Abstract
The valorization of agri-food wastes can provide value-added products, enzymes and biofuels. For the second-generation ethanol (2G) production, pulps rich in cellulose are desirable in order to release fermentable sugars. This study investigated the homemade biosynthesis of cellulases and hemicellulases via solid-state fermentation (SSF) using sugarcane bagasse (SB) and wheat bran (WB) for the growth of endophytic fungi (Beauveria bassiana, Trichoderma asperellum, Metarhizium anisopliae and Pochonia chlamydosporia). Cocktails with high enzymatic levels were obtained, with an emphasis for M. anisopliae in the production of β-glucosidase (83.61 U/g after 288 h) and T. asperellum for xylanase (785.50 U/g after 144 h). This novel M. anisopliae β-glucosidase demonstrated acidophile and thermotolerant properties (optimum activity at pH 5.5 and 60 °C and stability in a wide pH range and up to 60 °C), which are suitable for lignocellulose saccharifications. Hence, the M. anisopliae multi-enzyme blend was selected for the hydrolysis of raw and organosolv-pretreated corn straw (CS) and corncob (CC) using 100 CBU/g cellulose. After the ethanol/water (1:1) pretreatment, solid fractions rich in cellulose (55.27 in CC and 50.70% in CS) and with low concentrations of hemicellulose and lignin were found. Pretreated CC and CS hydrolysates reached a maximum TRS release of 12.48 and 13.68 g/L, with increments of 100.80 and 73.82% in comparison to untreated biomass, respectively, emphasizing the fundamental role of a pretreatment in bioconversions. This is the first report on β-glucosidase biosynthesis using M. anisopliae and its use in biomass hydrolysis. These findings demonstrated a closed-loop strategy for internal enzyme biosynthesis integrated to reducing sugar release which would be applied for further usage in biorefineries.
1. Introduction
The Brazilian economy is tightly based on the agriculture industry, with a high production of coffee, soybean, cereals and sugarcane, among others. However, one of the main bottlenecks in the Brazilian agroindustry is the amount of waste generated in the processing of these raw materials, whose accumulation is harmful to the environment [1]. On the other hand, these resources have great economic potential due to their low cost and high value and can be converted into biofuels and chemical products [2]. Hence, the reuse of lignocellulosic wastes, such as sugarcane bagasse, wheat bran, corn straw and corn cob, can contribute to the energy matrix and to the reduction in environmental problems such as forest deforestation and new areas of planting.
The lignocellulosic biomass is basically composed of lignin, cellulose and hemicelluloses, which can be converted into fermentable sugars and other compounds. The cellulose represents about 35 to 50% of the plant cell wall [3,4]. This polysaccharide is composed of β-D-glucose subunits, linked by β(1 → 4) glycosidic bonds with repetitive cellobiose units, presenting ordered (crystalline) and amorphous regions [4]. The hemicellulose is the second main component (26 to 36%), which consists of different heteropolysaccharides, composed by β-D-xylopyranoside monomers linked by β(1 → 4) glycosidic linkages, also known as xylans [5]. Unlike the cellulose, which contains regions with high and low crystallinity, hemicelluloses are formed in amorphous regions [4,6]. Lignin is the third major component, constituted by aromatic alcohols units (p-coumaryl, coniferyl and sinapyl), and represents around 20 to 30% of the cell wall [7]. Lignin provides rigidity to the plants and can be considered a “natural glue” that links the cellulose and hemicelluloses, as it is directly associated with the biomass complexity.
The efficient conversion of agro-industrial wastes into value-added products presents challenges due to the complex lignin–hemicellulose–cellulose association, which makes the plant fibers more resistant to degradation [8]. To enable the valorization of these materials, integration strategies have been proposed through sustainable techniques, focusing on the generation of biofuels and other inputs [9]. In this purpose, the pretreatment represents a key step in order to reduce lignocellulose recalcitrance [9]. Among the chemical pretreatments, the organosolv method has stood out due to the selective fractionation of the biomass, promoting an increase in porosity [10,11]. In this method, organic solvents can be used in self-catalyzed acid processes that lead to a greater extraction of the hemicellulosic fraction, due to its superior susceptibility to acid hydrolysis [12]. On the other hand, this process leads to the separation of hemicelluloses and lignin, giving rise to a solid fraction rich in cellulose. Thus, the pulp become more accessible to the enzymes during saccharification [13].
Enzymatic catalysis has been employed to release high sugar yields, which can be converted into different kinds of bioproducts [2,14]. However, the use of commercial enzymes elevates the cost of biorefineries. As an alternative, in-house cocktails containing extracellular enzymes, produced through solid-state fermentation (SSF), have been studied [15,16,17,18]. SSF is an economic and advantageous path for the management of residual biomass, transforming agro-industrial byproducts of wide availability with a low cost in enzymes and contributing to the mitigation of environmental impacts and to the circular economy [17]. In this process, some microorganisms, mainly filamentous fungi, are cultured in lignocellulosic wastes, acting as potential bio-factories for a wide range of enzymes, which can be applied in various sectors [17,18,19].
For the biosynthesis of cellulases and hemicellulases, the mostly employed fungal species in SSF include Aspergillus (A. niger and A. fumigatus), Thrichoderma (T. reesei and T. viride) and Thermoascus aurantiacus, with sugarcane bagasse (SB), wheat bran (WB) and corn wastes as the most commonly used carbon sources [17,18,19,20]. Nevertheless, the search for new fungal species capable of secreting large quantities of these cell wall-degrading enzymes has intensified in recent years.
The entomopathogenic and endophytic fungi Beauveria bassiana, Trichoderma asperellum, Metarhizium anisopliae and Pochonia chlamydosporia are well known as biocontrol agents and, recently, have been used as promoters of plant growth, improving the biochemical properties of the rhizosphere soil [21]. Prior studies have revealed that these species increase the plant mass and soil availability, solubilize nutrients and improve soil enzymatic activity [21,22]. Nonetheless, the literature is very scarce with respect to the use of these fungi for the biosynthesis of cellulases and hemicellulases via SSF. For example, the ability of a B. bassiana strain to produce extracellular hydrolases, such as amylases, cellulases, chitinases, glucanases, laccase, pectinases and xylanases, was demonstrated but under submerged fermentation conditions [23,24]. For M. anisopliae, only one study was found describing the production of chitinase, β-1,3-glucanase, endocellulase and exocellulase using SSF [25]. For P. chlamydosporia, there are currently no previous records on the biosynthesis of (hemi)cellulases using this species. Regarding T. asperellum, the majority of the studies have described the production of xylanases and glucanases via submerged fermentation [26] or through heterologous expression [27], but few studies have reported the synthesis of these enzymes using SSF by this fungus [28]. Moreover, as far as we know, the role of enzymatic blends from these fungal species has not been previously explored in the saccharification of lignocellulosic biomass. Based on these aspects, this work focused on the production of novel hydrolytic enzymes with these endophytic fungi through SSF, using sugarcane bagasse (SB) and wheat bran (WB) as substrates. Then, the obtained multienzyme preparation that presented the best enzymatic profile was selected for the saccharification of organosolv-pretreated pulps of corn straw (CS) and corn cob (CC), and the total reducing sugars (TRSs) release was evaluated, aiming at future applications in biorefineries.
2. Materials and Methods
2.1. Microorganisms
The fungal strains B. bassiana, M. anisopliae, P. chlamydosporia and T. asperellum were kindly donated by H.T.M. Comercio e Laboratórios de Corretivos do Solo Ltd.a—Biosag (Ituverava, SP, Brazil). These isolates were previously identified via gene sequencing using ribosomal RNA SR6R and LR1 primers [29] to confirm their taxonomical classification. The fungal isolates are stored at the Tropical Collection Culture of the Andre Tosello Foundation (http://fat.org.br/, accessed on 20 March 2024) under the codes CCT7827 (B. bassiana), CCT7829 (T. asperellum), CCT7828 (M. anisopliae) and CCT7830 (P. chlamydosporia). All cultures were preserved in Petri dishes containing the potato dextrose agar (PDA) culture medium at room temperature.
