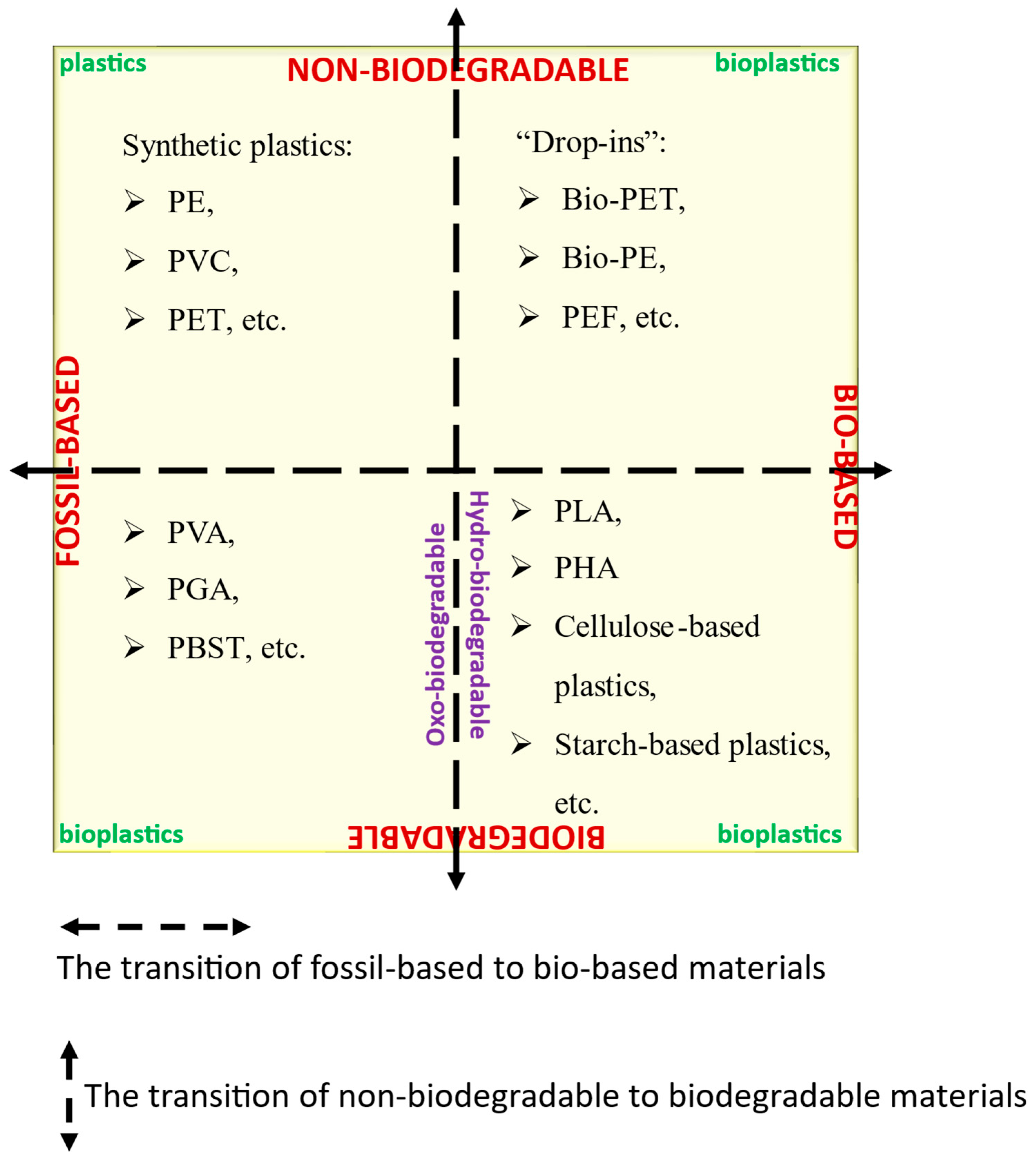
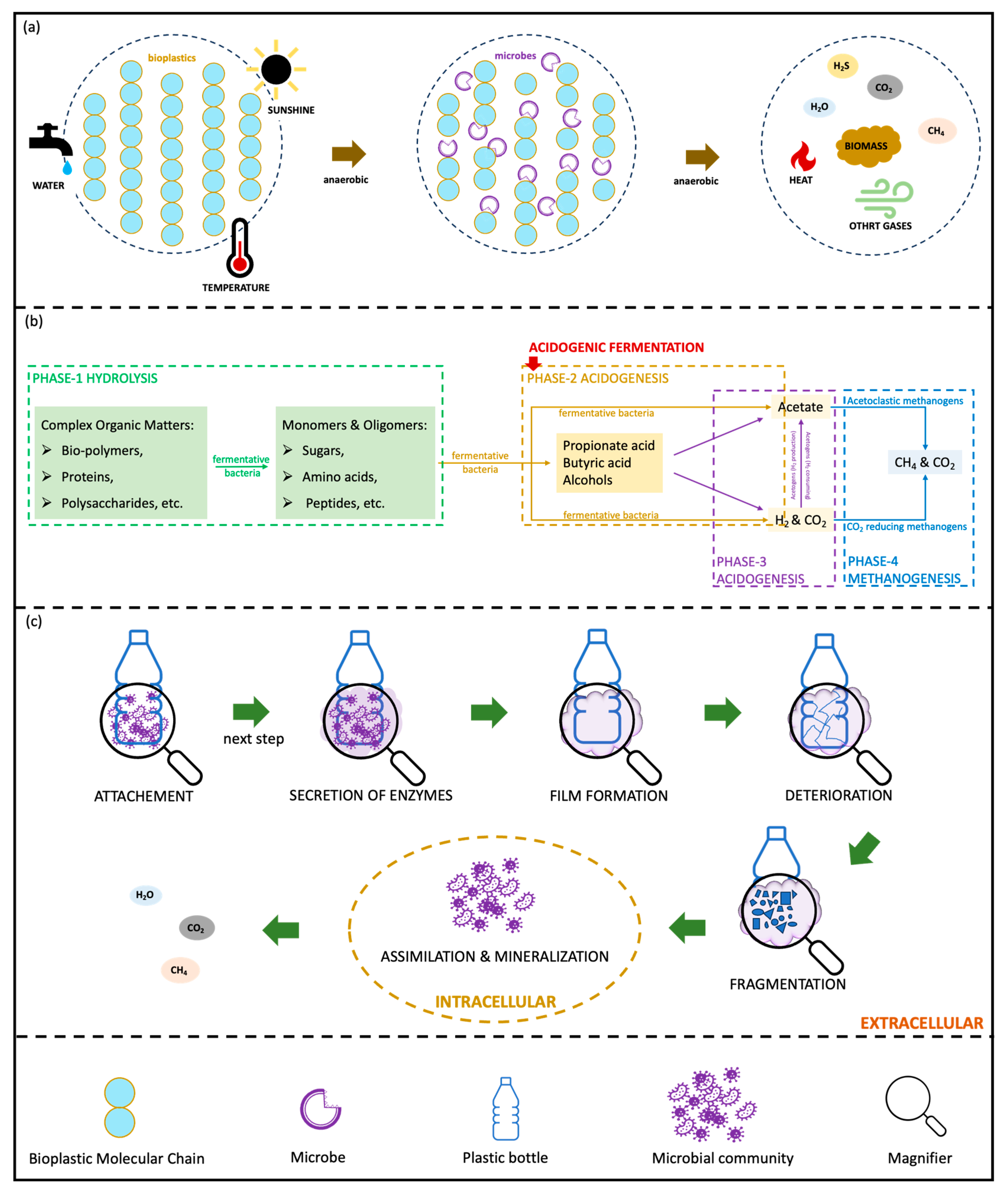
1. Introduction
Plastics, first developed in the 1950s, quickly became essential for both everyday life and high-end applications (e.g., packaging, toys, grocery bags, plastic cutlery, etc.) due to their high durability, low bulk density, excellent mechanical and barrier properties, low cost, and good processability [
1]. Thus, the global production has risen sharply, reaching 350 million tonnes in 2017 and 368 million tonnes in 2019 [
2,
3]. Conventional plastics are high molecular weight polymers derived from petroleum, with monomers such as ethylene and propylene produced from naphtha [
4,
5]. While cost-effective, this process generates significant pollutants and greenhouse gas emissions and produces nonbiodegradable materials that can persist for centuries [
6,
7]. It is estimated that approximately 100 million tonnes of plastic will have entered the oceans between 2010 and 2025 [
8,
9]. As plastics break down, they release micro- and nanoplastics [
10], which can negatively impact both aquatic ecosystems and human health [
11,
12]. Bioplastics (BPs), derived from renewable biomass such as corn, lignocellulosic, and starch, have emerged as sustainable alternatives to traditional petroleum-based plastics. While some, including polylactic acid (PLA), polyhydroxyalkanoates (PHA), and thermoplastic starch (TPS), are biodegradable and already applied in food packaging, medicinal implants, and building construction fields [
13], others, such as bio-based polyethylene and bio-based polyethylene terephthalate, are non-biodegradable and still contribute to environmental burdens [
5]. In 2021, the total production of BPs was about 2.36 million tonnes, of which about 1.55 million tonnes were degradable materials and about 860,000 tonnes were non-degradable materials [
14]. Despite decades of research, large-scale industrialization and waste management remain limited due to high costs and weaker mechanical properties compared to petrochemical plastics [
15]. Nevertheless, with the urgent global demand for efficient alternatives to petroleum-based plastics, the BPs market is expected to experience significant growth in the coming years.
However, switching from petroleum-based plastics to BPs does not automatically resolve the issue of plastic waste, since even biodegradable BPs require appropriate conditions to degrade. An effective waste management strategy is therefore essential to mitigate plastic pollution. Among current approaches, biotechnological recycling (bio-recycling) has emerged as a promising method, in which microorganisms and enzymes selectively depolymerize biodegradable BPs to recover monomers and other valuable chemicals [
3]. The biodegradable materials are converted into gas, nutrients, and energy through composting or anaerobic digestion; bio-recycling specifically targets controlled polymer breakdown and chemical recovery [
16]. The main technologies explored for BP degradation include anaerobic digestion (AD), acidogenic fermentation (AF), and enzymatic hydrolysis (EH) [
17,
18,
19]. However, problems such as low efficiency, long processing time, unstable product yields, and incomplete depolymerization still hinder their large-scale application and overall treatment effectiveness.
