Optimizing Fermentation of Morus nigra L. Residues with Schizophyllum commune to Enhance Anthocyanin Release and Anti-Inflammatory Activity via Pyroptosis Pathway Modulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Optimization of the SC–Morus nigra L. Extract Residue Through Bidirectional Fermentation of the Bacterial Mass
2.3. Detection of Total Anthocyanins
2.4. High-Performance Liquid Chromatography Detection of C3G Concentration
2.5. Cell Culture
2.6. Scratch Assay
2.7. Biochemical Analysis
2.8. RNA Isolation and Real-Time Quantitative PCR
2.9. Western Blotting
2.10. Molecular Docking of C3G with NLRP3
2.11. Statistical Analysis
3. Results
3.1. Response Surface Analysis and Optimization of Morus nigra L. Residue Following Fermentation with L. Schizophyllum commune
3.2. Analysis of Factor Interactions
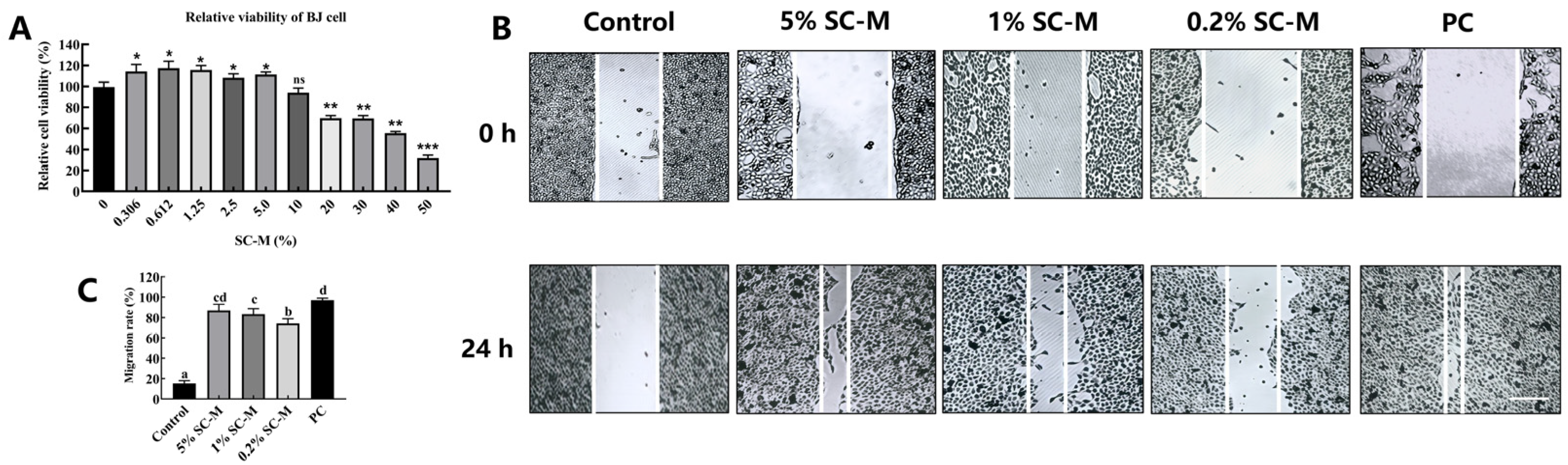
3.3. Effect of SC–M on BJ Cell Proliferation
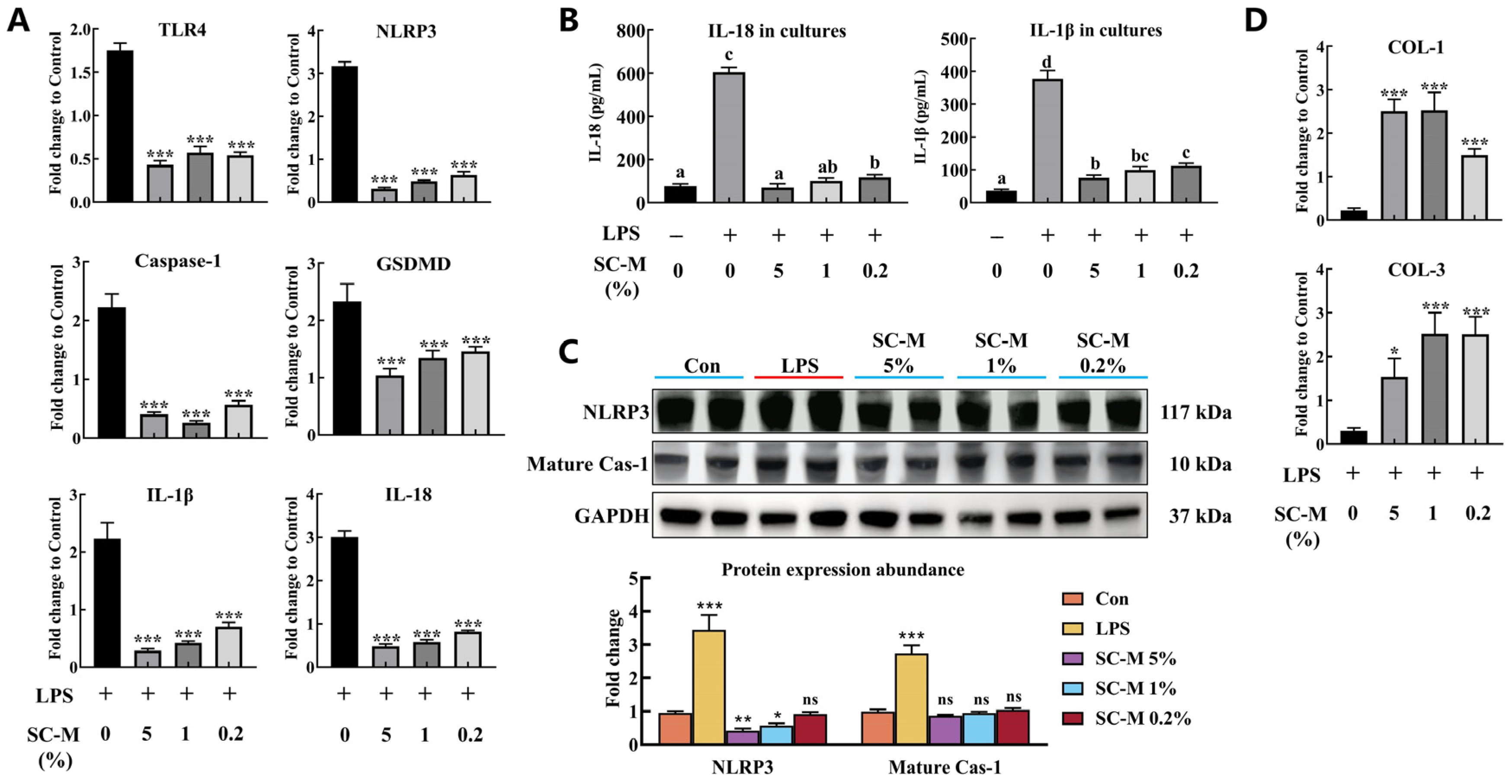
3.4. Anti-Inflammatory Effect of SC–M
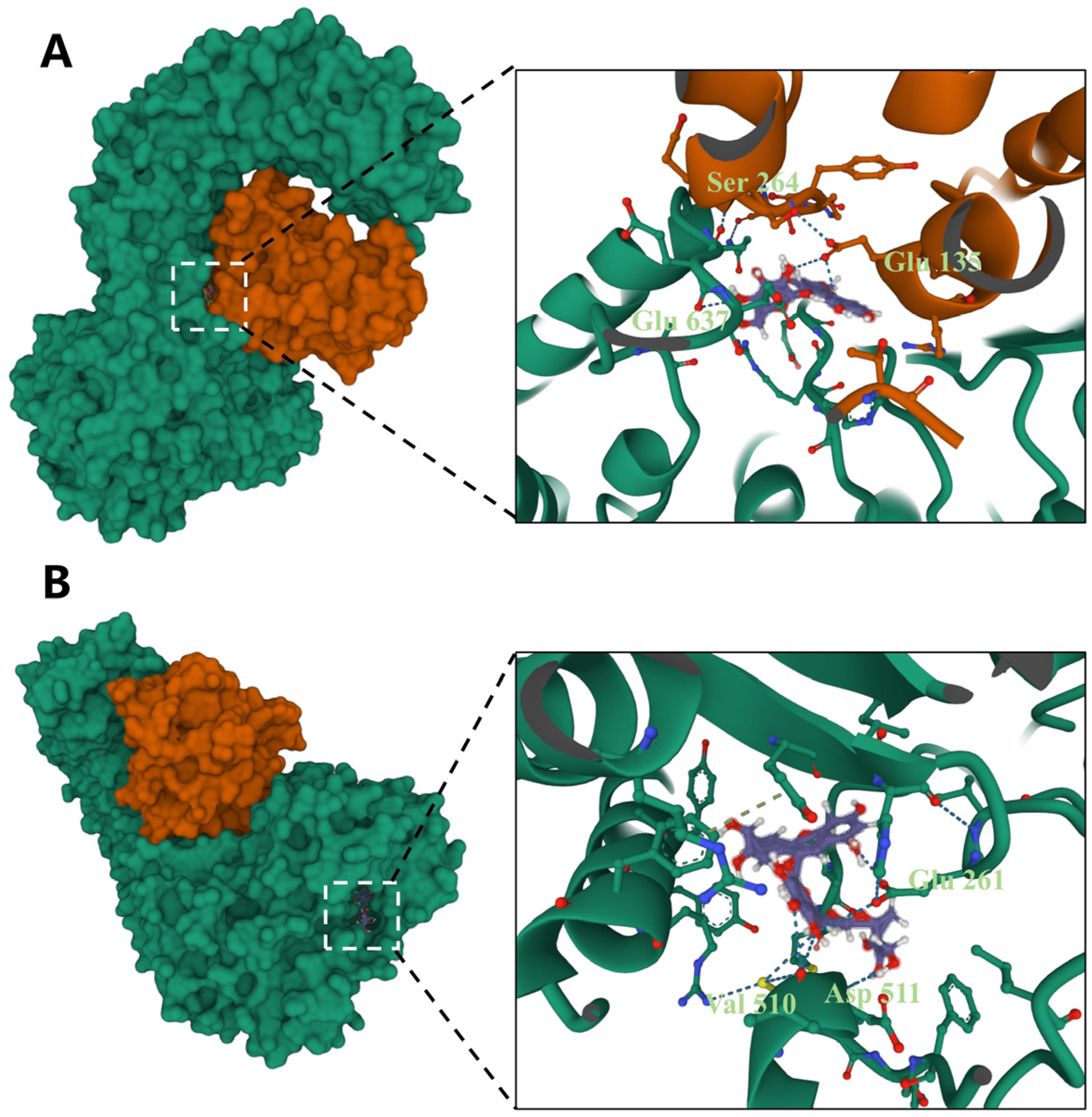
3.5. Docking of Different Anthocyanins with the NLRP3 Protein
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| C3G | Cyanidin-3-O-glucoside |
| C3R | Cyanidin-3-rutinoside |
| IL-1β | Interleukin-1β |
| LPS | Lipopolysaccharide |
| MR | Morus nigra L. extraction residue |
| SC | Schizophyllum commune |
| RSM | Response surface methodology |
| SC–M | Schizophyllum commune–Morus nigra L. fermentation filtrate |
| q-PCR | Quantitative polymerase chain reaction |
References
- Choi, K.H.; Lee, H.A.; Park, M.H.; Han, J.-S. Mulberry (Morus alba L.) Fruit Extract Containing Anthocyanins Improves Glycemic Control and Insulin Sensitivity via Activation of AMP-Activated Protein Kinase in Diabetic C57BL/Ksj-db/db Mice. J. Med. Food 2016, 19, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Gao, Y.; Xue, J.; Yang, Y.; Yin, J.; Wu, T.; Zhang, M. Phytochemicals, Pharmacological Effects and Molecular Mechanisms of Mulberry. Foods 2022, 11, 1170. [Google Scholar] [CrossRef]
- Guo, S.; Bai, L.; Ho, C.-T.; Bai, N. Characteristic Components, Biological Activities and Future Prospective of Fructus Mori: A Review. Curr. Pharmacol. Rep. 2018, 4, 210–219. [Google Scholar] [CrossRef]
