Artificial vs. Mechanical Daqu: Comparative Analysis of Physicochemical, Flavor, and Microbial Profiles in Chinese Baijiu Starter Cultures
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Physicochemical Properties and Enzyme Activity Analysis
2.3. Volatile Flavor Compound Analysis
2.4. Sample DNA Extraction and Microbial Community Structure Analysis
2.5. Bioinformatics Analysis
2.6. Alpha Diversity Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. The Impact of Qu-Making Methods on the Physicochemical Factors of Medium-High Temperature Daqu
3.2. The Impact of Qu-Making Methods on the Biochemical Factors of Medium-High Temperature Daqu
3.3. The Impact of Qu-Making Methods on the Flavor Components of Medium-High Temperature Daqu
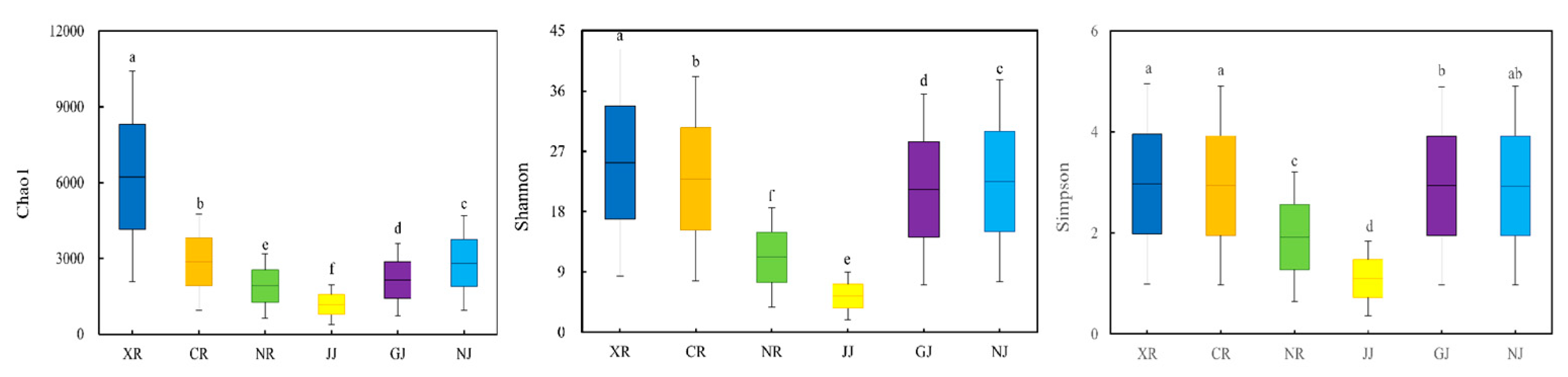
3.4. The Impact of Qu-Making Methods on the α-Diversity of Microbial Communities in Medium-High Temperature Daqu
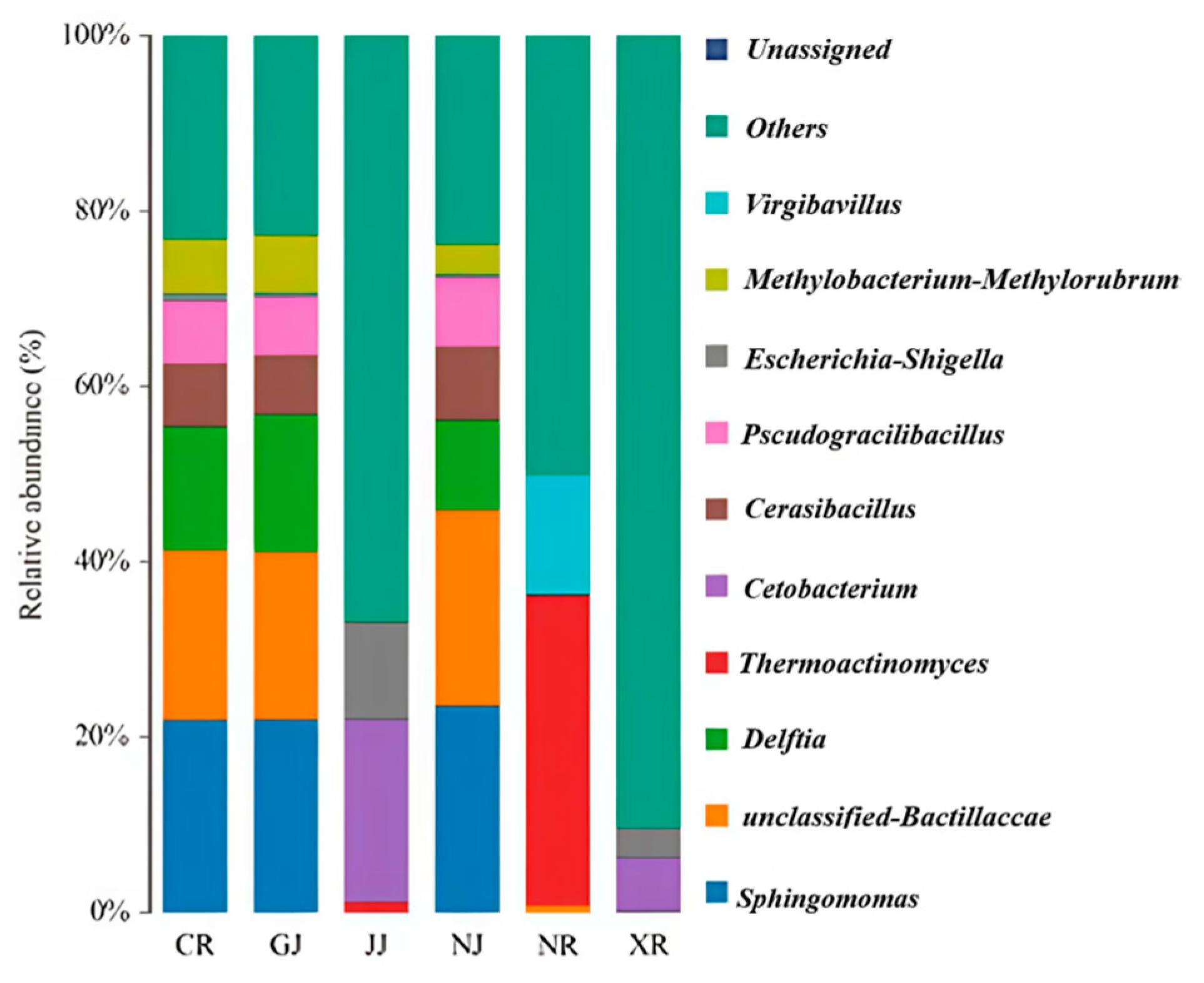
3.5. The Impact of Qu-Making Methods on the Structure of Microbial Communities in Medium-High Temperature Daqu
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tu, X.; Cao, Y.; Cheng, J.; Li, Y.; Zhang, W.; Wu, Q.; Xiang, W.; Shen, C.; Li, Q. Chinese Baijiu: The Perfect Works of Microorganisms. Front. Microbiol. 2022, 13, 919044. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xu, D. Complete Techniques of Luzhou-Style Liquor Production; China Light Industry Press: Beijing, China, 2011. [Google Scholar]
- Liu, M.; Zhao, J.; Bian, M. Research Progress on Microorganisms in High-Temperature Daqu. Brewing 2021, 48, 8–11. [Google Scholar]
- Cui, X.; Lyu, Z.; Zhang, M.; Liu, Y.; Qin, B.; Zhao, Q.; Li, X.; Li, P. Establishment of an Evaluation Model for Aroma Substances in Medium-High Temperature Daqu Based on Principal Component Analysis. J. Food Saf. Qual. Insp. 2023, 14, 279–287. [Google Scholar]
- Li, P.; Li, S.; Liu, X.; Luo, L.X.; Lin, W.T. Dynamic Changes of Volatile Components during Daqu Fermentation Studied by HS-SPME-GC-MS. Mod. Food Sci. Technol. 2016, 32, 271–276. [Google Scholar]
- Jian, X.; Shi, Y.; Wang, T. Comparative Analysis of Biological and Physicochemical Indicators between Mechanically Produced Daqu and Manually Produced Daqu. Liquor-Mak. Sci. Technol. 2014, 1, 55–58. [Google Scholar]