A bioelectrochemical system (BES) is an environmentally friendly and renewable electrochemical technology with wide applications (e.g., wastewater treatment, metal recovery, biosensor, etc.), which uses bacterial action to convert chemical energy into useful resources [
20]. Although they are not considered conventional bio-recycling methods, BESs can significantly enhance bio-recycling processes by accelerating biodegradation, improving product selectivity, and enabling energy recovery. The main BESs include microbial fuel cells (MFCs), microbial electrolysis cells (MECs), microbial desalination cells (MDCs), and enzymatic biofuel cells (EBCs) [
21,
22]. BES can also integrate with various technologies, such as photobioreactors [
23], constructed wetlands [
24], as well as anaerobic systems (ASs) [
25]. Such integrations not only strengthen degradation efficiency for complex wastes like plastics [
26] but also provide added benefits, including improved energy recovery, real-time monitoring capabilities, reduced environmental impact, and the potential production of value-added by-products [
26].
Over the past five years (2020–2025), approximately 831 review articles (Scopus data) have explored various aspects of BPs, including their classification, disposal options, biodegradation processes, recycling mechanisms, and environmental impacts evaluated through life cycle assessment (LCA) [
27,
28,
29,
30,
31,
32]. However, most of these reviews emphasize the production and application of BPs, providing broad overviews of their development and potential advantages, while offering limited discussion on waste treatment. In particular, biological conversion of BPs into energy and value-added chemicals has received little systematic attention, with the exception of Bátori et al. [
17], who focused solely on AD technology. Recent studies suggest that integrating BESs with ASs can enhance organic waste degradation by combining electrochemical and biological pathways [
25,
33,
34]. Yet, to date, no comprehensive review has evaluated the potential of BES-ASs, particularly in their potential application for the degradation of BPs. To address this gap, this review aims to (i) provide a comprehensive overview of the current biotechnological approaches for degradable BP waste, with a focus on BP removal efficiency, recovery potential, and by-products generated during treatment; (ii) summarize the principles and functional mechanisms of various BES configurations (e.g., microbial fuel cells, microbial electrolysis cells) and their integration with an AS; (iii) highlight recent research progress in the use of BES-ASs for BPs degradation, with a focus on performance comparisons, product recovery (e.g., volatile fatty acids, biogas), and energy conversion efficiency; and (iv) discuss the current limitations, knowledge gaps, and future perspectives, including potential optimization strategies, scale-up challenges, and the role of BES-AS in advancing sustainable BP waste management. This review aims to serve as a foundational reference for researchers and practitioners developing efficient, low-carbon, and value-added strategies for BP waste management.