- Kaewmool, C.; Udomruk, S.; Phitak, T.; Pothacharoen, P.; Kongtawelert, P. Cyanidin-3-O-Glucoside Protects PC12 Cells Against Neuronal Apoptosis Mediated by LPS-Stimulated BV2 Microglial Activation. Neurotox. Res. 2020, 37, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Xu, J.; Sheng, L.; Song, K. Comprehensive Utilization Technology of Aronia melanocarpa. Molecules 2024, 29, 1388. [Google Scholar] [CrossRef]
- Xue, H.; Zha, M.; Tang, Y.; Zhao, J.; Du, X.; Wang, Y. Research Progress on the Extraction and Purification of Anthocyanins and Their Interactions with Proteins. Molecules 2024, 29, 2815. [Google Scholar] [CrossRef] [PubMed]
- Tena, N.; Asuero, A.G. Up-To-Date Analysis of the Extraction Methods for Anthocyanins: Principles of the Techniques, Optimization, Technical Progress, and Industrial Application. Antioxidants 2022, 11, 286. [Google Scholar] [CrossRef]
- Kwaw, E.; Ma, Y.; Tchabo, W.; Apaliya, M.T.; Wu, M.; Sackey, A.S.; Xiao, L.; Tahir, H.E. Effect of lactobacillus strains on phenolic profile, color attributes and antioxidant activities of lactic-acid-fermented mulberry juice. Food Chem. 2018, 250, 148–154. [Google Scholar] [CrossRef]
- Dutta, R.; Ghosal, S.; Bhattacharyya, D.K.; Bhowal, J. Effect of Fungal Fermentation on Enhancement of Nutritional Value and Antioxidant Activity of Defatted Oilseed Meals. Appl. Biochem. Biotechnol. 2023, 195, 2172–2195. [Google Scholar] [CrossRef]
- Abd Razak, D.L.; Abd Ghani, A.; Mohd Lazim, M.I.; Khulidin, K.A.; Shahidi, F.; Ismail, A. Schizophyllum commune (Fries) mushroom: A review on its nutritional components, antioxidative, and anti-inflammatory properties. Curr. Opin. Food Sci. 2024, 56, 101129. [Google Scholar] [CrossRef]
- Ohm, R.A.; de Jong, J.F.; Lugones, L.G.; Aerts, A.; Kothe, E.; Stajich, J.E.; de Vries, R.P.; Record, E.; Levasseur, A.; Baker, S.E.; et al. Genome sequence of the model mushroom Schizophyllum commune. Nat. Biotechnol. 2010, 28, 957–963. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, Q.; Hu, L.; Liu, T.; Zheng, B.; Lu, D.; Guo, C.; Zhou, L. Enhanced exopolysaccharide yield and antioxidant activities of Schizophyllum commune fermented products by the addition of Radix Puerariae. RSC Adv. 2021, 11, 38219–38234. