The Optimization of the Nutrient Medium Composition for the Submerged Cultivation of the Mycolicibacterium neoaurum Strain VKM Ac-3067D in a 100 L Bioreactor Under Controlled Conditions by Mathematical Planning
Abstract
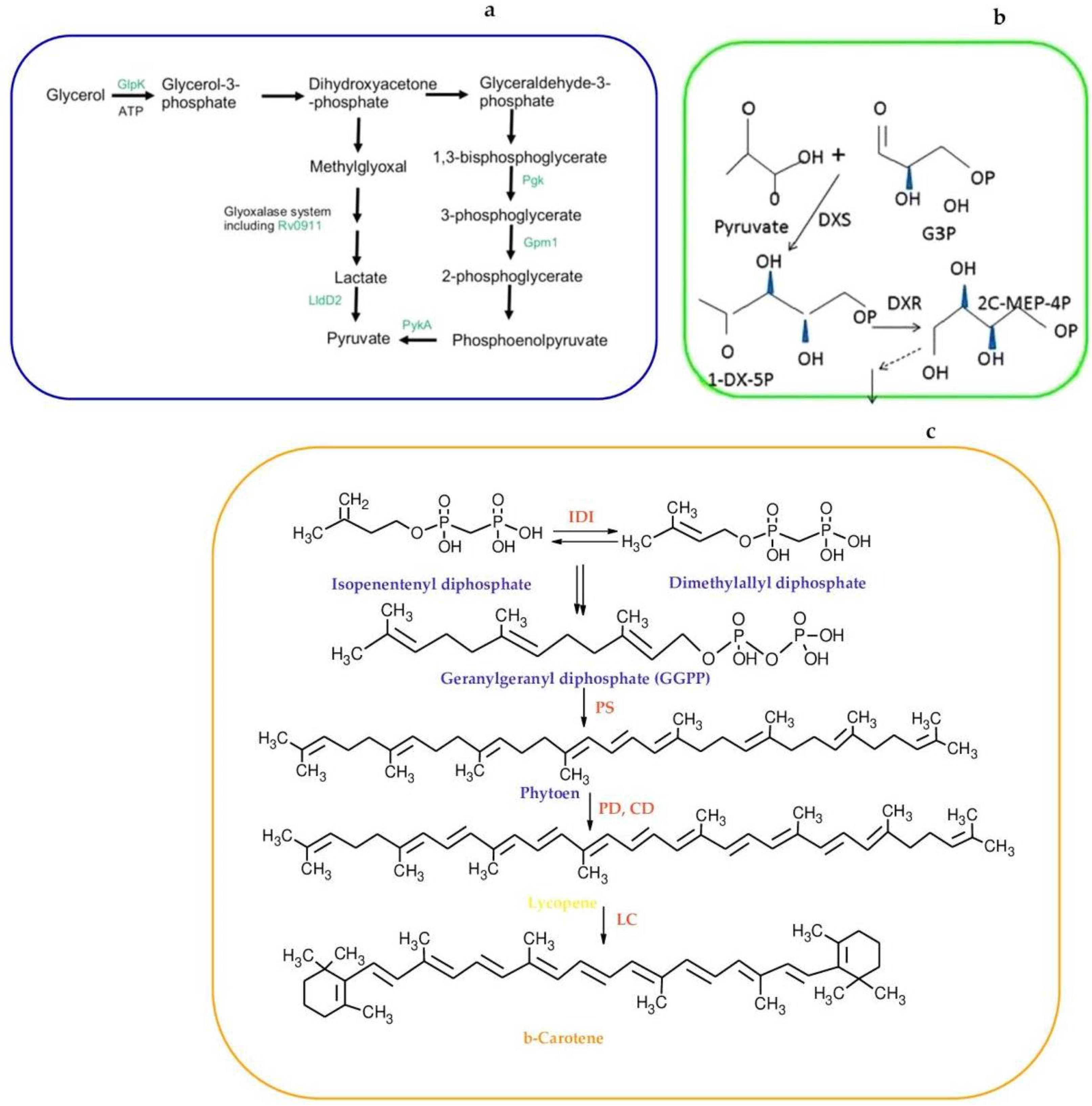
1. Introduction
2. Materials and Methods
2.1. Reagents and Media Components
2.2. Producer Strain
2.3. Nutrient Media for M. neoaurum Cultivation and Maintenance
2.4. Cultivation of M. neoaurum VKM Ac-3067D on a Liquid Nutrient Medium
2.5. Carotenoid Content Determination
2.6. Nutrient Medium Optimization Using the Mathematical Planning Method (Complete Factorial Experiment, CFE 23)
2.6.1. Nutrient Medium Optimization by a Complete Factorial Experiment (CFE)
2.6.2. Nutrient Medium Optimization Using the Steepest Ascent Method
2.7. M. neoaurum Fermentation in a 3 L Bioreactor
2.7.1. Inoculum Preparation
2.7.2. Bioreactor Preparation and Fermentation
2.8. M. neoaurum Fermentation in a 15 L Bioreactor
2.8.1. Inoculum Preparation
2.8.2. Bioreactor Preparation
2.8.3. M. neoaurum Fermentation
2.9. M. neoaurum Fermentation in a 100 L Bioreactor
2.9.1. Inoculum Preparation
2.9.2. Bioreactor Preparation
2.9.3. M. neoaurum Fermentation
3. Results
3.1. Optimization of Nutrient Medium Composition by CFE 23
3.2. The Optimization of the Nutrient Medium Composition by the Steepest Ascent Method
3.3. M. neoaurum Fermentation in a 3 L Bioreactor
3.4. M. neoaurum Fermentation in a 15 L Bioreactor
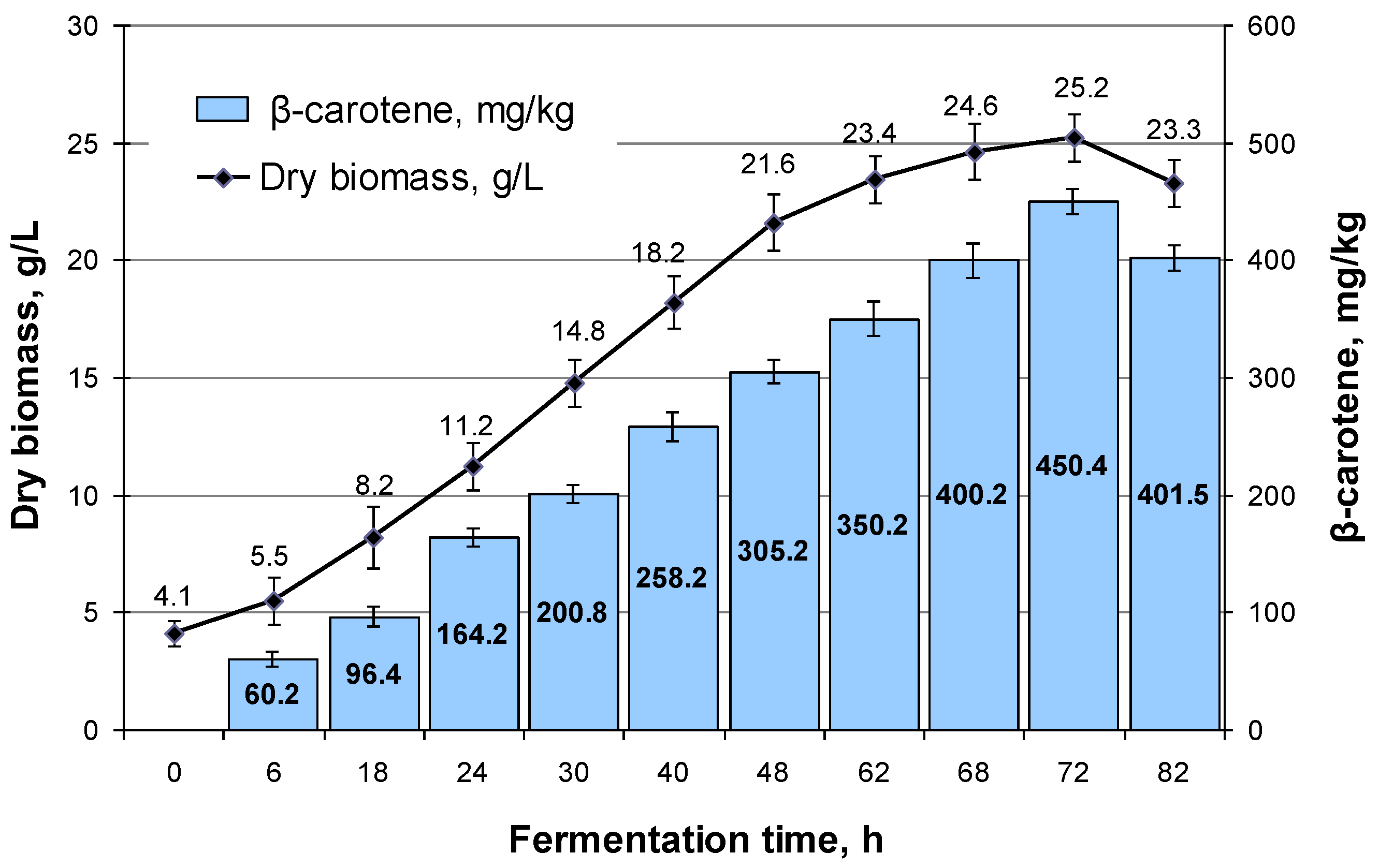
3.5. M. neoaurum Fermentation in a 100 L Bioreactor
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maoka, T. Carotenoids: Distribution, function in nature, and analysis using LC-photodiode array detector (DAD)-MS and MS/MS system. Mass Spectrom 2023, 12, A0133. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as natural functional pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef]
- Yaderets, V.V.; Karpova, N.V.; Glagoleva, E.V.; Petrova, K.S.; Shibaeva, A.S.; Dzhavakhiya, V.V. Carotenoids: Overview of the main methods and conditions of their preparation. Proc. Univ. Appl. Chem. Biotechnol. 2024, 14, 41–54. (In Russian) [Google Scholar] [CrossRef]
- Ashokkumar, V.; Flora, G.; Sevanan, M.; Sripriya, R.; Chen, W.H.; Park, J.H.; Rajesh Banu, J.; Kumar, G. Technological advances in the production of carotenoids and their applications—A critical review. Bioresour. Technol. 2023, 367, 128215. [Google Scholar] [CrossRef] [PubMed]