- Fu, J.; Wang, Y.; Luo, G. A New Type of Qu-Block Press for Distilleries. Packag. Food Mach. 2006, 4, 46–47. [Google Scholar]
- Wang, X.; Ning, M.; Cui, H. Analysis and Research on the Mechanical Production Process of Medium-High Temperature Baobao Qu in Northern China. Liquor Mak. 2012, 39, 36–38. [Google Scholar]
- Cui, H. Discussion on the Production Process of Mechanized Medium-High Temperature Baobao Qu. Liquor-Mak. Sci. Technol. 2013, 2, 3. [Google Scholar]
- Wang, X.; Qiu, S.; Li, P. Analysis of Microbial Community Structure in Traditional and Automated Moutai-Flavor Daqu. J. Am. Soc. Brew. Chem. 2019, 77, 140–146. [Google Scholar] [CrossRef]
- Jiang, S.; Qiu, S.; Zou, J.; Chen, L.; Luo, X.; Wang, X. Preliminary Study on Yeast in Traditional Maotai-Flavor Daqu and Mechanized Maotai-Flavor Daqu. China Brew. 2017, 36, 59–65. [Google Scholar]
- Lin, P.; Zhao, M.; Wu, S. Analysis of Differences in Daqu Cultivation between Manual Trampling and Mechanical Production. Liquor-Mak. Sci. Technol. 2012, 4, 70–71. [Google Scholar]
- Lyu, Y.; Sha, J. Study on the Influence of Different Cultivation Periods on the Physicochemical Indicators of Medium-High Temperature Daqu. Brewing 2017, 44, 67–69. [Google Scholar]
- Yang, Y.; Wang, S.-T.; Lu, Z.-M.; Zhang, X.-J.; Chai, L.-J.; Shen, C.-H.; Shi, J.-S.; Xu, Z.-H. Metagenomics Unveils Microbial Roles Involved in Metabolic Network of Flavor Development in Medium-Temperature Daqu Starter. Food Res. Int. 2021, 140, 110037. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Su, Z. Effect of Preparation Methods on the Physicochemical Properties and Bacterial Community of Chixiangxing Baijiu Qu. Food Sci. 2023, 44, 211–217. [Google Scholar] [CrossRef]
- Huang, P.; Jin, Y.; Liu, M.; Peng, L.; Yang, G.; Luo, Z.; Jiang, D.; Zhao, J.; Zhou, R.; Wu, C. Exploring the Successions in Microbial Community and Flavor of Daqu during Fermentation Produced by Different Pressing Patterns. Foods 2023, 12, 2603. [Google Scholar] [CrossRef]
- QB/T 4257-2011; General Analysis Methods for Brewing Daqu. Standardization Administration of China: Beijing, China, 2011.
