Spectroscopic Analysis of Selenium Nanoparticles Synthesized by Saccharomyces boulardii for the Production of Craft Beer
Abstract
1. Introduction
2. Materials and Methods
2.1. Selenization of S. boulardii
2.2. Beer Production
2.2.1. Wort
2.2.2. Fermentation
2.2.3. Physicochemical Parameters of Beer
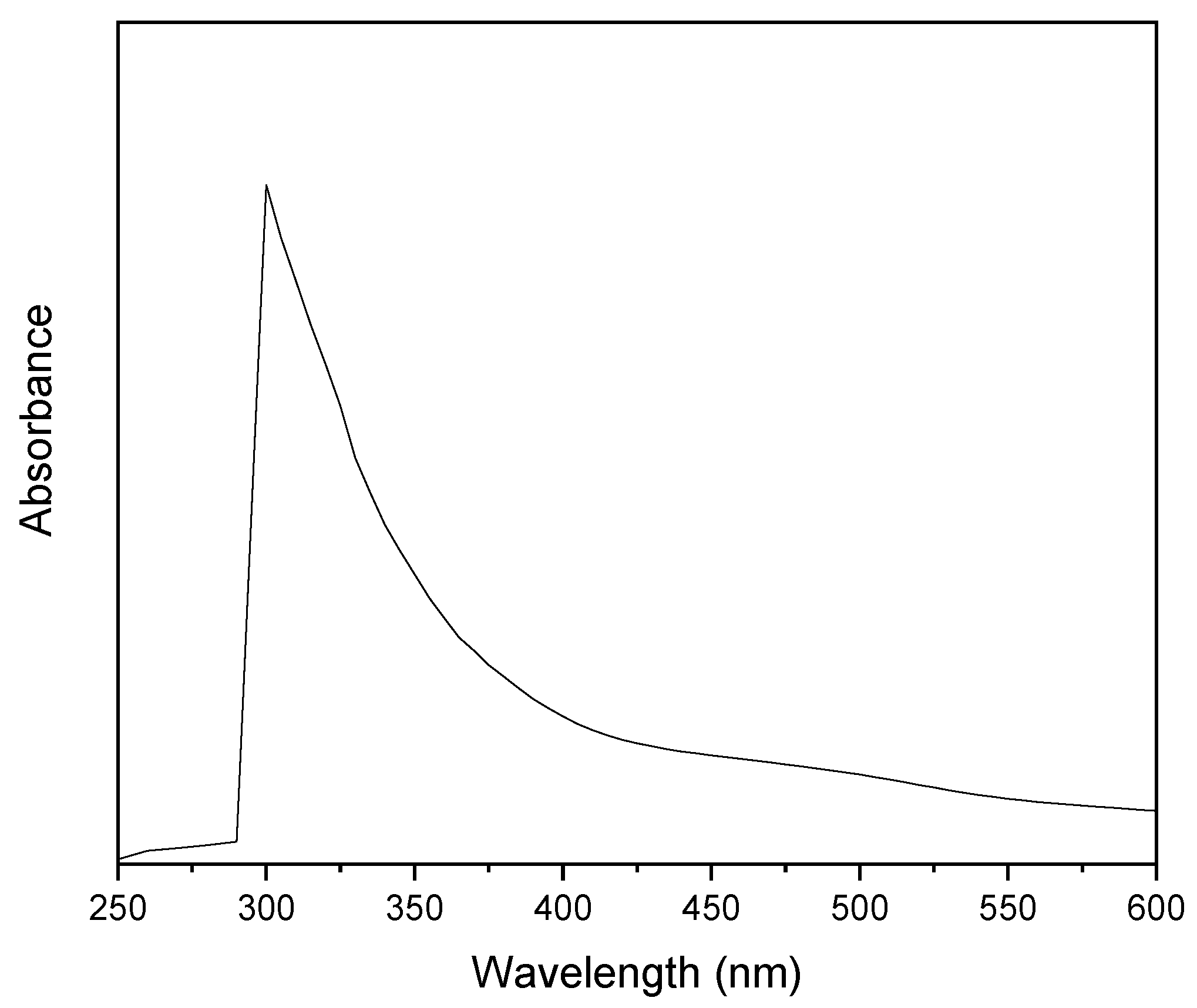
2.3. UV-Vis Spectroscopy
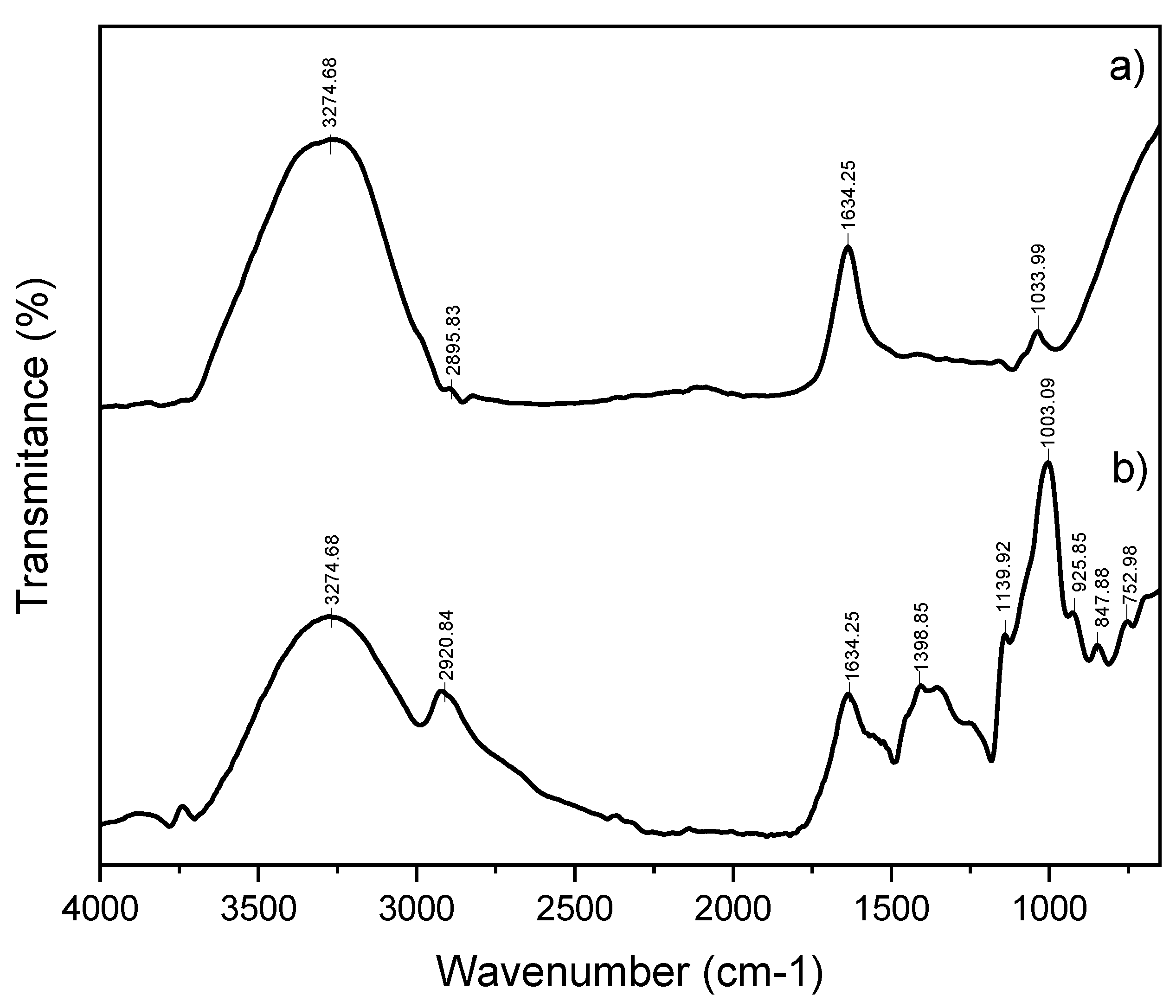
2.4. Fourier Transform Infrared Spectroscopy (FT-IR)
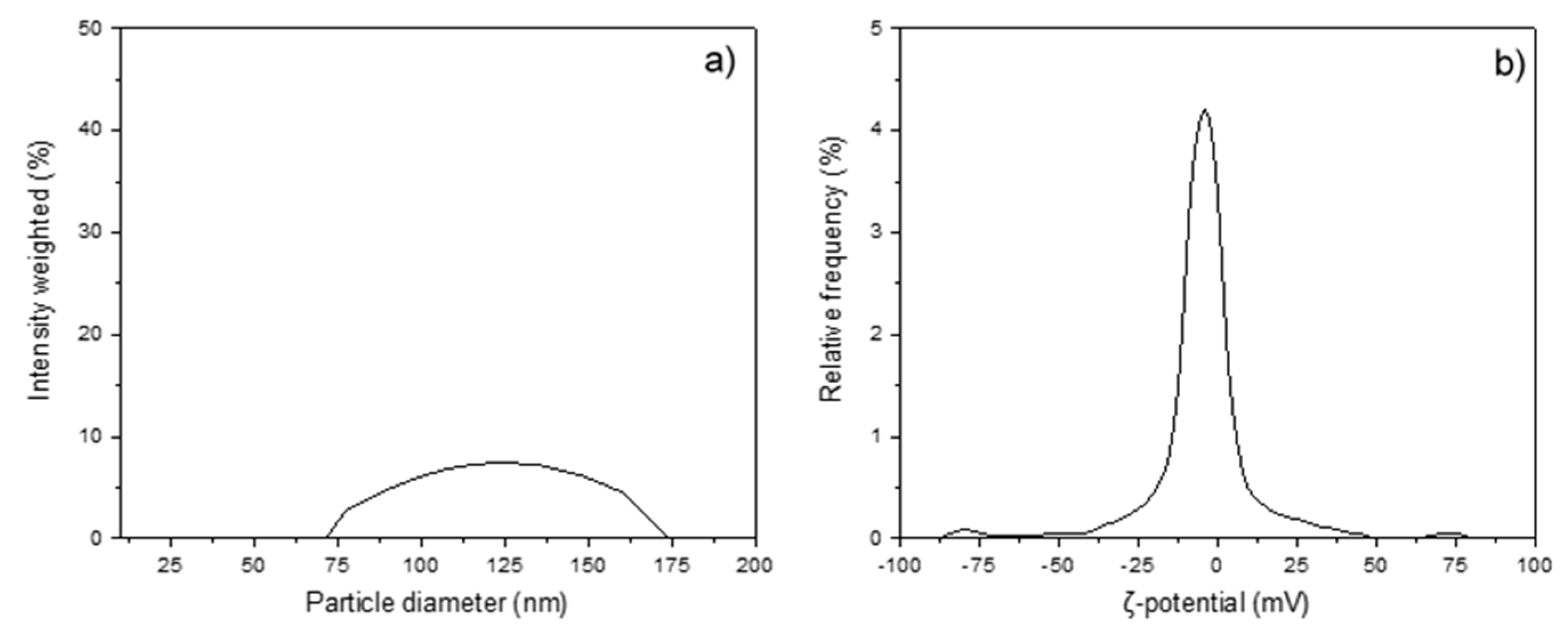
2.5. Dynamic Light Scattering (DLS) and ζ-Potential
2.6. Plasma Optical Emission Spectrometry (ICP-OES)
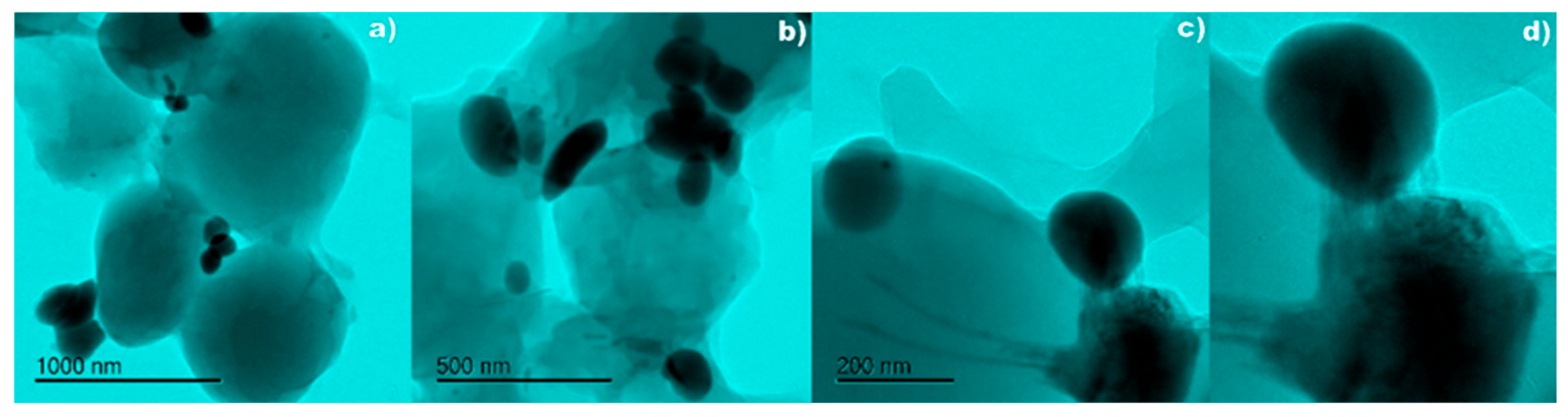
2.7. Transmission Electron Microscopy (TEM)
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- NIH. Selenio. 2024. Available online: https://ods.od.nih.gov/factsheets/Selenium-DatosEnEspanol/ (accessed on 9 December 2024).
- González-Salitre, L.; Román-Gutiérrez, A.; Contreras-López, E.; Bautista-Ávila, M.; Rodríguez-Serrano, G.M.; González-Olivares, L.G. Promising use of selenized yeast to develop new enriched food: Human health implications. Food Rev. Int. 2023, 39, 1594–1611. [Google Scholar] [CrossRef]