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Ma, W.; Liang, P.; Wang, X.; Zhang, S.; Han, Y.; Guo, Y. Anti-inflammatory potential of mycoprotein peptides obtained from fermentation of Schizophyllum commune DS1 with young apples. Int. J. Biol. Macromol. 2024, 281, 136638. [Google Scholar] [CrossRef] [PubMed]
- Song, M.H.; Bae, J.T.; Ko, H.; Jang, Y.M.; Lee, J.D.; Lee, G.S.; Pyo, H.B. Anti-Oxidant Effect and Anti-Inflammatory of Fermented Citrus unshiu Peel Extract by using Schizophyllum commune. J. Soc. Cosmet. Sci. Korea 2011, 37, 351–356. [Google Scholar]
- Wang, K.; Guan, L.; Gao, Y.; Yan, S.; Li, J.; Ji, N.; Li, B.; Zhou, Y. Optimization of Fermentation Process of Panax quinquefolius L. by Schizophyllum commune and Its Antioxidant Capacity in vitro. Sci. Technol. Food Ind. 2024, 45, 142–151. [Google Scholar] [CrossRef]
- Guo, W.; Mehrparvar, S.; Hou, W.; Pan, J.; Aghbashlo, M.; Tabatabaei, M.; Rajaei, A. Unveiling the impact of high-pressure processing on anthocyanin-protein/polysaccharide interactions: A comprehensive review. Int. J. Biol. Macromol. 2024, 6, 132042. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, J.; Liu, F.; Tong, L.; Chen, Z.; Chen, J.; He, H.; Xu, R.; Ma, Y.; Huang, C. Neuroprotective effects of anthocyanins and its major component cyanidin-3-O-glucoside (C3G) in the central nervous system: An outlined review. Eur. J. Pharmacol. 2019, 858, 172500. [Google Scholar] [CrossRef]
- Ye, L.; Chen, H.; Wang, J.; Tsim, K.W.K.; Wang, Y.; Shen, X.; Lei, H.; Liu, Y. Aflatoxin B1-induced liver pyroptosis is mediated by disturbing the gut microbial metabolites: The roles of pipecolic acid and norepinephrine. J. Hazard. Mater. 2024, 474, 134822. [Google Scholar] [CrossRef]
- Ye, L.; Chen, H.; Tsim, K.W.K.; Shen, X.; Li, X.; Li, X.; Lei, H.; Liu, Y. Aflatoxin B1 Induces Inflammatory Liver Injury via Gut Microbiota in Mice. J. Agric. Food Chem. 2023, 71, 10787–10797. [Google Scholar] [CrossRef]