- Foong, L.C.; Loh, C.W.L.; Ng, H.S.; Lan, J.C. Recent development in the production strategies of microbial carotenoids. World J. Microbiol. Biotechnol. 2021, 37, 12. [Google Scholar] [CrossRef] [PubMed]
- Ernst, H. Recent advances in industrial carotenoid synthesis. Pure Appl. Chem. 2002, 74, 2213–2226. [Google Scholar] [CrossRef]
- Lim, K.C.; Yusoff, F.M.; Shariff, M.; Kamarudin, M.S. Astaxanthin as feed supplement in aquatic animals. Rev. Aquac. 2017, 10, 738–773. [Google Scholar] [CrossRef]
- Botella-Pavía, P.; Rodríguez-Concepción, M. Carotenoid biotechnology in plants for nutritionally improved foods. Physiol. Plant. 2006, 126, 369–381. [Google Scholar] [CrossRef]
- Gong, M.; Bassi, A. Carotenoids from microalgae: A review of recent developments. Biotechnol. Adv. 2016, 34, 1396–1412. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Amaya, D.B. Update on natural food pigments – A mini-review on carotenoids, anthocyanins, and betalains. Food Res. Int. 2019, 124, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.S.; Kim, G.D. Production of carotenoids by bacteria: Carotenoid productivity and availability. J. Life Sci. 2022, 32, 411–419. [Google Scholar] [CrossRef]
- El Baky, H.H.A.; El Baroty, G.S.; Mostafa, E.M. Optimization growth of Spirulina (Arthrospira) platensis in photobioreactor under varied nitrogen concentration for maximized biomass, carotenoids and lipid contents. Recent Pat. Food. Nutr. Agric. 2018, 11, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Aneesh, P.A.; Ajeeshkumar, K.K.R.G.; Kumar Lekshmi, R.; Anandan, C.N.; Ravishankar, S.M. Bioactivities of astaxanthin from natural sources, augmenting its biomedical potential: A review. Trends Food Sci. Technol. 2022, 125, 81–90. [Google Scholar] [CrossRef]
- Frusciante, S.; Diretto, G.; Bruno, M.; Ferrante, P.; Pietrella, M.; Prado-Cabrero, A.; Rubio-Moraga, A.; Beyer, P.; Gomez-Gomez, L.; Al-Babili, S.; et al. Novel carotenoid cleavage dioxygenase catalyzes the first dedicated step in saffron crocin biosynthesis. Proc. Natl. Acad. Sci. USA 2014, 111, 12246–12251. [Google Scholar] [CrossRef]