4. Integration of Anaerobic and Bioelectrochemical Systems (BESs) for Bioplastic Degradation
BESs are a group of biotechnologies that combine electrochemical catalysis and microbial metabolism to convert chemical waste into valuable energy substances [
26]. They have been applied in various fields, such as biosensing, wastewater treatment, CH
4 reduction in rice paddies, etc. [
125,
126,
127]. BESs are also considered an advanced form of AD technology [
128,
129], offering advantages such as high sludge reduction, low sludge yield, effective pathogen elimination, and efficient net energy recovery. In particular, BESs can not only promote the degradation of organic waste by microorganisms, but also enhance microbial resistance to pollutants in wastewater, showing great potential in mitigating the toxic effects of microplastics [
130]. In general, BES can be categorized into electricity-producing microbial fuel cells (MFCs), which convert the chemical energy of organic matter into electrical energy [
22]; electricity-consuming microbial electrolytic cells (MECs), which require 0.2–1.0 V applied voltage in two-electrode configurations, and synthesizing value-added chemicals via a poised biocathode [
131]; water-purifying microbial desalination cells (MDCs), which convert seawater or brackish water into desalinated water through integrated bioelectrochemical and ion exchange processes [
132]; sustainably powered enzymatic biofuel cells (EBCs), which generate sustainable energy using renewable enzyme biocatalysts and organic fuels under mild working conditions; microbial electrosynthesis system (MES), which uses cathodic biocatalysis to convert CO
2 (or other substrates) into value-added products [
133,
134] (
Figure 3). Furthermore, BES can also be integrated with other technologies to achieve better performance because of its versatile ability, such as membrane-based technologies [
135], photobioelectrochemical system [
23], hydroponic agriculture [
136], and AD [
137,
138], etc. In comparison with the traditional microbial degradation process, the BES-anaerobic system (BES-AS) can enhance microbial metabolic activity, resulting in faster degradation and improved mineralization of pollutants in wastewaters [
139]. In addition, BES-AS can increase the resistance of microbes to unfavorable environmental conditions. For instance, electrochemical activity promotes the secretion of microbial extracellular polymeric substances (EPS), which can bind pollutants in wastewater and protect microbial cells from damage [
140]. Furthermore, combining BES with AD can increase the abundance of functional microbes and promote the expression of functional genes and the activity of key enzymes [
141]. Although the above advantages indicate that BES-AS holds great promise in alleviating BPs pollution, related research is still in its early exploratory stages, and existing studies remain limited. The following section aims to systematically review the current research progress on BES-AS in the field of BP degradation and to explore its potential future development directions.
4.1. Coupling BES with Acidogenic Fermentation (BES-AF)
4.1.1. Microelectrolysis Fuel Cell—Acidogenic Fermentation (MEC-AF)
MECs are also known as microbial electrohydrogenesis systems [
142], are used to produce biohydrogen from organic wastes by microorganisms with the assistance of an externally applied voltage [
143]. In MECs, the cathode chamber operates under anaerobic conditions, preventing water formation during the reduction reaction [
142]. Instead, protons are reduced to H
2 by electrons supplied from the anode (Equation (1)) [
142,
143,
144].
Given the capacity of MECs to produce biohydrogen, MECs can effectively utilize AF effluents, which are rich in VFAs [
145]. Chookaew et al. [
146] reported that MEC-AF integration enhanced H
2 yield to 0.55 mol H
2/mol glycerol (equivalent to 142 kJ/mol glycerol) under 1.0 V, with the COD removal efficiency increasing from 20% (single-stage AF) to 41% in the MEC-AF system. Similarly, Tuna et al. [
147] demonstrated that operational factors such as low pH, high voltage, and elevated VFA concentrations strongly influenced hydrogen output from wheat flour-based AF effluents. Further studies confirmed that under potentiostatic regulation, MEC-AF systems enable efficient mineralization of organic acids and residual carbon from various wastes (e.g., food waste [
148], cellobiose [
149], sugar [
150], cheese [
150], corn stalk [
151], waste peach pulp [
152], and beet juice [
153]), achieving hydrogen yields of up to 1608.6 ± 266.2 mL H
2/gCOD consumed and COD removal efficiencies approaching 78.5% [
150]. During this processing, VFAs generated by AF serve as electron donors for anodic electroactive bacteria, which oxidize them to release electrons and protons. The electrons are transferred to the cathode via an external circuit, while the protons (or other ions) migrate through an ion-exchange membrane in two-chamber configurations. At the cathode, these electrons and protons recombine to form H
2, thus converting VFAs into clean bioenergy [
153].
The integration of MEC with AF provides a promising approach to regulate microbial metabolism via electrochemical simulation [
154]. By applying the external voltage and altering oxidation-reduction potential (ORP), MEC-AF systems can direct microbial pathways, enhance the production of target products, modify metabolic pathways, and optimize microbial community structures [
155]. Unlike conventional microbial electrochemical systems, MEC-AF uses electrical current not only for energy recovery but also as a metabolic control tool to overcome thermodynamic limitations and guide fermentation to produce specific high-value products [
156,
157,
158]. Some studies have proved the effectiveness of MEC-AF in enhancing short and medium-chain fatty acid (SCFA and MCFA) production [
159]. For instance, Wu et al. [
160] produced MCFAs from waste biomass under 0.9 V, while Similarly, Mukherjee and Venkata Mohan [
161] showed that adjusting the poised potential of MEC-AF (from −0.8 V to +0.8 V vs. Ag/AgCl) could regulate the NADH/NAD
+ ratio in
Bacillus subtilis, thereby shifting metabolic pathways. The negative potential (−0.8 V) favored H
2 and fatty acid production, while the positive potential (+0.6 V) promoted succinate formation [
161]. Similarly, Kracke and Krömer reported that MFC-AF could significantly increase yields of economically valuable metabolites such as succinate and lysine compared to conventional AF [
162]. In addition, technologies such as gas recycling have further improved system efficiency. Zhou et al. [
163] found that in situ recycling of anodic off-gases (CO
2 and H
2) at the cathode increased VFA production by 48.70% and improved carbon-to-VFA conversion efficiency by 13.92%. This is because the recovery and reuse of CO
2 and H
2 produced during AF at the cathode not only alleviates gas inhibition in the anode headspace, but also promotes their conversion into VFAs, thereby significantly enhancing acid production efficiency and carbon conversion rate, while simultaneously mitigating gas accumulation-induced inhibition and shifting the fermentation pathway at the anode from single-acid to mixed-acid fermentation [
163].