- Chen, Z.; Lu, Y.; Dun, X.; Wang, X.; Wang, H. Research progress of selenium-enriched foods. Nutrients 2023, 15, 4189. [Google Scholar] [CrossRef] [PubMed]
- Tangjaidee, P.; Swedlund, P.; Xiang, J.; Yin, H.; Quek, S.Y. Selenium-enriched plant foods: Selenium accumulation, speciation, and health functionality. Front. Nutr. 2023, 9, 962312. [Google Scholar] [CrossRef]
- Kieliszek, M. Selenium–fascinating microelement, properties and sources in food. Molecules. 2019, 24, 1298. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohé, R. Selenium in human health and disease: An overview. In Selenium. Molecular and Integrative Toxicology; Springer: Cham, Switzerland, 2018; pp. 3–26. [Google Scholar]
- Zhang, C.; Wang, Y.; Ullah, H.; Rahman, A.U.; Wei, J.; Qin, Y.H.; Li, X. Saccharomyces boulardii (CNCM I-745) alleviates collagen-induced arthritis by partially maintaining intestinal mucosal integrity through TLR2/MYD88/NF-κB pathway inhibition. Int. Immunopharmacol. 2024, 139, 112738. [Google Scholar] [CrossRef]
- Babaei, F.; Navidi-Moghaddam, A.; Naderi, A.; Ghafghazi, S.; Mirzababaei, M.; Dargahi, L.; Nassiri-Asl, M. The preventive effects of Saccharomyces boulardii against oxidative stress induced by lipopolysaccharide in rat brain. Heliyon 2024, 10, e30426. [Google Scholar] [CrossRef]