- Asker, D. Isolation and characterization of a novel, highly selective astaxanthin-producing marine bacterium. Agric. Food Chem. 2017, 65, 101–9109. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.P.; Paixão, S.M.; Alves, L. Ability of Gordonia alkanivorans strain 1B for high added value carotenoids production. RSC Adv. 2018, 5, 58055. [Google Scholar] [CrossRef]
- Tran, T.; Dawrs, S.N.; Norton, G.J.; Virdi, R.; Honda, J.R. Brought to you courtesy of the red, white, and blue pigments of nontuberculous mycobacteria. AIMS Microbiol. 2020, 6, 434–450. [Google Scholar] [CrossRef] [PubMed]
- Igreja, W.S.; Maia, F.A.; Lopes, A.S.; Chisté, R.C. Biotechnological production of carotenoids using low-cost substrates is influenced by cultivation parameters: A review. Int. J. Mol. Sci. 2021, 22, 8819. [Google Scholar] [CrossRef] [PubMed]
- Pagels, F.; Vasconcelos, V.; Guedes, A.C. Carotenoids from Cyanobacteria: Biotechnological potential and optimization strategies. Biomolecules 2021, 11, 735. [Google Scholar] [CrossRef]
- Dyaa, A.; Soliman, H.; Abdelrazak, A.; Samra, B.N.; Khojah, E.; Ahmed, A.F.; El-Esawi, M.A.; Elsayed, A. Optimization of carotenoids production from Rhodotorula sp. strain ATL72 for enhancing its biotechnological applications. J. Fungi 2022, 8, 160. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W.; Niu, H.; Hua, M.; Su, Y.; Miao, X.; Chi, Y.; Xu, H.; Wang, J.; Sun, M.; et al. Study on the fermentation effect of Rhodotorula glutinis utilizing tofu whey wastewater and the influence of Rhodotorula glutinis on lying hens. Front. Nutr. 2023, 10, 1125720. [Google Scholar] [CrossRef]
- Siziya, I.N.; Yoon, D.J.; Kim, M.; Seo, M.J. Enhanced production of C30 carotenoid 4,4’-diaponeurosporene by optimizing culture conditions of Lactiplantibacillus plantarum subsp. plantarum KCCP11226T. J. Microbiol. Biotechnol. 2022, 32, 892–901. [Google Scholar] [CrossRef] [PubMed]
- Stanchev, V.; Georfiev, D.; Gargova, S. Mathematical modeling of the nutrient medium composition for the production of yeast phytase. Bulg. J. Agric. Sci. 2010, 16, 628–634. [Google Scholar]
- Husseiny, S.M.; Abdelhafez, A.A.; Ali, A.A.; Sand, H.M. Optimization of b-carotene production from Rhodotorula glutinis ATCC 4054 growing on agro-industrial substrate using Plackett–Burman design. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2017, 88, 1637–1646. [Google Scholar] [CrossRef]