2.2. Enzyme Production via Solid State Fermentation (SSF)
Raw samples of wheat bran (WB) and sugarcane bagasse (SB) were used as substrates in SSF for fungal development and induction of enzymatic synthesis. WB was purchased from a local cereal market (Uberlandia, MG, Brazil), and SB was supplied by the Jatiboca Sugar and Ethanol Plant (Urucania, Minas Gerais, Brazil). Both carbon sources were washed multiple times under running tap water until the wash water was clear to remove all the residues. Then, they were air-dried for 48 h, crushed in a blender, sieved to a particle size of approximately 0.6–1.0 cm and stored in a desiccator. WB was composed of 45.2% of cellulose, 28.8% of hemicellulose and 19.3% of lignin [30], and SB contained 52.11% of cellulose, 28.43% of hemicelluloses and 22.38% of lignin [2].
The preinocula of each fungal strain were prepared in Petri dishes containing the PDA medium and incubated for seven days at 28 °C. SSF was performed in monocultures with six mycelial disks of approximately 6 mm in diameter of each preinoculum to investigate the biosynthesis of cellulases (endoglucanase/avicelase, exoglucanase/carboxymethycellulase and β-glucosidase) and hemicellulases (xylanase and β-xylosidase). Each fungal strain was cultivated in 250 mL Erlenmeyer flasks containing 5 g of a 1:1 (m/m) mixture of SB and WB as substrates for 14 days at 28 °C [17]. The substrates were supplemented with 5 mL of a sterile nutrient solution (65% of moisture) containing 0.35% (NH4)2SO4, 0.3% KH2PO4, 0.05% Mg2SO4·7H2O and 0.05% CaCl2. All SSF procedures were carried out in triplicates. Every 24 h, three flasks were taken from the incubator, added with 50 mL of distilled water and subjected to orbital agitation at 150 rpm for 60 min for enzymatic extraction. The material was then filtered through nylon fabric and centrifuged for 10 min at 10,000 rpm (8760 g). The supernatants containing the crude enzymatic extracts were aliquoted at −20 °C for further enzymatic quantifications.
2.3. Determination of Enzymatic Activities
Xylanase activity was quantified using 10 μL of each crude enzymatic cocktail and 90 μL of sodium acetate buffer (0.05 mol/L, pH 4.8) containing 1% (w/v) of xylan (Sigma, St. Louis, MO, USA) as the substrate at 50 °C for 10 min. The reaction was stopped by the addition of 100 μL of 3.5 dinitrosalicylic acid (DNS) and boiled in a boiling water bath for 10 min [31]. Then, it was cooled in an ice bath, and 800 μL of distilled water was added. The released reducing sugars (TRSs) were quantified at 540 nm [17]. An enzymatic activity unit was defined as the quantity of enzyme required to liberate 1 μmol of β-D-xylose per minute under assay conditions. Exoglucanase (avicelase) and Endoglucanase (CMCase) activities were measured using the same methodology, but 1.0% avicel and carboxymethylcellulose (CMC, from Sigma) were used as substrates, respectively [17]. One unit of enzyme activity was defined as the amount of enzyme required to release 1 μmol of glucose per minute of reaction from a glucose standard curve.
β-Glucosidase activity was measured using p-nitrophenyl-β-D-glucopyranoside (4 mmol/L, PNPG) as the substrate in a sodium citrate buffer solution (0.05 mol/L, pH 4.8) at 50 °C for 10 min [17]. The reaction was stopped with the addition of 2 mL of a 2 M sodium carbonate (Na2CO3) solution. The amount of released p-nitrophenol (pNP) was measured at 410 nm, and one enzymatic activity unit was determined as the amount of enzyme required to release 1 μmol of pNP per minute [17]. β-xylosidase activity was quantified using the same method, but p-nitrophenyl-β-D-xylopyranoside (4 mmol/L, PNPX) was used as the substrate.
The temperature of 50 °C and a pH of 4.8 were selected for the enzymatic assays based on the common conditions applied for hydrolytic processes employing cellulases and hemicellulases, providing a balance between structural stability and catalytic activity [32,33]. In addition, the chosen temperature and pH values fall within the optimal activity range for the evaluated hydrolytic enzymes and reduce the probability of microbiological contamination, improving experimental control [15,34].
After the determination of the enzymatic profiles of each fungal strain, a statistical analysis was performed among the produced cocktails, based on the activity peaks through the Scott–Knott test (Sisvar 5.6) at a probability level of 0.05, to verify if there were significant differences between the extracts produced for each enzyme.
2.4. β-Glucosidase Characterization
After the determination of the enzymatic profile of each fungal strain, the extract which displayed the maximum β-glucosidase biosynthesis value was chosen for physico-chemical characterization. Then, the optimum pH, optimum temperature, stability pH and thermostability of β-glucosidase were evaluated [15]. For the determination of optimum pH, 50 μL of the crude enzymatic solution was incubated in 250 μL of PNPG (4 mmol/L), varying the buffer pH from 3.5 to 8.0 at 50 °C for 10 min. The buffer solutions used were 0.05 mol/L sodium citrate (from 3.5 to 7.0) and 0.05 mol/L sodium phosphate (7.5 and 8.0), and the reactions were stopped by adding 2 mL of 2 M Na2CO3. The optimum temperature was determined through the incubation of 50 μL of crude enzymatic extract at the optimum pH, but at temperatures varying from 30 to 80 °C, under the same previously described experimental conditions. The stability pH was evaluated via the incubation of the enzymatic extract for 24 h at room temperature in variable pH solutions from 3.5 to 8.0. After this period, residual β-glucosidase activity was quantified under the respective optimum pH and temperature. At last, thermostability was examined through the incubation of the enzymatic extract at different temperatures (from 30 to 80 °C) for 1 h. Afterwards, the enzymatic activity was quantified at the optimum pH and temperature. All experiments were performed in triplicate.
2.5. Organosolv Pretreatment
Raw corn cob (CC) was provided by JC Rações and Insumos Siderúrgicos Ltd.a (Uberlândia, MG, Brazil), and corn straw (CS) was obtained from the Bom Jardim Colombo farm (Araguari, MG, Brazil). In order to ensure that the biomass particles had the same particle size, 50 g of each biomass was sieved with stirring for 20 min in sieves with mesh sizes between 106 and 250 mm. The organosolv pretreatment of CC and CS was performed in a high-pressure reactor with a capacity of 7 L, a pressure of 190 to 200 bar and control of agitation and heating. The reaction occurred with 500 g (dry mass) of biomass and 5 L of the ethanol/water solution (1:1) at 180 °C for 2 h in a static system with a solid–liquid ratio fixed at 1:10 (m/v) without agitation. The obtained pulps were dispersed in a defibrator and vacuum filtered to remove the liquor. Then, the pulps were washed with plenty of water until the water did not present residues, vacuum filtered and stored. The assays were carried out in triplicate.
Raw and organosolv-pretreated CC and CS were chemically analyzed following the Technical Association of the Pulp and Paper Industry (TAPPI) and National Renewable Energy Laboratory (NREL) standard methods. For Klason lignin determination, the samples were hydrolyzed with 72% sulfuric acid (H2SO4) at room temperature for two hours. The acid was diluted to 3%, and the mixture was heated at ~100 °C under reflux for 1 h (TAPPI T 222 om-88). The residual material was cooled and filtered through porous glass filter number 3. The solids were dried to a constant weight at 105 °C. The cellulose and hemicellulose contents were determined using a CR7A SHIMADZU chromatograph with an R10-6A detector refraction index and an Aminex HPX 87 H column (300 × 7.8 mm BIO-RAD). The eluent used was 0.005 mol/L H2SO4 with a flux of 0.6 mL/minute (NREL LAP-002).