- Fu, H.; Chen, Z.; Teng, W.; Du, Z.; Zhang, Y.; Ye, X.; Pi, X. Effects of fructooligosaccharides and Saccharomyces boulardii on the compositional structure and metabolism of gut microbiota in students. Microbiol. Res. 2024, 285, 127741. [Google Scholar] [CrossRef]
- Kaźmierczak-Siedlecka, K.; Ruszkowski, J.; Fic, M.; Folwarski, M.; Makarewicz, W. Saccharomyces boulardii CNCM I-745: A non-bacterial microorganism used as probiotic agent in supporting treatment of selected diseases. Curr. Microbiol. 2020, 77, 1987–1996. [Google Scholar] [CrossRef]
- González-Salitre, L.; Castaneda-Ovando, A.; Basilio-Cortés, U.A.; del Carmen García-Contreras, A.; Serrano, G.R.; Cardelle-Cobas, A.; González-Olivares, L.G. Biogenic production of seleno-amino acids and seleno-nanoparticles by Saccharomyces boulardii. Food Biosci. 2023, 53, 102552. [Google Scholar] [CrossRef]
- Hyrslova, I.; Kana, A.; Nesporova, V.; Mrvikova, I.; Doulgeraki, A.I.; Lampova, B.; Krausova, G. In vitro digestion and characterization of selenized Saccharomyces cerevisiae, Pichia fermentans and probiotic Saccharomyces boulardii. J. Trace Elem. Med. Biol. 2024, 83, 127402. [Google Scholar] [CrossRef]
