Optimization, Scale-Up, and Economic Analysis of the Ethanol Production Process Using Sargassum horneri
Abstract
:1. Introduction
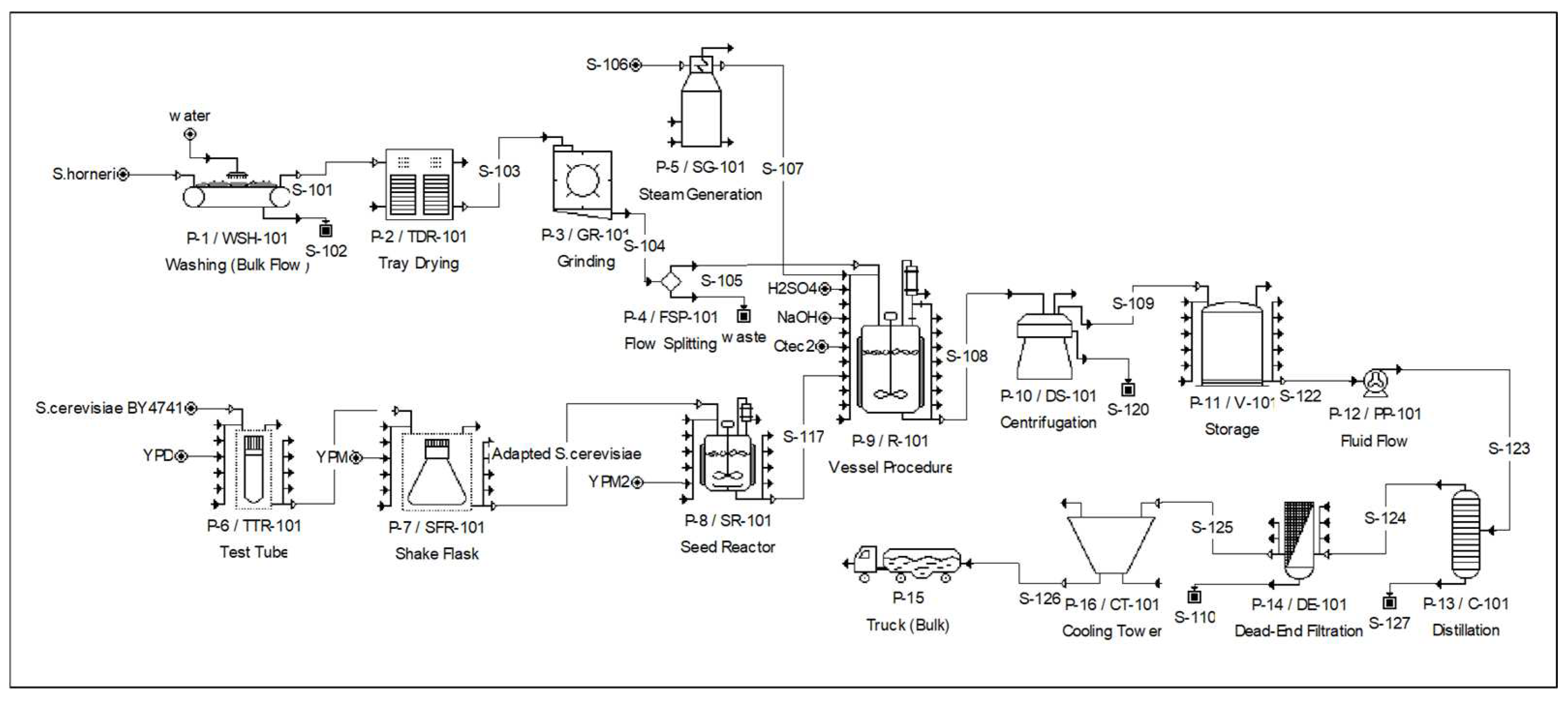
2. Materials and Methods
2.1. Biomass
2.2. Optimization of Thermal Acid Hydrolysis and Enzymatic Saccharification Conditions
2.3. Adaptive Evolution of Saccharomyces cerevisiae BY4741 to Mannitol
2.4. Ethanol Fermentation in an Erlenmeyer Flask and 5 L and 500 L Fermenters
2.5. Aanlysis
2.5.1. Fourier Transform Infrared Spectroscopy
2.5.2. UV Spectrophotometer
2.5.3. High Performance Liquid Chromatography (HPLC)
2.6. Economic Analysis of Bioethanol Production
2.7. Statistical Analysis
3. Results
3.1. Characterization of the Biomass
3.2. Optimization of Thermal Acid Hydrolysis and Enzymatic Saccharification
3.3. Adaptive Evolution of S. cerevisiae BY4741 to Mannitol
3.4. Continuous Hydrolysis, Enzymatic Saccharification, and Fermentation with Various Scales
3.5. Economic Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martin, L.M.; Taylor, M.; Huston, G.; Goodwin, D.S.; Schell, J.M.; Siuda, A.N. Pelagic Sargassum morphotypes support different rafting motile epifauna communities. Mar. Biol. 2021, 168, 115. [Google Scholar] [CrossRef]
- Peng, Z.; Guo, Z.; Wang, Z.; Zhang, R.; Wu, Q.; Gao, H.; Wang, Y.; Shen, Z.; Lek, S.; Xiao, J. Species-specific bioaccumulation and health risk assessment of heavy metal in seaweeds in tropic coasts of South China Sea. Sci. Total Environ. 2022, 832, 155031. [Google Scholar] [CrossRef]
- Wang, M.; Hu, C.; Barnes, B.B.; Mitchum, G.; Lapointe, B.; Montoya, J.P. The great Atlantic sargassum belt. Science 2019, 365, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Rotter, A.; Barbier, M.; Bertoni, F.; Bones, A.M.; Cancela, M.L.; Carlsson, J.; Carvalho, M.F.; Cegłowska, M.; Chirivella-Martorell, J.; Conk Dalay, M. The essentials of marine biotechnology. Front. Mar. Sci. 2021, 8, 158. [Google Scholar] [CrossRef]
- Greene, J.M.; Gulden, J.; Wood, G.; Huesemann, M.; Quinn, J.C. Techno-economic analysis and global warming potential of a novel offshore macroalgae biorefinery. Algal Res. 2020, 51, 102032. [Google Scholar] [CrossRef]
- Nazemi, F.; Karimi, K.; Denayer, J.F.; Shafiei, M. Techno-economic aspects of different process approaches based on brown macroalgae feedstock: A step toward commercialization of seaweed-based biorefineries. Algal Res. 2021, 58, 102366. [Google Scholar] [CrossRef]
- Yusoff, M.; Zulkifli, N.; Masum, B.; Masjuki, H. Feasibility of bioethanol and biobutanol as transportation fuel in spark-ignition engine: A review. RSC Adv. 2015, 5, 100184–100211. [Google Scholar] [CrossRef]
- Sudhakar, M.; Arunkumar, K.; Perumal, K. Pretreatment and process optimization of spent seaweed biomass (SSB) for bioethanol production using yeast (Saccharomyces cerevisiae). Renew. Energy 2020, 153, 456–471. [Google Scholar] [CrossRef]