- DB 34/T 3085-2018; Operating Procedures for Testing Strong-Flavor Daqu. Anhui Provincial Bureau of Quality and Technical Supervision: Hefei, China, 2018.
- NY/T 912-2020; Determination of Cellulase Activity in Feed Additives—Spectrophotometric Method. Ministry of Agriculture and Rural Affairs of the People’s Republic of China: Beijing, China, 2020.
- GB/T 23874-2009; Determination of Xylanase Activity in Feed Additives—Spectrophotometric Method. Standardization Administration of China: Beijing, China, 2009.
- GB 5009.7-2016; National Food Safety Standard—Determination of Reducing Sugars in Foods. National Health Commission of the People’s Republic of China: Beijing, China, 2016.
- Tang, H.; Liang, H.; Song, J.; Lin, W.; Luo, L. Comparison of Microbial Community and Metabolites in Spontaneous Fermentation of Two Types Daqu Starter for Traditional Chinese Vinegar Production. J. Biosci. Bioeng. 2019, 128, 307–315. [Google Scholar] [CrossRef]
- Wang, X.Y.; Zhu, X.G.; Bi, Y.L. Dynamics of Microbial Community and Changes of Metabolites during Production of Type Ι Sourdough Steamed Bread Made by Retarded Sponge-Dough Method. Food Chem. 2020, 330, 127316. [Google Scholar] [CrossRef] [PubMed]
- Magoč, T.; Salzberg, S.L. FLASH: Fast Length Adjustment of Short Reads to Improve Genome Assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Callahan, B.; McMurdie, P.; Rosen, M.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Huang, Z.G.; Hou, H.B.; Luo, H.B. Study on the Changes of Starch and Reducing Sugar during the Fermentation of Strong-Flavor Baijiu Fermented Grains. China Brew. 2012, 31, 107–110. [Google Scholar]
- Wang, Y.; Gai, J.; Hou, Q.; Zhao, H.; Shan, C.; Guo, Z. Ultra-High-Depth Macrogenomic Sequencing Revealed Differences in Microbial Composition and Function between High Temperature and Medium-High Temperature Daqu. World J. Microbiol. Biotechnol. 2023, 39, 337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, S.; Sun, Z. Discussion and Analysis of Existing Methods for Determining Moisture Content in Pure Grain Solid-State Daqu. Brewing 2020, 47, 72–75. [Google Scholar]
- Liu, C.; He, P.; Luo, M.Y. Comparative Study on Main Biological and Physicochemical Indicators of Medium-High Temperature Mechanically Produced and Manually Produced Daqu. Liquor-Mak. Sci. Technol. 2022, 7, 52–59. [Google Scholar]
- Du, X.J.; Ming, H.M.; Ma, H.; Jiang, D.C.; Zhu, H.Q.; Chen, Y.; Xu, J. Microbial Community Structure Differences and Key Influencing Factors among Different Parts of Mechanical and Artificial Daqu. Food Ferment. Ind. 2023, 49, 83–91. [Google Scholar]
- Xiang, G.X.; Chen, Y.Q.; Shen, Y.; Wang, X.; Zhang, Y.D.; Luo, H.B.; Huang, D. Comparative Analysis of Microbial Community Structure and Physicochemical Properties of Different Grades of Nongxiangxing Daqu. Food Sci. 2022, 43, 184–191. [Google Scholar]
- Ren, F.; Zhu, W.Y.; Cheng, W.H. Effects of Acidity on the Fermentation of Strong-Flavor Daqu Liquor and Its Control Measures. China Brew. 2021, 40, 14–17. [Google Scholar]
- Kitamoto, K. Molecular Biology of the Koji Molds. Adv. Appl. Microbiol. 2002, 51, 129–153. [Google Scholar] [CrossRef]