- González-Salitre, L.; González-Olivares, L.G.; Basilio-Cortes, U.A. Humulus lupulus L. a potential precursor to human health: High hops craft beer. Food Chem. 2023, 405, 134959. [Google Scholar] [CrossRef]
- Seo, S.H.; Kim, E.J.; Park, S.E.; Park, D.H.; Park, K.M.; Na, C.S.; Son, H.S. GC/MS-based metabolomics study to investigate differential metabolites between ale and lager beers. Food Biosci. 2020, 36, 100671. [Google Scholar] [CrossRef]
- Carisma, N.A.S.; Calingacion, M.N. Metabolomics and (craft) beers–recent advances. Food Res. Int. 2025, 205, 116010. [Google Scholar] [CrossRef]
- González-Salitre, L.; Basilio-Cortés, U.A.; Rodríguez-Serrano, G.M.; Contreras-López, E.; Cardelle-Cobas, A.; González-Olivares, L.G. Physicochemical and microbiological parameters during the manufacturing of a beer-type fermented beverage using selenized Saccharomyces boulardii. Heliyon 2023, 9, e21190. [Google Scholar] [CrossRef]
- Buiatti, S.; Tat, L.; Natolino, A.; Passaghe, P. Biotransformations performed by yeasts on aromatic compounds provided by hop—A Review. Fermentation 2023, 9, 327. [Google Scholar] [CrossRef]