- da Costa Cardoso, L.A.; Kanno, K.Y.F.; Karp, S.G. Microbial production of carotenoids—A review. Afr. J. Biotechnol. 2017, 16, 139–146. [Google Scholar] [CrossRef]
- Kerr, S.; Calé, C.; Cabral, J.M.; van Keulen, F. Factors enhancing lycopene production by a new Mycobacterium aurum mutant. Biotechnol. Lett. 2004, 26, 103–108. [Google Scholar] [CrossRef]
- Yaderets, V.; Karpova, N.; Glagoleva, E.; Shibaeva, A.; Dzhavakhiya, V. Enhanced β-carotene production in Mycolicibacterium neoaurum Ac-501/22 by combining mutagenesis, strain selection, and subsequent fermentation optimization. Fermentation 2023, 9, 1007. [Google Scholar] [CrossRef]
- Rozhnov, E.D. Modeling of Biotechnological Processes: Methodological Recommendations for Performing Laboratory Work, Conducting Practical Classes and Organizing Independent Work of Students for the Speciality 04/19/2011—Biotechnology; Altai State Technical University: Biysk, Russia, 2019; pp. 40–96. [Google Scholar]
- Zhernosekova, I.V.; Chernogor, N.P.; Tymchuk, A.A.; Vinnikov, A.I. Methods of experiments planning by optimization of the nutrient medium for Streptomycete. Visnyk Dnipropetrovsk Univ. Biol. Ecol. 2001, 18, 20–28. [Google Scholar] [CrossRef]
- Zeni, J.; Colet, R.; Cence, K.; Tiggeman, L.; Toniazzo, G.; Cansian, R.L.; Di Luccio, M.; Oliveira, D.; Valduga, E. Screening of microorganisms for production of carotenoids. CyTA J. Food 2011, 9, 160–166. [Google Scholar] [CrossRef]
- Mata-Gómez, L.C.; Montañez, J.C.; Méndez-Zavala, A.; Aguilar, C. Biotechnological production of carotenoids by yeasts: An overview. Microb. Cell Fact. 2014, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Bindea, M.; Rusu, B.; Rusu, A.; Trif, M.; Leopold, L.F.; Dulf, F.; Vodnar, D.C. Valorification of crude glycerol for pure fractions of docosahexaenoic acid and β-carotene production by using Schizochytrium limacinum and Blakeslea trispora. Microb. Cell Fact. 2018, 17, 97. [Google Scholar] [CrossRef] [PubMed]
- Suwaleerat, T.; Thanapimmetha, A.; Srisaiyoot, M.; Chisti, Y.; Srinophakun, P. Enhanced production of carotenoids and lipids by Rhodococcus opacus PD630. J. Chem. Technol. Biotechnol. 2018, 93, 2160–2169. [Google Scholar] [CrossRef]
- Baughn, A.D.; Rhee, K.Y. Metabolomics of central carbon metabolism in Mycobacterium tuberculosis. Microbiol. Spectr. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Paniagua-Michel, J.; Olmos-Soto, J.; Ruiz, M.A. Pathways of carotenoid biosynthesis in bacteria and microalgae. Methods Mol. Biol. 2012, 892, 1–12. [Google Scholar] [CrossRef]