- Han, P.-J.; Luo, L.-J.; Han, Y.; Song, L.; Zhen, P.; Han, D.-Y.; Wei, Y.-H.; Zhou, X.; Wen, Z.; Qiu, J.-Z.; et al. Microbial Community Affects Daqu Quality and the Production of Ethanol and Flavor Compounds in Baijiu Fermentation. Foods 2023, 12, 2936. [Google Scholar] [CrossRef]
- Hu, P.P.; Wang, J.; Ali, U.; Aziz, T.; Sameeh, M.Y.; Feng, C.P. Comparative Study on Physicochemical Properties, Microbial Composition, and the Volatile Component of Different Light Flavor Daqu. Food Sci. Nutr. 2023, 11, 5174–5187. [Google Scholar] [CrossRef]
- Yuan, X.; Huang, D.; Peng, J. Isolation of Dominant Protease-Producing Bacteria in Strong-Flavor Daqu and Study on Enzyme Production Conditions. Liquor-Mak. Sci. Technol. 2012, 8, 54–57. [Google Scholar]
- Chen, Y.; Li, K.; Liu, T.; Li, R.; Fu, G.; Wan, Y.; Zheng, F. Analysis of Difference in Microbial Community and Physicochemical Indices between Surface and Central Parts of Chinese Special-Flavor Baijiu Daqu. Front. Microbiol. 2021, 12, 592421. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Luo, B.X.; Zhang, L.L.; Yao, Y.L.; Guo, Y.; Huang, Z.G.; Ren, Z.Q. Research on Physicochemical Properties and Microbial Community Structure of Daqu Made from Different Grains. Food Ferment. Ind. 2020, 46, 74–79. [Google Scholar]
- Feng, J.T.; Lu, Z.M.; Shi, W. Effects of Different Cultivation Temperatures on Microbial Community Structure, Enzyme Activity, and Volatile Compounds in Daqu. J. Appl. Environ. Biol. 2021, 27, 760–767. [Google Scholar]
- Nie, X.; Jia, X.; Zhu, K.; Ling, Z.; Chen, H.; Xie, J.; Ao, Z.; Song, C.; Shen, C.; Zhu, C.; et al. Dynamic Changes and Potential Correlations between Microbial Diversity and Volatile Flavor Compounds in Chinese Medium-Temperature Daqu during Manufacturing. Molecules 2024, 29, 4851. [Google Scholar] [CrossRef]
- Yang, S.B.; Fu, J.J.; He, J.H.; Zhang, X.J.; Chai, L.J.; Shi, J.S.; Wang, S.T.; Zhang, S.Y.; Shen, C.H.; Lu, Z.M.; et al. Decoding the Qu-Aroma of Medium-Temperature Daqu Starter by Volatilomics, Aroma Recombination, Omission Studies, and Sensory Analysis. Food Chem. 2024, 457, 140186. [Google Scholar] [CrossRef]
- Thapa, N.; Pal, J.; Tamang, J.P. Microbial Diversity in Ngari, Hentaak and Tungtap, Fermented Fish Products of Northeast India. World J. Microbiol. Biotechnol. 2007, 23, 1775–1780. [Google Scholar] [CrossRef]
- Liu, W.; Liu, G.; Zhang, R.; Zheng, L.; Lu, Z.; Zhang, X.; Wang, S.; Shen, C.; Shi, J.; Xu, Z.; et al. Metagenomics Unveils the Differences in the Functions of Microbial Community of Medium-Temperature Daqu before and after Maturation. Chin. J. Biotechnol. 2024, 40, 877–894. [Google Scholar]
- Kang, J.; Chen, X.; Han, B.-Z.; Xue, Y. Insights into the Bacterial, Fungal, and Phage Communities and Volatile Profiles in Different Types of Daqu. Food Res. Int. 2022, 158, 111488. [Google Scholar] [CrossRef]
- Bal, J.; Yun, S.H.; Yeo, S.H.; Kim, J.M.; Kim, D.H. Metagenomic Analysis of Fungal Diversity in Korean Traditional Wheat-Based Fermentation Starter Nuruk. Food Microbiol. 2016, 60, 73–83. [Google Scholar] [CrossRef]