- Mertens, S.; Steensels, J.; Saels, V.; De Rouck, G.; Aerts, G.; Verstrepen, K.J. A large set of newly created interspecific Saccharomyces hybrids increases aromatic diversity in lager beers. Appl. Environ. Microbiol. 2015, 81, 8202–8214. [Google Scholar] [CrossRef]
- Zastrow, C.R.; Hollatz, C.; De Araujo, P.S.; Stambuk, B.U. Maltotriose fermentation by Saccharomyces cerevisiae. J. Ind. Microbiol. Biotechnol. 2001, 27, 34–38. [Google Scholar] [CrossRef]
- Castro, L.F.; Affonso, A.D.; Lehman, R.M. Impact of specialty malts on wort and beer characteristics. Fermentation 2021, 7, 137. [Google Scholar] [CrossRef]
- Cutaia, A.J.; Reid, A.J.; Speers, R.A. Examination of the relationships between original, real and apparent extracts, and alcohol in pilot plant and commercially produced beers. J. Inst. Brew. 2009, 115, 318–327. [Google Scholar] [CrossRef]
- Norma Oficial Mexicana NOM-199-SCFI-2017. Bebidas Alcohólicas-Denominación, Especificaciones Fisicoquímicas, Información Comercial y Métodos de Prueba. 2017. Available online: https://www.dof.gob.mx/nota_detalle.php?codigo=5502882&fecha=30/10/2017#gsc.tab=0 (accessed on 26 January 2025).