So far, there is no literature reporting the degradation of BPs using the MEC-AF system. However, as previously summarized, the AF system has been shown to successfully degrade BPs. Therefore, based on existing studies on MEC-AF systems, it can be speculated that integrating MEC may enhance the degradation rate of BPs during the AF process, improve both biohydrogen production and COD removal efficiency. In addition, due to the different electrochemical reactions produced by the anode and cathode of MECs, the degradation products of BPs during the AF process can also be regulated by applying either an anodic or a cathodic voltage.
Nevertheless, current findings are not fully consistent across different feedstocks. For instance, sugar- and glycerol-based substrates often yield higher hydrogen production rates and COD removal efficiencies, whereas lignocellulosic or protein-rich wastes (e.g., corn stalk, cheese wastewater) show slower but more stable performance due to mass transfer limitations and complex degradation intermediates. These variations suggest that substrate composition, buffering capacity, and biodegradability play critical roles in determining system stability and electrochemical efficiency. Nevertheless, current findings are not fully consistent across different feedstocks. For instance, sugar- and glycerol-based substrates often yield higher hydrogen production rates and COD removal efficiencies, whereas lignocellulosic or protein-rich wastes (e.g., corn stalk and cheese wastewater) show slower but more stable performance due to mass transfer limitations and complex degradation intermediates. These variations suggest that substrate composition, buffering capacity, and biodegradability play critical roles in determining system stability and electrochemical efficiency. Technical trade-offs also emerge between maximizing hydrogen yield and maintaining process stability. While higher applied voltages and VFA concentrations can boost H2 production and accelerate organic conversion, they may also increase internal resistance, cause pH fluctuations, and lead to microbial inhibition or electrode fouling over time. Conversely, operating under lower voltages enhances long-term stability but results in lower energy recovery and slower degradation kinetics. Therefore, optimizing operational parameters (e.g., voltage, pH, and substrate loading) remains essential to balance yield and stability across various feedstocks.
4.1.2. Microbial Fuel Cell—Acidogenic Fermentation (MFC-AF)
The end-product VFAs produced from the AF process can be directly utilized by MFCs for bioelectricity production without requiring any pretreatment, which provides an advantage over other bioprocesses [
164]. In MFCs, these VFAs are oxidized at the anode by EBA into CO
2, protons, and electrons [
143,
165,
166] (Equation (2)). These electrons flow through an external circuit to the cathode, generating electricity, while the protons move through the semipermeable membrane to the cathode and react with oxygen to form water (Equation (3)).
Several studies have confirmed the feasibility and efficiency of coupling AF with MFCs for enhanced bioenergy recovery. Cardar et al. [
167] used the VFAs produced by the AF process as the substrate for an air-cathode MFC and achieved a maximum power density of 5.9 W/m
3. Pasupuleti et al. [
168] compared bioelectricity production from AF wastewater using batch, semi-continuous, and continuous MFCs, and semi-continuous mode reaching the highest power density of 19.1 mW/m
2. Raychaudhuri et al. [
169] proved that the VFAs enriched in the substrate from AF improved the availability of electron donors at the anode of MFCs, thereby enhancing the power generation efficiency in comparison with unfermented substrate. Rozsenberszki et al. [
170] evaluated the potential of the MFC-AF system using pressing wastewater and observed an increase in bioenergy yield. Similarly, Schievano et al. [
171] coupled AF with MFC and achieved enhanced bioenergy production from a mixture of pig manure and rice bran. The increased bioelectricity production from these studies is mainly due to that exoelectrogenic bacteria preferentially utilizing simple dissolved organic acids (primarily acetate) as the carbon source in MFC-AF systems.
In addition, MFCs also contributed to further degradation and stabilization of AF process, as indicated by the increased COD removal [
170,
172]. Likewise, Pasupuleti et al. [
168] observed that 82.0% of the COD in AF effluent was removed using MFC treatment. Similarly, Chookaew et al. [
146] reported that COD removal efficiency increased from 20% with AF alone to 50% in the combined MFC-AF system. This is because the anode of MFCs worked as electron acceptor for EAB, promoting the further degradation of organic matter (mainly VFAs) produced by AF and releasing electrons, thereby achieving deep mineralization of pollutants [
146,
168]. In addition, the synergistic effect of the diverse microbial community, system pH buffering, and current output drive also contributed to the significant reduction in COD [
146,
168]. Beyond pollutant removal and energy recovery, recent studies have also explored the potential of MFCs as real-time monitoring tools for the AF process. For example, Finch et al. [
173] demonstrated that
C. acetobutylicum can generate current in MFC-AF system without the addition of redox mediators, and that the current output can be used to monitor both acidogenic and solventogenic metabolic phases.
Although many works have reported the potential application of MFC in the AF process, there has been no systematic study or clear report on the role of the MFC-AF system in the degradation of BPs. As mentioned above, existing studies have preliminarily explored the interaction between MPs and MFC; however, the effects of BPs on the operating performance of MFC, as well as their degradation behavior in the MFC system are still almost blank. Considering that BPs have a certain biodegradability, especially in AF conditions, where they can be converted into intermediate metabolites VFAs, it is reasonable to speculate that BPs also possess degradation potential in the MFC-AF systems. On the one hand, electroactive microorganisms enriched in the MFC may promote further metabolic conversion of BPs, thereby improving the degradation rate and stability of the AF system; on the other hand, the VFAs produced during the AF process can serve as high-quality electron donors for the MFC anode, thus improving the power generation efficiency of the system. Furthermore, based on the findings of Finch et al. [
173], it is suggested that changes in the MFC output electrical signals (such as voltage, current density, etc.) can indirectly reflect the degree of decomposition of BPs and their metabolic stage during AF process, offering a possible path for the subsequent development of electrochemical monitoring strategies for AF of BPs. Therefore, building upon the existing research foundation of MFC-AF coupling system, exploring its applicability in the process of resource utilization of BPs holds significant scientific value and offers innovative solutions and technical support for the sustainable management of BPs pollution. But the results also vary according to different stocks, such as substrates derived from simple carbohydrates or food waste typically yield higher power densities and faster COD removal, whereas lignocellulosic or protein-rich feedstocks (e.g., pig manure and rice bran) often exhibit lower but more stable electricity generation due to slower hydrolysis and accumulation of complex intermediates. These differences indicate that substrate composition, biodegradability, and buffering capacity play decisive roles in determining the electrochemical and microbial performance of MFC-AF systems. In addition, clear technical trade-offs exist between maximizing bioelectricity output and maintaining long-term system stability. High VFA concentrations and strong anodic oxidation favor higher power density and faster pollutant removal, but can also lead to pH imbalance, microbial inhibition, and electrode fouling, thereby compromising system stability. Conversely, operating at lower substrate concentrations or under more moderate conditions enhances operational robustness and biofilm durability, though bioenergy yield decreases accordingly. Therefore, optimizing operational parameters such as feedstock type, organic loading rate, and applied potential is essential to balance yield and stability across diverse feedstocks in MFC-AF systems.