- Chen, H.; Zhou, D.; Luo, G.; Zhang, S.; Chen, J. Macroalgae for biofuels production: Progress and perspectives. Renew. Sustain. Energy Rev. 2015, 47, 427–437. [Google Scholar] [CrossRef]
- Tan, I.S.; Lee, K.T. Enzymatic hydrolysis and fermentation of seaweed solid wastes for bioethanol production: An optimization study. Energy 2014, 78, 53–62. [Google Scholar] [CrossRef]
- Maurya, D.P.; Singla, A.; Negi, S. An overview of key pretreatment processes for biological conversion of lignocellulosic biomass to bioethanol. 3 Biotech 2015, 5, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.J.; Jin, Y.-S. Engineering of Saccharomyces cerevisiae for efficient fermentation of cellulose. FEMS Yeast Res. 2020, 20, foz089. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Jameel, A.; Xing, X.-H.; Zhang, C. Advanced strategies and tools to facilitate and streamline microbial adaptive laboratory evolution. Trends Biotechnol. 2022, 40, 38–59. [Google Scholar] [CrossRef]
- Sandberg, T.E.; Salazar, M.J.; Weng, L.L.; Palsson, B.O.; Feist, A.M. The emergence of adaptive laboratory evolution as an efficient tool for biological discovery and industrial biotechnology. Metab. Eng. 2019, 56, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ishizaki, H.; Hasumi, K. Ethanol production from biomass. Res. Approaches Sustain. Biomass Syst. 2014, 243–258. [Google Scholar]
- Kassim, M.A.; Meng, T.K.; Kamaludin, R.; Hussain, A.H.; Bukhari, N.A. Bioprocessing of Sustainable Renewable Biomass for Bioethanol Production. In Value-Chain of Biofuels; Elsevier: Amsterdam, The Netherlands, 2022; pp. 195–234. [Google Scholar]
- Santos, F.; Eichler, P.; de Queiroz, J.H.; Gomes, F. Production of Second-Generation Ethanol from Sugarcane. In Sugarcane Biorefinery, Technology and Perspectives; Elsevier: Amsterdam, The Netherlands, 2020; pp. 195–228. [Google Scholar]
- Soccol, C.R.; Faraco, V.; Karp, S.; Vandenberghe, L.P.; Thomaz-Soccol, V.; Woiciechowski, A.; Pandey, A. Lignocellulosic bioethanol: Current status and future perspectives. Biofuels 2011, 101–122. [Google Scholar]
- Visioli, L.J.; Stringhini, F.M.; Salbego, P.R.; Chielle, D.P.; Ribeiro, G.V.; Gasparotto, J.M.; Aita, B.C.; Klaic, R.; Moscon, J.M.; Mazutti, M.A. Use of Agroindustrial Residues for Bioethanol Production. In Bioenergy Research: Advances and Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 49–56. [Google Scholar]
- Horwitz, W. Official Methods of Analysis; Association of Official Analytical Chemists: Washington, DC, USA, 1975; Volume 222. [Google Scholar]