- Senkarcinova, B.; Dias, I.A.G.; Nespor, J.; Branyik, T. Probiotic alcohol-free beer made with Saccharomyces cerevisiae var. boulardii. LWT 2019, 100, 362–367. [Google Scholar] [CrossRef]
- Edwards-Ingram, L.; Gitsham, P.; Burton, N.; Warhurst, G.; Clarke, I.; Hoyle, D.; Stateva, L. Genotypic and physiological characterization of Saccharomyces boulardii, the probiotic strain of Saccharomyces cerevisiae. Appl. Environ. Microbiol. 2007, 73, 2458–2467. [Google Scholar] [CrossRef]
- Du, L.P.; Hao, R.X.; Xiao, D.G.; Guo, L.L.; Gai, W.D. Research on the Characteristics and Culture Conditions of Saccharomyces boulardii. Adv. Mater. Res. 2012, 343, 594–598. [Google Scholar] [CrossRef]
- Faramarzi, S.; Anzabi, Y.; Jafarizadeh-Malmiri, H. Nanobiotechnology approach in intracellular selenium nanoparticle synthesis using Saccharomyces cerevisiae—Fabrication and characterization. Arch. Microbiol. 2020, 202, 1203–1209. [Google Scholar] [CrossRef]
- Ojeda, J.J.; Dittrich, M. Fourier transform infrared spectroscopy formolecular análisis of microbial cells. In Microbial Systems Biology: Methods and Protocols; Methods in Molecular Biology Series; Navid, A., Ed.; Springer: New York, NY, USA, 2012; Volume 881, Chapter 8; pp. 187–211. [Google Scholar] [CrossRef]
- Tugarova, A.V.; Mamchenkova, P.V.; Dyatlova, Y.A.; Kamnev, A.A. FTIR and Raman spectroscopic studies of selenium nanoparticles synthesised by the bacterium Azospirillum thiophilum. Spectrochim. Acta Part A 2018, 192, 458–463. [Google Scholar] [CrossRef]
- Panghal, A.; Janghu, S.; Virkar, K.; Gat, Y.; Kumar, V.; Chhikara, N. Potential non-dairy probiotic products—A healthy approach. Food Biosci. 2018, 21, 80–89. [Google Scholar] [CrossRef]
- Silanikove, N.; Leitner, G.; Merin, U. The interrelationships between lactose intolerance and the modern dairy industry: Global perspectives in evolutional and historical backgrounds. Nutrients 2015, 7, 7312–7331. [Google Scholar] [CrossRef]
- Sánchez-Martínez, M.; da Silva EG, P.; Pérez-Corona, T.; Cámara, C.; Ferreira, S.L.; Madrid, Y. Selenite biotransformation during brewing. Evaluation by HPLC–ICP-MS. Talanta 2012, 88, 272–276. [Google Scholar] [CrossRef]
- Martiniano, S.E.; Philippini, R.R.; Franco-Marcelino, P.R.; da Silva, S.S. Effect of selenium uptake on growth metabolism in yeasts for the production of enriched single-cell protein using agro-industrial by-products. Biomass Convers. Biorefin. 2020, 12, 3975–3983. [Google Scholar] [CrossRef]
- Capece, A.; Romaniello, R.; Pietrafesa, A.; Siesto, G.; Pietrafesa, R.; Zambuto, M.; Romano, P. Use of Saccharomyces cerevisiae var. boulardii in co-fermentations with S. cerevisiae for the production of craft beers with potential healthy value-added. Int. J. Food Microbiol. 2018, 284, 22–30. [Google Scholar] [CrossRef]
- Senthamarai, M.D.; Hillary, V.E.; Rajan, M.R.; Ceasar, S.A. Biosynthesis of selenium nanoparticles and its biological applications: A systematic review. Nano-Struct. Nano-Objects 2024, 39, 101261. [Google Scholar] [CrossRef]