- Plikus, M.V.; Wang, X.; Sinha, S.; Forte, E.; Thompson, S.M.; Herzog, E.L.; Driskell, R.R.; Rosenthal, N.; Biernaskie, J.; Horsley, V. Fibroblasts: Origins, definitions, and functions in health and disease. Cell 2021, 184, 3852–3872. [Google Scholar] [CrossRef]
- Wu, T.; Yin, J.; Zhang, G.; Long, H.; Zheng, X. Mulberry and cherry anthocyanin consumption prevents oxidative stress and inflammation in diet-induced obese mice. Mol. Nutr. Food Res. 2016, 60, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Tovar-Herrera, O.E.; Martha-Paz, A.M.; Pérez-Llano, Y.; Aranda, E.; Tacoronte-Morales, J.E.; Pedroso-Cabrera, M.T.; Arévalo-Niño, K.; Folch-Mallol, J.L.; Batista-García, R.A. Schizophyllum commune: An unexploited source for lignocellulose degrading enzymes. Microbiol. Open 2018, 7, e00637. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Q.; Yang, Y.; Zhang, X.; Wang, J.; Jia, J.; Wu, Q. Bidirectional fermentation of Monascus and Mulberry leaves enhances GABA and pigment contents: Establishment of strategy, studies of bioactivity and mechanistic. Prep. Biochem. Biotechnol. 2024, 54, 73–85. [Google Scholar] [CrossRef]
- Song, Z.; Geng, J.; Wang, D.; Fang, J.; Wang, Z.; Wang, C.; Li, M. Reparative effects of Schizophyllum commune oat bran fermentation broth on UVB-induced skin inflammation via the JAK/STAT pathway. Bioresour. Bioprocess. 2024, 11, 73. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, J.; Shao, G.; Chang, X.; Liu, Y.; Xiang, T.; Zhu, Q.; Ren, A.; Jiang, A.; He, Q. Bidirectional Solid-State Fermentation of Highland Barley by Edible Fungi to Improve Its Functional Components, Antioxidant Activity and Texture Characteristics. Plant Foods Hum. Nutr. 2024, 79, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, M.; Liu, Y.; Liu, J.; Zheng, T.; Li, Y.; He, S.; Jiang, M.; Wu, L.; Liu, F. Influence of fermentation with lactic bacteria on the structure, functional properties and antioxidant activity of flaxseed gum. Int. J. Biol. Macromol. 2024, 281, 136133. [Google Scholar] [CrossRef]
- Chen, J.; Xu, B.; Sun, J.; Jiang, X.; Bai, W. Anthocyanin supplement as a dietary strategy in cancer prevention and management: A comprehensive review. Crit. Rev. Food Sci. Nutr. 2022, 62, 7242–7254. [Google Scholar] [CrossRef]