- Sunwoo, I.Y.; Sukwong, P.; Park, Y.R.; Jeong, D.Y.; Kim, S.R.; Jeong, G.-T.; Kim, S.-K. Enhancement of galactose uptake from Kappaphycus alvarezii using Saccharomyces cerevisiae through deletion of negative regulators of GAL genes. Appl. Biochem. Biotechnol. 2021, 193, 577–588. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, R.T.; DuPré, E.F.; Mitcham, D. Applications of infrared absorption spectroscopy to investigations of cotton and modified cottons: Part I: Physical and crystalline modifications and oxidation. Text. Res. J. 1958, 28, 382–392. [Google Scholar] [CrossRef]
- Nelson, M.L.; O’Connor, R.T. Relation of certain infrared bands to cellulose crystallinity and crystal lattice type. Part II. A new infrared ratio for estimation of crystallinity in celluloses I and II. J. Appl. Polym. Sci. 1964, 8, 1325–1341. [Google Scholar] [CrossRef]
- Gengiah, K.; Rajendran, N.; Al-Ghanim, K.A.; Govindarajan, M.; Gurunathan, B. Process and technoeconomic analysis of bioethanol production from residual biomass of marine macroalgae Ulva lactuca. Sci. Total Environ. 2023, 868, 161661. [Google Scholar] [CrossRef]
- Panahi, H.K.S.; Dehhaghi, M.; Guillemin, G.J.; Gupta, V.K.; Lam, S.S.; Aghbashlo, M.; Tabatabaei, M. Bioethanol production from food wastes rich in carbohydrates. Curr. Opin. Food Sci. 2022, 43, 71–81. [Google Scholar] [CrossRef]
- Badrinathan, S.; Suneeva, S.; Shiju, T.; Girish Kumar, C.; Pragasam, V. Exploration of a novel hydroxyl radical scavenger from Sargassum myriocystum. J. Med. Plants Res. 2011, 5, 1997–2005. [Google Scholar]
- Matanjun, P.; Mohamed, S.; Mustapha, N.M.; Muhammad, K. Nutrient content of tropical edible seaweeds, Eucheuma cottonii, Caulerpa lentillifera and Sargassum polycystum. J. Appl. Phycol. 2009, 21, 75–80. [Google Scholar] [CrossRef]
- Heo, S.-J.; Park, P.-J.; Park, E.-J.; Kim, S.-K.; Jeon, Y.-J. Antioxidant activity of enzymatic extracts from a brown seaweed Ecklonia cava by electron spin resonance spectrometry and comet assay. Eur. Food Res. Technol. 2005, 221, 41–47. [Google Scholar] [CrossRef]
- Kumar, S.; Sahoo, D.; Levine, I. Assessment of nutritional value in a brown seaweed Sargassum wightii and their seasonal variations. Algal Res. 2015, 9, 117–125. [Google Scholar] [CrossRef]
- Belattmania, Z.; Bentiss, F.; Jama, C.; Barakate, M.; Katif, C.; Reani, A.; Sabour, B. Biosynthesis and characterization of silver nanoparticles using sodium alginate from the invasive macroalga Sargassum muticum. Bionanoscience 2018, 8, 617–623. [Google Scholar] [CrossRef]
- Kannan, S. FT-IR and EDS analysis of the seaweeds Sargassum wightii (brown algae) and Gracilaria corticata (red algae). Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 341–351. [Google Scholar]
- Zhang, J.; Hori, N.; Takemura, A. Thermal and time regularities during oilseed rape straw liquefaction process to produce bio-polyol. J. Clean. Prod. 2020, 277, 124015. [Google Scholar] [CrossRef]