- Kamnev, A.A.; Mamchenkova, P.V.; Dyatlova, Y.A.; Tugarova, A.V. FTIR spectroscopic studies of selenite reduction by cells of the rhizobacterium Azospirillum brasilense Sp7 and the formation of selenium nanoparticles. J. Mol. Struct. 2017, 1140, 106–112. [Google Scholar] [CrossRef]
- Bahrami, R.; Ebrahimi, B.; Awlqadr, F.H.; Rouhi, M.; Paimard, G.; Sarlak, Z.; Mohammadi, R. Assessment of selenium-enriched deactivated probiotic yeast efficiency in patulin detoxification in apple juice. Food Control. 2025, 168, 110880. [Google Scholar] [CrossRef]
- Ye, S.; Shen, F.; Jiao, L.; Xu, Z.; Wang, F. Biosynthesis of selenoproteins by Saccharomyces cerevisiae and characterization of its antioxidant activities. Int. J. Biol. Macromol. 2020, 164, 3438–3445. [Google Scholar] [CrossRef]
- Li, Y.; Wang, T.; Liu, D.; Zhang, C.; Wu, W.; Yi, H.; Zhang, J. Biotransformation of inorganic selenium into selenium nanoparticles and organic selenium by Lactiplantibacillus plantarum CXG4. Food Biosci. 2025, 65, 106060. [Google Scholar] [CrossRef]
- Akçay, F.A.; Avcı, A. Effects of process conditions and yeast extract on the synthesis of selenium nanoparticles by a novel indigenous isolate Bacillus sp. EKT1 and characterization of nanoparticles. Arch. Microbiol. 2020, 202, 2233–2243. [Google Scholar] [CrossRef]
- Sadeghi, A.; Ebrahimi, M.; Shahryari, S.; Kharazmi, M.S.; Jafari, S.M. Food applications of probiotic yeasts; focusing on their techno-functional, postbiotic and protective capabilities. Trends Food Sci Technol. 2022, 128, 278–295. [Google Scholar] [CrossRef]
- Puligundla, P.; Mok, C.; Park, S. Advances in the valorization of spent brewer’s yeast. Innovative Food Sci. Emerg. Technol. 2020, 62, 102350. [Google Scholar] [CrossRef]
- Salem, S.S. Bio-fabrication of selenium nanoparticles using Baker’s yeast extract and its antimicrobial efficacy on food borne pathogens. Appl. Biochem. Biotechnol. 2022, 194, 1898–1910. [Google Scholar] [CrossRef]
- Wu, Z.; Ren, Y.; Liang, Y.; Huang, L.; Yang, Y.; Zafar, A.; Hasan, M.; Yang, F.; Shu, X. Synthesis, characterization, immune regulation, and antioxidative assessment of yeast-derived selenium nanoparticles in cyclophosphamide-induced rats. ACS Omega 2021, 6, 24585–24594. [Google Scholar] [CrossRef]
- Lian, S.; Diko, C.S.; Yan, Y.; Li, Z.; Zhang, H.; Ma, Q.; Qu, Y. Characterization of biogenic selenium nanoparticles derived from cell-free extracts of a novel yeast Magnusiomyces ingens. 3 Biotech 2019, 9, 221. [Google Scholar] [CrossRef]
- Hunter, R.J. Zeta Potential in Colloid Science: Principles and Applications; Academic Press: Sydney, Australia, 1981. [Google Scholar]