4.2. Coupling BES with Anaerobic Digestion (BES-AD)
4.2.1. Microbial Electrolysis Cell—Anaerobic Digestion (MEC-AD)
MECs are the most commonly used type of BES to assist AD processes. They accelerate organic matter degradation, shorten the recovery period, and improve the overall system stability [
174,
175]. The electron exchange pathway and rate play a critical role in determining the performance of MEC-AD systems. Physical separation of electrodes can hinder electron transfer, while excessively high electrode potentials may inhibit microbial activity by suppressing electrode-colonizing species [
176]. While high electrode potentials may exert electrochemical stress and inhibit microbial activity, selection of overly low anodic potentials can also reduce the kinetics of bioanodic oxidation and therefore limit hydrogen production or current generation. These trade-offs have been observed in comparative MEC studies (e.g., working potential +0.30 V vs. SHE) [
177]. Numerous studies have highlighted the importance of direct interspecies electron transfer (DIET) between electrogenic bacteria and methanogenic archaea in enhancing biomethane production efficiency during AD process [
178,
179]. In MEC-AD systems, the application of an external voltage promotes the enrichment of electroactive microorganisms on electrode surfaces and in the bulk solution, thereby facilitating DIET and accelerating the hydrolysis of refractory substrates [
180]. Unlike traditional AD processes, MEC-AD enables enhanced methanogenesis through external energy input and efficient electron transfer between electrochemically active microbes [
181,
182]. Choi et al. [
183] observed the highest CH
4 yields (408.3 mL/g COD-glucose) at 1.0 V among the tested voltages of 0.5, 0.7, 1.0, and 1.5 V, while Flores-Rodriguez et al. [
184] investigated anaerobic acetate degradation under voltages ranging from 0.5 to 1.5 V, and obtained a maximum CH
4 yield of 0.351 L CH
4/g COD at 1.0 V. This enhancement is attributed to direct methanogenesis at the electrode surface, where methanogenic archaea convert CO
2, electrons, and protons into CH
4 [
185]. Meanwhile, oxygen generated at the anode under microaerobic conditions can accelerate substrate hydrolysis, further boosting CH
4 yield [
183]. Building upon these insights, recent studies extended MEC-AD to BPs. Liu et al. [
186] found that the MEC-AD system reduced polylactic acid (PLA) digestion time by 60% and significantly increased CH
4 production, particularly under an applied voltage of 0.3 V.
Traditional AD often becomes unstable due to organic overload or is inhibited by certain compounds, such as VFA [
187] and ammonia sulfide [
188], leading to reduced biogas production or lower CH
4 content. In contrast, the application of voltage in MEC-AD systems promotes the enrichment of electroactive bacteria, accelerating the VFA degradation and other organic compounds, thereby improving process stability and boosting CH
4 yields [
189]. Kondaveeti et al. [
190] demonstrated that MECs can enhance the AD process by converting VFAs into various alcohols, including ethanol, butanol, and propanol, with alcohol yields increasing in proportion to the reduction current. This is because externally applied voltage drives electrons through electrodes or mediators to electroactive microorganisms, enabling the reduction of VFAs into alcohols and gases as high-value biofuels [
190]. MEC-AD also achieves high ammonia removal efficiency, reaching up to 60% during the continuous mixed VFA pulse stage [
191]. This is attribute to microorganisms in the system oxidize organic matter to generate current, which drives NH
4+ migration from the anode to the cathode, where it is converted into NH
3 under high pH conditions, thereby enabling ammonia nitrogen recovery. Although previous studies have shown that BPs produce VFAs during AD process [
48,
53], research on the transformation behavior and regulatory mechanisms of these VFAs remains limited. Given the potential of MECs to modulate VFA dynamics, exploring their regulatory role in VFA changes during the AD of BPs is both significant and promising, and warrants further in-depth investigation. The available findings suggest that while MEC-AD consistently improves CH
4 yield and stability across different feedstocks, such as PLA, PHA, and mixed microplastics, the magnitude of improvement varies depending on substrate composition, conductivity, and biodegradability. For instance, highly crystalline or hydrophobic BPs tend to show slower degradation kinetics even under electrochemical stimulation, indicating that material properties strongly influence MEC-AD performance.
In natural environments, BPs typically occur as mixtures. MEC-AD systems have shown potential in degrading both single and mixed BPs, thereby supporting the treatment of complex waste streams. Wang et al. [
192] presented the first comprehensive evaluation of the MEC-AD system for wastewater co-contaminated with antibiotics and mixed microplastics (including PLA). Compared to conventional AD, the MEC-AD system improved wastewater treatment efficiency (14.39%) and CH
4 recovery (14.32%), while also revealing complex microbial responses and functional adaptations under composite pollutant stress [
192]. This study points out that electrical stimulation in MECs can enhance metabolic activity, enrich functional microbial communities, optimize metabolic pathways, alleviate stress, and regulate the microecological structure, thereby improving the system’s capacity to handle complex microplastics and enhancing overall process stability and CH
4 production [
192].