- Wen, Z.; Han, P.J.; Han, D.Y.; Song, L.; Wei, Y.H.; Zhu, H.Y.; Chen, J.; Guo, Z.X.; Bai, F.Y. Microbial Community Assembly Patterns at the Species Level in Different Parts of the Medium Temperature Daqu during Fermentation. Curr. Res. Food Sci. 2024, 9, 100883. [Google Scholar] [CrossRef]
- Ding, L.; Zhao, M.M.; Zhao, X.F.; Chen, G.Y.; Jiang, Q.T.; Liu, M.; Xiong, Y.; Zhang, X.; Wang, X.J.; Wei, Y.M.; et al. Evaluation of the Spatial Distribution and Dynamic Succession of Microbial Community and Quality Properties during Fermentation in Chinese Medium-Temperature Daqu. J. Food Process. Preserv. 2022, 46, e17272. [Google Scholar] [CrossRef]
- Deng, L.; Mao, X.; Liu, D.; Ning, X.Q.; Shen, Y.; Chen, B.; Nie, H.F.; Huang, D.; Luo, H.B. Comparative Analysis of Physicochemical Properties and Microbial Composition in High-Temperature Daqu With Different Colors. Front. Microbiol. 2020, 11, 588117. [Google Scholar] [CrossRef]
- Li, P.; Aflakpui, F.W.K.; Yu, H.; Luo, L.; Lin, W.T. Characterization of Activity and Microbial Diversity of Typical Types of Daqu for Traditional Chinese Vinegar. Ann. Microbiol. 2015, 65, 2019–2027. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, Y.; Hu, X.; Huang, Y. Analysis of Bacterial Diversity and Functional Differences of Jiang-Flavored Daqu Produced in Different Seasons. Front. Nutr. 2023, 9, 1078132. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yan, X.; Zou, S.; Ji, C.; Dong, L.; Zhang, S.; Liang, H.; Lin, X. Analysis of Fungal Diversity, Physicochemical Properties and Volatile Organic Compounds of Strong-Flavor Daqu from Seven Different Areas. Foods 2024, 13, 1263. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Y.; Qu, D.; Zhao, H.; Tian, L.; Zhou, J.; Guo, Z. Microbial Communities, Functional, and Flavor Differences among Three Different-Colored High-Temperature Daqu: A Comprehensive Metagenomic, Physicochemical, and Electronic Sensory Analysis. Food Res. Int. 2024, 184, 114257. [Google Scholar]




| Flavor Components | XR | CR | NR | JJ | GJ | NJ |
|---|---|---|---|---|---|---|
| Alcohols | ||||||
| 2-Pentanol | 0.005 ± 0.001d | 0.024 ± 0.001c | 0.081 ± 0.005a | 0.036 ± 0.003b | 0.027 ± 0.001a | nd |
| 3-Methyl-1-butanol | 0.042 ± 0.001c | 0.031 ± 0.003c | 0.045 ± 0.003c | 0.289 ± 0.050a | 0.134 ± 0.024b | 0.028 ± 0.004c |
| 1-Pentanol | 0.102 ± 0.003a | 0.010 ± 0.001b | nd | 0.012 ± 0.002b | nd | nd |
| 1-Hexanol | 0.110 ± 0.006a | 0.043 ± 0.007c | 0.101 ± 0.003a | 0.086 ± 0.013b | 0.027 ± 0.004d | 0.036 ± 0.005cd |
| 3-Octenol | 0.010 ± 0.001c | 0.018 ± 0.002b | 0.020 ± 0.001b | 0.029 ± 0.005a | 0.005 ± 0.001d | 0.019 ± 0.002c |
| 1-Heptanol | 0.025 ± 0.003a | 0.008 ± 0.001d | 0.015 ± 0.002b | 0.013 ± 0.002bc | 0.012 ± 0.002bc | 0.009 ± 0.001cd |
| 2-Decanol | nd | 0.013 ± 0.000b | nd | 0.029 ± 0.002a | nd | nd |
| 1-Octanol | 0.074 ± 0.002a | 0.013 ± 0.001d | 0.047 ± 0.003b | nd | nd | 0.025 ± 0.005c |
| 2,3-Butanediol | 0.601 ± 0.067 | nd | nd | nd | nd | nd |
| (E)-2-Octenol | 0.011 ± 0.001 | nd | nd | nd | nd | nd |