2.6. Enzymatic Hydrolysis
The solid fractions rich in cellulose (pretreated pulps) were hydrolyzed using an on-site enzymatic cocktail with the highest β-glucosidase activity (previously selected in the SSF step). Before saccharifications, the homemade enzyme blend was concentrated using a freeze-drying method in order to increase the enzyme activity. The sample was freeze-dried in a Liotop L101 freeze dryer for 24 h at a temperature of −48 °C ± 2. The freeze-dried extract was resuspended in 10 mL of distilled water. Then, the β-glucosidase activity in the concentrated cocktail was quantified again.
The hydrolytic assays of raw and organosolv-pretreated CS and CC were carried out in 50 mL Erlenmeyer flasks containing 10% (w/v) of total solids, a β-glucosidase enzymatic load of 100 CBU/g of cellulose and a sodium citrate buffer (0.05 mol/L, pH 4.8), with a final volume of 10 mL. The saccharifications were assayed in triplicates at 150 rpm and 50 °C for 72 h. The hydrolysates containing the released sugars were centrifuged at 10,000 rpm (8760 G) for 10 min and membrane filtered (0.20 μm, Chromafil® Xtra CA-20/25). The released total reducing sugars (TRSs) were quantified via the 3,5-dinitrosalicylic acid (DNS) method using 100 µL of hydrolysate and 100 µL of the DNS solution, which were heated to 100 °C for 10 min in a water bath. Then, 800 µL of distilled water was added to the reaction mixture, and the absorbance was determined using a UV-Vis spectrophotometer (Metash, model UV-5100, Shanghai, China) at a wavelength of 540 nm [2].
2.7. Data Analysis
The experiments were performed in triplicate and reported as the mean and standard deviation. In order to evaluate if there was a significant statistical difference between the produced enzymatic cocktails, analysis of variance (ANOVA) and the Scott–Knott test were performed [35], with a 5% significance level. The statistical analyses were conducted using Action 2.9 (for ANOVA) and Sisvar 5.6 (for the Scott–Knott test) software [36].
3. Results
3.1. Production of Cellulases and Hemicellulases
After SSF, time course production curves were constructed to identify the biosynthetic potential of each endophytic fungus and select the cocktail with the greatest enzymatic profile for further saccharifications. The M. anisopliae, B. bassiana, P. chlamydosporia and T. asperellum strains were initially cultivated via SSF using SB and WB as substrates (1:1), and the activities of cellulases (Figure 1) and hemicellulases (Figure 2) were determined in the crude enzyme solutions. Among the cellulases, the highest results were recorded by T. asperellum HTM for endoglucanase (CMCase), with 75.32 U/g at 144 h of cultivation, followed by M. anisopliae HTM for avicelase (30.25 U/g at 144 h) and β-glucosidase (83.61 U/g among 288–336 h of SSF). For the hemicellulases, the maximum xylanase activity (785.49 U/g) was produced by T. asperellum HTM at 144 h of fermentation (Figure 2), and for β-xylosidase, M. anisopliae HTM exhibited the highest concentration (31.83 U/g at 288 h). These data indicate that M. anisopliae and T. asperellum displayed better potential for future applications in microbial bioprospecting processes than the other investigated strains. These findings are potentially noteworthy since although there are several studies on the enzyme production via SSF in the literature [17,30], few reports have described enzyme synthesis with the fungal endophyte species explored in the current work [25,28].
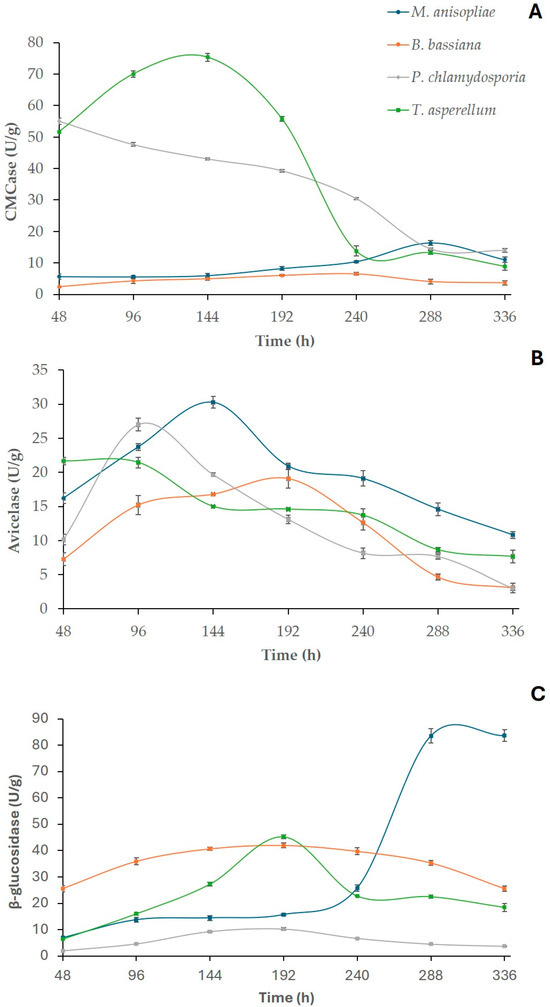
Figure 1.
Cellulase production with M. anisopliae, B. bassiana, P. chlamydosporia and T. asperellum monocultures using sugarcane bagasse (SB) and wheat bran (WB) as substrates. CMCase (A), avicelase (B) and β-glucosidase (C) activities are expressed as U/g. Values represent the mean values of the experiments performed in triplicate (p < 0.05). Error bars indicate the standard deviation of the mean.
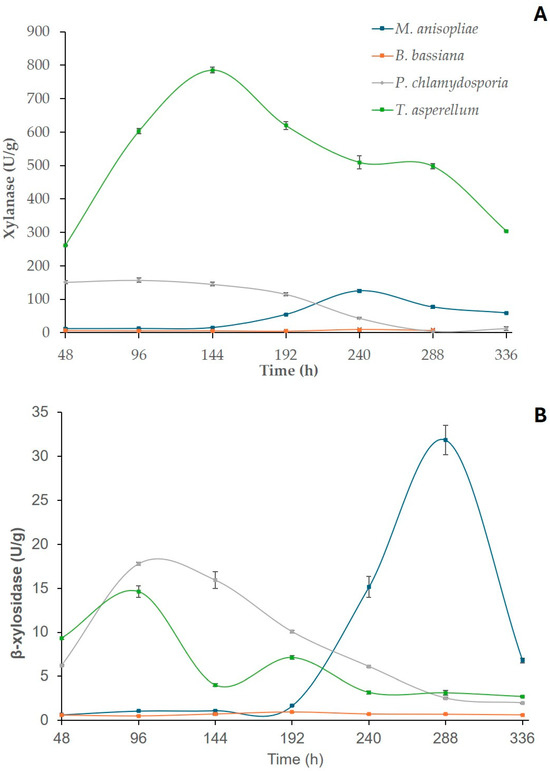
Figure 2.
Hemicellulases production with M. anisopliae, B. bassiana, P. chlamydosporia and T. asperellum monocultures using sugarcane bagasse (SB) and wheat bran (WB) as substrates. Xylanase (A) and β-xylosidase (B) activities are expressed as U/g. Values represent the mean values of the experiments performed in triplicate (p < 0.05). Error bars indicate the standard deviation of the mean.
Among the examined genera, the genus Trichoderma is well known for its ability to synthesize lignocellulose-degrading enzymes under SSF, with an emphasis for the species T. koningii, T. pseudokoningii, T. harzianum, T. longiflorum and T. reesei [37,38]. However, the use of T. asperellum for a laboratory-scale cellulase and hemicellulase biosynthesis process using SSF is a relatively novel approach [28,39]. For example, Ezeilo et al. [28] studied the enzyme production of T. asperellum UC1 using oil palm frond leaves (OPFLs) as the substrate and reported CMCase, β-glucosidase and xylanase activities of 136.16, 130.09 and 255.01 U/g, respectively. Despite these authors obtaining higher values of CMCase and β-glucosidase than in the present study, we observed xylanase activity almost three times higher using SB/WB as carbon sources (Table 1). In addition, as far as we are aware, this is the first report of avicelase (exoglucanase) secretion with T. asperellum using SSF. Comparing the current data to a recent study carried out with the same T. asperellum HTM strain, our results are greater than those obtained by Correa et al. [30], who detected 32.80, 109.41 and 3.79 U/g of β-glucosidase, xylanase and β-xylosidase activities using only WB compared to 45.26, 785.49 and 14.61 U/g, respectively, when SB was added to WB. These findings indicate that the association of SB and WB in the SSF culture medium improved the enzymatic biosynthesis of this fungal species. In addition, these results highlight the heterogeneity in the production of these enzymes, depending on the carbon source used, as evidenced in Table 1, which presents a comparison of enzyme synthesis with T. asperellum through SSF using different lignocellulosic biomasses.