- Janisch, N.; Levendosky, K.; Budell, W.C.; Quadri, L.E.N. Genetic underpinnings of carotenogenesis and light-induced transcriptome remodeling in the opportunistic pathogen Mycobacterium kansasii. Pathogens 2023, 12, 86. [Google Scholar] [CrossRef]
- Zheng, X.; Hu, R.; Chen, D.; Chen, J.; He, W.; Huang, L.; Lin, C.; Chen, H.; Chen, Y.; Zhu, J.; et al. Lipid and carotenoid production by the Rhodosporidium toruloides mutant in cane molasses. Biores. Technol. 2021, 326, 124816. [Google Scholar] [CrossRef] [PubMed]
- Dzhavakhiya, V.; Savushkin, V.; Ovchinnikov, A.; Glagolev, V.; Savelyeva, V.; Popova, E.; Novak, N.; Glagoleva, E. Scaling up a virginiamycin production by a high-yield Streptomyces virginiae VKM Ac-2738D strain using adsorbing resin addition and fed-batch fermentation under controlled conditions. 3 Biotech 2016, 6, 240. [Google Scholar] [CrossRef]
- Schmidt, F.R. Optimization and scale up of industrial fermentation processes. Appl. Microbiol. Biotechnol. 2005, 68, 425–435. [Google Scholar] [CrossRef]
- Liu, W.C.; Gong, T.; Wang, Q.H.; Liang, X.; Chen, J.J.; Zhu, P. Scaling-up fermentation of Pichia pastoris to demonstration-scale using new methanol-feeding strategy and increased air pressure instead of pure oxygen supplement. Sci. Rep. 2016, 6, 18439. [Google Scholar] [CrossRef]
- Jing, Y.; Wang, J.; Gao, H.; Jiang, Y.; Jiang, W.; Jiang, M.; Xin, F.; Zhang, W. Enhanced β-carotene production in Yarrowia lipolytica through the metabolic and fermentation engineering. J. Ind. Microbiol. Biotechnol. 2023, 50, kuad009. [Google Scholar] [CrossRef]
- Liu, L.; Qu, Y.L.; Dong, G.R.; Wang, J.; Hu, C.Y.; Meng, Y.H. Elevated β-carotene production using codon-adapted CarRA&B and metabolic balance in engineered Yarrowia lipolytica. Front. Microbiol. 2021, 12, 627150. [Google Scholar] [CrossRef]
- Hunter, G.J. The oxidation of glycerol by Mycobacteria. Biochem. J. 1953, 55, 320–328. [Google Scholar] [CrossRef] [PubMed]


| Factors | X1 | X2 | X3 |
|---|---|---|---|
| Variables | Glycerol | SMP | Urea |
| Concentration, g/L | 20.0 | 10.0 | 1.0 |
| δ, g/L | 2.5 | 1.5 | 0.15 |
| Low level (–1), g/L | 17.5 | 7.5 | 0.85 |
| High level (+1), g/L | 22.5 | 11.5 | 1.15 |
| Parameter | Value |
