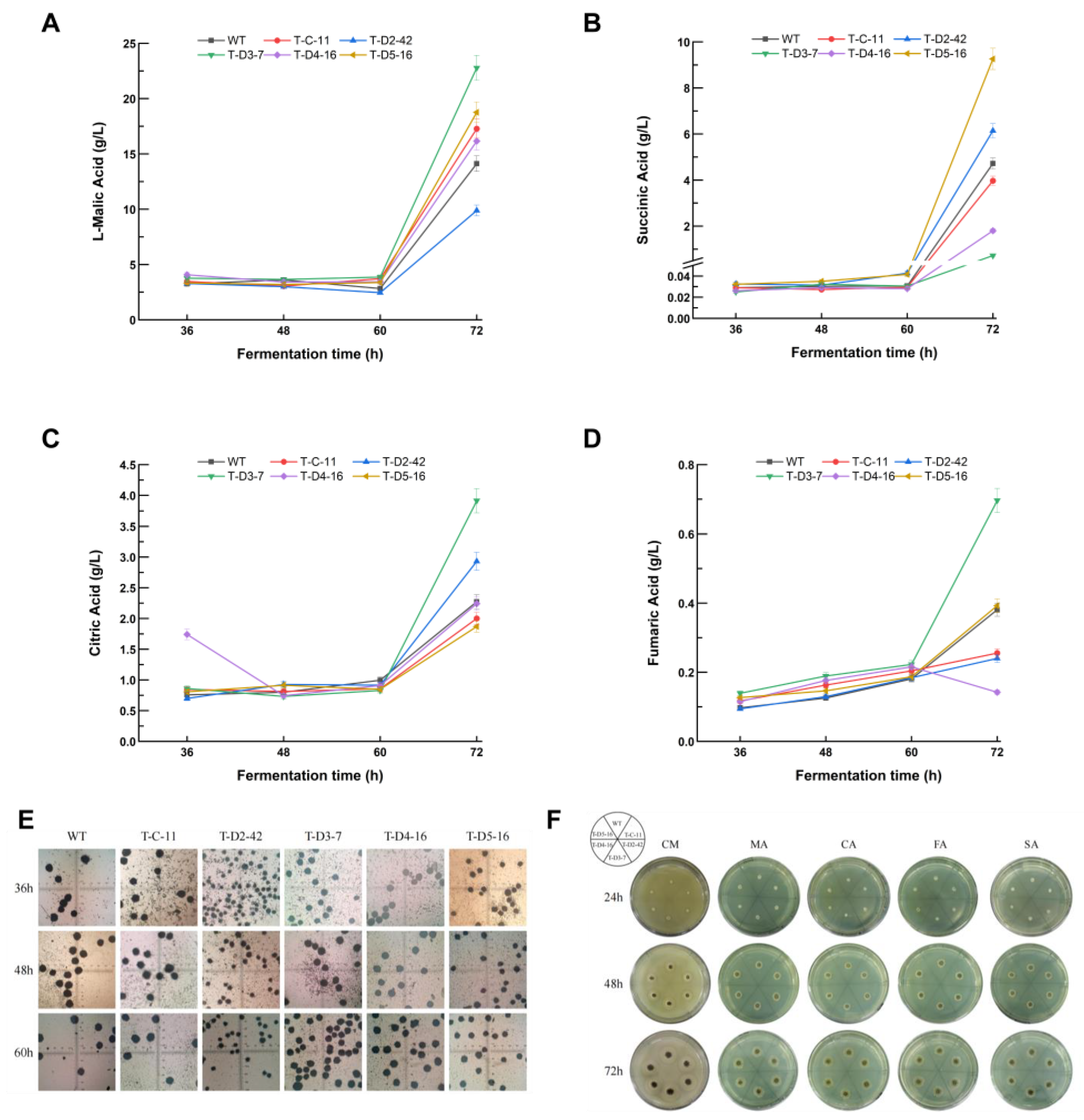
3.4. Activating C4-Dicarboxylate Transporter for Improving Organic Acid Production
3.5. Observation on the Morphology of Mycelium Pellets and Plate Colonies
3.6. qRT-PCR Analysis of Key Genes in Metabolic Pathway
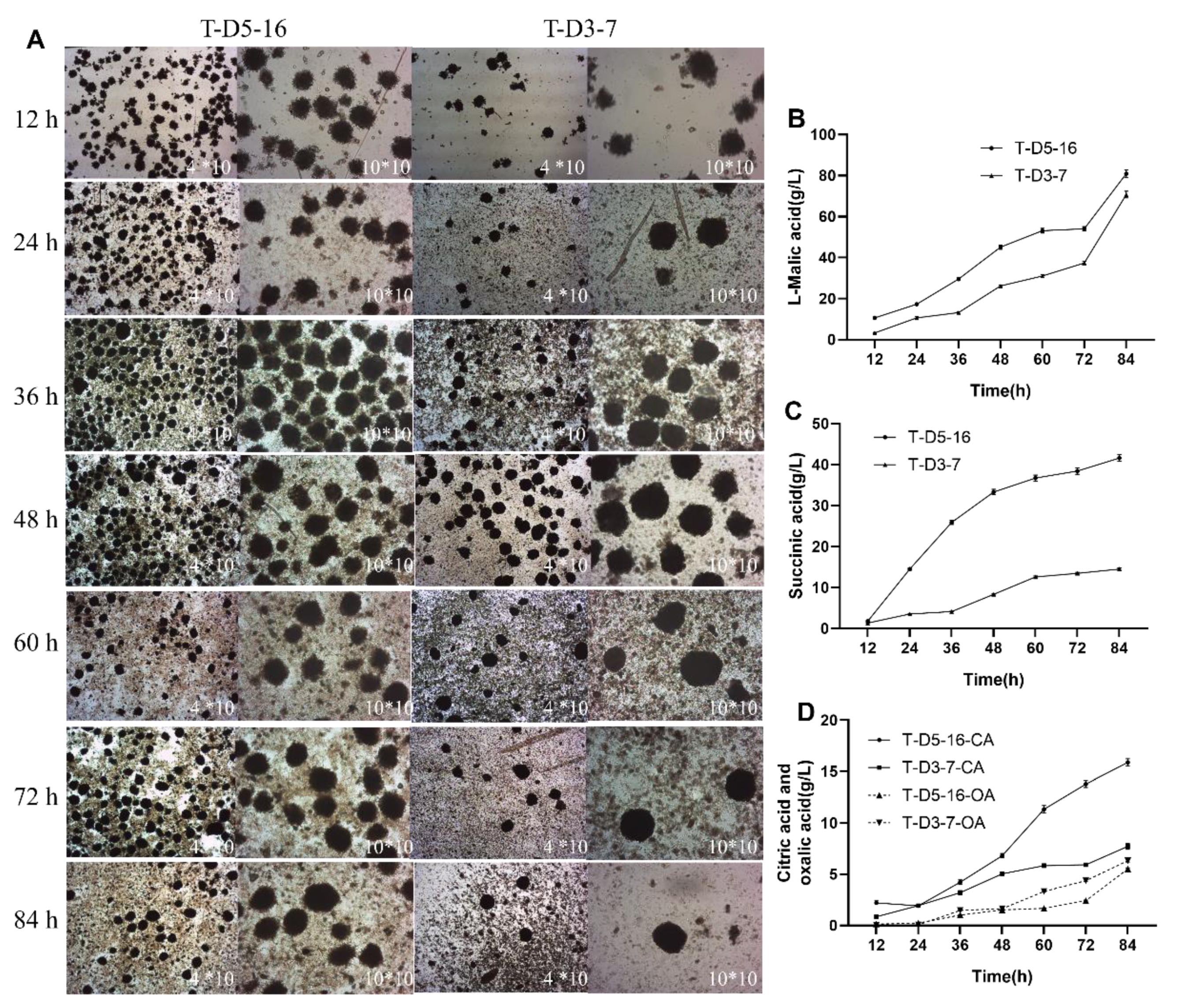
3.7. The Acid Production Capacity of the Transformants in 30 L Bioreactor and Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Luo, Y.; Yan, X.; Xia, Y.; Cao, Y. Tetracarboxylic acid transporter regulates growth, conidiation, and carbon utilization in Metarhizium acridum. Appl. Microbiol. Biotechnol. 2023, 107, 2969–2982. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tian, D.; Zhang, X.; Han, M.; Cheng, X.; Ye, X.; Zhang, C.; Gao, H.; Li, Z. The Regulation of Phosphorus Release by Penicillium chrysogenum in Different Phosphate via the TCA Cycle and Mycelial Morphology. J. Microbiol. 2023, 61, 765–775. [Google Scholar] [PubMed]
- Zhou, S.; Ding, N.; Han, R.; Deng, Y. Metabolic engineering and fermentation optimization strategies for producing organic acids of the tricarboxylic acid cycle by microbial cell factories. Bioresour. Technol. 2023, 379, 128986. [Google Scholar]
- Wu, J.; Li, Y.; Yin, J.; Wang, C.; Qi, X.; Zhou, Y.; Liu, H.; Wu, P.; Zhang, J. Mutation breeding of high-stress resistant strains for succinic acid production from corn straw. Appl. Microbiol. Biotechnol. 2024, 108, 278. [Google Scholar] [CrossRef]
- Khandelwal, R.; Srivastava, P.; Bisaria, V.S. Recent advances in the production of malic acid by native fungi and engineered microbes. World J. Microbiol. Biotechnol. 2023, 39, 217. [Google Scholar]
- Sebastian, J.; Dominguez, K.V.; Brar, S.K.; Rouissi, T. Fumaric acid production using alternate fermentation mode by immobilized Rhizopus oryzae-a greener production strategy. Chemosphere 2021, 281, 130858. [Google Scholar]
- Chu, Q.; Sun, S.; Xing, X. Transcriptional analysis and key genes associated with sugar, amino acid and organic acid metabolism in Saccharomyces cerevisiae during wine-making combined with Torulaspora delbrueckii. LWT 2024, 198, 116007. [Google Scholar]
- Rendulić, T.; Perpelea, A.; Ortiz, J.P.R.; Casal, M.; Nevoigt, E. Mitochondrial membrane transporters as attractive targets for the fermentative production of succinic acid from glycerol in Saccharomyces cerevisiae. FEMS Yeast Res. 2024, 24, foae009. [Google Scholar]