The above research confirms the potential of MECs to enhance the performance of AD by MEC systems for BPs degradation. As shown in
Table 3, compared with conventional AD, MEC-AD systems improve degradation efficiency, methane yield, and system stability. However, these improvements are accompanied by notable technical trade-offs. Higher applied voltages can accelerate electron transfer and increase CH
4 yield, but they may also cause local pH shifts, oxygen leakage from the anode, and inhibitory effects on methanogens, compromising long-term stability. In addition, maintaining electrode performance and controlling energy input remain critical challenges for large-scale implementation. Thus, their application to BPs remains at an early stage, with only a limited number of studies available. Future research should therefore focus on system optimization, mechanistic elucidation, and evaluation of long-term stability and scalability under real-world conditions (
Table 3).
4.2.2. Microbial Fuel Cell—Anaerobic Digestion (MFC-AD)
The microbial fuel cell-assisted anaerobic digestion (MFC-AD) system represents another promising BES-AD technology for BP degradation. A notable variant is the photosynthetic microbial fuel cell (PMFC), which generates bioelectricity by photosynthetic bacteria [
193]. Qi et al. [
194] utilized the PMFC-AD system to degrade PLA materials and observed a maximum weight loss of 26.67%. In this process, anoxygenic photosynthetic bacteria (APB) were activated by light and gelatin wastewater to secrete PLA-degrading enzymes, thereby cleaving polymer bonds and releasing metabolizable intermediates. These intermediates were subsequently utilized via anaerobic metabolism to generate electrons, enabling continuous power output and forming a coupled degradation—metabolism—electricity generation process. Even without external carbon supplementation, the system maintained a stable current density of 4.4 ± 0.2 mA/cm
2 [
194]. In a follow-up study, weak infrared (IR) light (850 nm) from a light-emitting diode (LED) further stimulated APB activity in activated sludge, thereby promoting the anaerobic biodegradation of PLA in MFC systems [
195].
Beyond degradation, MFCs have been widely explored as biosensors due to their portability, short hydraulic retention times, and suitability for continuous monitoring [
196]. Electrical signals generated by MFCs can reflect dynamic changes in AD performance, providing a real-time monitoring tool [
197]. For example, Liu et al. [
197] installed an MFC in the recirculation loop of a bench-scale upflow anaerobic fixed-bed (UAFB) reactor and demonstrated strong correlations between MFC outputs and pH, gas flow rate, and COD levels over six months. Similarly, Kaur et al. [
198] reported that the current generation from a dual-chamber MFC coupled to VFA oxidation showed a linear increase along with the VFA concentrations (<80 mg/L) and therefore could be used for quantitative detection of VFA. Sun et al. [
199] designed an innovative dual-chamber MFC biosensor for monitoring VFA during AD, which exhibited a linear response at low concentrations (<14 mM) and a nonlinear increase at higher concentrations, and its applicability was validated using effluent from the digester startup stage. This principle also applies to BP degradation monitoring. As BPs are decomposed anaerobically into intermediates and ultimately into CH
4, CO
2, and H
2O, their metabolic conversion partially translates into electrical signals in MFCs [
200]. Fluctuations in BP concentration and intermediates thus directly affect microbial activity and voltage output. Based on this mechanism, MFC-AD systems have been proposed as real-time biosensors for BP degradation. Dong et al. [
201] reported that MFC-AD-based sensors can effectively and accurately monitor the anaerobic biodegradation of P34HB films in real time, with a strong linear correlation (R
2 = 0.998) between coulombic yield biodegradability (Q-biodegradability) and weight loss, offering a reliable alternative to conventional assessment methods.
To date, although there are limited studies on the use of MFC-AD systems to treat BPs, several investigations have demonstrated the potential of MFCs in the degradation of microplastics (MPs). Wang et al. [
202] observed that the addition of polyethylene (PE) MPs inhibited the activity of electrogenic biofilms in MFCs, increased electrode resistance, reduced microbial richness and MP-related OTUs, and lowered the abundance of electron transfer-related genes such as
pilA,
mtrC, and
cytochrome c. Similarly, Yuan et al. [
203] confirmed that PE MP accumulation in MFCs caused a series of changes in substrate properties (e.g., oxygen mass transfer rate, and internal resistance), microbial community structure (e.g., denitrifying bacteria), and microbial activity, thereby affecting both pollutant removal efficiency and power generation performance. MFC offers several advantages as an MP treatment technology, requires no energy and chemicals, generates bioelectricity, and is adaptable to various MP-polluted substrates [
26]. Based on the above characteristics, the MFC-AD system may exhibit synergistic effects. On the one hand, the organic intermediates produced during the AD process can serve as substrates for electroactive microorganisms in the MFC, enhancing bioelectricity generation. On the other hand, the electrochemical environment within the MFC can improve substrate conversion efficiency, thereby accelerating the degradation of BPs (
Table 4). However, current findings are not yet fully consistent across different feedstocks or BPs. For example, systems treating starch-based or protein-containing feedstocks often exhibit higher bioelectricity yields but reduced stability due to rapid acidification and microbial community shifts; otherwise, PLA- or PHA-based systems show slower but more stable degradation under comparable operating conditions. These differences indicated that the MFC-AD performance strongly depends on substrate characteristics, microbial adaptability, and reactor configuration.
4.3. BES—Enhanced Enzymatic Hydrolysis
An enzymatic biofuel cell (EBC) is a special kind of BES that utilizes a biological enzyme as an electrode catalyst for fuel oxidation and oxidant reduction, thereby converting biochemical energy into electrical energy [
204,
205]. The enzymes used in EBCs exhibit high catalytic activity and are harmless to living organisms [
206,
207]. Similarly to most conventional BESs, EBCs also consist of an anode and a chamber with electrodes, substrates, and some have a proton-selective membrane [
208]. The electrochemical connection between the electrode surface and the enzyme is a key factor influencing the performance of EBCs [
209]. EBCs include compatibility with a wide range of fuel sources and the ability to support appropriate and efficient enzymatic reactions [
204], which makes EBCs suitable for energy storage, implantable energy supply devices, and self-powered biosensors [
210,
211,
212].