|---|---|
| Medium volume | 10.0 L |
| Temperature | 35 ± 1 °C |
| Aeration | 0.1 L/min |
| Stirring rate | 250 rpm |
| pO2 level | 100% of saturation |
| Pressure within the bioreactor | 0.03–0.05 MPa |
| Medium pH | 6.8–7.2 |
| Parameter | Value |
|---|---|
| Temperature | 35 ± 1 °C |
| Aeration | 5–10 L/min |
| pH | 6.8–7.2 |
| Stirring rate | 400–450 rpm to maintain the required level of dissolved oxygen |
| Feeding addition | Glycerol (2.5 g/L) after 30 h of fermentation |
| Fermentation time | 72–76 h |
| Process control | The first sampling to control the culture purity and crude biomass weight is performed after 18–24 h of fermentation. The further sampling is performed as required, but at least one time a day. |
| Parameter | Value |
|---|---|
| Medium volume | 70.0 L |
| Temperature | 35 ± 1 °C |
| Aeration | 3.5 L/min |
| Stirring rate | 250 rpm |
| pO2 level | 100% of saturation |
| Pressure within the bioreactor | 0.03–0.05 MPa |
| Medium pH | 6.8–7.2 |
| Parameter | Value |
|---|---|
| Temperature | 35 °C |
| Aeration | 35–70 L/min |
| pH | Maintained at 6.8–7.2 using a sterile 10% HCl solution |
| Stirring rate | 400–450 rpm to maintain the required level of dissolved oxygen |
| Feeding addition | 50% glycerol solution (2.5 g/L of medium) after 30 h of fermentation |
| Fermentation time | 72–80 h |
| Experiment No. | Encoded Factors | Natural-Scale Factors, g/L | β-Carotene Yield, mg/kg | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| x1 | x2 | x3 | x1 | x2 | x3 | y1 * | y2 * | y3 * | ȳu ** | |
| 1 | −1 | −1 | −1 | 17.5 | 8.5 | 0.85 | 182.5 | 170.4 | 186.2 | 179.70 |
| 2 | +1 | −1 | −1 | 22.5 | 8.5 | 0.85 | 206.2 | 196.5 | 188.4 | 197.03 |
| 3 | −1 | +1 | −1 | 17.5 | 11.5 | 0.85 | 190.6 | 198.5 | 188.6 | 192.57 |
| 4 | +1 | +1 | −1 | 22.5 | 11.5 | 0.85 | 248.3 | 220.4 | 229.5 | 232.73 |
| 5 | −1 | −1 | +1 | 17.5 | 8.5 | 1.15 | 180.8 | 188.5 | 183.8 | 184.37 |
| 6 | +1 | −1 | +1 | 22.5 | 8.5 | 1.15 | 204.3 | 227.4 | 214.5 | 215.40 |
| 7 | −1 | +1 | +1 | 17.5 | 11.5 | 1.15 | 196.6 | 202.6 | 220.7 | 206.63 |
| 8 | +1 | +1 | +1 | 22.5 | 11.5 | 1.15 | 216.7 | 200.8 | 218.7 | 212.07 |
| u | yul | ∑(yul)2 | (∑yul)2 | ||||
|---|---|---|---|---|---|---|---|
| 1 | 182.5 | 170.4 | 186.2 | 97,012.85 | 290,628.81 | 96,876.27 | 68.29 |
| 2 | 206.2 | 196.5 | 188.4 | 116,625.25 | 349,399.21 | 116,466.40 | 79.42 |