- Li, K.; Li, C.; Zhao, X.Q.; Liu, C.G.; Bai, F.W. Engineering Corynebacterium glutamicum for efficient production of succinic acid from corn stover pretreated by concentrated-alkali under steam-assistant conditions. Bioresour. Technol. 2023, 378, 128991. [Google Scholar]
- Khandelwal, R.; Srivastava, P.; Bisaria, V.S. Expression of Escherichia coli malic enzyme gene in Zymomonas mobilis for production of malic acid. J. Biotechnol. 2022, 351, 23–29. [Google Scholar]
- Xiao, H.; Shao, Z.; Jiang, Y.; Dole, S.; Zhao, H. Exploiting Issatchenkia orientalis SD108 for succinic acid production. Microb. Cell Fact. 2014, 13, 121. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, G.; Xu, N.; Zou, W.; Zhu, P.; Liu, L.; Chen, J. Metabolic engineering of Torulopsis glabrata for malate production. Metab. Eng. 2013, 19, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhou, Y.; Cao, W.; Liu, H. Improved Production of Malic Acid in Aspergillus niger by Abolishing Citric Acid Accumulation and Enhancing Glycolytic Flux. ACS Synth. Biol. 2020, 9, 1418–1425. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Xu, Y.; Mo, L.; Shi, M.; Wu, N.; Lu, L.; Xue, F.; Xu, Q.; Zhang, C. Enhancing L-Malic Acid Production in Aspergillus niger via Natural Activation of sthA Gene Expression. J. Agric. Food Chem. 2024, 72, 4869–4879. [Google Scholar] [CrossRef]
- Wu, N.; Wu, X.; Zhang, M.; Zhang, C.; Xu, Q. Metabolic engineering of Aspergillus niger for accelerated malic acid biosynthesis by improving NADPH availability. Biotechnol. J. 2024, 19, 2400014. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, N.; Zhang, C.; Xu, Q. Investigation on the influence of GPI-AP for the production of malic acid in Aspergillus niger. Process Biochem. 2024, 147, 371–380. [Google Scholar] [CrossRef]
- Zhang, C.; Shi, M.; Xu, Y.; Yang, D.; Lu, L.; Xue, F.; Xu, Q. Conditional expression of FumA in Aspergillus niger enhances synthesis of L-malic acid. Appl. Environ. Microbiol. 2024, 90, e00008–e00024. [Google Scholar] [CrossRef]
- Liu, J.; Li, J.; Liu, L. Reconstruction of the cytoplasmic pathway and regulation of transport of L-malate in Aspergillus oryzae Chinese Society of Bioengineering Youth Working Committee. In Abstracts of the Second Youth Science and Technology Forum and the First Annual Meeting of the Youth Working Committee of the Chinese Society of Bioengineering; Key Laboratory of Industrial Biotechnology, Ministry of Education, Jiangnan University: Wuxi, China, 2017; Volume 70. [Google Scholar]
- Yang, L.; Christakou, E.; Vang, J.; Lübeck, M.; Lübeck, P.S. Overexpression of a C4-dicarboxylate transporter is the key for rerouting citric acid to C4-dicarboxylic acid production in Aspergillus carbonarius. Microb. Cell Fact. 2017, 16, 43. [Google Scholar] [CrossRef]