- Zhong, X.; Wang, G.; Li, F.; Fang, S.; Zhou, S.; Ishiwata, A.; Tonevitsky, A.G.; Shkurnikov, M.; Cai, H.; Ding, F. Immunomodulatory effect and biological significance of β-glucans. Pharmaceutics 2023, 4, 1615. [Google Scholar] [CrossRef]
- Luo, F.; Zhang, Z.; Lu, F.; Li, D.; Zhou, C.; Li, Y.; Niu, L.; Xu, Y.; Feng, L.; Dai, Z.; et al. Ultrasound modification of pectin and the mechanism of its interaction with cyanidin-3-O-glucoside. Food Hydrocoll. 2024, 152, 109898. [Google Scholar] [CrossRef]
- Aboonabi, A.; Aboonabi, A. Anthocyanins reduce inflammation and improve glucose and lipid metabolism associated with inhibiting nuclear factor-kappaB activation and increasing PPAR-γgene expression in metabolic syndrome subjects. Free Radic. Biol. Med. 2020, 150, 30–39. [Google Scholar] [CrossRef]
- Ma, Z.; Du, B.; Li, J.; Yang, Y.; Zhu, F. An Insight into Anti-Inflammatory Activities and Inflammation Related Diseases of Anthocyanins: A Review of Both In Vivo and In Vitro Investigations. Int. J. Mol. Sci. 2021, 22, 11076. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Guan, R.; Huang, H.; Yang, K.; Wang, L.; Wu, Y. Anti-inflammatory activity of cyanidin-3-O-glucoside and cyanidin-3-O-glucoside liposomes in THP-1 macrophages. Food Sci. Nutr. 2021, 9, 6480–6491. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.; Correia, P.; Pereira, A.R.; Araújo, P.; Mateus, N.; de Freitas, V.; Oliveira, J.; Fernandes, I. Exploring the Applications of the Photoprotective Properties of Anthocyanins in Biological Systems. Int. J. Mol. Sci. 2020, 21, 7464. [Google Scholar] [CrossRef] [PubMed]
- Zang, Z.; Tang, S.; Li, Z.; Chou, S.; Shu, C.; Chen, Y.; Chen, W.; Yang, S.; Yang, Y.; Tian, J.; et al. An updated review on the stability of anthocyanins regarding the interaction with food proteins and polysaccharides. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4378–4401. [Google Scholar] [CrossRef]


| Factors | Levels | ||
|---|---|---|---|
| A: Addition of Morus nigra L. residue (g/L) | 25 | 30 | 35 |
| B: Addition of Schizophyllum commune (%) | 5.0 | 7.5 | 10.0 |
| C: Fermentation time (day) | 2 | 3 | 4 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value |