- Wu, S.; Shi, S.; Liu, R.; Wang, C.; Li, J.; Han, L. The transformations of cellulose after concentrated sulfuric acid treatment and its impact on the enzymatic saccharification. Biotechnol. Biofuels Bioprod. 2023, 16, 36. [Google Scholar] [CrossRef]
- Kostas, E.T.; White, D.A.; Cook, D.J. Bioethanol production from UK seaweeds: Investigating variable pre-treatment and enzyme hydrolysis parameters. Bioenergy Res. 2020, 13, 271–285. [Google Scholar] [CrossRef]
- Sunwoo, I.; Kwon, J.E.; Jeong, G.-T.; Kim, S.-K. Optimization of hyper-thermal acid hydrolysis and enzymatic saccharification of Ascophyllum nodosum for ethanol production with mannitol-adapted yeasts. Bioprocess Biosyst. Eng. 2019, 42, 1255–1262. [Google Scholar] [CrossRef]
- Hong, I.K.; Jeon, H.; Lee, S.B. Comparison of red, brown and green seaweeds on enzymatic saccharification process. J. Ind. Eng. Chem. 2014, 20, 2687–2691. [Google Scholar] [CrossRef]
- Schultze-Jena, A.; Vroon, R.; Macleod, A.; Hreggviðsson, G.; Adalsteinsson, B.; Engelen-Smit, N.; de Vrije, T.; Budde, M.; van der Wal, H.; López-Contreras, A. Production of acetone, butanol, and ethanol by fermentation of Saccharina latissima: Cultivation, enzymatic hydrolysis, inhibitor removal, and fermentation. Algal Res. 2022, 62, 102618. [Google Scholar] [CrossRef]
- Chujo, M.; Yoshida, S.; Ota, A.; Murata, K.; Kawai, S. Acquisition of the ability to assimilate mannitol by Saccharomyces cerevisiae through dysfunction of the general corepressor Tup1-Cyc8. Appl. Environ. Microbiol. 2015, 81, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, T.; Osório, C.; Sousa, M.J.; Franco-Duarte, R. Contributions of Adaptive Laboratory Evolution towards the Enhancement of the Biotechnological Potential of Non-Conventional Yeast Species. J. Fungi 2023, 9, 186. [Google Scholar] [CrossRef] [PubMed]
- Offei, F.; Mensah, M.; Thygesen, A.; Kemausuor, F. Seaweed bioethanol production: A process selection review on hydrolysis and fermentation. Fermentation 2018, 4, 99. [Google Scholar] [CrossRef]
- Huang, Y.; Dehkordy, F.M.; Li, Y.; Emadi, S.; Bagtzoglou, A.; Li, B. Enhancing anaerobic fermentation performance through eccentrically stirred mixing: Experimental and modeling methodology. Chem. Eng. J. 2018, 334, 1383–1391. [Google Scholar] [CrossRef]
- Clark, I.C.; Zhang, R.H.; Upadhyaya, S.K. The effect of low pressure and mixing on biological hydrogen production via anaerobic fermentation. Int. J. Hydrogen Energy 2012, 37, 11504–11513. [Google Scholar] [CrossRef]
- Roesijadi, G.; Jones, S.B.; Snowden-Swan, L.J.; Zhu, Y. Macroalgae as a Biomass Feedstock: A Preliminary Analysis; Pacific Northwest National Lab.(PNNL): Richland, WA, USA, 2010.