Table 1.
Comparison of cellulase and hemicellulase production under SSF with T. asperellum in the present work with other previous studies. Substrates: sugarcane bagasse: SB, wheat bran: WB, grape pomace: GP, pressed oil palm petiole fiber: POPPF, oil palm frond leaf: OPFL and oil palm empty fruit bunch: OPEFB.
Concerning M. anisopliae, this fungus has been well explored as a biopesticide agent for plant protection [43], and SSF was applied for conidia production using different agro-industrial wastes [44]. However, there is a lack of studies involving the production of hydrolytic enzymes under SSF with this species, with only one report found in the literature [25]. In the previous study, sugarcane bagasse was the most appropriate substrate for enzyme biosynthesis, with maximum activities of 20.87 and 22.30 U/g for endocellulase and exocellulase, respectively, compared to 16.33 and 30.25 U/g, respectively, with SB and WB (1:1) in the current work. The comparison of these results to those by Aita et al. [25] suggests that the addition of WB to SB positively contributed to the enhancement of exoglucanase synthesis by M. anisopliae HTM. Furthermore, it is worth mentioning that this is the first report on the production of β-glucosidase, β-xylosidase and xylanase by M. anisopliae under SSF (Table 2), with remarkable β-glucosidase activity (83.61 U/g).

Table 2.
Comparison of cellulase and hemicellulase production under SSF by M. anisopliae from the present work with other previous studies. Substrates: sugarcane bagasse: SB and Wheat bran: WB.
Regarding P. chlamydosporia HTM, this soil fungus is well known for its bionematicide properties [45]. Nevertheless, its potential for the biosynthesis of cellulases and hemicellulases has not been described. In our work, this strain revealed notable cellulase activities, with highlights for CMCase (55.07 U/g) and avicelase (26.99 U/g), as it is the second fungal strain with the highest activities for these enzymes among the investigated species (Figure 1). For hemicellulases, it did not exhibit significant values. Nonetheless, these findings are very promising, since, to date, this is the first study which describes the production of lignocellulose-degrading enzymes through SSF by this species.
Finally, the last entomopathogenic/endophytic fungal species investigated was B. bassiana. This fungus has been cultivated using SSF for the production of conidia due to its biopesticide properties [46]. Nevertheless, the ability of B. bassiana to produce cellulases and hemicellulases has been almost exclusively reported under submerged fermentation conditions [23,24,47], with an absence of comparative studies employing SSF for this purpose. In the present work, B. bassiana HTM was able to produce celullases and hemicellulases via SSF, with attention for avicelase (19.05 U/g) and β-glucosidase (40.82 U/g) under the evaluated conditions. These results are of relevance since according to the available literature, this is the primary study which explored the potential of this species in the production of cellulolytic and hemicellulolytic enzymes via SSF using SB and WB as substrates. Only one prior work reporting the production of exoglucanase and endoglucanase via SSF was found, but it used other carbon sources [48]. These authors obtained 42.54 and 17.18 U/g of these enzymes, respectively, when rice was used as the substrate. When sugarcane bagasse was employed as the sole carbon source, they detected 44.20 and 6.93 U/g, respectively. Comparing these data with the present ones, it was observed that in the previous study, a higher production of exoglucanase was obtained using rice as a substrate than in the current work. However, β-glucosidase production was not reported in any prior study, reinforcing the relevance of our data.
After the screening of enzymatic profiles of each fungal strain, a statistical analysis was carried out based on the activity peaks among the produced extracts (Table 3). For avicelase, no significant difference (p ≥ 0.05) was observed among the M. anisopliae and P. chlamydosporia HTM cocktails. For the other enzymes, there were significant differences in the production peaks (p < 0.05), with an emphasis for T. asperellum HTM in the synthesis of CMCase and xylanase and M. anisopliae HTM for β-glucosidase and β-xylosidase activities.

Table 3.
Maximum enzymatic activities (U/g) in the cocktails produced from each fungal strain.
3.2. Effects of pH and Temperature in M. anisopliae β-Glucosidase
β-glucosidase is a key enzyme in lignocellulose saccharifications, as it is responsible for the final cleavage of cellobiose in free glucose monomers. Among the produced enzyme preparations, the M. anisopliae HTM cocktail presented the highest β-glucosidase activity and also provided relatively high activity levels for the other enzymes. Then, it was selected to continue the study, and its physico-chemical characteristics were investigated. The temperature and pH are the chief factors for the hydrolytic effectiveness, which can trigger alterations in the enzyme conformation, leading to denaturation and the loss of activity [15,30]. In this study, the β-glucosidase from M. anisopliae HTM showed optimum activity at 60 °C and pH 5.5, achieving a total of 962.7 U/g. Moreover, it exhibited stability in a wide pH range and up to 60 °C (Figure 3), indicating that this β-glucosidase presents a thermotolerant and acidophile nature. These results are in accordance with previous studies which reported other fungal β-glucosidases with acidophilic and thermophilic properties [30,34].
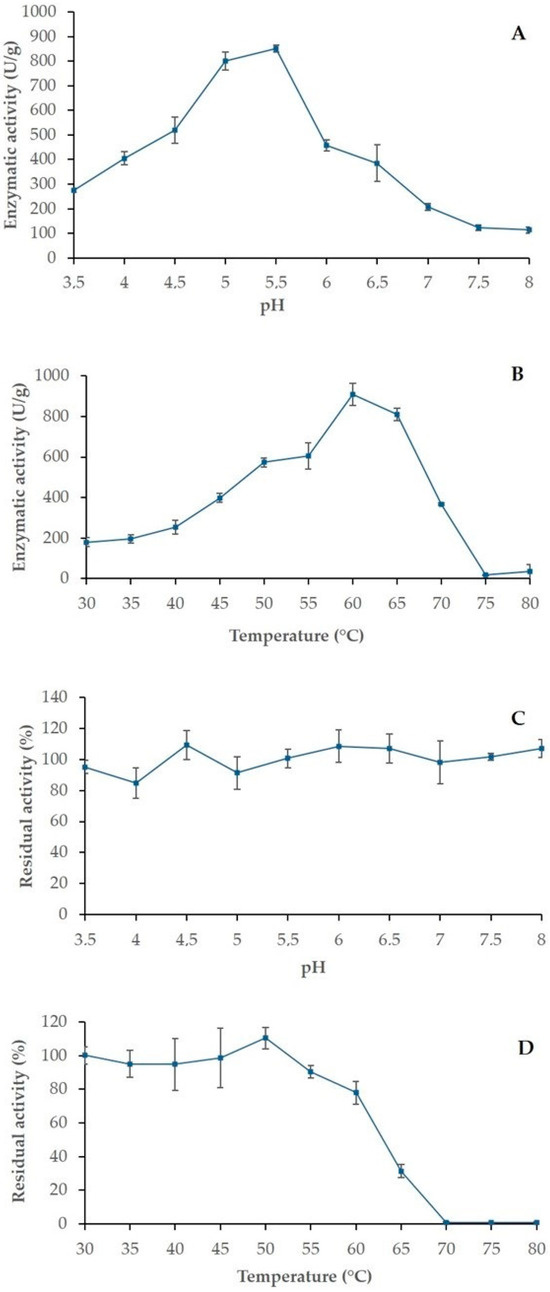
Figure 3.