- Cao, W.; Yan, L.; Li, M.; Liu, X.; Xu, Y.; Xie, Z.; Liu, H. Identification and engineering a C4-dicarboxylate transporter for improvement of malic acid production in Aspergillus niger. Appl. Microbiol. Biotechnol. 2020, 104, 9773–9783. [Google Scholar] [CrossRef]
- Yang, L.; Henriksen, M.M.; Hansen, R.S.; Lübeck, M.; Vang, J.; Andersen, J.E.; Bille, S.; Lübeck, P.S. Metabolic engineering of Aspergillus niger via ribonucleoprotein-based CRISPR–Cas9 system for succinic acid production from renewable biomass. Biotechnol. Biofuels. 2020, 13, 206. [Google Scholar] [CrossRef]
- Meng, J.; Mäkelä, M.R.; de Vries, R.P. Identification of an l-Arabitol Transporter from Aspergillus niger. Biomolecules 2023, 13, 188. [Google Scholar] [CrossRef] [PubMed]
- Alves, J.; Sousa-Silva, M.; Soares, P.; Sauer, M.; Casal, M.; Soares-Silva, I. Structural characterization of the Aspergillus niger citrate transporter CexA uncovers the role of key residues S75, R192 and Q196. Comput. Struct. Biotechnol. J. 2023, 21, 2884–2898. [Google Scholar] [CrossRef] [PubMed]
- Sloothaak, J.; Odoni, D.I.; de Graaff, L.H.; Martins Dos Santos, V.A.P.; Schaap, P.J.; Tamayo-Ramos, J.A. Aspergillus niger membrane-associated proteome analysis for the identification of glucose transporters. Biotechnol. Biofuels 2015, 8, 150. [Google Scholar]
- Chen, H.; Du, Y.; Guo, H. Construction of a high-efficiency gene-targeting system in brewing-wine Aspergillus oryzae industrial strain used in direct xylitol conversion from xylan. Acta. Biochim. Biophys. Sin. 2018, 50, 723–726. [Google Scholar]
- Papanikolaou, S.; Rontou, M.; Belka, A.; Athenaki, M.; Gardeli, C.; Mallouchos, A.; Kalantzi, O.; Koutinas, A.A.; Kookos, I.K.; Zeng, A.P.; et al. Conversion of biodiesel-derived glycerol into biotechnological products of industrial significance by yeast and fungal strains. Eng. Life Sci. 2017, 17, 262–281. [Google Scholar]
- Moses, G.S.; Otero, C.R.; Grange, L.D.; Rensburg, P.; Pretorius, I.S. Different genetic backgrounds influence the secretory expression of the LKA1-encoded Lipomyces kononenkoae α-amylase in industrial strains of Saccharomyces cerevisiae. Biotechnol. Lett. 2002, 24, 651–656. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, R.; Bi, F.; Chen, Y.; Xue, X.; Wang, D. Overexpression of kojR and the entire koj gene cluster affect the kojic acid synthesis in Aspergillus oryzae 3.042. Gene 2023, 892, 147852. [Google Scholar]