- Fasahati, P.; Woo, H.C.; Liu, J.J. Industrial-scale bioethanol production from brown algae: Effects of pretreatment processes on plant economics. Appl. Energy 2015, 139, 175–187. [Google Scholar] [CrossRef]
- Jang, J.-S.; Cho, Y.; Jeong, G.-T.; Kim, S.-K. Optimization of saccharification and ethanol production by simultaneous saccharification and fermentation (SSF) from seaweed, Saccharina japonica. Bioprocess Biosyst. Eng. 2012, 35, 11–18. [Google Scholar] [CrossRef]
- McDermid, K.J.; Stuercke, B. Nutritional composition of edible Hawaiian seaweeds. J. Appl. Phycol. 2003, 15, 513–524. [Google Scholar] [CrossRef]
- Valdivia, M.; Galan, J.L.; Laffarga, J.; Ramos, J.L. Biofuels 2020: Biorefineries based on lignocellulosic materials. Microb. Biotechnol. 2016, 9, 585–594. [Google Scholar] [CrossRef]
- Baral, P.; Munagala, M.; Shastri, Y.; Kumar, V.; Agrawal, D. Cost reduction approaches for fermentable sugar production from sugarcane bagasse and its impact on techno-economics and the environment. Cellulose 2021, 28, 6305–6322. [Google Scholar] [CrossRef]
- Kityo, M.K.; Sunwoo, I.; Kim, S.H.; Park, Y.R.; Jeong, G.-T.; Kim, S.-K. Enhanced bioethanol fermentation by sonication using three yeasts species and Kariba weed (Salvinia molesta) as biomass collected from Lake Victoria, Uganda. Appl. Biochem. Biotechnol. 2020, 192, 180–195. [Google Scholar] [CrossRef]



| Composition (%) | Moisture | Crude Protein | Crude Lipid | Crude Fiber | Crude Ash | Carbohydrate |
|---|---|---|---|---|---|---|
| 12.64 | 13.00 | 1.13 | 8.59 | 20.74 | 43.90 |
| Glucose (g/L) | Galactose (g/L) | Mannitol (g/L) | Total Sugar (g/L) | EAE (%) | ||
|---|---|---|---|---|---|---|
| Thermal acid hydrolysis | H2SO4 | 1.35 ± 0.13 | 1.56 ± 0.26 | 1.91 ± 0.16 | 4.82 | 11 |
| HNO3 | 0.84 ± 0.01 | 1.41 ± 0.07 | 1.33 ± 0.03 | 3.59 | 8 | |
| HCl | 0.76 ± 0.11 | 1.22 ± 0.06 | 1.97 ± 0.20 | 3.94 | 9 | |
| H2O | 1.24 ± 0.01 | 1.23 ± 0.06 | 0.62 ± 0.02 | 3.10 | 7 | |
| Enzymatic saccharification | H2SO4 | 19.85 ± 0.03 | 1.56 ± 0.26 | 2.11 ± 0.07 | 23.53 | 54 |
| HNO3 | 15.04 ± 0.07 | 1.47 ± 0.11 | 1.37 ± 0.14 | 17.88 | 41 | |
| HCl | 13.03 ± 0.06 | 1.35 ± 0.01 | 1.91 ± 0.06 | 16.29 | 37 | |
| H2O | 10.03 ± 0.06 | 1.33 ± 0.01 | 0.94 ± 0.07 | 12.31 | 28 |
| Thermal Acid Hydrolysis and Enzymatic Saccharification | Glucose (g/L) | Galactose (g/L) | Mannitol (g/L) | Total Sugar (g/L) | EAE (%) |
|---|---|---|---|---|---|
| 0.05 L | 19.85 ± 0.03 | 1.56 ± 0.26 | 2.11 ± 0.07 | 23.52 | 53.58 |
| 5 L | 13.32 ± 0.05 | 5.72 ± 0.05 | 1.83 ± 0.01 | 20.87 | 47.54 |
| 500 L | 10.33 ± 0.04 | 5.18 ± 1.75 | 2.16 ± 0.14 | 17.67 | 40.24 |
| 0.05 L | 0.00 | 0.00 | 0.83 ± 0.48 | 10.06 | 0.43 |
| 5 L | 0.00 | 0.23 ± 0.01 | 0.62 ± 0.01 | 10.56 | 0.51 |
| 500 L | 0.00 | 1.05 ± 0.40 | 0.07 ± 0.01 | 7.88 | 0.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sunwoo, I.; Kim, Y.; Kim, J.; Cho, H.; Jeong, G.-T. Optimization, Scale-Up, and Economic Analysis of the Ethanol Production Process Using Sargassum horneri. Fermentation 2023, 9, 1004. https://doi.org/10.3390/fermentation9121004
Sunwoo I, Kim Y, Kim J, Cho H, Jeong G-T. Optimization, Scale-Up, and Economic Analysis of the Ethanol Production Process Using Sargassum horneri. Fermentation. 2023; 9(12):1004. https://doi.org/10.3390/fermentation9121004
Chicago/Turabian StyleSunwoo, InYung, Yoojin Kim, Jieun Kim, HyunJin Cho, and Gwi-Taek Jeong. 2023. "Optimization, Scale-Up, and Economic Analysis of the Ethanol Production Process Using Sargassum horneri" Fermentation 9, no. 12: 1004. https://doi.org/10.3390/fermentation9121004
APA StyleSunwoo, I., Kim, Y., Kim, J., Cho, H., & Jeong, G.-T. (2023). Optimization, Scale-Up, and Economic Analysis of the Ethanol Production Process Using Sargassum horneri. Fermentation, 9(12), 1004. https://doi.org/10.3390/fermentation9121004