Effects of pH and temperature on the β-glucosidase activity of the M. anisopliae HTM cocktail. (A) optimum pH, (B) optimum temperature, (C) pH stability and (D) thermostability. The results are expressed as the average ± standard deviation (n = 3).
3.3. Chemical Composition of Raw and Organosolv-Pretreated Biomasses
The chemical composition of raw and organosolv-pretreated biomasses were investigated. In the raw samples, the percentages of cellulose varied from 31.97 (CS) to 33.18% (CC), hemicelluloses from 34.30 (CS) to 35.15% (CC) and lignin from 19.04 (CC) to 21.80% (CS), corroborating previous studies with these lignocellulosic residues [12,49]. After the organosolv pretreatment, the chemical compositions of pulps were determined, and the mass balance was obtained from the sum of the percentages of cellulose (glucans), hemicelluloses (xylan, arabinan and acetyl) and lignin (Table 4). The pulp yields (solid fractions) were 52.37% in CS and 49.41% in CC, with a mass loss of 47.63 and 50.59%, respectively. These mass losses in the pretreated biomasses can be due to the partial removal of hemicelluloses and lignin and due to the solubilization of these components, but with the preservation of cellulose. The highest hemicellulose reduction was observed in pretreated CC (47.65%). For lignin, the maximum removal was observed in pretreated CS (19.68%), indicating that the pretreatment was more efficient for the removal of the hemicellulosic fraction. These results are in agreement with previous studies that also found a significant decrease in the hemicellulosic fraction after organosolv pretreatment [50,51]. On the other hand, the cellulosic fraction increased in all the pretreated biomasses, chiefly in CC (66.57%), demonstrating the preservation of this polysaccharide after the pretreatment. The selective and significant removal of hemicelluloses, mainly in CC, indicates that the organosolv pretreatment promoted the fractionation of the biomasses, resulting in solid fractions enriched with cellulose. These data can positively contribute to the improvement of the bioconversion process, enabling cellulosic fractions to be more accessible to the enzymatic attack.

Table 4.
Chemical composition (%) of raw and organosolv-pretreated (PT) corn straw (CS) and corn cob (CC).
3.4. Enzymatic Hydrolysis
Due to the suitable physico-chemical properties of M. anisopliae HTM β-glucosidase, the potential of this new enzymatic blend was investigated in the saccharification of raw and organosolv-pretreated CS and CC. Before hydrolysis, the M. anisopliae HTM strain was freeze-dried, and a total of 962.69 U/g of β-glucosidase activity was obtained (at the previously determined optimal pH and temperature). Then, the solid fractions of organosolv-pretreated CC and CS were hydrolyzed with 100 CBU/g of cellulose from this concentrated β-glucosidase solution using the same previously described conditions (Section 2.6).
In the hydrolysates of raw CS and CC, the TRS concentrations were 7.87 and 6.21 g/L, respectively. On the other hand, after the saccharification of organosolv-pretreated CS and CC, the TRS release significantly increased, reaching 13.67 and 12.47 g/L, respectively (Table 5). This enhancement was of 100.80 and 73.82% in pretreated CS and CC, in comparison to raw CS and CC. This notable improvement in the hydrolysis effectiveness can probably be attributed to the significant removal of hemicelluloses after this pretreatment (Table 1), which could have favored the accessibility of the cellulases to the cellulosic fraction. In addition, the partial removal of lignin after the pretreatment could have also contributed to the improvement of enzymatic hydrolysis. These results demonstrated that the organosolv pretreatment positively affected the efficiency of the enzymatic saccharifications in CS and CC, emphasizing the importance of a pretreatment step for the bioconversion of the lignocellulosic biomass.

Table 5.
Concentrations of total reducing sugars (TRSs) after the enzymatic hydrolysis of raw and organosolv-pretreated corn straw (CS) and corncob (CS) with the M. anisopliae-concentrated β-glucosidase solution. Assays carried out with 10% solids and 100 CBU/g biomass at 50 °C for 72 h. CE: conversion efficiency (%).
Currently, the use of high-cost commercial enzyme cocktails has been the main bottleneck in the valorization of agro-industrial wastes for the production of high-value-added products, limiting the widespread reutilization of these biomasses [52,53]. On the other hand, saccharifications carried out with low-cost enzymes produced via SSF represent sustainable and promising alternatives [2,54]. However, the few available prior studies that have investigated the role of homemade preparations in enzymatic hydrolysis have employed cocktails synthesized by other fungal species and other kinds of lignocellulosic substrates [25,49], limiting the comparison of the present data with the literature.
Due to the absence of preceding investigations on the enzymatic bioconversions of lignocellulosic residues carried out with enzymatic extracts produced by M. anisopliae, the obtained data were compared to reports of hydrolysis executed with in-house enzymes from other fungal species. For example, Rodrigues et al. [19] employed a crude Aspergillus niger enzyme cocktail in the saccharification of hydrothermal (HP)-pretreated sugarcane bagasse and obtained 10.8 g/L of glucose and a bioconversion rate of 16.2%. These same authors also pretreated SB with HP–soda, followed by hydrolysis using an extract produced by an A. niger, G. lucidum and Pleurotus ostreatus consortium and achieved 11.92 g/L of glucose. Pereira et al. [14] analyzed the hydrolytic effectiveness of SB pretreated with ozone using a Myceliophthora thermophila JCP 1–4 extract in an enzymatic load of a 10 FPU/g substrate and found 4.19 g/L of glucose. Pereira Scarpa et al. [18] tested a Pycnoporus sanguineus MCA16 enzyme cocktail in the hydrolysis of alkaline-pretreated SB and detected 7.32 g/L of glucose using 260 U of endoglucanase/g cellulose. Lei et al. [55] investigated the potential of an on-site A. costaricensis LS18 enzyme extract produced via SSF using lycium barbarum leaves (LBLs) as the substrate. The saccharification of LBL carried out with this cocktail reached a TRS concentration of 8.17 g/L. Teixeira et al. [56] investigated the role of a T. asperellum PEC-6/P cocktail containing xylanase and β-glucosidase activities in the saccharification of pineapple crown waste and obtained a conversion rate of 13.93%. In the present study, higher and promising yields of cellulose bioconversion were achieved in the hydrolysates of pretreated CS and CC after hydrolysis with this novel on-site M. anisopliae enzyme solution (Table 5). In addition, superior TRS concentrations were reached in the hydrolysates of organosolv-pretreated CS and CC using this innovative M. anisopliae enzymatic extract, in comparison to these earlier studies.
The TRS release in pretreated CS and CC hydrolyzed with this M. anisopliae HTM extract was also superior than in some studies carried out with commercial enzymes. For example, Klein et al. [57] evaluated hydrolytic efficiency in H2SO4-pretreated banana peel waste employing a commercial cellulase (Sigma-Aldrich), and the highest yield of TRS was 11.88 g/L, and Zhu et al. [58] acquired 13.18 g/L of reducing sugars after the enzymatic saccharification of wheat straw pretreated with ultrasound and dilute alkali cooking (RU) using 20FPU/g residue of cellulase (C2730). These data reinforce the promising role of this M. anisopliae enzyme cocktail in lignocellulose bioconversions.
Techno-economic analyses have suggested that the usage of internal enzymes in saccharifications, such as those in this present study, can be a valuable tool to expand the viability of biorefineries. For example, around 43% of the total production expense of 2G ethanol can be due to the high cost of the enzymatic cocktails [59]. On the other hand, the innovative role of low-cost enzymatic blends in biomass bioconversions, such as those in this present work, has also been highlighted and can contribute to a circular economy. Therefore, these preliminary data are of great relevance, representing a closed-loop and sustainable approach for integrated enzyme biosynthesis and highlighting its use in the saccharifications of agri-food byproducts. Moreover, it is important to emphasize that the SSF carried out for enzymatic biosynthesis was performed without any kind of nutritional supplementation and conditions optimization. Likewise, the saccharification of CS and CC was executed with low enzymatic and solid loadings (only 100 CBU/g of cellulose and 10% of biomass). Future studies on the optimization of SSF (supplementations, raw materials and pH adjustment, among others) and saccharification conditions (other kinds of pretreatment, other lignocellulosic biomasses, fed-batch mode and variable enzyme concentrations) might be developed in order to take full advantage of this novel M. anisopliae HTM enzymatic preparation.