It has been reported that enzymes such as lipases, proteases, and cutinase play a crucial role in the degradation of BPs; they can specifically target the structure and degrade it into monomers [
213]. Furthermore, protein engineering and immobilization techniques (e.g., reinforcing the bonding between the active site of the enzyme and substrate, increasing enzyme activity, improving enzyme and substrate interaction, etc.) have been utilized to enhance enzyme stability and predict protein structures [
214] (
Table 5). Although there are currently no reports on the application of BPs-degrading enzymes in EBC systems, based on the working principles of EBCs, it can be inferred that this strategy represents a promising method for BPs removal. Compared with MEC and MFC systems, EBCs exhibit several distinct performance characteristics. Specifically, EBCs operate under milder conditions (ambient temperature, neutral pH, and no external voltage), achieving higher catalytic selectivity but generally lower power output. In contrast, MECs and MFCs can handle complex organic feedstocks and real waste effluents, producing both electricity and valuable byproducts (e.g., H
2 and VFAs), yet they often require active microbial consortia, longer startup times, and are more sensitive to substrate fluctuations. EBCs, on the other hand, rely on purified enzymes, enabling faster electron transfer and cleaner conversion but facing challenges in enzyme deactivation, stability, and cost under real waste conditions. Notably, while MFCs and MECs have already demonstrated measurable degradation of biodegradable plastics and even microplastics, EBCs remain at a conceptual or proof-of-principle stage regarding BP treatment. Thus, EBCs currently lag behind MEC/MFC systems in practical applicability and robustness but hold promise for targeted, low-energy, and selective degradation when appropriate enzyme polymer pairs and immobilization support are developed.
The findings regarding EBC performance are generally consistent across different types of biodegradable polymers, although the reaction efficiency may vary depending on the enzyme substrate affinity and the molecular structure of the feedstock. For instance, polymers with more complex backbones or higher crystallinity tend to exhibit slower degradation rates, leading to lower current densities. In terms of technical trade-offs, EBC systems achieving higher catalytic activity and energy yield often face challenges related to enzyme stability and lifetime, especially under continuous operation or fluctuating environmental conditions. Conversely, immobilized enzyme systems typically offer improved long-term stability but may suffer from reduced electron transfer efficiency and lower peak power output. Balancing these factors, maximizing yield while maintaining enzyme durability, remains a key direction for future optimization of EBC-based BPs degradation. Moreover, future research should focus on identifying and engineering enzymes that can efficiently process a broader range of polymeric feedstocks while preserving operational stability, to ensure consistent EBC performance under diverse real-world conditions.
4.4. Additional Benefits of BES Integration
Microbial desalination cells (MDCs) represent a novel type of bioelectrochemical system designed for simultaneous wastewater treatment, seawater desalination, and renewable energy production without the need for external electrical or thermal energy [
215]. They can be used independently or integrated with various water treatment technologies to enhance the overall purification process. Typically, an MDC consists of three chambers: an anaerobic anodic chamber, a desalination chamber, and a cathodic chamber, along with an anion exchange membrane (AEM) and a cation exchange membrane (CEM) [
216,
217]. Among these, the AEM and CEM are critical components, as they not only separate the three chambers but also selectively allow the passage of ions, thereby contributing to the water purification process. Jin et al. [
218] found that MDC can monitoring of VFAs during AD process. The system demonstrated a wide linear detection range (1–200 mM), high specificity, and strong correlation with gas chromatography results [
218]. By separating the anode and VFA-containing chamber with an AEM, the sensor enables selective transport and detection of ionized VFAs while excluding interference from non-ionic compounds like proteins and lipids [
218]. In addition, the ion-selective membrane structure and internal bioelectrochemical potential of MDC system enable it to achieve the initial separation of target fermentation products (such as VFAs) in AD or AF process. Specifically, negatively charged small molecules such as VFAs can preferentially migrate to the anode chamber through the AEM under potential drive, thereby achieving initial separation from complex organic substrates. This process not only has the advantages of energy self-sufficiency and environmental friendliness, but also provides a good pretreatment basis for subsequent high-value utilization. However, in actual application, the separation efficiency of MDC is often limited by factors such as interference from competing ions in the solution and membrane fouling caused by long-term operation. These problems will significantly reduce the selectivity and transmission efficiency of the membrane, affecting the stability of the system and the separation effect. Therefore, it is difficult for a single MDC system to achieve high-purity and high-recovery target product acquisition. To overcome the above limitations and achieve efficient extraction of fermentation products, the MDC system needs to be used in combine with other separation technologies. For example, it can be integrated with a bioelectrosynthesis system (MES) to further realize the conversion of VFA into high value-added compounds (such as ethanol, butanol, butyrate, etc.) based on selective migration; or combined with a microbial electrodialysis system (MEDS) to achieve higher purity VFA enrichment by enhancing ion migration and graded separation capabilities. This multi-technology integration strategy not only improves the overall recovery efficiency, but also expands the application prospects of MDC in resource recovery and wastewater deep treatment (
Table 6).
MFC-driven-MEC (MFC-MEC) system is also be suggested. Up to date, MEC-MFC systems have been widely explored for the recovery of energy and resources (e.g., H
2 and CH
4) from diverse types of wastewater [
219,
220,
221]. Notably, Li et al. [
222] demonstrated that coupling MEC and MFC, with acetate supplied as the substrate, significantly enhanced the anaerobic anammox process, resulting in over 30% improvement in total nitrogen (TN) removal efficiency compared to traditional anammox systems. This is because the MFC-MEC system enables a synergistic mechanism involving self-power supply, external electric field stimulation, and microbial activity, which effectively accelerates TN removal, particularly the oxidation of NH
4+, thus significantly enhancing the efficiency and sustainability of the anammox process. It can be seen that the MFC-MEC system has a promoting effect on the microbial metabolic process. Therefore, three different kinds of MFC-MEC systems can be explored: (i) A simple MFC-MEC conversion system can be connected to an anaerobic system to degrade BPs, allowing the generation of different by-products by switching between MFC and MEC modes, which can help degrade mixed BPs. This setup can also be used to monitor the degradation process. (ii) Both MFC and MEC are integrated with the anaerobic system, where the MFC supplies external power to the MEC to lower the overall cost. (iii) The MEC is connected to the anaerobic system to degrade BPs, and the resulting solution is then fed into the MFC to monitor VFA production.
MDC and MFC-MEC systems exhibit distinct strengths and limitations. MDCs excel in desalination and selective ion separation, providing an environmentally friendly and energy-autonomous means of removing salts while enabling partial wastewater treatment. However, their performance is generally limited when treating complex organic waste streams or high-strength industrial effluents, as membrane fouling, ion competition, and slow biofilm development reduce long-term stability and overall efficiency. In contrast, MFC-MEC systems demonstrate strong potential for energy recovery and pollutant degradation due to their synergistic bioelectrochemical and electrohydrogenic processes. These systems can achieve higher power output, enhanced substrate utilization, and deeper organic conversion than standalone MDCs. Nonetheless, they are more technologically complex, requiring multiple electrodes, controlled voltage regulation, and costly materials, leading to higher operational and maintenance expenses.