- Xue, X.; Bi, F.; Liu, B.; Li, J.; Zhang, L.; Zhang, J.; Gao, Q.; Wang, D. Improving citric acid production of an industrial Aspergillus niger CGMCC 10142: Identification and overexpression of a high-affinity glucose transporter with different promoters. Microb. Cell Fact. 2021, 20, 168. [Google Scholar]
- Başkan, K.S.; Tütem, E.; Akyüz, E.; Özen, S.; Apak, R. Spectrophotometric total reducing sugars assay based on cupric reduction. Talanta 2016, 147, 162–168. [Google Scholar]
- Fanciullino, A.L.; Dhuique-Mayer, C.; Luro, F.; Morillon, R.; Ollitrault, P. Carotenoid biosynthetic pathway in the citrus genus: Number of copies and phylogenetic diversity of seven genes. J. Agric. Food Chem. 2007, 55, 7405–7417. [Google Scholar] [CrossRef]
- Shrestha, S.; Tang, J.; Kaslow, R.A. Gene copy number: Learning to count past two. Nat. Med. 2009, 15, 1127–1129. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.J.; Hong, P.H.; Da, Y.Y.; Li, L.K.; Stephanopoulos, G. Metabolic engineering of Escherichia coli for the production of L-malate from xylose. Metab. Eng. 2018, 48, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Ruijter, G.J.G.; van de Vondervoort, P.J.I.; Visser, J. Oxalic acid production by Aspergillus niger: An oxalate-non-producing mutant produces citric acid at pH 5 and in the presence of manganese. Microbiology 1999, 145 Pt 9, 2569–2576. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Linde, T.; Hossain, A.H.; Lübeck, M.; Punt, P.J.; Lübeck, P.S. Disruption of a putative mitochondrial oxaloacetate shuttle protein in Aspergillus carbonarius results in secretion of malic acid at the expense of citric acid production. BMC Biotechnol. 2019, 19, 72. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, G.P.; Li, X.; Liu, J.; Gao, C.; Liu, L.Y. Research Progress. on Microbial Cell Metabolism and Environmental Adaptation Regulation. Chin. J. Biotechnol. 2024, 1–21. [Google Scholar]
- Liu, J.; Li, J.; Liu, Y.; Shin, H.D.; Ledesma-Amaro, R.; Du, G.; Chen, J.; Liu, L. Synergistic Rewiring of Carbon Metabolism and Redox Metabolism in Cytoplasm and Mitochondria of Aspergillus oryzae for Increased L-Malate Production. ACS Synth. Biol. 2018, 7, 2139–2147. [Google Scholar] [CrossRef]
- Sun, W.; Jiang, B.; Zhang, Y.; Guo, J.; Zhao, D.; Pu, Z.; Bao, Y. Enabling the biosynthesis of malic acid in Lactococcus lactis by establishing the reductive TCA pathway and promoter engineering. Biochem. Eng. J. 2020, 161, 107645. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, C.; Pei, L.; Qian, Q.; Lu, L. Production of L-Malic Acid by Metabolically Engineered Aspergillus nidulans Based on Efficient CRISPR-Cas9 and Cre-loxP Systems. J. Fungi. 2023, 9, 719. [Google Scholar] [CrossRef]