4. Conclusions
New on-site (hemi)cellulolytic cocktails from endophytic fungi were obtained via SSF. Among them, a novel high-producing β-glucosidase M. anisopliae strain demonstrated desirable industrial properties, such as tolerance to a wide pH range and thermostability, suggesting its future applicability in biorefineries. The organosolv pretreatment of corn straw and corn cob was effective for the removal of hemicelluloses and lignin while preserving the cellulose fraction, facilitating the enzymatic accessibility and contributing to the hydrolysis enhancement. The M. anisopliae HTM extract was able to efficiently hydrolyze pretreated CS and CC into reducing sugars. The saccharification yields have not been attained in any previous report with homemade cocktails, demonstrating the importance of prospecting new fungal enzymes towards sustainable and economical bioconversions. As far as we know, this study is a pioneering research on the integrated production of an enzymatic cocktail from M. anisopliae and its essential role in lignocellulose saccharification. In this sense, the findings provide valuable insights for further applications of this original M. anisopliae HTM cellulase blend in biofuel and value-added product industries, reducing environmental issues and contributing to the circular economy.
Author Contributions
Conceptualization, P.d.O.R., M.A.B. and D.P.; methodology, P.d.O.R., M.A.B., A.G.C. and D.P.; validation, M.A.B. and D.P.; formal analysis, M.A.B. and D.P.; investigation, P.d.O.R., M.A.B., A.G.C. and D.P.; resources, M.A.B., L.C.B.d.A. and D.P.; data curation, M.A.B., L.C.B.d.A. and D.P writing—original draft preparation, P.d.O.R., M.A.B.; writing—review and editing, M.A.B.; visualization, M.A.B.; supervision, M.A.B. and D.P.; project administration, M.A.B. and D.P.; funding acquisition, M.A.B., L.C.B.d.A. and D.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Minas Gerais Research Funding Foundation (FAPEMIG), grant number APQ-03286-21 and Brazilian National Council for Scientific and Technological Development (CNPq), grant number 307905/2022-5.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article; further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors are grateful to Federal University of Uberlandia (UFU) and Coordination for the Improvement of Higher Education Personnel (CAPES) for their technical and administrative supports. The authors also thank the H.T.M. (Biosag—Comercio e Serviços Agrícolas Ltd.a) for donations in kind (materials for experiments) and the reviewers for their suggestions for the improvement of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Baêta, B.E.L.; Lima, D.R.S.; Filho, J.G.B.; Adarme, O.F.H.; Gurgel, L.V.A.; de Aquino, S.F. Evaluation of Hydrogen and Methane Production from Sugarcane Bagasse Hemicellulose Hydrolysates by Two-Stage Anaerobic Digestion Process. Bioresour. Technol. 2016, 218, 436–446. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Rodrigues, P.; Moreira, F.S.; Cardoso, V.L.; Santos, L.D.; Gurgel, L.V.A.; Pasquini, D.; Baffi, M.A. Combination of High Solid Load, On-Site Enzyme Cocktails and Surfactant in the Hydhydrolysis of Hydrothermally Pretreated Sugarcane Bagasse and Ethanol Production. Waste Biomass Valorization 2022, 13, 3085–3094. [Google Scholar] [CrossRef]
- Bayu, A.; Abudula, A.; Guan, G. Reaction Pathways and Selectivity in Chemo-Catalytic Conversion of Biomass-Derived Carbohydrates to High-Value Chemicals: A Review. Fuel Process. Technol. 2019, 196, 106162. [Google Scholar] [CrossRef]
- Wan Azelee, N.I.; Mahdi, H.I.; Cheng, Y.S.; Nordin, N.; Illias, R.M.; Rahman, R.A.; Shaarani, S.M.; Bhatt, P.; Yadav, S.; Chang, S.W.; et al. Biomass Degradation: Challenges and Strategies in Extraction and Fractionation of Hemicellulose. Fuel 2023, 339, 126982. [Google Scholar] [CrossRef]
- Capetti, C.C.d.M.; Pellegrini, V.O.A.; Espirito Santo, M.C.; Cortez, A.A.; Falvo, M.; Curvelo, A.A.d.S.; Campos, E.; Filgueiras, J.G.; Guimaraes, F.E.G.; de Azevedo, E.R.; et al. Enzymatic Production of Xylooligosaccharides from Corn Cobs: Assessment of Two Different Pretreatment Strategies. Carbohydr. Polym. 2023, 299, 120174. [Google Scholar] [CrossRef]
- Geng, W.; Venditti, R.A.; Pawlak, J.J.; Chang, H.M. Effect of Delignification on Hemicellulose Extraction from Switchgrass, Poplar, and Pine and Its Effect on Enzymatic Convertibility of Cellulose-Rich Residues. Bioresources 2019, 13, 4946–4963. [Google Scholar] [CrossRef]
- Park, J.; Riaz, A.; Insyani, R.; Kim, J. Understanding the Relationship between the Structure and Depolymerization Behavior of Lignin. Fuel 2018, 217, 202–210. [Google Scholar] [CrossRef]
- Choi, J.H.; Jang, S.K.; Kim, J.H.; Park, S.Y.; Kim, J.C.; Jeong, H.; Kim, H.Y.; Choi, I.G. Simultaneous Production of Glucose, Furfural, and Ethanol Organosolv Lignin for Total Utilization of High Recalcitrant Biomass by Organosolv Pretreatment. Renew. Energy 2019, 130, 952–960. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Taherzadeh, M.J. Improving the Economy of Lignocellulose-Based Biorefineries with Organosolv Pretreatment. Bioresour. Technol. 2020, 299, 122695. [Google Scholar] [CrossRef]
- Das, A.; Mohanty, K. Optimization of Lignin Extraction from Bamboo by Ultrasound-Assisted Organosolv Pretreatment. Bioresour. Technol. 2023, 376, 128884. [Google Scholar] [CrossRef]
- Jomnonkhaow, U.; Sittijunda, S.; Reungsang, A. Assessment of Organosolv, Hydrothermal, and Combined Organosolv and Hydrothermal with Enzymatic Pretreatment to Increase the Production of Biogas from Napier Grass and Napier Silage. Renew. Energy 2022, 181, 1237–1249. [Google Scholar] [CrossRef]
- Zhong, L.; Zhang, X.; Tang, C.; Chen, Y.; Shen, T.; Zhu, C.; Ying, H. Hydrazine Hydrate and Organosolv Synergetic Pretreatment of Corn Stover to Enhance Enzymatic Saccharification and Co-Production of High-Quality Antioxidant Lignin. Bioresour. Technol. 2018, 268, 677–683. [Google Scholar] [CrossRef]
- Mahmood, H.; Moniruzzaman, M.; Iqbal, T.; Khan, M.J. Recent Advances in the Pretreatment of Lignocellulosic Biomass for Biofuels and Value-Added Products. Curr. Opin. Green. Sustain. Chem. 2019, 20, 18–24. [Google Scholar] [CrossRef]