| 2-Furfuryl alcohol | 0.038 ± 0.007a | 0.006 ± 0.001c | 0.023 ± 0.003b | 0.025 ± 0.003b | 0.013 ± 0.002c | 0.021 ± 0.002b |
| α-Methylphenethyl alcohol | 0.010 ± 0.001c | nd | 0.023 ± 0.004b | nd | nd | 0.048 ± 0.006a |
| Benzyl alcohol | 0.083 ± 0.003b | 0.058 ± 0.009c | 0.135 ± 0.005a | 0.062 ± 0.009c | 0.120 ± 0.015a | 0.028 ± 0.004d |
| Linoleic alcohol | 0.060 ± 0.001c | nd | 0.057 ± 0.011b | 0.445 ± 0.009a | nd | 0.065 ± 0.008b |
| Phenylethyl Alcohol | 1.313 ± 0.054d | 1.308 ± 0.017d | 1.785 ± 0.241c | 3.157 ± 0.119b | 3.728 ± 0.191a | 0.99 ± 0.047e |
| 1-Undecanol | 0.005 ± 0.001c | 0.018 ± 0.001c | 0.043 ± 0.008c | nd | 0.685 ± 0.129b | nd |
| β-Ethylphenethyl alcohol | 0.005 ± 0.001b | 0.005 ± 0.001b | nd | nd | 0.013 ± 0.001a | nd |
| Cedrol | 0.545 ± 0.021a | 0.006 ± 0.001b | 0.017 ± 0.020b | nd | nd | 0.008 ± 0.001b |
| 1-Tetradecanol | nd | 0.012 ± 0.001c | 0.050 ± 0.006a | 0.034 ± 0.002b | nd | 0.045 ± 0.003a |
| Sclareol | nd | 0.008 ± 0.001 | nd | nd | nd | nd |
| Aldehydes | ||||||
| Hexanal | 0.034 ± 0.003a | nd | nd | 0.017 ± 0.001b | 0.014 ± 0.002b | nd |
| Nonanal | 0.199 ± 0.010a | 0.015 ± 0.001e | 0.049 ± 0.008d | 0.129 ± 0.008c | 0.153 ± 0.005b | 0.016 ± 0.002e |
| Decanal | 0.086 ± 0.010b | 0.009 ± 0.002e | nd | 0.159 ± 0.005a | 0.037 ± 0.002d | 0.064 ± 0.009c |
| Benzaldehyde | 0.129 ± 0.006a | 0.044 ± 0.002c | 0.032 ± 0.002c | 0.069 ± 0.001b | 0.059 ± 0.007b | 0.120 ± 0.013a |
| (E)-2-Nonenal | 0.014 ± 0.002c | 0.006 ± 0.001d | nd | 0.028 ± 0.004b | 0.015 ± 0.002c | 0.034 ± 0.001a |
| 5-Methyl furfural | 0.053 ± 0.008a | nd | 0.012 ± 0.002b | nd | 0.012 ± 0.002b | 0.010 ± 0.002b |
| Undecyl aldehyde | 0.013 ± 0.002b | nd | 0.017 ± 0.001a | nd | nd | nd |
| Benzeneacetaldehyde | nd | nd | 0.129 ± 0.013b | 0.112 ± 0.008b | 0.061 ± 0.005c | 0.316 ± 0.013a |
| cis-Citral | 0.042 ± 0.006a | nd | nd | nd | nd | 0.017 ± 0.002b |
| Tridecanal | nd | 0.022 ± 0.003b | nd | nd | 0.025 ± 0.003b | 0.076 ± 0.004a |
| 4-Methylsalicylaldehyde | 0.139 ± 0.005a | nd | 0.054 ± 0.006b | 0.054 ± 0.003b | nd | nd |
| 2-phenyl-2-Butenal | 0.09 ± 0.008a | nd | nd | 0.073 ± 0.001b | 0.036 ± 0.002c | 0.008 ± 0.001d |
| Pentadecanal | nd | nd | 0.062 ± 0.010a | nd | nd | 0.058 ± 0.006a |
| Farnesylacetaldehyde | nd | nd | 0.047 ± 0.055a | 0.039 ± 0.007a | 0.016 ± 0.003a | 0.017 ± 0.002a |
| Ketones | ||||||
| 2-Octanone | 0.165 ± 0.021a | 0.063 ± 0.008c | 0.036 ± 0.002d | 0.09 ± 0.015b | 0.071 ± 0.004bc | 0.087 ± 0.001b |
| Methyl heptenone | 0.048 ± 0.004a | 0.009 ± 0.001c | 0.018 ± 0.003b | 0.017 ± 0.003b | 0.014 ± 0.001bc | 0.015 ± 0.003b |
| 2-Undecanone | 0.027 ± 0.003a | nd | 0.007 ± 0.001b | nd | 0.007 ± 0.001b | 0.032 ± 0.005a |
| Acetophenone | 0.019 ± 0.001c | 0.023 ± 0.001c | 0.031 ± 0.003c | 0.279 ± 0.048a | 0.226 ± 0.010b | 0.029 ± 0.005c |
| Geranylacetone | 0.110 ± 0.007b | 0.005 ± 0.001d | 0.252 ± 0.028a | 0.068 ± 0.028c | 0.015 ± 0.002d | 0.051 ± 0.008c |
| γ-Nonanolactone | 0.061 ± 0.007a | 0.015 ± 0.002d | 0.013 ± 0.002d | 0.043 ± 0.003bc | 0.042 ± 0.005c | 0.051 ± 0.005b |
| Perhydrofarnesyl acetone | 0.059 ± 0.007a | nd | 0.027 ± 0.004b | nd | 0.026 ± 0.002b | 0.030 ± 0.002b |