- Xu, Y.; Shan, L.; Zhou, Y.; Xie, Z.; Ball, A.S.; Cao, W.; Liu, H. Development of a Cre-loxP-based genetic system in Aspergillus niger ATCC1015 and its application to construction of efficient organic acid-producing cell factories. Appl. Microbiol. Biotechnol. 2019, 103, 8105–8114. [Google Scholar] [CrossRef]
- Yin, X.; Li, J.; Shin, H.D.; Du, G.; Liu, L.; Chen, J. Metabolic engineering in the biotechnological production of organic acids in the tricarboxylic acid cycle of microorganisms: Advances and prospects. Biotechnol. Adv. 2015, 33, 830–841. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Li, P.; Wang, Y.; Li, S.; Gao, M.; Song, Y. Enhanced lipid production by addition of malic acid in fermentation of recombinant Mucor circinelloides Mc-MT-2. Sci. Rep. 2021, 11, 12674. [Google Scholar]
- Ramu, E.; Sreelatha, N.; Vijay, K.; Ravi, V.; Srinivas, R.A. ChemInform Abstract: Green Approach for the Efficient Synthesis of Quinolines Promoted by Citric Acid. Chem. Inform. 2009, 40. [Google Scholar] [CrossRef]
- Kang, N.K.; Lee, J.W.; Ort, D.R.; Jin, Y.S. L-malic acid production from xylose by engineered Saccharomyces cerevisiae. Biotechnol. J. 2021, 17, e2000431. [Google Scholar] [CrossRef] [PubMed]
- Fayin, Z.; Ka-Yiu, S.; George, N. Bennett Metabolic engineering of Escherichia coli for malate production with a temperature sensitive malate dehydrogenase. Biochem. Eng. J. 2020, 164, 107762. [Google Scholar]
- Dai, Z.; Zhou, H.; Zhang, S.; Gu, H.; Yang, Q.; Zhang, W.; Dong, W.; Ma, J.; Fang, Y.; Jiang, M.; et al. Current advance in biological production of malic acid using wild type and metabolic engineered strains. Bioresour. Technol. 2018, 258, 345–353. [Google Scholar]
- Xi, Y.; Xu, H.; Zhan, T.; Qin, Y.; Fan, F.; Zhang, X. Metabolic engineering of the acid-tolerant yeast Pichia kudriavzevii for efficient L-malic acid production at low pH. Metab. Eng. 2022, 75, 170–180. [Google Scholar]
- Skorokhodova, Y.A.; Gulevich, Y.A.; Debabov, G.V. Anaerobic biosynthesis of intermediates of reductive branch of tricarboxylic acids cycle by Escherichia coli strains with inactivated frdA and sdhAB genes. Appl. Biochem. Microbiol. 2016, 52, 679–684. [Google Scholar]