- de Cassia Pereira, J.; Travaini, R.; Paganini Marques, N.; Bolado, S.; Bocchini Martins, D.A. Saccharification of Ozonated Sugarcane Bagasse Using Enzymes from Myceliophthora Thermophila JCP 1-4 for Sugars Release and Ethanol Production. Bioresour. Technol. 2016, 204, 122–129. [Google Scholar] [CrossRef]
- de Oliveira Rodrigues, P.; de Cássia Pereira, J.; dos Santos, D.Q.; Gurgel, L.V.A.; Pasquini, D.; Baffi, M.A. Synergistic Action of an Aspergillus (Hemi-)Cellulolytic Consortium on Sugarcane Bagasse Saccharification. Ind. Crops Prod. 2017, 109, 173–181. [Google Scholar] [CrossRef]
- Marques, N.P.; de Cassia Pereira, J.; Gomes, E.; da Silva, R.; Araújo, A.R.; Ferreira, H.; Rodrigues, A.; Dussán, K.J.; Bocchini, D.A. Cellulases and Xylanases Production by Endophytic Fungi by Solid State Fermentation Using Lignocellulosic Substrates and Enzymatic Saccharification of Pretreated Sugarcane Bagasse. Ind. Crops Prod. 2018, 122, 66–75. [Google Scholar] [CrossRef]
- Rodrigues, P.d.O.; Gurgel, L.V.A.; Pasquini, D.; Badotti, F.; Góes-Neto, A.; Baffi, M.A. Lignocellulose-Degrading Enzymes Production by Solid-State Fermentation through Fungal Consortium among Ascomycetes and Basidiomycetes. Renew. Energy 2020, 145, 2683–2693. [Google Scholar] [CrossRef]
- Pereira Scarpa, J.d.C.; Paganini Marques, N.; Alves Monteiro, D.; Martins, G.M.; de Paula, A.V.; Boscolo, M.; da Silva, R.; Gomes, E.; Alonso Bocchini, D. Saccharification of Pretreated Sugarcane Bagasse Using Enzymes Solution from Pycnoporus Sanguineus MCA 16 and Cellulosic Ethanol Production. Ind. Crops Prod. 2019, 141, 111795. [Google Scholar] [CrossRef]
- de Oliveira Rodrigues, P.; da Silva Barreto, E.; Brandão, R.L.; Gurgel, L.V.A.; Pasquini, D.; Baffi, M.A. On-Site Produced Enzyme Cocktails for Saccharification and Ethanol Production from Sugarcane Bagasse Fractionated by Hydrothermal and Alkaline Pretreatments. Waste Biomass Valorization 2022, 13, 95–106. [Google Scholar] [CrossRef]
- Khaswal, A.; Mishra, S.K.; Chaturvedi, N.; Saini, S.; Pletschke, B.; Kuhad, R.C. Microbial Enzyme Production: Unlocking the Potential of Agricultural and Food Waste through Solid-State Fermentation. Bioresour. Technol. Rep. 2024, 27, 101880. [Google Scholar] [CrossRef]
- Alves, G.S.; Bertini, S.C.B.; Barbosa, B.B.; Pimentel, J.P.; Ribeiro Junior, V.A.; Mendes, G.d.O.; Azevedo, L.C.B. Fungal Endophytes Inoculation Improves Soil Nutrient Availability, Arbuscular Mycorrhizal Colonization and Common Bean Growth. Rhizosphere 2021, 18, 100330. [Google Scholar] [CrossRef]
- Barra-Bucarei, L.; González, M.G.; Iglesias, A.F.; Aguayo, G.S.; Peñalosa, M.G.; Vera, P.V. Beauveria Bassiana Multifunction as an Endophyte: Growth Promotion and Biologic Control of Trialeurodes Vaporariorum, (Westwood) (Hemiptera: Aleyrodidae) in Tomato. Insects 2020, 11, 591. [Google Scholar] [CrossRef] [PubMed]
- Amobonye, A.; Bhagwat, P.; Singh, S.; Pillai, S. Enhanced Xylanase and Endoglucanase Production from Beauveria Bassiana SAN01, an Entomopathogenic Fungal Endophyte. Fungal Biol. 2021, 125, 39–48. [Google Scholar] [CrossRef]
- Amobonye, A.; Bhagwat, P.; Mthethwa, N.; Kwenda, S.; Ismail, A.; Kumari, S.; Singh, S.; Pillai, S. Transcriptomic Profiling of Beauveria Bassiana SAN01, an Endophytic Fungal Entomopathogen, for the Production of Lignocellulosic Enzymes. Biocatal. Agric. Biotechnol. 2023, 54, 102918. [Google Scholar] [CrossRef]
- Aita, B.C.; Spannemberg, S.S.; Schmaltz, S.; Zabot, G.L.; Tres, M.V.; Kuhn, R.C.; Mazutti, M.A. Production of Cell-Wall Degrading Enzymes by Solid-State Fermentation Using Agroindustrial Residues as Substrates. J. Environ. Chem. Eng. 2019, 7, 103193. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, L.; Yu, D.; Lin, H.; Shen, Q.; Zhao, Y. Excellent Waste Biomass-Degrading Performance of Trichoderma Asperellum T-1 during Submerged Fermentation. Sci. Total Environ. 2017, 609, 1329–1339. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Chen, J.; Wang, J.; Zhuang, H. Transformation of Corncob into High-Value Xylooligosaccharides Using Glycoside Hydrolase Families 10 and 11 Xylanases from Trichoderma Asperellum ND-1. Bioresour. Technol. 2024, 394, 130249. [Google Scholar] [CrossRef]
- Ezeilo, U.R.; Lee, C.T.; Huyop, F.; Zakaria, I.I.; Wahab, R.A. Raw Oil Palm Frond Leaves as Cost-Effective Substrate for Cellulase and Xylanase Productions by Trichoderma Asperellum UC1 under Solid-State Fermentation. J. Environ. Manag. 2019, 243, 206–217. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar] [CrossRef]
- Corrêa, A.G.; de Oliveira Rodrigues, P.; de Azevedo, L.C.B.; Pasquini, D.; Baffi, M.A. Reuse of Grape Pomace and Wheat Bran for Biosynthesis of On-Site Lignocellulose-Degrading Enzymes by Trametes Villosa and Trichoderma Asperellum Under Solid State Fermentation. Waste Biomass Valorization 2024, 15, 4747–4760. [Google Scholar] [CrossRef]
- Miller, G.L. Use of DinitrosaIicyIic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- De Cassia Pereira, J.; Paganini Marques, N.; Rodrigues, A.; Brito de Oliveira, T.; Boscolo, M.; Da Silva, R.; Gomes, E.; Bocchini Martins, D.A. Thermophilic Fungi as New Sources for Production of Cellulases and Xylanases with Potential Use in Sugarcane Bagasse Saccharification. J. Appl. Microbiol. 2015, 118, 928–939. [Google Scholar] [CrossRef]
- Baffi, M.A.; Tobal, T.; Henrique, J.; Lago, G.; Leite, R.S.R.; Boscolo, M.; Gomes, E.; Da-Silva, R. A Novel β-Glucosidase FromSporidiobolus Pararoseus: Characterization and Application in Winemaking. J. Food Sci. 2011, 76, 997–1002. [Google Scholar] [CrossRef]
- Dos Santos, B.S.L.; Gomes, A.F.S.; Franciscon, E.G.; De Oliveira, J.M.; Baffi, M.A. Thermotolerant and Mesophylic Fungi from Sugarcane Bagasse and Their Prospection for Biomass-Degrading Enzyme Production. Braz. J. Microbiol. 2015, 46, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.J.; Knott, M. A cluster analysis method for grouping means in the analysis of variance. Biometrics 1974, 30, 507–512. [Google Scholar] [CrossRef]
- Ferreira, D.F. Sisvar: A Computer Statistical Analysis System. Ciência E Agrotecnologia 2011, 35, 1039–1042. [Google Scholar] [CrossRef]