| 3 | 190.6 | 198.5 | 188.6 | 111,300.57 | 333,737.29 | 111,245.76 | 27.40 |
| 4 | 248.3 | 220.4 | 229.5 | 162,899.3 | 487,483.24 | 162,494.41 | 202.44 |
| 5 | 180.8 | 188.5 | 183.8 | 102,003.33 | 305,919.61 | 101,973.20 | 15.06 |
| 6 | 204.3 | 227.4 | 214.5 | 139,459.5 | 417,574.44 | 139,191.48 | 134.01 |
| 7 | 196.6 | 202.6 | 220.7 | 128,406.81 | 384,276.01 | 128,092.00 | 157.40 |
| 8 | 216.7 | 200.8 | 218.7 | 135,109.22 | 404,750.44 | 134,916.81 | 96.20 |
| ∑ = 780.24 | |||||||
| u | yul | ȳu | ȳu − yu | (ȳu − yu)2 |
|---|---|---|---|---|
| 1 | 182.38 | 179.70 | −2.68 | 7.18 |
| 2 | 205.87 | 197.03 | −8.84 | 78.10 |
| 3 | 199.25 | 192.57 | −6.69 | 44.72 |
| 4 | 222.75 | 232.73 | 9.99 | 99.75 |
| 5 | 182.38 | 184.37 | 1.99 | 3.95 |
| 6 | 205.87 | 215.40 | 9.53 | 90.81 |
| 7 | 199.25 | 206.63 | 7.38 | 54.45 |
| 8 | 222.75 | 212.07 | −10.68 | 114.04 |
| ∑ = 493.00 |
| Experiment No. | Factors at a Natural Scale, g/L | Encoded Factors | Auxiliary Graphs in a CFE 23 Matrix | Β-Carotene Yield, mg/kg | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| x1 | x2 | x3 | x1 | x2 | x3 | x1x2 | x2x3 | x1x3 | x1x2x3 | y1 * | y2 * | y3 * | ȳu ** | |
| 1 | 17.5 | 8.5 | 0.85 | −1 | −1 | −1 | +1 | +1 | +1 | −1 | 182.5 | 170.4 | 186.2 | 179.70 |
| 2 | 22.5 | 8.5 | 0.85 | +1 | −1 | −1 | −1 | +1 | −1 | + | 206.2 | 196.5 | 188.4 | 197.03 |
| 3 | 17.5 | 11.5 | 0.85 | −1 | +1 | −1 | −1 | −1 | +1 | +1 | 190.6 | 198.5 | 188.6 | 192.57 |
| 4 | 22.5 | 11.5 | 0.85 | +1 | +1 | −1 | +1 | −1 | −1 | −1 | 248.3 | 220.4 | 229.5 | 232.73 |
| 5 | 17.5 | 8.5 | 1.15 | −1 | −1 | +1 | +1 | −1 | −1 | + | 180.8 | 188.5 | 183.8 | 184.37 |
| 6 | 22.5 | 8.5 | 1.15 | +1 | −1 | +1 | −1 | −1 | +1 | −1 | 204.3 | 227.4 | 214.5 | 215.40 |
| 7 | 17.5 | 11.5 | 1.15 | −1 | +1 | +1 | −1 | +1 | −1 | −1 | 196.6 | 202.6 | 220.7 | 206.63 |
| 8 | 22.5 | 11.5 | 1.15 | +1 | +1 | +1 | +1 | +1 | +1 | +1 | 216.7 | 200.8 | 218.7 | 212.07 |
| u | yul | ȳu | ȳu − yu | (ȳu − yu)2 |
|---|---|---|---|---|
| 1 | 194.49 | 179.70 | −14.79 | 218.67 |
| 2 | 193.76 | 197.03 | 3.27 | 10.70 |
| 3 | 187.15 | 192.57 | 5.42 | 29.39 |
| 4 | 234.85 | 232.73 | −2.12 | 4.50 |
| 5 | 170.27 | 184.37 | 14.10 | 198.69 |
| 6 | 217.98 | 215.40 | −2.58 | 6.65 |
| 7 | 211.36 | 206.63 | −4.73 | 22.37 |
| 8 | 210.64 | 212.07 | 1.43 | 2.04 |
| ∑ = 493.00 |