- Steinsiek, S.; Frixel, S.; Stagge, S.; Bettenbrock, K. Characterization of E. coli MG1655 and frdA and sdhC mutants at various aerobiosis levels. J. Biotechnol. 2011, 154, 35–45. [Google Scholar] [CrossRef]
- Meijer, S.; Otero, J.; Olivares, R.; Andersen, M.R.; Olsson, L.; Nielsen, J. Overexpression of isocitrate lyase-glyoxylate bypass influence on metabolism in Aspergillus niger. Metab. Eng. 2009, 11, 107–116. [Google Scholar] [CrossRef]
- Wakai, S.; Arazoe, T.; Ogino, C.; Kondo, A. Future insights in fungal metabolic engineering. Bioresour. Technol. 2017, 245 Pt B, 1314–1326. [Google Scholar] [CrossRef]
- Yang, L.; Lübeck, M.; Lübeck, P.S. Aspergillus as a versatile cell factory for organic acid production. Fungal. Biol. Rev. 2017, 31, 33–49. [Google Scholar] [CrossRef]



| Gene ID | Gene Name | Length (aa) | Enzyme | FPKM | |||
|---|---|---|---|---|---|---|---|
| MA48 | MA72 | MA120 | |||||
| 4989224 | ANI_1_2040144 | dct1 | 416 | C4-dicarboxylate transporter/malic acid transport protein | 687.92 | 164.78 | 206.71 |
| 4988409 | ANI_1_1316144 | dct2 | 374 | C4-dicarboxylate transporter/malic acid transport protein | 223.13 | 268.80 | 545.86 |
| 4986875 | ANI_1_54124 | dct3 | 479 | C4-dicarboxylate/malic acid transporter | 185.13 | 289.40 | 206.71 |
| 4980153 | ANI_1_1070034 | dct4 | 403 | Malic acid transport protein | 65.28 | 20.60 | 39.84 |
| 4985437 | ANI_1_130104 | dct5 | 395 | Sulfite efflux pump SSU1 | 297.19 | 86.51 | 708.43 |
| -- | AO090023000318 | c4t318 | 380 | C4-dicarboxylate transporter/malic acid transport | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, Y.; Liu, S.; Wu, S.; Wang, X.; Wang, D.; Xue, X. Improving Organic Acid Secretion of Aspergillus niger by Overexpression C4-Dicarboxylic Acid Transporters. Fermentation 2025, 11, 156. https://doi.org/10.3390/fermentation11030156
Tan Y, Liu S, Wu S, Wang X, Wang D, Xue X. Improving Organic Acid Secretion of Aspergillus niger by Overexpression C4-Dicarboxylic Acid Transporters. Fermentation. 2025; 11(3):156. https://doi.org/10.3390/fermentation11030156
Chicago/Turabian StyleTan, Yiyang, Shutong Liu, Sheng Wu, Xiaolu Wang, Depei Wang, and Xianli Xue. 2025. "Improving Organic Acid Secretion of Aspergillus niger by Overexpression C4-Dicarboxylic Acid Transporters" Fermentation 11, no. 3: 156. https://doi.org/10.3390/fermentation11030156
APA StyleTan, Y., Liu, S., Wu, S., Wang, X., Wang, D., & Xue, X. (2025). Improving Organic Acid Secretion of Aspergillus niger by Overexpression C4-Dicarboxylic Acid Transporters. Fermentation, 11(3), 156. https://doi.org/10.3390/fermentation11030156