| 2-Heptadecanone | 0.025 ± 0.004a | 0.005 ± 0.001c | 0.006 ± 0.001c | 0.012 ± 0.003b | nd | 0.022 ± 0.002a |
| Esters | ||||||
| Methyl caproate | 0.041 ± 0.002a | 0.014 ± 0.000b | nd | nd | nd | nd |
| Ethyl caproate | 0.105 ± 0.002a | 0.031 ± 0.003d | 0.085 ± 0.009b | 0.072 ± 0.008c | 0.032 ± 0.002d | 0.014 ± 0.002e |
| Ethyl lactate | nd | 0.008 ± 0.000b | nd | nd | nd | 0.032 ± 0.004a |
| Ethyl octanoate | 0.012 ± 0.001c | nd | 0.055 ± 0.008a | 0.042 ± 0.003b | nd | nd |
| Butyrolactone | nd | 0.005 ± 0.001 | nd | nd | nd | nd |
| (E)-Methyl oleate | 0.26 ± 0.017a | 0.038 ± 0.004d | 0.182 ± 0.009b | nd | 0.016 ± 0.002e | 0.104 ± 0.001c |
| γ-Hexanolactone | 0.02 ± 0.003b | 0.122 ± 0.014a | 0.005 ± 0.001b | nd | nd | nd |
| Methyl phenylacetate | 0.009 ± 0.001b | nd | 0.035 ± 0.006a | nd | nd | 0.041 ± 0.002a |
| Ethyl phenylacetate | 0.035 ± 0.003 | nd | nd | nd | nd | nd |
| (Z)-Methyl oleate | nd | 0.021 ± 0.002c | 0.135 ± 0.011a | 0.064 ± 0.003b | nd | nd |
| Methyl linoleate | 0.840 ± 0.056b | nd | 1.294 ± 0.160a | 0.755 ± 0.009b | 0.610 ± 0.453bc | 0.318 ± 0.040c |
| Methyl myristate | 0.024 ± 0.004c | 0.021 ± 0.003c | 0.035 ± 0.004b | 0.051 ± 0.002a | 0.026 ± 0.001c | 0.036 ± 0.001b |
| γ-Nonalactone | 0.075 ± 0.007a | 0.061 ± 0.008b | 0.042 ± 0.003c | nd | nd | nd |
| Methyl pentadecanoate | 0.050 ± 0.002b | 0.013 ± 0.002d | 0.069 ± 0.006a | 0.037 ± 0.001c | 0.037 ± 0.005c | 0.069 ± 0.010a |
| Methyl palmitate | 1.096 ± 0.092a | 0.431 ± 0.064c | 0.260 ± 0.015d | 1.020 ± 0.121a | 0.605 ± 0.097b | nd |
| Isopropyl palmitate | 0.822 ± 0.090a | 0.432 ± 0.063b | 0.469 ± 0.069b | nd | nd | 0.984 ± 0.100a |
| Methyl hexadec-9-enoate | 0.052 ± 0.008b | 0.015 ± 0.002c | 0.002 ± 0.003d | 0.054 ± 0.002b | 0.077 ± 0.004a | 0.069 ± 0.012a |
| Ethyl palmitate | 0.371 ± 0.046a | 0.035 ± 0.004b | 0.025 ± 0.027b | 0.051 ± 0.004b | 0.053 ± 0.003b | 0.018 ± 0.002b |
| Diethyl Phthalate | 0.049 ± 0.004c | 1.739 ± 0.285a | 1.066 ± 0.140b | 0.065 ± 0.004c | 0.012 ± 0.002c | 0.026 ± 0.005c |
| Acids | ||||||
| Acetic acid | 0.177 ± 0.007b | 0.037 ± 0.007d | 0.068 ± 0.007c | 0.195 ± 0.004a | 0.075 ± 0.003c | 0.029 ± 0.004d |
| Propanoic acid | 0.040 ± 0.005a | nd | 0.057 ± 0.006b | nd | 0.048 ± 0.004ab | nd |
| Isobutyric acid | 0.166 ± 0.008b | 0.411 ± 0.028a | 0.187 ± 0.021b | 0.022 ± 0.004c | nd | nd |
| Isovaleric acid | 0.760 ± 0.038b | 0.021 ± 0.001d | 1.122 ± 0.060a | 0.064 ± 0.002cd | nd | 0.128 ± 0.014c |
| Pentanoic acid | 0.027 ± 0.004b | 0.008 ± 0.001b | 0.661 ± 0.092a | nd | nd | 0.084 ± 0.008b |
| 4-Methylvaleric acid | 0.045 ± 0.003b | nd | 0.063 ± 0.009a | nd | nd | nd |
| Hexanoic acid | 0.348 ± 0.061a | 0.030 ± 0.002b | 0.023 ± 0.002b | 0.034 ± 0.002b | 0.053 ± 0.006b | 0.045 ± 0.003b |
| 5-Methylhexanoic acid | 0.035 ± 0.004b | nd | 0.045 ± 0.008a | nd | nd | nd |
| Heptanoic acid | 0.100 ± 0.006bc | 0.147 ± 0.019b | nd | 0.155 ± 0.006b | 1.026 ± 0.074a | 0.038 ± 0.003c |
| Phenethyl isovalerate | 0.026 ± 0.003a | nd | 0.022 ± 0.003a | nd | nd | — |
| Octanoic acid | 0.159 ± 0.002a | 0.005 ± 0.001b | nd | nd | nd | 0.007 ± 0.001b |