| Factor and Experiment Characteristics | X1 | X2 |
|---|---|---|
| Glycerol Concentration | SPM Concentration | |
| Base level, g/L | 22.5 | 11.5 |
| Maximum level, g/L | 30.0 | 15.0 |
| Stock, Δi | 7.5 | 4.5 |
| Variation interval (δi) | 2.5 | 1.5 |
| Coefficient of regression (bi) | 11.75 | 8.44 |
| Production Li = biδi | 29.34 | 12.66 |
| Coefficient (γi) | 0.26 | 0.59 |
| Steepest ascent step (hi), g/L | 1.5 | 0.65 |
| Experiment No. | X1 (Glycerol Concentration, g/L) | X2 (SMP Concentration, g/L) | β-Carotene Yield, mg/kg |
|---|---|---|---|
| 1 (initial medium) | 22.5 | 11.5 | 248.3 ± 5.7 |
| 2 | 24.0 | 12.15 | 284.5 ± 8.2 |
| 3 | 25.5 | 12.80 | 318.4 ± 8.3 |
| 4 | 27.0 | 13.45 | 292.4 ± 6.4 |
| 5 | 28.5 | 14.10 | 252.1 ± 5.5 |
| 6 | 30.0 | 14.75 | 224.6 ± 8.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaderets, V.V.; Karpova, N.V.; Glagoleva, E.V.; Shibaeva, A.S.; Dzhavakhiya, V.V. The Optimization of the Nutrient Medium Composition for the Submerged Cultivation of the Mycolicibacterium neoaurum Strain VKM Ac-3067D in a 100 L Bioreactor Under Controlled Conditions by Mathematical Planning. Fermentation 2025, 11, 82. https://doi.org/10.3390/fermentation11020082
Yaderets VV, Karpova NV, Glagoleva EV, Shibaeva AS, Dzhavakhiya VV. The Optimization of the Nutrient Medium Composition for the Submerged Cultivation of the Mycolicibacterium neoaurum Strain VKM Ac-3067D in a 100 L Bioreactor Under Controlled Conditions by Mathematical Planning. Fermentation. 2025; 11(2):82. https://doi.org/10.3390/fermentation11020082
Chicago/Turabian StyleYaderets, Vera V., Nataliya V. Karpova, Elena V. Glagoleva, Alexandra S. Shibaeva, and Vakhtang V. Dzhavakhiya. 2025. "The Optimization of the Nutrient Medium Composition for the Submerged Cultivation of the Mycolicibacterium neoaurum Strain VKM Ac-3067D in a 100 L Bioreactor Under Controlled Conditions by Mathematical Planning" Fermentation 11, no. 2: 82. https://doi.org/10.3390/fermentation11020082
APA StyleYaderets, V. V., Karpova, N. V., Glagoleva, E. V., Shibaeva, A. S., & Dzhavakhiya, V. V. (2025). The Optimization of the Nutrient Medium Composition for the Submerged Cultivation of the Mycolicibacterium neoaurum Strain VKM Ac-3067D in a 100 L Bioreactor Under Controlled Conditions by Mathematical Planning. Fermentation, 11(2), 82. https://doi.org/10.3390/fermentation11020082