In summary, MDCs are advantageous for desalination and selective separation but less suited for treating complex or solid-laden wastewaters (
Table 6), whereas MFC-MEC systems offer greater versatility and energy yield but face challenges in scalability and cost-efficiency (
Table 7). Future research should aim to integrate the selective ion migration capability of MDCs with the high bioenergy recovery efficiency of MFC-MEC systems to develop multifunctional platforms for simultaneous desalination, waste degradation, and resource recovery.
5. Challenges and Future Perspectives
As a clean energy and environmental protection technology that integrates microbial metabolic processes with electrochemical reaction mechanisms, BESs show great potential in the treatment of complex pollutants [
126]. As mentioned above, BESs exhibit promising application prospects in assisting AS for the degradation of BPs, particularly in enhancing degradation rates, facilitating intermediate conversion, and enabling energy recovery. As shown in
Table 3, selecting the appropriate type of BES (e.g., MFC, MEC, MDC, etc.) is crucial for achieving optimal treatment performance. Different systems vary in terms of energy input and output, electrode materials, biofilm formation capabilities, and adaptability to intermediate compounds. Therefore, a reasonable matching between BES configuration and operating parameters is a key prerequisite for advancing its practical application. However, it is worth noting that there is currently no systematic study on the direct application of BES-ASs for the degradation of BPs, indicating that this area remains in the exploratory stage and has broad development potential. Consequently, future research can be further deepened in the following directions:
The research foundation in this field remains weak, with a lack of systematic exploration. Although some studies have reported the application of BES in microplastic degradation [
223,
224], there is still no direct evidence demonstrating their role in the degradation of BPs. In particular, after coupling BES with AS, the potential synergistic effects between the two in degrading BPs have not been thoroughly investigated. Mechanistic insights and supporting experimental data are also very limited. Therefore, future research can focus on the following directions: (i) Construct standardized experimental models to systematically compare the degradation efficiency of BES, AS, and BES-ASs across different types of BPs. (ii) Investigate the performance differences among various BES configurations. The degradation efficiency, energy conversion efficiency, and by-product characteristics of MFC, MEC, MDC, and their integrated forms in the anaerobic degradation of BP need to be systematically compared. (iii) Screen and enrich functional microbial communities. Employ genetic engineering, high-throughput screening, or enrichment cultivation to identify efficient electroactive microorganisms, AD bacteria, or digestive enzymes suitable for BP degradation, and construct composite microbial consortia with synergistic metabolic capabilities. (iv) Investigate microbial community succession and system stability. Integrate artificial intelligence and other modeling tools to develop dynamic operational models that simulate long-term microbial community shifts, explore their relationship with system performance variability, and identify key microbial factors that influence system stability. (v) Develop novel functional electrode materials. Evaluate the adaptability and long-term performance of materials with high conductivity, anti-biofouling properties, and biocompatibility (e.g., doped carbon materials, nanocomposites, and conductive polymers) for BPs degradation. In addition, thoroughly explore the applicability, performance differences, and optimal configurations (e.g., series, parallel, or coupled) of various BES types in supporting anaerobic degradation. (vi) Analyze the relationship between bioplastic structure and degradation characteristics. Establish models that link the chemical structures of different BPs (e.g., main chain types, polarity, crystallinity) with their degradability by electroactive microorganisms. (vii) Construct mixed degradation systems for multiple types of BPs. Simulate real-world scenarios of mixed plastic pollution and investigate the synergistic or antagonistic effects among different BPs during co-degradation.
The mechanism of action is still unclear, and research on electron transfer and intermediate metabolic pathways remains insufficient. Currently, the specific mechanism by which BES promotes the degradation of BPs in AS has not been fully studied. In particular, the pathways of electron transfer from organic matter to the anode, the roles of key metabolic enzymes in the degradation process [
225], and the influence of electrode potential on microbial metabolic regulation remain poorly understood. Moreover, the types of intermediate metabolites formed during the degradation of BPs in the BES-AS, their transformation pathways, and their potential promoting or inhibiting effects within the system are still unclear. Therefore, future research can focus on the following areas: (i) Up to date, many studies have used 16S rRNA amplicon sequencing to characterize microbiomes in BESs, and metagenomic approaches have revealed multispecies catabolic pathways in these systems [
226]. Therefore, future research can conduct molecular-level studies using omics technologies (e.g., metabolomics, transcriptomics, and proteomics) to identify key metabolic pathways and functional enzymes, and to elucidate the coupling mechanisms between electron transfer and BPs degradation. (ii) Develop in situ monitoring and tracing technologies (e.g., stable isotope labeling, intermediate product enrichment, etc.) to detect key intermediates and track their dynamic changes during the degradation of BPs. Tian et al. [
227] successfully applied 14C isotope tracing technology to the quantitative analysis of polystyrene plastic degradation, indicating that this technology also has great potential for application in BP degradation research. (iii) Systematically evaluate the regulatory mechanisms of electrode potential. Since variations in current intensity can alter the performance and microbial community diversity in BESs [
228], it is important to elucidate how different electrode potentials influence the BP degradation rate, intermediate accumulation, and microbial community structure. Such understanding will contribute to optimizing the energy-driven strategy of the BES–AS.
The analysis of degradation products and resource recovery pathways is unclear. BPs degraded through the BES-AS can yield valuable resource products such as VFAs, alcohols, and biogas. However, existing studies pay limited attention to the specific composition, yield, and transformation pathways of these products. Furthermore, the subsequent resource utilization strategies, such as the extraction of VFAs or the energy-efficient conversion of biogas, remain underexplored, and the full value of resource recovery has yet to be realized. Future research should consider the following directions: (i) Establish a comprehensive degradation product spectrum database, use advanced analytical techniques (e.g., gas chromatography-mass spectrometry, liquid chromatography-mass spectrometry, high-performance liquid chromatography, etc.) to systematically identify and quantify intermediate and final products from the degradation of various BPs. (ii) Investigate metabolic pathways and influencing factors, clarify how key enzymes, environmental conditions (e.g., temperature, pH, redox potential, etc.), and reaction parameters affect the types and proportions of degradation products formed. (iii) Evaluate the energy efficiency and economic viability of resource recovery. Use life cycle assessments (LCA) or techno-economic analyses to assess the sustainability and feasibility of resource recovery pathways.