| Arachidonic acid | 0.120 ± 0.009c | 0.069 ± 0.007c | 0.664 ± 0.089a | 0.105 ± 0.005c | nd | 0.298 ± 0.016b |
| Nonanoic acid | 0.051 ± 0.008b | 0.014 ± 0.002c | nd | nd | nd | 0.082 ± 0.009a |
| 9-Decenoic acid | 0.027 ± 0.002c | nd | 1.080 ± 0.164a | 0.275 ± 0.016b | nd | 0.074 ± 0.005c |
| Alkanes | ||||||
| Dodecane | 0.004 ± 0.001e | 0.031 ± 0.003b | 0.026 ± 0.003c | 0.072 ± 0.003a | 0.016 ± 0.001d | 0.017 ± 0.002d |
| Tetradecane | 0.027 ± 0.003b | 0.015 ± 0.001c | 0.026 ± 0.004a | nd | 0.008 ± 0.001e | 0.010 ± 0.001d |
| Pentadecanal- | 0.02 ± 0.003a | 0.013 ± 0.002b | 0.026 ± 0.005b | 0.023 ± 0.001a | nd | 0.025 ± 0.004a |
| Hexadecane | 0.032 ± 0.003b | 0.585 ± 0.081a | 0.026 ± 0.006b | 0.026 ± 0.004b | 0.040 ± 0.007b | 0.040 ± 0.007b |
| Heneicosane | 0.023 ± 0.002a | 0.007 ± 0.001c | 0.026 ± 0.007c | nd | nd | 0.013 ± 0.002b |
| 2-Methyloctacosane | 0.008 ± 0.001d | 0.027 ± 0.002b | nd | nd | 0.016 ± 0.002c | 0.039 ± 0.004a |
| Pyrazines | ||||||
| 2-Methylpyrazine | 0.021 ± 0.003b | 0.027 ± 0.005b | 0.026 ± 0.010b | 0.040 ± 0.003a | 0.023 ± 0.004b | 0.041 ± 0.006a |
| 2,6-Dimethylpyrazine | 0.158 ± 0.010a | 0.047 ± 0.005c | 0.026 ± 0.011b | 0.147 ± 0.015ab | 0.032 ± 0.004c | 0.039 ± 0.007c |
| 2,3-Dimethylpyrazine | 0.084 ± 0.002bc | 0.076 ± 0.002c | 0.026 ± 0.012d | 0.087 ± 0.005b | 0.114 ± 0.011a | 0.015 ± 0.001e |
| 2-ethyl-6-methyl-Pyrazine | 0.013 ± 0.002d | 0.009 ± 0.001d | 0.026 ± 0.013a | 0.046 ± 0.005b | 0.026 ± 0.315c | 0.066 ± 0.012a |
| 2,3,5-Trimethylpyrazine | 0.886 ± 0.036a | 0.049 ± 0.005e | 0.026 ± 0.014c | 0.565 ± 0.040b | 0.601 ± 0.010b | 0.136 ± 0.004d |
| 2,3,5,6-Tetramethylpyrazine | 1.459 ± 0.145a | 0.093 ± 0.009cd | 0.026 ± 0.015b | 0.143 ± 0.017cd | 0.018 ± 0.002d | 0.179 ± 0.017c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, H.; Zhang, Z.; Ding, L.; Jiang, Q.; Li, Q.; Huang, J.; Wang, S.; Li, L.; Nan, G.; Lou, K. Artificial vs. Mechanical Daqu: Comparative Analysis of Physicochemical, Flavor, and Microbial Profiles in Chinese Baijiu Starter Cultures. Fermentation 2025, 11, 135. https://doi.org/10.3390/fermentation11030135
Yuan H, Zhang Z, Ding L, Jiang Q, Li Q, Huang J, Wang S, Li L, Nan G, Lou K. Artificial vs. Mechanical Daqu: Comparative Analysis of Physicochemical, Flavor, and Microbial Profiles in Chinese Baijiu Starter Cultures. Fermentation. 2025; 11(3):135. https://doi.org/10.3390/fermentation11030135
Chicago/Turabian StyleYuan, Huawei, Zhong Zhang, Liping Ding, Qin Jiang, Qian Li, Jie Huang, Songtao Wang, Li Li, Guohui Nan, and Kai Lou. 2025. "Artificial vs. Mechanical Daqu: Comparative Analysis of Physicochemical, Flavor, and Microbial Profiles in Chinese Baijiu Starter Cultures" Fermentation 11, no. 3: 135. https://doi.org/10.3390/fermentation11030135
APA StyleYuan, H., Zhang, Z., Ding, L., Jiang, Q., Li, Q., Huang, J., Wang, S., Li, L., Nan, G., & Lou, K. (2025). Artificial vs. Mechanical Daqu: Comparative Analysis of Physicochemical, Flavor, and Microbial Profiles in Chinese Baijiu Starter Cultures. Fermentation, 11(3), 135. https://doi.org/10.3390/fermentation11030135