|---|---|---|---|---|---|
| Model | 3374.15 | 9 | 374.91 | 50.82 | <0.0001 |
| A | 826.69 | 1 | 826.69 | 112.05 | <0.0001 |
| B | 1.63 | 1 | 1.63 | 0.2215 | 0.6522 |
| C | 636.97 | 1 | 636.97 | 86.34 | <0.0001 |
| AB | 3.48 | 1 | 3.48 | 0.4713 | 0.5145 |
| AC | 33.51 | 1 | 33.51 | 4.54 | 0.0705 |
| BC | 770.19 | 1 | 770.19 | 104.4 | <0.0001 |
| A2 | 339.89 | 1 | 339.89 | 46.07 | 0.0003 |
| B2 | 698.55 | 1 | 698.55 | 94.68 | <0.0001 |
| C2 | 0.2357 | 1 | 0.2357 | 0.0319 | 0.8632 |
| Residual | 51.64 | 7 | 7.38 | - | - |
| Lack of Fit | 36.14 | 3 | 12.05 | 3.11 | 0.151 |
| Pure Error | 15.5 | 4 | 3.88 | - | - |
| Cor Total | 3425.79 | 16 | - | - | - |
| Anthocyanin | Best Docking Score (kcal/mol) | Number of Hydrogen Bonds | Interacting Amino Acids |
|---|---|---|---|
| Cyanidin-3-O-glucoside | −11.013 | 5 | Ser264, Glu637, Glu135 |
| Cyanidin-3-O-rutinoside | −9.746 | 6 | Glu261, Val510, Asp511 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, L.; Hu, Q.; Lin, Y.; Guo, C. Optimizing Fermentation of Morus nigra L. Residues with Schizophyllum commune to Enhance Anthocyanin Release and Anti-Inflammatory Activity via Pyroptosis Pathway Modulation. Fermentation 2025, 11, 145. https://doi.org/10.3390/fermentation11030145
Ye L, Hu Q, Lin Y, Guo C. Optimizing Fermentation of Morus nigra L. Residues with Schizophyllum commune to Enhance Anthocyanin Release and Anti-Inflammatory Activity via Pyroptosis Pathway Modulation. Fermentation. 2025; 11(3):145. https://doi.org/10.3390/fermentation11030145
Chicago/Turabian StyleYe, Lin, Qin Hu, Ying Lin, and Chaowan Guo. 2025. "Optimizing Fermentation of Morus nigra L. Residues with Schizophyllum commune to Enhance Anthocyanin Release and Anti-Inflammatory Activity via Pyroptosis Pathway Modulation" Fermentation 11, no. 3: 145. https://doi.org/10.3390/fermentation11030145
APA StyleYe, L., Hu, Q., Lin, Y., & Guo, C. (2025). Optimizing Fermentation of Morus nigra L. Residues with Schizophyllum commune to Enhance Anthocyanin Release and Anti-Inflammatory Activity via Pyroptosis Pathway Modulation. Fermentation, 11(3), 145. https://doi.org/10.3390/fermentation11030145