- Jain, R.; Jordan, N.; Weiss, S.; Foerstendorf, H.; Heim, K.; Kacker, R.; Lens, P.N. Extracellular polymeric substances govern the surface charge of biogenic elemental selenium nanoparticles. Environ. Sci. Technol. 2015, 49, 1713–1720. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Ramírez, M.C.; Rodríguez-Serrano, G.M.; Salazar-Pereda, V.; Castañeda-Ovando, A.; Pérez-Escalante, E.; Jaimez-Ordaz, J.; González-Olivares, L.G. Biogenic production of selenocysteine by Enterococcus faecium ABMC-05: An indigenous lactic acid bacterium from fermented Mexican beverage. Food Sci. Technol. 2022, 43, e63622. [Google Scholar] [CrossRef]
- Tugarova, A.V.; Mamchenkova, P.V.; Khanadeev, V.A.; Kamnev, A.A. Selenite reduction by the rhizobacterium Azospirillum brasilense, synthesis of extracellular selenium nanoparticles and their characterisation. New Biotechnol. 2020, 58, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Tugarova, A.V.; Vladimirova, A.A.; Dyatlova, Y.A.; Kamnev, A.A. Raman spectroscopic and TEM monitoring of selenite and selenate reduction by the bacterium Azospirillum thiophilum with the formation of selenium (0) nanoparticles: Effects of sulfate. Spectrochim. Acta Part A 2025, 329, 125463. [Google Scholar] [CrossRef]
- Ge, M.; Zhou, S.; Li, D.; Song, D.; Yang, S.; Xu, M. Reduction of selenite to selenium nanoparticles by highly selenite-tolerant bacteria isolated from seleniferous soil. J. Hazard. Mater. 2024, 472, 134491. [Google Scholar] [CrossRef]
| Parameter | S. boulardii (selenized) | S. boulardii |
|---|---|---|
| pH | 4.5 ± 0.022 a | 4.8 ± 0.031 b |
| %Alc. Vol. | 2.40 ± 0.004 a | 3.51 ± 0.009 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Salitre, L.; González-Olivares, L.G.; Salazar-Navarro, A.A.; Cervantes-García, D.; Durán-Hernández, D.; Torres-Ramos, R.; Beleño-Cabarcas, M.T.; Basilio-Cortes, U.A. Spectroscopic Analysis of Selenium Nanoparticles Synthesized by Saccharomyces boulardii for the Production of Craft Beer. Fermentation 2025, 11, 144. https://doi.org/10.3390/fermentation11030144
González-Salitre L, González-Olivares LG, Salazar-Navarro AA, Cervantes-García D, Durán-Hernández D, Torres-Ramos R, Beleño-Cabarcas MT, Basilio-Cortes UA. Spectroscopic Analysis of Selenium Nanoparticles Synthesized by Saccharomyces boulardii for the Production of Craft Beer. Fermentation. 2025; 11(3):144. https://doi.org/10.3390/fermentation11030144
Chicago/Turabian StyleGonzález-Salitre, Lourdes, Luis Guillermo González-Olivares, Alexis Alejandro Salazar-Navarro, David Cervantes-García, Dagoberto Durán-Hernández, Ricardo Torres-Ramos, Mary Triny Beleño-Cabarcas, and Ulin Antobelli Basilio-Cortes. 2025. "Spectroscopic Analysis of Selenium Nanoparticles Synthesized by Saccharomyces boulardii for the Production of Craft Beer" Fermentation 11, no. 3: 144. https://doi.org/10.3390/fermentation11030144
APA StyleGonzález-Salitre, L., González-Olivares, L. G., Salazar-Navarro, A. A., Cervantes-García, D., Durán-Hernández, D., Torres-Ramos, R., Beleño-Cabarcas, M. T., & Basilio-Cortes, U. A. (2025). Spectroscopic Analysis of Selenium Nanoparticles Synthesized by Saccharomyces boulardii for the Production of Craft Beer. Fermentation, 11(3), 144. https://doi.org/10.3390/fermentation11030144