- Sanguine, I.S.; Cavalheiro, G.F.; Garcia, N.F.L.; dos Santos, M.V.; Gandra, J.R.; de Tonissi e Buschinelli de Goes, R.H.; da Paz, M.F.; Fonseca, G.G.; Leite, R.S.R. Xylanases of Trichoderma Koningii and Trichoderma Pseudokoningii: Production, Characterization and Application as Additives in the Digestibility of Forage for Cattle. Biocatal. Agric. Biotechnol. 2022, 44, 102482. [Google Scholar] [CrossRef]
- Noguchi, T.; Nishiyama, R.; Shimokawa, T.; Yamada, K.; Kagawa, Y. Simultaneous Production of Cellobiose and Xylobiose from Alkali-Treated Bagasse Using Cellulase Secreted by Fe-Ion-Irradiated Trichoderma Reesei Mutant. J. Biosci. Bioeng. 2022, 134, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Ajijolakewu, K.A.; Leh, C.P.; Lee, C.K.; Wan Nadiah, W.A. Characterization of Novel Trichoderma Hemicellulase and Its Use to Enhance Downstream Processing of Lignocellulosic Biomass to Simple Fermentable Sugars. Biocatal. Agric. Biotechnol. 2017, 11, 166–175. [Google Scholar] [CrossRef]
- Mohamad Ikubar, M.R.; Abdul Manan, M.; Md. Salleh, M.; Yahya, A. Solid-State Fermentation of Oil Palm Frond Petiole for Lignin Peroxidase and Xylanase-Rich Cocktail Production. 3 Biotech 2018, 8, 259. [Google Scholar] [CrossRef]
- Raghuwanshi, S.; Deswal, D.; Karp, M.; Kuhad, R.C. Bioprocessing of Enhanced Cellulase Production from a Mutant of Trichoderma Asperellum RCK2011 and Its Application in Hydrolysis of Cellulose. Fuel 2014, 124, 183–189. [Google Scholar] [CrossRef]
- Marx, I.J.; Van Wyk, N.; Smit, S.; Jacobson, D.; Viljoen-Bloom, M.; Volschenk, H. Comparative Secretome Analysis of Trichoderma Asperellum S4F8 and Trichoderma Reesei Rut C30 during Solid-State Fermentation on Sugarcane Bagasse. Biotechnol Biofuels 2013, 29, 172. [Google Scholar] [CrossRef]
- García Riaño, J.L.; Barrera, G.P.; Hernández, L.C.; Villamizar, L.F. Microsclerotia from Metarhizium Robertsii: Production, Ultrastructural Analysis, Robustness, and Insecticidal Activity. Fungal Biol. 2024, 128, 1643–1656. [Google Scholar] [CrossRef] [PubMed]
- Santos, P.d.S.; Abati, K.; Mendoza, N.V.R.; Mascarin, G.M.; Delalibera Júnior, I. Nutritional Impact of Low-Cost Substrates on Biphasic Fermentation for Conidia Production of the Fungal Biopesticide Metarhizium Anisopliae. Bioresour. Technol. Rep. 2021, 13, 100619. [Google Scholar] [CrossRef]
- de Gouveia, A.S.; Monteiro, T.S.A.; Balbino, H.M.; de Magalhães, F.C.; Ramos, M.E.S.; Moura, V.A.S.; Luiz, P.H.D.; de Oliveira, M.G.A.; de Freitas, L.G.; de Ramos, H.J.O. Inoculation of Pochonia Chlamydosporia Triggers a Defense Response in Tomato Roots, Affecting Parasitism by Meloidogyne Javanica. Microbiol. Res. 2023, 266, 127242. [Google Scholar] [CrossRef] [PubMed]
- Sala, A.; Artola, A.; Barrena, R.; Sánchez, A. Harnessing Packed-Bed Bioreactors’ Potential in Solid-State Fermentation: The Case of Beauveria Bassiana Conidia Production. Fermentation 2024, 10, 481. [Google Scholar] [CrossRef]
- Magwaza, B.; Amobonye, A.; Bhagwat, P.; Pillai, S. Biochemical and in Silico Structural Properties of a Thermo-Acid Stable β-Glucosidase from Beauveria Bassiana. Heliyon 2024, 10, e28667. [Google Scholar] [CrossRef]
- Alves, E.A.; Schmaltz, S.; Tres, M.V.; Zabot, G.L.; Kuhn, R.C.; Mazutti, M.A. Process Development to Obtain a Cocktail Containing Cell-Wall Degrading Enzymes with Insecticidal Activity from Beauveria Bassiana. Biochem. Eng. J. 2020, 156, 107484. [Google Scholar] [CrossRef]
- Park, Y.C.; Kim, T.H.; Kim, J.S. Flow-through Pretreatment of Corn Stover by Recycling Organosolv to Reduce Waste Solvent. Energies 2018, 11, 879. [Google Scholar] [CrossRef]
- Rabelo, S.C.; Nakasu, P.Y.S.; Scopel, E.; Araújo, M.F.; Cardoso, L.H.; da Costa, A.C. Organosolv Pretreatment for Biorefineries: Current Status, Perspectives, and Challenges. Bioresour. Technol. 2023, 369, 128331. [Google Scholar] [CrossRef]
- Sulbarán-Rangel, B.; Aguirre, J.S.A.; Breton-Deval, L.; del Real-Olvera, J.; Tun, K.J.G. Improvement of Anaerobic Digestion of Hydrolysed Corncob Waste by Organosolv Pretreatment for Biogas Production. Appl. Sci. 2020, 10, 2785. [Google Scholar] [CrossRef]
- Taiwo, A.E.; Tom-James, A.; Falowo, O.A.; Okoji, A.; Adeyi, O.; Olalere, A.O.; Eloka-Eboka, A. Techno-Economic Analysis of Cellulase Production by Trichoderma Reesei in Submerged Fermentation Processes Using a Process Simulator. S. Afr. J. Chem. Eng. 2022, 42, 98–105. [Google Scholar] [CrossRef]
- Carpio, R.R.; Secchi, S.G.; Barros, R.O.; Oliveira, R.A.; Queiroz, S.; Teixeira, R.S.S.; Bon, E.P.S.; Secchi, A.R. Techno-Economic Evaluation of Second-Generation Ethanol from Sugarcane Bagasse: Commercial versus on-Site Produced Enzymes and Use of the Xylose Liquor. J. Clean. Prod. 2022, 369, 133340. [Google Scholar] [CrossRef]
- Knesebeck, M.; Schäfer, D.; Schmitz, K.; Rüllke, M.; Benz, J.P.; Weuster-Botz, D. Enzymatic One-Pot Hydrolysis of Extracted Sugar Beet Press Pulp after Solid-State Fermentation with an Engineered Aspergillus Niger Strain. Fermentation 2023, 9, 582. [Google Scholar] [CrossRef]
- Lei, Z.; Chen, X.; Cao, F.; Guo, Q.; Wang, J. Efficient Saccharification of Lycium Barbarum Leaf Biomass by Using Enzyme Cocktails Produced by a Novel Fungus Aspergillus Costaricensis LS18. J. Environ. Manag. 2022, 321, 115969. [Google Scholar] [CrossRef]
- Teixeira, W.F.A.; Batista, R.D.; do Amaral Santos, C.C.A.; Júnior, A.C.F.; Terrasan, C.R.F.; de Santana, M.W.P.R.; de Siqueira, F.G.; de Paula-Elias, F.C.; de Almeida, A.F. Minimal Enzymes Cocktail Development by Filamentous Fungi Consortia in Solid-State Cultivation and Valorization of Pineapple Crown Waste by Enzymatic Saccharification. Waste Biomass Valorization 2021, 12, 2521–2539. [Google Scholar] [CrossRef]
- Klein, G.H.; Longo, V.D.; Romani, L.C.; Saldanha, L.F.; Fornari, A.C.; Bazoti, S.F.; Camargo, A.F.; Alves, S.L.; Treichel, H. Utilization of Banana Peel Waste for the Production of Bioethanol and Other High-Value-Added Compounds. Food Humanit. 2024, 3, 100376. [Google Scholar] [CrossRef]
- Zhu, J.; Song, W.; Chen, X.; Sun, S. Integrated Process to Produce Biohydrogen from Wheat Straw by Enzymatic Saccharification and Dark Fermentation. Int. J. Hydrogen Energy 2023, 48, 11153–11161. [Google Scholar] [CrossRef]
- Verma, N.; Kumar, V.; Bansal, M.C. Valorization of Waste Biomass in Fermentative Production of Cellulases: A Review. Waste Biomass Valorization 2020, 12, 613–640. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).